Abstract
Background
Both alloplastic 3-D dynamic titanium mesh implants and Autogenous split calvarial cortico-cancellous bone grafts have been extensively used for cranial defect reconstruction. Whether either method is procedurally, cosmetically or therapeutically superior to the other, has rarely been studied or evaluated.
Aim
The aim of the study was to objectively examine, assess, evaluate and compare the procedural ease, convenience, safety and versatility of cranioplasty performed using titanium mesh implants versus split calvarial grafts and to compare the intra- and post-operative complications encountered, and the cosmetic and therapeutic outcomes achieved using these two cranioplasty techniques.
Material and Methods
A retrospective analysis was carried out on 40 patients with large post-craniectomy defects, who underwent cranioplasty between 2012 and 2016. Twenty patients underwent cranioplasty with titanium mesh implants and 20 with split calvarial cortico-cancellous bone grafts. Post-operative follow-up ranged from 1 to 5 years and the patients were observed (clinically as well as by means of radiographs and CT scans) for cosmetic, functional and neurological improvements.
Results
Titanium mesh cranioplasty afforded more benefits, such as a shorter operating time, ease in manipulation, absence of donor-site morbidity, usefulness in previously infected or compromised recipient sites, absence of the risk of graft resorption or rejection, and a ready means to aspirate any post-operative epidural collection through its mesh structure. It also compared favorably when the cranial defects were large, owing to its, so to speak, limitless supply viz a viz, the relative paucity of harvestable split calvarial bone autograft.
Conclusion
Both modalities have their pros and cons. Split calvarial grafting is the more physiologic and less expensive option, useful for small- to medium-sized defects, while titanium mesh is the safer, more versatile, reliable and often preferred option, particularly when the cranial defects are large and also in severe head injury patients in whom harvesting calvarial bone could further compromise the already traumatized calvarium with possible stress fractures, further endangering its vital contents.
Keywords: Calvarial defect, Cranioplasty, Alloplasts, Autogenous grafts, Neurosensory deficits, Titanium mesh, Split thickness cortico-cancellous graft (CCG), Surgical site infection (SSI), Motor Trephine Syndrome (MTS), Extradural hematoma (EDH), Subdural hematoma (SDH)
Introduction
Cranioplasty is the surgical repair, reconstruction and replacement of a missing part of the cranium, thus restoring its shape, symmetry, contour and continuity, which is extremely important from a cosmetic as well as a psychosocial point of view. At the same time, it also restores the cranial shield or barrier, thus affording protection to the underlying brain that would otherwise be vulnerable to injury. In this way, cranioplasty brings about a morphological as well as functional rehabilitation of the cranial vault [1]. Large cranial defects also allow the atmospheric pressure to compress the unprotected intracranial contents giving it a typical kidney bean appearance on CT, and more importantly adversely affecting brain perfusion, leading to sensory-motor deficits and a variety of symptoms such as headache, dizziness, seizures, anxiety attacks, depression, memory loss and mood swings, all typical features of the “Motor Trephine” or “Sinking Skin Flap” syndrome [2].
Cranioplasty is a well-accepted neurosurgical procedure, which has applications in a wide range of post-craniectomy defects resulting from cases of cerebral decompressive procedures following Traumatic Brain Injury (TBI), ablative surgical resection of tumors, and so on. Continuing advances in cranioplasty techniques have enabled the repair of large and increasingly complicated calvarial defects; however, the optimal reconstructive material for different clinical scenarios still remains unclear and debateable.
Given the varied need for both autologous and synthetic cranial graft materials in different clinical situations and scenarios, it is important to compare and establish benefits and shortfalls of both and compare rates of procedural complications and post-operative short and long-term outcomes between these two major cranioplasty techniques [2–4].
Both alloplastic 3-D dynamic titanium mesh implants and Autogenous Split thickness cortico-cancellous calvarial bone grafts have been used extensively for cranial defect reconstruction. In the past, the choice of method has usually been subjective, based almost solely on the surgeon’s preference and the cost factor involved. It has remained unclear, however, whether one method is superior to the other, as neither retrospective studies nor prospective randomized trials to evaluate and compare the safety and efficacy of these two methods have ever been conducted. This study objectively examines, assesses and compares the ease and versatility in the intraoperative use of these two materials, the relative outcomes of cranioplasty performed using each of them; and also whether either method is procedurally, cosmetically or therapeutically superior to the other.
Material and Methods
A retrospective analysis of 40 patients, who had undergone cranioplasty between 2011 and 2016, was carried out. These patients had presented with large post-craniectomy defects of varying sizes (ranging from 17 cm × 11 cm to 9 cm × 7 cm) and at different locations, most of them involving the fronto-temporo-Parietal region of the cranium.
Twenty-eight of these patients had undergone decompressive craniectomy for intractable brain swelling, to relieve raised intracranial pressure (ICP), secondary to traumatic brain injury (TBI), and for evacuation of extradural/subdural/intracranial hematomas, with or without contusectomy of affected portions of the brain; three had sustained comminuted/depressed skull fractures from trauma, three were repeat cranioplasties in whom previous attempts at cranial reconstruction had failed due to various causes such as graft/implant rejection or surgical site infection (SSI), while the remaining six had undergone craniectomy either for ablative tumor resection (such as for cranial meningiomas) or for accessing intracranial brain tumors, including meningiomas and astrocytomas (Table 1). In addition to the obvious cosmetic deformity, these patients also exhibited a variety of neurological and sensorimotor deficits secondary to large cranial defects, including features of the “Motor Trephine/Sinking Skin Flap” Syndrome.
Table 1.
Causes of craniectomy defects and cranioplasty technique employed
| S. no. | Cause of cranial defect | No. of patients | % age of cases | Cranioplasty material/technique employed | |
|---|---|---|---|---|---|
| Titanium mesh implant | Split calvarial graft | ||||
| 1. | Decompressive craniectomy to relieve raised intracranial pressure and to evacuate EDH, SDH, and intracranial bleed and for contusectomy | 28 | 70 | 10 | 12 |
| 2. | Comminuted/depressed skull fractures from trauma | 3 | 07.5 | 2 | 2 |
| 3. | Previously failed cranioplasty | 3 | 07.5 | 3 | 1 |
| 4. | Ablative resection for tumors | 2 | 05 | 1 | 3 |
| 5. | To gain access to intracranial tumors | 4 | 10 | 4 | 2 |
| Total no. of patients | 40 | – | 20 | 20 | |
In this case series, 20 patients underwent titanium mesh cranioplasty, and the remaining 20 patients, cranioplasty with split calvarial cortico-cancellous bone grafts, and they were according placed in two Groups, Group A and Group B, respectively. Post-operative follow-up ranged from 1 to 5 years, and all patients were observed carefully (clinically and by means of radiographs and CT scans) for cosmetic, functional and neurological improvements.
Results
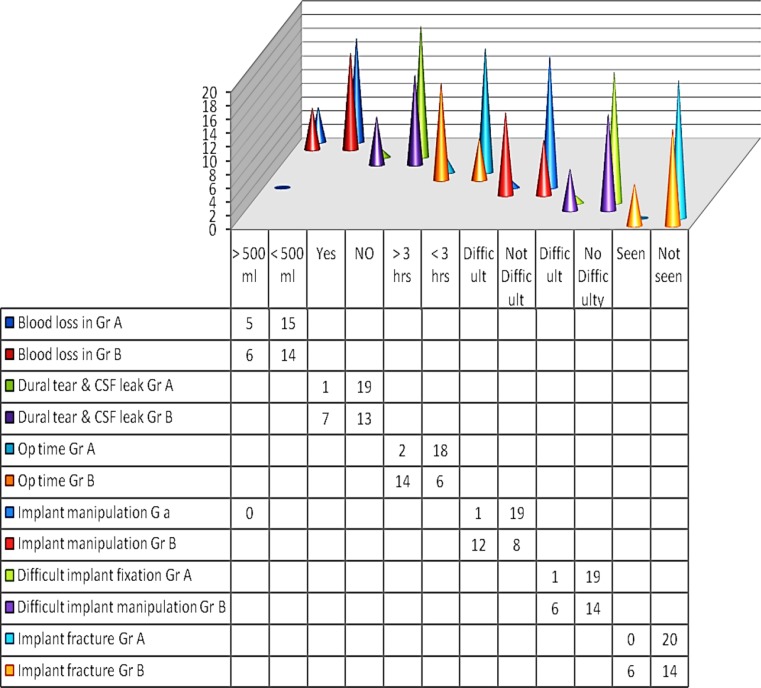
Cranioplasty performed using titanium mesh implants (Group A) demonstrated ease in implant manipulation, absence of any donor-site morbidity, fewer intraoperative risks and post-operative complications, much shorter operating time, quicker post-operative recovery period and predictably stable and long-lasting results, as compared to cranioplasty using split calvarial bone grafts (Group B) (Fig. 1).
Fig. 1.
Comparative analysis of intra- and post-operative complications in the two groups (Statistical Analysis—Kind courtesy Ashish Chakranarayan, INHS Kalyani, Visakhapatnam 530005)
In all patients but one, dural closure post-craniectomy, had been meticulously accomplished, and wherever there was destruction or dural loss, the dura mater had been reconstructed with Dural Substitute. In one patient of Group A, as the initial craniectomy had been carried out in an emergency, post-trauma scenario by an inexperienced General surgeon, the dura had not been adequately closed following craniectomy, which had led to adhesions developing between the brain tissue and the undersurface of the scalp flap (Fig. 2a–d). So, when this patient was taken up for cranioplasty 4 months later (defect size 11 cm × 9 cm), there was exposure of brain tissue with considerable hemorrhage (Fig. 2e–h). In such cases, where the situation is already compromised, titanium mesh cranioplasty was found to be the safest, quickest and most effective treatment option (Fig. 2i–l). Though the intracranial clots took some time to resolve postoperatively, there was no other adverse outcome and the cranioplasty was successful from both an esthetic as well as functional standpoint (Fig. 2m–p).
Fig. 2.
a–d A large fronto-temporo-parietal cranial defect resulting from a decompressive craniectomy carried out in an emergency post-trauma scenario. e, f Adhesions noted between the brain tissue and the overlying scalp flap at the time of cranioplasty, owing to inadequate dural repair and closure at the time of initial craniectomy. g, h 3-D dynamic titanium mesh implant cranioplasty, a quick and efficient means to restore the cranial defect in this compromised situation, following meticulous dural grafting. i–l Restoration of the cranial shield and a good calvarial contour evidenced on NCCT. m–p Cranioplasty a success, achieving both functional and morphological rehabilitation of the cranial vault
A 39-year-old male patient from Group A had reported with history of multiple surgical attempts at cranioplasty at different hospitals, for reconstruction of a large left-sided fronto-temporo-parietal cranial defect (defect size 17 cm × 11 cm). The patient had undergone decompressive craniectomy 2 years ago to relieve a subdural hemorrhage sustained in a road traffic accident (RTA). Two separate attempts at cranioplasty, carried out elsewhere, had failed. The first attempt at cranioplasty was by reimplantation of the autologous bone flap that had been stored in the subcutaneous pocket of his abdominal wall, which following cranioplasty, got infected resulting in subdural empyema, therefore necessitating its removal. The second attempt at cranioplasty repair of the defect was by using a large polymethyl methacrylate (PMMA) implant. When the patient reported to us, the implant had become dislodged, had descended down into the temporal region and was lying freely mobile under the scalp (Fig. 3a–d), thereby offering little neuroprotection for the cranial contents (Fig. 3e–h). The PMMA implant was removed, the area was thoroughly debrided and irrigated (Fig. 3i–l), and the defect was then restored with two overlapping titanium mesh implants (measuring 12 cm × 12 cm/144 cm2 each) bridging the large cranial defect (Fig. 3m–p). There was an excellent restoration of the calvarial contour (Fig. 3q–t), and esthetic and cosmetic results were gratifying with nil post-operative complications in the entire 4 years post-operative follow-up period, with no requirement for any further intervention (Fig. 3u–x).
Fig. 3.
a–d 39-year-old patient, with a history of multiple failed attempts at cranioplasty, presently reported with a freely mobile polymethylmethacrylate implant, which had become dislodged and had descended down into the temporal region. e–h NCCT showing the large fronto-temporo-parietal cranial defect with inadequate neuroprotection afforded by the PMMA implant. i–k Removal of the PMMA implant followed by thorough debridement and irrigation of the area. l–p A single titanium mesh implant found inadequate, so the defect was spanned bridged using two overlapping meshes. q–t NCCT confirming a good restoration of Calvarial contour as well structural integrity. u–x Gratifying esthetic results
A 33-year-old patient (Gp A) had undergone craniectomy for gaining access to a large intracranial meningioma (Fig. 4a–c). The excised bone flap had been preserved in the subcutaneous pocket of his abdominal wall for 9 months, before being reimplanted (Fig. 4c). However, considerable resorption then occurred all along its margins resulting in inadequate bridging of the 9 cm × 8 cm defect, leading to a residual deformity (Fig. 4d, e). So, the resorbed bone flap was removed and replaced with a titanium mesh, with excellent results (Fig. 4f–i).
Fig. 4.
a–c Craniectomy carried out in a 33-year-old patient to treat a large intracranial meningioma with preservation of the excised autologous bone flap in the subcutaneous pocket of his abdominal wall for 9 months prior to its replacement. d, e Resorbed bone flap resulting in a residual cranial deformity. f–i Resorbed bone flap removed and the defect successfully restored with a titanium mesh implant
A 34-year-old patient (Gp A) had undergone craniectomy to treat an astrocytoma. The resultant defect size was 9 cm × 7 cm. Autologous bone flap cranioplasty was performed 6 months later, using the excised cranial flap which had been stored in the subcutaneous pocket of his abdominal wall, following which he developed a surgical site infection (SSI) with abscess formation in the subgaleal plane (Fig. 5a–c). The bone flap was removed and discarded, the fluid collection was drained, the area was thoroughly debrided, and an immediate titanium mesh cranioplasty was successfully performed, with nil post-operative complications thereafter (Fig. 5d–f). A possible advantage of titanium mesh over bone flaps or grafts is its mesh structure which can permit aspiration of fluid collections such as hematomas or epidural effusions that could develop in the post-operative period, without having to remove the implant altogether.
Fig. 5.
a–d Surgical site infection (SSI) with abscess formation in the subgaleal plane following an autologous bone flap cranioplasty. e, f Fluid drained, bone flap removed and discarded, and area thoroughly debrided followed by titanium mesh cranioplasty, with no post-operative complications thereafter
A 50-year-old patient in Gp A had developed a left-sided hemiparesis and was confined to a wheelchair, following a Rt. fronto-temporo-parietal craniectomy to treat a spontaneous intracranial hemorrhage. In addition to the motor deficit, he had also developed symptoms such as headache, dizziness, seizures, anxiety attacks, depression, memory loss and mood swings, all typical features of the “Motor Trephine” Syndrome attributable to the large cranial defect. Effect of the atmospheric pressure on the unprotected intracranial contents gave it the typical kidney bean appearance on CT, further evidence of the “Sinking Skin Flap” syndrome. Titanium mesh was chosen for cranioplasty as the cranial defect was quite large (12 cm × 10 cm). Soon after cranioplasty, this patient began to regain strength in his left limbs and began to walk using a crutch. Titanium mesh cranioplasty serves not merely as a cosmetic procedure, the esthetic results being quite gratifying; in addition, it serves as a definite therapeutic procedure as well, by reversing the sensorimotor deficits and neurological deterioration characteristic of the syndrome.
Yet another patient, who had undergone a failed attempt at autologous bone flap cranioplasty, reported with a residual cranial defect (11.5 cm × 10 cm in size) (Fig. 6a–d). NCCT revealed resorbed fragments of the bone flap lying suspended over the cranial defect with the fixation implants in situ (Fig. 6e–h). The fragments were removed, the area was thoroughly debrided, and the cranial defect was restored with a single titanium mesh implant (Fig. 6i–l), thus successfully restoring both the structural integrity and the contour of the cranial vault (Fig. 6m–t).
Fig. 6.
a–d Failed autologous bone flap cranioplasty with a residual large fronto-temporo-parietal defect. e–h NCCT revealing resorbed fragments of the bone flap lying suspended over the cranial defect with fixation plates and screws in situ. i–l Bone pieces and implants removed and cranial defect restored using titanium mesh implant. m to t Excellent restoration of form and function following the titanium mesh cranioplasty
The spilt calvarial cortico-cancellous bone graft is typically harvested as narrow strips, around 7 cm long and 2 cm wide, to prevent them from cracking or fracturing (Fig. 7i–p). Then, multiple strips are laid across together to span the defect. As compared to the titanium mesh cranioplasty, it is a much more laborious and time-taking procedure, needing a great deal of surgeon skill, dexterity and experience (Fig. 7). The osteogenic potential and osteoinductive influence of the cortico-cancellous strips recruits and induces pluripotent cells of the dura and pericranium to differentiate into osteoblasts and to lay down bone in the gaps in between, with graft consolidation which takes about a year. A disadvantage is that, in this intervening period, the strength, rigidity and impact resistance of both, the donor as well as recipient sites are compromised. An attractive feature of split calvarial grafts is that the curvature of the grafts harvested has an excellent match with the contour desired at the defect site, hence producing excellent esthetic results restoring the shape, contour and symmetry of the head (Fig. 7q–aa). A drawback is that the quantity of Calvarial graft that can be harvested is limited and may be inadequate to restore large cranial defects.
Fig. 7.
a to h Large post-craniectomy defect. i to p Spilt calvarial cortico-cancellous bone graft typically harvested as narrow strips, which are then laid across the cranial defect. Osteogenic potential and osteoinductive influence of the CCG bring about bone fill and graft consolidation after a period of time. q to aa Close match between the contour of the donor and recipient sites, resulting in a good esthetic outcome following cranioplasty
In Group B patients, the incidence of solid fusion between the skull and bone grafts as well as that of abnormal resorption of the grafts, were evaluated at 6, 12, 18 and 24 months using follow-up imaging studies. These were also be evaluated in a comparative fashion with Group A patients, using the edge apposition of the titanium mesh implants, with the calvarial defect edges.
The largest cranial defect in this study is reconstructed using the 3-D dynamic titanium mesh implant measured 17 cm × 11 cm in dimension. Two titanium meshes (12 cm × 12 cm each) were overlapped and used to span and bridge the large cranial defect successfully. The largest cranial defect in this series is reconstructed using split thickness calvarial bone graft measured 10 cm × 7 cm in size.
Incidences of intra- and post-operative complications in the two groups were evaluated and compared (Table 2).
Table 2.
Evaluation of intraoperative complications in Group A patients (Y = Yes, N = No)
| Case no. | Cranial defect size | Blood loss (>500 ml) | Dural tear and CSF leak | Operating time (>3 h) | Difficulty in implant manipulation and contouring | Difficulty in implant fixation | Fracture of implant |
|---|---|---|---|---|---|---|---|
| 1. | 10 cm × 8 cm | N | N | N | N | N | N |
| 2. | 11 cm × 9 cm | N | N | N | N | N | N |
| 3. | 15 cm × 10 cm | N | N | N | N | N | N |
| 4. | 12 cm × 11 cm | N | N | N | N | N | N |
| 5. | 9 cm × 7 cm | Y | N | N | N | N | N |
| 6. | 10 cm × 8 cm | N | N | N | N | N | N |
| 7. | 7 cm × 6 cm | N | N | N | N | N | N |
| 8. | 11.5 cm × 10 cm | N | N | N | N | N | N |
| 9. | 17 cm × 11 cm | N | N | Y | Y | Y | N |
| 10. | 13 cm × 11 cm | N | Y | N | N | N | N |
| 11. | 9 cm × 7 cm | N | N | N | N | N | N |
| 12. | 11 cm × 9 cm | N | N | N | N | N | N |
| 13. | 10 cm × 8 cm | N | N | N | N | N | N |
| 14. | 9 cm × 8 cm | N | N | N | N | N | N |
| 15. | 11 cm × 7 cm | N | N | Y | N | N | N |
| 16. | 10 cm × 8 cm | N | N | N | N | N | N |
| 17. | 9 cm × 5 cm | N | N | N | N | N | N |
| 18. | 10 cm × 8 cm | N | N | N | N | N | N |
| 19. | 13 cm × 5 cm | N | N | N | N | N | N |
| 20. | 10 cm × 7 cm | N | N | N | N | N | N |
Discussion
An ideal cranioplasty material should be biocompatible, inert, non-thermo conductive, radio-transparent, nonmagnetic, lightweight, rigid, easily applicable and inexpensive. No graft or implant material qualifies all these prerequisites in totality. However, autogenous split calvarial grafts and alloplastic titanium mesh implants have proved their worth as the best choices in the world literature [5, 6].
The calvarial bone is somewhat like a sandwich, composed of two parallel layers of cortical bone separated by a layer of cancellous bone called diploe. The outer table can be safely harvested [7], by splitting along the diploe, leaving the inner table intact. Calvarial cortico-cancellous bone supplies living, immunocompatible bony cells that integrate fully with the skull bone [8, 9]. The graft’s numerous desirable characteristics include its osteoconductive and osteoinductive capability, excellent contour, close proximity to the surgical field, and reasonable amounts of harvestable bone. Its inherent advantages of perfect histocompatibility and the ability to be incorporated and integrated as biologically active and dynamic living tissue with potential for revascularization and consolidation allows its successful use in previously infected or other compromised recipient sites [10]. It is the more physiologic and less expensive option [11] as compared with alloplastic implants such as 3-D dynamic titanium mesh [12].
Nevertheless, 3-D titanium mesh implant has always been a very close competitor, whose biocompatibility and property to osseointegrate often makes it preferable even to autologous bone flaps and split calvarial grafts. Although an alloplast, hence a foreign body, the titanium mesh implant possesses an inert nature and excellent biocompatibility and corrosion resistance. It demonstrates durability with no fear of resorption, rejection or infection, features which are often noted with bone flaps and grafts. Being a non-ferromagnetic metal, titanium is CT and MRI safe, causing hardly any scatter, artifacts or image degradation; and its special radiolucency permits safe imaging of the brain in long-term follow-ups (Figs. 2i–l; 3q–t; 6m–p; 8c–h). Although it is lightweight, titanium mesh is sufficiently rigid to maintain the calvarial contour and has adequate impact resistance to provide protection to the cranial contents. The operating time is short, with minimal blood loss, eliminating the need for blood transfusions. The recovery period is quick, and results are stable and long lasting.
Fig. 8.
a to h Titanium mesh, an ideal reconstructive material for the cosmetically and functionally challenging forehead region, providing a smooth surface and even contour, with no fear of graft resorption or loss
Titanium mesh cranioplasty can serve not only as a merely cosmetic procedure, but as a definite therapeutic procedure as well, by reversing the subjective and objective features of the MTS, bringing about a definite reversal in the neurological deterioration and sensorimotor deficits that often develop following large craniectomy procedures. By restoring the cranial defect, split calvarial grafting too can reverse pre-cranioplasty sensorimotor and neurological deficits [13].
The 3-D dynamic titanium mesh implant is easily and quickly moldable and ready to apply over the cranial defect once the defect is exposed, involving a comparatively simple, speedy and straightforward surgical procedure. Its malleable and pliable nature makes it easy to handle, manipulate and adapt to the desired shape and contour intraoperatively. It can easily be cut into the desired shape and size and fixed using titanium micro-screws. On the other hand, the split calvarial graft is relatively much more laborious and time taking to harvest, needing a great deal of surgeon’s skill, dexterity, care and experience. The split calvarial strips cannot be bent or contoured and being quite delicate, can fracture while harvesting, handling and fixing, if meticulous care and attention to detail is not given.
Titanium mesh cranioplasty is associated with much fewer intraoperative risks, complications and difficulties. On the other hand, split calvarial grafting is often accompanied by risks such as fracture of inner cortical table of the donor site, dural tear and breach, CSF leak and intracranial hemorrhage. The split calvarial grafts are usually harvested from the cranium from the side contralateral to the defect. There is the risk of propagating and radiating stress fractures of the calvarium, at the time of harvesting these grafts, especially in previously traumatized and compromised head injury cases (Tables 3, 4, 5, 6, 7).
Table 3.
Evaluation of intraoperative complications in Group B patients (Y = Yes, N = No)
| Case no. | Cranial defect size | Blood loss (>500 ml) | Dural tear and CSF leak | Operating time (>3 h) | Difficulty in graft harvesting and manipulation | Difficulty in graft fixation | Fracture of graft strip/s |
|---|---|---|---|---|---|---|---|
| 1. | 7 cm × 8 cm | Y | N | Y | Y | Y | Y |
| 2. | 5 cm × 4 cm | N | N | N | N | N | N |
| 3. | 5 cm × 4 cm | N | N | Y | Y | Y | N |
| 4. | 8 cm × 6 cm | N | N | N | N | N | Y |
| 5. | 9 cm × 7 cm | Y | Y | Y | Y | Y | Y |
| 6. | 10 cm × 7 cm | N | Y | Y | Y | Y | Y |
| 7. | 7 cm × 6 cm | N | N | Y | Y | Y | N |
| 8. | 5 cm × 4 cm | N | N | Y | N | N | N |
| 9. | 7 cm × 5 cm | N | N | Y | Y | Y | N |
| 10. | 8 cm × 9 cm | N | Y | N | N | N | N |
| 11. | 9 cm × 7 cm | N | N | Y | Y | Y | N |
| 12. | 10 cm × 8 cm | Y | Y | Y | Y | Y | N |
| 13. | 10 cm × 7 cm | Y | Y | N | N | Y | Y |
| 14. | 9 cm × 8 cm | N | N | Y | Y | Y | N |
| 15. | 8 cm × 7 cm | N | N | Y | Y | N | N |
| 16. | 10 cm × 7 cm | Y | Y | Y | Y | Y | Y |
| 17. | 9 cm × 5 cm | N | N | N | N | Y | N |
| 18. | 8 cm × 7 cm | Y | Y | Y | Y | Y | N |
| 19. | 5 cm × 4 cm | N | N | N | N | N | N |
| 20. | 6 cm × 5 cm | N | N | Y | Y | Y | N |
Table 4.
Evaluation of post-operative complications in Group A patients (Y = Yes, N = No)
| Case no. | Unsatisfactory esthetic result due to mild flattening of contour | Lack of edge apposition with visible/palpable gaps between implant and defect edge | Irregular contour of reconstructed site | Extrusion/displacement/mobility of implant | Surgical site infection (SSI) | Deterioration of esthetic result with time (due to implant rejection) | Continuing/increased sensorimotor deficits | Donor site morbidity |
|---|---|---|---|---|---|---|---|---|
| 1. | N | N | N | N | N | N | N | N |
| 2. | N | N | N | N | N | N | N | N |
| 3. | N | N | N | N | N | N | N | N |
| 4. | N | N | N | N | N | N | N | N |
| 5. | N | N | N | N | N | N | N | N |
| 6. | N | N | N | N | N | N | N | N |
| 7. | N | N | N | N | N | N | N | N |
| 8. | Y | N | Y | N | N | N | N | N |
| 9. | N | N | N | N | N | N | Y | N |
| 10. | N | N | N | N | N | N | N | N |
| 11. | Y | N | N | N | N | N | N | N |
| 12. | N | N | N | N | N | N | N | N |
| 13. | N | N | N | N | N | N | N | N |
| 14. | N | N | N | N | N | N | N | N |
| 15. | Y | N | N | N | N | N | N | N |
| 16. | N | N | N | N | N | N | N | N |
| 17. | N | N | N | N | N | N | N | N |
| 18. | N | N | N | N | N | N | N | N |
| 19. | Y | N | N | N | N | N | N | N |
| 20. | N | N | N | N | N | N | N | N |
Table 5.
Evaluation of post-operative complications in Group B patients (Y = Yes, N = No)
| Case no. | Unsatisfactory esthetic result | Lack of edge apposition with visible/palpable gaps between graft and defect edges | Irregular contour of reconstructed site | Extrusion/displacement/mobility of graft | Surgical site infection (SSI) | Deterioration of esthetic result with time (due to Graft resorption/rejection) | Continuing/increased sensorimotor deficits | Donor site morbidity |
|---|---|---|---|---|---|---|---|---|
| 1. | N | N | N | N | N | N | N | Y |
| 2. | Y | Y | Y | N | N | N | N | Y |
| 3. | N | N | N | N | N | N | N | Y |
| 4. | N | Y | Y | N | N | Y | N | Y |
| 5. | N | N | N | N | N | N | Y | Y |
| 6. | N | N | Y | N | N | N | N | Y |
| 7. | N | N | N | N | N | N | N | Y |
| 8. | Y | Y | Y | Y | Y | Y | Y | Y |
| 9. | N | N | Y | N | N | N | N | Y |
| 10. | Y | Y | Y | N | N | N | Y | Y |
| 11. | N | N | N | N | N | N | N | Y |
| 12. | Y | Y | Y | Y | N | Y | Y | Y |
| 13. | N | N | N | N | N | N | N | Y |
| 14. | N | Y | Y | N | N | Y | N | Y |
| 15. | Y | N | N | N | N | N | N | Y |
| 16. | N | Y | Y | N | Y | Y | Y | Y |
| 17. | N | N | N | N | N | N | N | Y |
| 18. | N | N | Y | N | N | Y | N | Y |
| 19. | Y | Y | Y | N | N | N | N | Y |
| 20. | N | N | N | N | N | N | N | Y |
Table 6.
Comparative evaluation of intra- and post-operative complications in the two groups
| Complications | 3-D dynamic titanium mesh cranioplasty (20 patients) | Spit thickness calvarial bone grafting (20 patients) | |||
|---|---|---|---|---|---|
| Number of pts | % age of pts | Number of pts | % age of pts | ||
| (A) Intraoperative complications | |||||
| 1. | Hemorrhage/excessive intraoperative blood loss (>500 ml) | 01 | 05 | 06 | 30 |
| 2. | Prolonged operating time (>3 h) | 02 | 10 | 14 | 70 |
| 3. | Dural tear with CSF leak | 01 | 05 | 07 | 35 |
| 4. | Implant/graft fracture | 0 | 0 | 06 | 30 |
| 5. | Difficulty in implant/graft manipulation | 01 | 05 | 13 | 65 |
| 6. | Difficulty in implant/graft fixation | 01 | 05 | 14 | 70 |
| (B) Post-operative complications | |||||
| 1. | Unsatisfactory esthetic result | 03 | 15 | 06 | 30 |
| 2. | Lack of edge apposition with visible/palpable gaps between graft/implant and defect edge | 0 | 0 | 08 | 40 |
| 3. | Irregular contour of reconstructed site | 01 | 05 | 11 | 55 |
| 4. | Extrusion/displacement/mobility of implant/graft | 0 | 0 | 02 | 10 |
| 5. | Surgical site infection (SSI) | 0 | 0 | 02 | 10 |
| 6. | Deterioration of esthetic result with time (due to implant rejection/graft resorption) | 0 | 0 | 06 | 30 |
| 7. | Continuing/increased sensorimotor deficits | 01 | 05 | 05 | 25 |
| 8. | Increased vulnerability of remaining part of cranium due to donor site morbidity | 0 | 0 | 20 | 100 |
Table 7.
Statistical analysis (Kind courtesy Ashish Chakranarayan, INHS Kalyani, Visakhapatnam 530005 AP)
| Statistics | Group A | Group B |
|---|---|---|
| Mean | 90.60 | 50.7500 |
| Std. deviation | 36.582 | 2.04602E1 |
| Std. error of mean | 8.180 | 4.57503 |
The data were analyzed using SPSS 16.0 for Windows. At 95% confidence interval, the mean defect size for Group A was 90.60 cm sq, with a standard deviation of 36.582 and the standard error of mean was 8.180, whereas the mean for the Group B was 50.75 cm sq and the standard deviation was 2.046 with the standard error of mean at 4.575
Ease in intraoperative modeling of the titanium mesh helps to establish an appropriate curvature of the implant matching with the rest of the cranial contour, thus producing excellent esthetic results. On occasions, a mild flattening of the head contour is noted especially if the defect is large and the mesh has been stretched across it without adequate contouring, compromising the convexity in its shape. In such cases where the defect is very large, two meshes may be overlapped to span the defect, with good results. Split calvarial grafts, although rigid and non-malleable, provide an excellent and well-matched contour to the restored defect as the donor site is usually the opposite side of the cranium. Hence, the symmetry, shape and contour of the head are restored.
Although not as rigid as bone itself, the titanium mesh implant is sufficiently rigid to maintain the calvarial contour and provides adequate protection to the cranial contents. Its impact resistance, though not been tested, is probably less than that of an acrylic plate or skull bone. The integrity and neuroprotective properties of titanium mesh implants have been demonstrated to be more than autologous bone flaps [2]. As there is no donor site, there is no compromise of the other areas of the skull. The split calvarial graft, on the other hand, comprises of strips of the outer table of the calvarial bone harvested from the opposite side of the cranium; hence, its strength and impact resistance is definitely less [14]. The transposed strips of bone take years to regenerate and consolidate, till which time rigidity and strength of the reconstructed site is quite reduced. Further, it compromises strength of calvarial bone at the donor site as well [15].
Calvarial cortico-cancellous bone supplies living, immunocompatible bone cells that integrate fully with the skull bone, although these grafts may take years to fully consolidate. Their potential for revascularization and subsequent integration and consolidation allows their successful use in previously infected or other compromised recipient sites [16, 17].The 3-D titanium mesh implants, being totally inert and biocompatible, fully integrating with the rest of the body tissues, are the much simpler but equally effective option for use in previously infected or other compromised recipient sites. In fact, owing to their mesh structure, they also allow aspiration of any epidural collections or hematomas developing thereafter, without having to remove the implant altogether.
Titanium mesh implants can be used comfortably to reconstruct even large calvarial defects. Either the larger 20 × 20 cm meshes can be used or two meshes of 12 × 12 cm dimension can be overlapped and spanned over extensive defects comfortably. When significantly large-sized cranial defects are present, the alloplastic implants are the material of choice as they are available in a limitless supply. Harvestable calvarial cortico-cancellous bone is usually available only in limited quantities, and owing to their relative paucity, may not be able to restore large calvarial post-craniectomy defects.
Long-term results following titanium mesh cranioplasty are stable and reliable, and these implants can be used confidently for large craniectomy defects without fear of resorption or surgical site infection (SSI), unlike what is often seen with autologous bone flaps and grafts. Bone graft resorption is a disastrous and often frequent late post-operative complication of cortico-cancellous grafting (CCG).
Cranioplasty of the frontal region can be cosmetically and functionally challenging, the forehead being the dominant and most noticeable part of the face. Also skin of the forehead is thin, delicate and exposed to view, unlike the scalp which is thick and hidden by the hair. Cranioplasty or onlay grafting using split calvarial bone grafts may not be suitable in this region due to the irregular and uneven surface caused by the bone strips. Another possible complication is the risk of bone graft resorption, leading to forehead contour irregularities, so titanium mesh is the better option providing a smooth and even contour, with assured long-term stability (Fig. 8).
An added advantage of titanium mesh is its mesh structure, which can permit aspiration of any epidural collections or hematomas that might develop in the post-operative period, without having to remove the implant altogether. This is not possible with bone grafts.
Titanium mesh cranioplasty demonstrates predictable long-term durability, and stable long-lasting results, with little risk of graft displacement, dislodgement, rejection or resorption. Resorption of the bone strips and failure of graft consolidation often compromises long-term stability and durability of split calvarial grafting [18]. Although Autologous split calvarial bone is a more physiologic option as compared with the titanium mesh, graft resorption and failure of consolidation between the skull and the graft was observed on long-term follow-up in many patients of Group B in this case series.
Being a relatively simple and straightforward surgical procedure, titanium mesh cranioplasty involves a much shorter operating time, less intraoperative blood loss with no need for blood transfusions. The post-operative period for recovery is quicker. As the surgical procedure of split calvarial grafting is much more time taking and laborious, with more blood loss and intraoperative risks involved, post-operative recovery may be correspondingly longer.
Restoring the integrity of the cranial vault structure allows better dissipation of traumatic energy. Titanium mesh implant allows this to be done without compromising the structural integrity of the remaining part of the cranial structure and there is nil donor site morbidity. In sharp contrast, harvesting autogenous calvarial bone graft can potentially weaken the donor site making it vulnerable to trauma. Deformational stresses have been shown to accumulate around donor site defect edges and can serve as seed points for calvarial fractures at much lower energy levels as compared with untouched/untampered with calvarial bone [19].
In difficult scenarios, such cases complicated by post-craniotomy surgical site infection (SSI), infected or compromised recipient sites; or in cases of failed cranioplasties due to either foreign body reactions or rejection of the graft/implant, implant removal and debridement followed by immediate titanium mesh cranioplasty is advocated. This provides an effective, efficient, safe and stable outcome with a permanent resolution no recurrence of infection [19]. In such cases, split calvarial grafting would not be a viable option, for fear of graft infection and resorption as well as risk of a fulminant infection due to prolonged operating time in an already infected site [20].
Conclusion
This retrospective study brings out the fact that though both split calvarial graft and titanium mesh are viable options for reconstruction of a calvarial bone defect, the choice of the reconstructive material to be used, has to be carefully decided based on factors like experience of the surgeon, suitability of the donor site, size/location of the recipient site, availability of materials/equipment etc. Although in the study the author has reconstructed defects as large as 80 sq cm using the split calvarial graft, however, based on the associated risks and intra/post-operative complications, it is recommended that the defect of 70 sq cm or less may be relatively safely taken up for reconstruction using this modality. This size value is not sacrosanct, and the choice of procedure would ultimately depend on the factors cited above based on the merits of the case. However, for larger defects, where distraction osteogenesis is not possible, titanium mesh cranioplasty has been observed to be the safer, versatile, long-lasting and operator friendly alternative for the kind of defects cited in the study.
Acknowledgements
Statistical Analysis (Kind courtesy Ashish Chakranarayan, INHS Klayani).
Compliance with Ethical Standards
Conflict of interest
The author of this article has not received any research grant, remuneration, or speaker honorarium from any company or committee whatsoever, and neither owns any stock in any company. The author declares that she does not have any conflict of interest.
Human and Animal Rights Statement
All procedures performed on the patients (human participants) involved were in accordance with the ethical standards of the institution and/or national research committee, as well as with the 1964 Helsinki declaration and its later amendments and comparable ethical standards.
Ethical Approval
This article does not contain any new studies with human participants or animals performed by the author.
Informed Consent
Informed consent was obtained from all the individual participants in this study.
References
- 1.Aciduman A, Belen D. The earliest document regarding the history of cranioplasty from the Ottoman era. Surg Neurol. 2007;68:349–353. doi: 10.1016/j.surneu.2006.10.073. [DOI] [PubMed] [Google Scholar]
- 2.Wallace RD, Craig MD, Konofaos P. Comparison of autogenous and alloplastic cranioplasty materials following impact testing. J Craniofac Surg. 2015;26:1551–1557. doi: 10.1097/SCS.0000000000001882. [DOI] [PubMed] [Google Scholar]
- 3.Klinger DR, Madden C, Beshav J, White J, Gambrell K, Rickert K. Autologous and acrylic cranioplasty: a review of 10 years and 258 cases. World Neurosurg. 2014;82(3–4):e525–e530. doi: 10.1016/j.wneu.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Al Tamimi YZ, Sinha P, Trivedi M, et al. Comparison of acrylic and titanium cranioplasty. Br J Neurosurg. 2012;26:510–513. doi: 10.3109/02688697.2011.633640. [DOI] [PubMed] [Google Scholar]
- 5.Bogris Elephterios N, Chiriac DA. Titanium mesh cranioplasty for patients with large cranial defects—technical notes. Rom Neurosurg. 2010;17:456–460. [Google Scholar]
- 6.Prolo DJ, Oklund SA. The use of bone grafts and allo-plastic materials in cranioplasty. Clin Orthop Relat Res. 1991;268:270–278. [PubMed] [Google Scholar]
- 7.Casanova R, Cavalcante D, Grotting JC, Vasconez LO, Psillakis JM. Anatomic basis for vascularized outertable calvarial bone flaps. Plast Reconstr Surg. 1986;78:300–308. doi: 10.1097/00006534-198609000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Agrawal A, Garg LN. Split calvarial bone graft for the reconstruction of skull defects. J Surg Tech Case Rep. 2011;3:13–16. doi: 10.4103/2006-8808.78465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Artico M, Ferrante L, Pastore FS, Ramundo EO, Cantarelli D, Scopelliti D. Bone autografting of the calvaria and craniofacial skeleton: historical background, surgical results in a series of 15 patients, and review of the literature. Surg Neurol. 2003;60:71–79. doi: 10.1016/S0090-3019(03)00031-4. [DOI] [PubMed] [Google Scholar]
- 10.Inoue A, Satoh S, Sekiguchi K, Ibuchi Y, Katoh S, Ota K. Cranioplasty with split thickness calvarial bone. Neurol Med Chir (Tokyo) 1995;35:804–807. doi: 10.2176/nmc.35.804. [DOI] [PubMed] [Google Scholar]
- 11.Hayward RD. Cranioplasty: don’t forget the patient’s own bone is cheaper than titanium. Br J Neurosurg. 1999;13:490–491. doi: 10.1080/02688699908540624. [DOI] [PubMed] [Google Scholar]
- 12.Tessier P. Autogenous bone grafts taken from the calvarium for facial and cranial applications. Clin Plast Surg. 1982;9:531–538. [PubMed] [Google Scholar]
- 13.Alibhai MK, Balasundaram I, Bridle C, Holmes SB. Is there a therapeutic role for cranioplasty? Int J Oral Maxillofac Surg. 2013;42:559–561. doi: 10.1016/j.ijom.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Kline RM, Jr, Wolfe SA. Complications associated with the harvesting of cranial bone grafts. Plast Reconstr Surg. 1995;95:5–13. doi: 10.1097/00006534-199501000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Prolo DJ, Burres KP, McLaughlin WT, Christensen AH. Autogenous skull cranioplasty: fresh and preserved (frozen), with consideration of the cellular response. Neurosurgery. 1979;4:18–29. doi: 10.1227/00006123-197901000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Artico M, Ferrante L, Pastore FS, Ramundo EO, Cantarelli D, Scopelliti D. Bone autografting of the calvaria and cran-iofacial skeleton: historical background, surgical results in a series of 15 patients, and review of the literature. Sur Neurol. 2003;60:71–79. doi: 10.1016/S0090-3019(03)00031-4. [DOI] [PubMed] [Google Scholar]
- 17.Valentini V, Cassoni A, Marianetti TM. Reconstruction of craniofacial bony defects using autogenous bone grafts: a retrospective study on 233 patients. J Craniofac Surg. 2007;18:953–958. doi: 10.1097/scs.0b013e3180690123. [DOI] [PubMed] [Google Scholar]
- 18.Kuttenberger JJ, Hardt N. Longterm results following reconstruction of craniofacial defects with titanium micromesh systems. J Craniomaxillofac Surg. 2001;29:75–81. doi: 10.1054/jcms.2001.0197. [DOI] [PubMed] [Google Scholar]
- 19.Fares B, Christian EA. Biomechanical effects of cranioplasty. J craniofac Surg. 2012;23:e152–e153. doi: 10.1097/SCS.0b013e31824cdc0d. [DOI] [PubMed] [Google Scholar]
- 20.Scloeker B, Trummer M. Prediction parameters of bone flap resorption following cranioplasty with autologous bone. Clin Neurol Neurosurg. 2014;120:64–67. doi: 10.1016/j.clineuro.2014.02.014. [DOI] [PubMed] [Google Scholar]