Tokarz et al. review the cell biology of insulin physiology throughout the body, from synthesis to the delivery, action, and final degradation of insulin.
Abstract
Insulin is the paramount anabolic hormone, promoting carbon energy deposition in the body. Its synthesis, quality control, delivery, and action are exquisitely regulated by highly orchestrated intracellular mechanisms in different organs or “stations” of its bodily journey. In this Beyond the Cell review, we focus on these five stages of the journey of insulin through the body and the captivating cell biology that underlies the interaction of insulin with each organ. We first analyze insulin’s biosynthesis in and export from the β-cells of the pancreas. Next, we focus on its first pass and partial clearance in the liver with its temporality and periodicity linked to secretion. Continuing the journey, we briefly describe insulin’s action on the blood vasculature and its still-debated mechanisms of exit from the capillary beds. Once in the parenchymal interstitium of muscle and adipose tissue, insulin promotes glucose uptake into myofibers and adipocytes, and we elaborate on the intricate signaling and vesicle traffic mechanisms that underlie this fundamental function. Finally, we touch upon the renal degradation of insulin to end its action. Cellular discernment of insulin’s availability and action should prove critical to understanding its pivotal physiological functions and how their failure leads to diabetes.
Introduction
Preceded by valiant efforts in Berlin, Strasbourg, Baltimore, and Bucharest, insulin was discovered in Toronto in 1921 by Fredrick Banting and Charles Best, with auspicious advice and support from John Macleod, and its purification was made possible by James Collip. The story of its discovery is legendary and was awarded the Nobel Prize in Physiology or Medicine in 1923 (Karamitsos, 2011), but the journey of this hormone in the body has not been “romanced” as much. Insulin is the paramount anabolic hormone (promoting dietary carbon source deposition), and its synthesis, quality control, delivery, and action are exquisitely regulated in different organs or “stations” of its bodily journey. These functions are enacted by highly orchestrated intracellular mechanisms, starting with production in the β-cells of the pancreas, on to its partial clearance by the liver hepatocytes, followed by its delivery and action on the vascular endothelium and its functions at level of the brain, muscle fibers, and adipocytes (major action sites), and ending with insulin degradation in the kidney. As such, the journey of insulin in the body is a superb example of integrated cellular physiology.
In this Beyond the Cell review, we focus on five stages of the journey of insulin through the body and the captivating cell biology that underlies its connections with each organ. We analyze insulin’s biosynthesis in and release from β-cells of the pancreas, its first pass and partial clearance in the liver, its action on the blood vasculature and exit from the capillary beds, its action in the central nervous system in brief, followed by its stimulation of muscle and adipose cell glucose uptake, and its degradation in the kidney to finalize its action (Fig. 1).
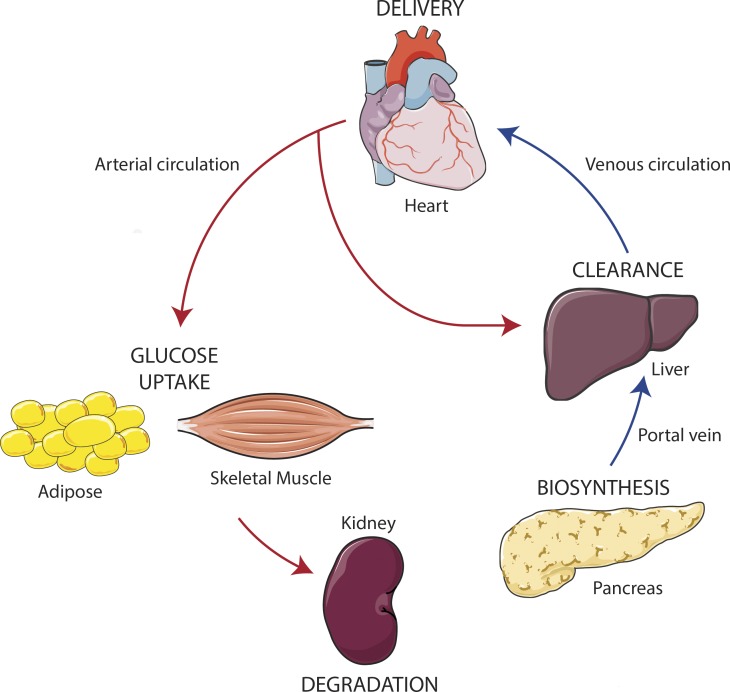
Figure 1.
Journey of insulin in the body. Insulin is transcribed and expressed in the β-cells of the pancreas, from whence it is exported through the portal circulation to the liver. During this first pass, over 50% of insulin is cleared by the hepatocytes in the liver. The remaining insulin exits the liver via the hepatic vein, where it follows the venous circulation to the heart. Insulin is distributed to the rest of the body through the arterial circulation. Along the arterial tree, insulin promotes vasodilation. Arterially delivered insulin exerts its metabolic actions in the liver and is further cleared (second pass). Insulin exits the circulation at the level of the microvasculature, reaching muscle and fat cells, where it stimulates GLUT4 translocation and glucose uptake. Remaining circulating insulin is delivered to and finally degraded by the kidney. This review analyzes the cellular processes at each stage of this journey. This figure was created using Servier Medical Art (available at https://smart.servier.com/).
By necessity, many aspects of the metabolic actions of insulin are not reviewed here; rather, we present the most current picture of each phenomenon, highlighting up-to-date concepts and spatial-temporal coordinates. By applying a cell biology lens to the five fundamental stages in insulin’s journey in the body, we hope to render an integrated view of insulin “within and beyond the cell.” Of major relevance, though not individually discussed here, defects in each station of the hormone’s journey in the body have been correlated and often causally related to insulin resistance, hypertension, and type 2 diabetes (Taniguchi et al., 2006; Hoehn et al., 2008; Odegaard and Chawla, 2013; Boucher et al., 2014; DeFronzo et al., 2015; Samuel and Shulman, 2016; Haeusler et al., 2018; also see other important highlights in the text box).
Selected examples of mechanistic defects in the five stages of the journey of insulin, associated with insulin resistance and type 2 diabetes
• Defective insulin exocytosis from diabetic β-cells (Ferdaoussi and MacDonald, 2017; Gandasi et al., 2017) and impaired pulsatile secretion of insulin in diabetic individuals (Lang et al., 1981; Hollingdal et al., 2000; Laedtke et al., 2000)
• Reduced hepatic insulin clearance (Jung et al., 2018) and CEACAM1 expression (Lee, 2011) in obesity
• Impaired vasoactive effects of insulin during insulin resistance, including capillary recruitment (de Jongh et al., 2004; Clerk et al., 2006; Keske et al., 2009); reduced insulin delivery to muscle in obesity and diabetes (Broussard et al., 2016)
• Diminished GLUT4 translocation to the muscle membrane in diabetic rodents and humans (Klip et al., 1990; Zierath et al., 1996; Garvey et al., 1998; Hoehn et al., 2008; Czech, 2017) and lowered expression of Rac1 (Sylow et al., 2013) as well as a number of proteins of the GLUT4 vesicle fusion machinery (Aslamy and Thurmond, 2017); the underlying defects include alterations in the maintenance of the storage compartment (Foley et al., 2011; Samuel and Shulman, 2012) and in the insulin-derived signals that trigger GLUT4 vesicle release from storage and interaction with the plasma membrane
• Compromised glomerular function in obesity (Kanasaki et al., 2013) that may alter insulin bioavailability; sodium retention and down-regulation of the natriuretic peptide system in insulin resistance (Spoto et al., 2016) that may herald hypertension
Biosynthesis and export of insulin in pancreatic β-cells
Insulin synthesis, processing, and packaging in pancreatic β-cells
Humans have a single insulin gene, INS (rodents have two, ins1 and ins2), located on chromosome 11, the transcription of which is controlled largely by upstream enhancer elements that bind key transcription factors that include IDX1 (PDX1), MafA, and NeuroD1 along with numerous coregulators (Artner and Stein, 2008). In the insulin-producing pancreatic β-cells, these are required for insulin gene expression and contribute to the regulation of INS transcription in response to glucose and autocrine insulin signaling (Andrali et al., 2008). Given the role of these enhancer elements, transcription factors, and their coregulators in controlling the expression of insulin and many additional components of the β-cell secretory pathway, such as glucose transporter 2 (GLUT2) and the insulin processing enzyme PC1/3, they are key defining contributors to the establishment and maintenance of β-cell identity (Gao et al., 2014).
Insulin is translated initially as a preproinsulin (Fig. 2 A), which is then processed to proinsulin in the RER upon cleavage of its signal sequence by a signal peptidase. In the RER, proinsulin is folded and stabilized in its 3D proinsulin configuration, linking the semihelical A domain and helical B domain via the formation of three disulfide bonds. After transit to the Golgi apparatus, the properly folded proinsulin is sorted into still-immature secretory granules where it is processed via the prohormone convertases PC1/3 and PC2, which cleave the C-peptide. Subsequently, carboxypeptidase E removes C-terminal basic amino acids from the resulting peptide chains, yielding mature insulin consisting of A- and B-peptide chains linked by disulfide bonds (Hutton, 1994).
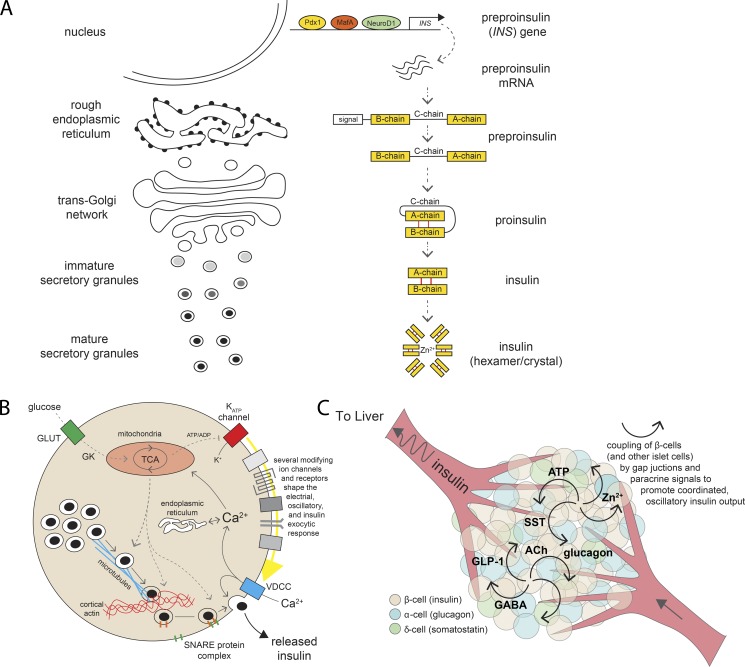
Figure 2.
Insulin biosynthesis and secretion. (A) Insulin maturation along the granule secretory pathway. Preproinsulin mRNA is transcribed from the INS gene and translated to preproinsulin peptide. As this transits through the RER and TGN, the prepropeptide is processed to its mature form and ultimately stored as hexameric insulin/Zn2+ crystals within mature secretory granules. (B) Glucose sensing and metabolic signals leading to insulin granule secretion. The release of insulin via exocytosis of secretory granules from pancreatic β-cells is controlled by a series of metabolic and electrical signals arising as a result of glucose entry through GLUTs, phosphorylation by GK, and entry into the TCA cycle. The closure of ATP-dependent K+ (KATP) channels triggers electrical events that culminate in Ca2+ entry through voltage-dependent Ca2+ channels (VDCCs), which triggers exocytosis mediated by SNARE complex proteins. The overall secretory response is modulated by numerous receptors, channels, intracellular Ca2+ stores, metabolic signals, and cytoskeletal elements. (C) Islet communication for coordinated pulsatile insulin secretion. Within an islet, β-cells communicate with each other and with glucagon-producing α-cells and somatostatin (SST)–producing δ-cells to coordinate their activity. Many putative intraislet messengers have been implicated, including ATP, Zn2+, γ-aminobutyric acid (GABA), glucagon-like peptide-1 (GLP-1), acetylcholine (ACh), and others. These, along with electrical coupling via gap junctions, are likely important for the physiological coordination of pulsatile insulin secretion.
Transit of immature secretory granules through the TGN, and their subsequent budding and maturation, is controlled by a host of regulatory proteins, including newly identified vesicle-sorting by proteins such as SORCS1 (Kebede et al., 2014) and HID-1 (Du et al., 2016). Insulin biosynthesis in this manner is generally rapid (less than ∼2 h) and efficient, with only 1–2% of the protein remaining as proinsulin within mature secretory granules where insulin couples with Zn2+ and exists as a hexameric crystal with the cation. Transport of the insulin hexamer into the secretory granules is thought to be mediated by ZnT8 or related zinc transporters (Lemaire et al., 2009).
Insulin granule pools and their intracellular traffic
Most insulin granules (perhaps 75–95% of an estimated 10,000) are stored within the β-cell cytoplasm at some distance away from the cell membrane (Rorsman and Renström, 2003). The remainder move to the cell periphery along microtubule networks in an AMPK- and kinesin1-dependent manner (McDonald et al., 2009). To reach the plasma membrane, however, granules must cross a cortical actin network that acts as a physical barrier to insulin secretion (Li et al., 1994). Actin reorganization is therefore an important component of the early journey of insulin before it can exit the β-cell. The process is coordinated by the action of several small G-proteins and their activating nucleotide exchange factors. This includes the glucose- and Cdc42-dependent activation of Rac1, which, when released from an inhibitory RhoGDI and in its GTP-bound form, promotes cortical actin remodeling, perhaps via an interaction with gelsolin (Kalwat and Thurmond, 2013). Finally, secretory granules must dock at the plasma membrane and be chemically “primed” in response to an intracellular Ca2+ signal (Fig. 2 B).
The coordinated interaction of exocytic machinery proteins in association with Ca2+ channels (Gandasi et al., 2017) ensures assembly of an insulin granule–exocytic site complex that is “ready to go” when needed. These events likely underlie the well-described biphasic nature of glucose-evoked insulin secretion seen in vitro: a rapid first phase resulting from fusion and secretion by already “docked and primed” secretory granules that in human lasts up to 10 min, and a subsequent second-phase secretion that is associated with actin reorganization thought to allow granule recruitment to the plasma membrane (Wang and Thurmond, 2009).
The orderly arrival, priming, docking, and fusion of granules is exquisitely coordinated in response to physiological inputs initiated by glucose and decoded by the β-cell, as described next.
Sensing glucose: Metabolism-controlled electrical signals and Ca2+ activity
Glucose is the paramount metabolic signal eliciting insulin secretion, and a consensus model reveals a relay of chemical to electrical on to mechanical signals (Fig. 2 B). In brief, glucose enters through the cell membrane glucose transporters GLUT2 in rodents and GLUT1 in humans (McCulloch et al., 2011). Glucose is rapidly phosphorylated by glucokinase (GK) to generate glucose-6-phosphate, which, through glycolysis, feeds the mitochondrial TCA cycle. GK, an isoform of hexokinase, effectively generates downstream signaling metabolites (i.e., ATP and pyruvate) within a range of glucose concentrations that matches the normal physiological range for plasma glucose homeostasis (Meglasson et al., 1983). For this reason, the tandem GLUT1/2 and GK is often referred to as a glucose-sensor controlling blood sugar levels. Mutations that alter the glucose-dependent activity of GK effectively adjust the set point for whole-body glucose homeostasis (Gloyn et al., 2003).
Pyruvate generated from glycolysis enters the mitochondria via mitochondrial pyruvate carriers (Patterson et al., 2014), where the TCA cycle–dependent generation of NADH promotes the export of H+ from the mitochondrial matrix by the electron transport chain, and then generation of ATP from ADP by ATP synthase, which itself appears dependent on mitochondrial Ca2+ uptake (Tarasov et al., 2013). Subsequent increases in the cytosolic ATP/ADP ratio control cell membrane potential by inhibiting ATP-sensitive K+ (KATP) channels, eliciting a membrane depolarization that is modulated by a number of additional ion channels (Fig. 2 B). This represents the conversion of chemical to electrical signaling. When the membrane potential depolarizes sufficiently (approximately −50 mV), the activation of voltage-dependent Na+ and Ca2+ channels cause repetitive action potential spiking and a rise in intracellular Ca2+ (Rorsman et al., 2012). Ca2+ thus becomes the “currency" that triggers granule fusion with the plasma membrane. The increase in cytosolic Ca2+ is rapidly reversed by very active Ca2+ pumps such as the ER sarco-ER Ca2+-ATPase (SERCA), and the use of ATP in the entire process might feed back to activate AMPK and promote insulin granule migration toward the cell periphery. Additional important feedback between Ca2+ and intracellular signals should be noted. For example, feedback from oscillatory Ca2+ signals controls mitochondrial ATP generation (Tarasov et al., 2012), and recent work shows that Ca2+ oscillations and the ER protein TMEM24 interact at ER–plasma membrane contact sites to maintain phosphatidylinositol levels required for β-cell signaling and insulin secretion (Lees et al., 2017).
Importantly, cells across the entire islet, and islets across the entire pancreas, coordinate their Ca2+ signals to effect insulin secretion that occurs as rhythmic oscillations. Although pancreatic β-cells are electrically excitable in response to glucose, they do not work in isolation: they talk to each other. The electrical and Ca2+-responses of β-cells within an islet are synchronized (Zarkovic and Henquin, 2004) and perhaps even coordinated by pacemaker (or “hub”) β-cells within the islet (Johnston et al., 2016). Gap junction coupling between β-cells via connexin36 plays a critical role, the loss of which results in dysregulation of insulin secretion (Ravier et al., 2005). Paracrine and autocrine signaling among β-cells also likely contributes to the coordination and amplification of electrical and Ca2+ responses. Transmitter molecules secreted by β-cells themselves, such as ATP (Gylfe et al., 2012), among others, likely modulate the excitatory activity of nearby β-cells, thus controlling islet Ca2+ and insulin secretory responses (Fig. 2 C).
Thus, communication between cells within an islet likely contributes to the well-described phenomenon of insulin secretory oscillations, which occur in healthy humans with a periodicity of 5–10 min (Satin et al., 2015). The electrical activity and intracellular Ca2+ responses in β-cells within rodent and human islets also oscillates, ranging from tens of seconds to ∼5 min (Dean and Matthews, 1970; Henquin et al., 1982). Further information is provided in Fig. 2 B and in recent modeling that integrates metabolic, electrical, and Ca2+ feedback to produce these oscillations (Bertram et al., 2018). Importantly, this translates into oscillations of insulin secretion from isolated islets, again with a periodicity of 1–5 min (Bergsten et al., 1994).
On top of this, the translation of this single-islet oscillatory activity into a pulsatile release of insulin from the whole pancreas in vivo requires coordination among many individual islets (perhaps a million within a human pancreas). It is not entirely clear how islets within a pancreas communicate in order to synchronize their oscillations. Strong recent evidence suggests a key role for an intrapancreatic neural network, which could coordinate activity among disparate islet populations. This “neuroinsular network” was most recently demonstrated by elegant 3D imaging techniques in rodent (Tang et al., 2018a) and human (Tang et al., 2018b) pancreata.
The glucose-dependent increase in cytosolic ATP/ADP, closure of KATP channels, and initiation of electrical activity to increase Ca2+ and trigger insulin exocytosis has been a useful consensus model for regulated insulin secretion for more than 35 yr. However, this model oversimplifies the physiological regulation of insulin secretion. It has been long recognized that additional signals from gut-derived hormones, autonomic inputs, glucose metabolism itself, and paracrine signals from neighboring α- and δ-cells impinge on this model to exert important control on insulin secretion (Fig. 2 C). Many of these signals “amplify” the secretory response, either by modulating the electrical/Ca2+ responses of β-cells or by controlling the efficacy of Ca2+-triggered insulin exocytosis. For example, the gut-derived hormones glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide together mediate the “incretin” effect whereby nutrient sensing in the gut signals to islets to augment the insulin secretory response to glucose (Drucker et al., 2017). These hormones act on classical G-protein–coupled receptors via Gαs-activation of adenylate cyclase to increase cAMP, which causes PKA-dependent phosphorylation of the exocytic machinery and PKA-independent effects mediated by Epac2A to promote the release of Ca2+ from intracellular stores (Kolic and MacDonald, 2015). Recently, Epac2A was also shown to regulate insulin granule priming (Alenkvist et al., 2017).
Although a glucose-dependent rise in the intracellular ATP/ADP ratio is critical for eliciting β-cell electrical and Ca2+ responses, other mitochondria-derived signals are also important determinants of the secretory response to that Ca2+ rise. Hence, glucose not only controls the Ca2+ signals that trigger insulin secretion but also generates signals that improve the efficacy of Ca2+ on the secretory process, likely by acting on the Ca2+ sensitivity of diverse components in the pathway (Henquin, 2000). This is likely behind the glucose-dependent improvement in insulin granule docking-priming, which is also promoted by additional metabolism-derived signals such as glutamate, the fatty acid metabolite monoacylglycerol, and NADPH (Ferdaoussi and MacDonald, 2017). Together, these inputs interact with various elements of the downstream signaling machinery to effectively amplify secretory responses to a Ca2+ signal. The metabolic signals controlling electrical activity and exocytic function ultimately determine the timing and magnitude of insulin secretion. This concerted mechanism accounts for the first phase insulin that takes place with 30 min (during a glucose tolerance test in humans). A second phase lasting up to 120 min ensues that may involve new insulin synthesis.
Insulin granule exocytosis
Insulin granules in apposition to the plasma membrane dock with the membrane through the coordinated interaction and recruitment of exocytic SNARE proteins that include SNAP-25, VAMP-8, and syntaxins 1A and 3 (Gaisano, 2017). Loss of key SNARE proteins results in impaired insulin secretion (Liang et al., 2017). The formation and fidelity of the SNARE complex mediating granule docking is regulated by a number of proteins such as Munc18 and syntaxin isoforms (Gandasi and Barg, 2014; Zhu et al., 2015). Assembly of the exocytic site in β-cells includes the association of insulin granules with L-type Ca2+ channels (Gandasi et al., 2017), which ensures efficient delivery of Ca2+ to the secretory vesicle Ca2+ sensor, synaptotagmin VII. Collectively, these mechanisms trigger the fusion of the insulin granule bilayer with the plasma membrane, with subsequent release of insulin.
Insulin release occurs directly into the interstitial space of the pancreas, which is surrounded by a fenestrated endothelial vasculature. In this way, released insulin readily finds its way into the portal circulation to be delivered directly to the liver for “first pass.”
Insulin clearance by the liver: Its receptor-mediated endocytosis and degradation
Pulsatile delivery of insulin through the portal vein to the liver
The liver is the first organ that insulin encounters along its journey. Accordingly, the liver is uniquely exposed to higher concentrations of insulin than other insulin-responsive tissues such as muscle and fat. The portal vein delivers insulin from the pancreas to the liver in discrete pulses that occur every ∼5 min (Song et al., 2000), where the amplitude of these insulin pulses is 0.5–1 nmol/liter in the fasted state and rises to ∼5 nmol/liter after a meal (Pørksen et al., 1995; Song et al., 2000). Pulsatile insulin delivery to the liver is an important physiological signal that regulates both hepatic insulin action (Matveyenko et al., 2012) and insulin clearance (Meier et al., 2005), although the cellular underpinnings of how hepatocytes sense pulsatility are unknown.
The liver acts as a gatekeeper that regulates the amount of insulin reaching peripheral tissues through a process called insulin clearance, which was first observed in dogs (Stevenson et al., 1985). The concentration of insulin arriving to the liver by the portal vein can be up to 10-fold higher than the concentration in the peripheral circulation (Horwitz et al., 1975), and the maintenance of this portal-systemic gradient is mediated by substantial insulin degradation by the liver. In humans, simultaneous measurements of portal vein and peripheral vein insulin concentrations during constant glucose infusion revealed that upwards of 50% and possibly even 80% of insulin arriving to the liver by the portal vein is degraded during first-pass hepatic clearance (Meier et al., 2005), and ∼25% of the circulating insulin is degraded upon its second pass through the liver, so that the circulating concentration of insulin is one third that in the portal circulation (Stevenson et al., 1985). This degradation is coupled to pulsatile delivery, such that the liver preferentially clears insulin that arrives in pulses (Meier et al., 2005). Although it seems counterintuitive that so much insulin would be disposed of, degradation appears to be the default mechanism that is however modulated by demand to achieve the insulin concentration required at the periphery. Indeed, adaptive decreases in the rate of hepatic insulin clearance (Ader et al., 2014; Jung et al., 2018) have been observed during insulin resistance and act to compensate for decreased insulin sensitivity (Jung et al., 2018). Moreover, although insulin internalization is not required for many of the metabolic actions of insulin in the liver, the internalized insulin receptor (IR) continues to signal at least within early endosomes (Bevan et al., 1995). Endosomal signaling may have a differential impact from that emanating exclusively from the cell surface, akin to the differential location-based signaling of the EGF receptor (Bergeron et al., 2016).
The portal circulation delivers insulin into the capillaries of the sinusoids, which are not supported by a basement membrane and their endothelial cells contain fenestrations (Wisse, 1970; Braet et al., 1995), together permitting the exchange of contents between the blood and the surrounding liver cells. The unique structure of the hepatic sinusoids allows insulin to easily diffuse out of the circulation and into the perisinusoidal space, where it comes into contact with hepatocytes (Fig. 3 A).
Figure 3.
Insulin clearance in the liver. (A) Insulin is delivered to the hepatic sinusoid, where it freely accesses the liver hepatocytes through the fenestrated sinusoidal endothelium. (B) Proposed mechanism for insulin degradation in hepatocytes. Insulin binds to the IR and forms a complex with CEACAM1. Prior to internalization, extracellular IDE begins to degrade receptor-bound insulin. After internalization, endosomal IDE degrades receptor-bound insulin and, once the endosome acidifies and the complex dissociates, also frees insulin. Any remaining insulin or insulin fragments progress toward lysosomes for their complete proteolytic degradation.
Hepatocytes are the major site of insulin clearance. Early electron microscopy studies revealed that IRs bind 125I-insulin on microvilli (interdigitations) of the hepatocyte membrane (Carpentier et al., 1985). After binding, 125I-insulin–IR complexes move to the base of the microvilli, where they associate with clathrin-coated pits (Pilch et al., 1983) and internalize by clathrin-mediated endocytosis (Fehlmann et al., 1982). Although still unknown for hepatocytes, IR autophosphorylation is required for insulin uptake by CHO cells (Carpentier et al., 1992). Consistent with earlier studies, liver-specific IR knockout mice provided direct evidence that receptor-mediated degradation regulates systemic insulin levels and that impairments in this process lead to severe hyperinsulinemia that, in turn, contributes to whole-body insulin resistance (Michael et al., 2000). Of note, mice lacking in the liver the IR substrates 1 and 2 (IRS1,2; adaptor proteins that can bind to the IR to initiate signal transduction) have less severe hyperinsulinemia (Dong et al., 2008) than mice lacking the IR (Michael et al., 2000; Cohen et al., 2007), suggesting that canonical insulin signaling via IRS1,2 may not participate in insulin clearance. However, there is no direct mechanistic proof of this or of the involvement of other classical insulin signals.
After insulin binds to its receptor on the hepatocyte surface, endocytosis of the receptor–ligand complex causes a concomitant loss of surface IR (Goodner et al., 1988), which is followed by rapid recycling and reinsertion of intact, unbound IRs in the plasma membrane (Goodner et al., 1988). These findings are concordant with the physiological intervals of pulsatile delivery, suggesting that hepatocyte IR internalization and reinsertion into the membrane is adaptively entrained to insulin delivery (Meier et al., 2005). In contrast to IR recycling, the fate of internalized insulin differs, as we will describe.
CEACAM1 and insulin-degrading enzyme (IDE): Hepatic molecules driving insulin clearance
Although hepatocytes are not exclusive in their ability to internalize insulin, they highly express the transmembrane glycoprotein CEACAM1 (carcinoembryonic antigen–related cell adhesion molecule 1), which mediates rapid and effective IR-mediated insulin endocytosis (Najjar, 2002). Mechanistically, CEACAM1 is phosphorylated by the IR, enhancing the formation of an insulin–IR–CEACAM1 complex (Najjar et al., 1995; Fig. 3 B). It is hypothesized that a so-far-unidentified adaptor protein targets the tripartite complex to the AP2 adaptor complex for clathrin-mediated endocytosis (Najjar, 2002). Tests in nonhepatic cells, on the other hand, show that the IR target protein SHC binds dynamin (a GTPase required for the scission of endocytic vesicles), and this complex contributes to IR internalization (Baron et al., 1998). It is tempting to hypothesize that, in hepatocytes, SHC might be the protein linking the IR to CEACAM1 and thus brings the complex to dynamin-rich regions prone for endocytosis. Consistent with the crucial role of CEACAM1 in hepatic insulin clearance, impairments in insulin-stimulated hepatic CEACAM1 phosphorylation or whole-body depletion of hepatic CEACAM1 cause severe hyperinsulinemia and, consequently, insulin resistance and hyperglycemia (Poy et al., 2002; Russo et al., 2017). Notably, hepatocytes from these mice have impaired insulin-dependent IR endocytosis, which can be rescued by liver-specific reexpression of CEACAM1 (Poy et al., 2002; Russo et al., 2017). Hence, the level of CEACAM1 at the plasma membrane and its phosphorylation may impart physiological fine-tuning regulation to the process of insulin clearance.
The majority of insulin that binds to hepatic IRs is degraded (Duckworth, 1988). The degradation process begins on the membrane immediately after insulin binding, where some insulin is reported to be partially degraded by extracellular IDE before internalization (Yokono et al., 1982). After internalization, additional IDE is thought to be targeted to endosomal membranes through its interaction with phosphatidylinositol phosphates (Song et al., 2017), where it begins to degrade receptor-bound insulin in endosomes (Yonezawa et al., 1988) before acidification occurs (Hamel et al., 1991). As endosomes acidify, any remaining insulin or partially degraded insulin that escaped complete degradation by IDE dissociates from the IR (Murphy et al., 1984; Fig. 3 B). Ultimately, these degradation products and any remaining intact insulin are delivered to lysosomes for complete proteolysis (Duckworth, 1988), although lysosomal degradation of insulin is thought to play a minor role in insulin clearance (Duckworth et al., 1981).
Although the in vitro data strongly indicate that IDE is essential for hepatocyte insulin degradation, the role of IDE in hepatic insulin clearance in vivo is controversial, as some studies report that loss of IDE results in hyperinsulinemia (Farris et al., 2003; Abdul-Hay et al., 2011), whereas others observed no changes in systemic circulating insulin in the absence of the enzyme (Steneberg et al., 2013).
Insulin that is not degraded in the liver exits through the hepatic vein, reaching the heart, which pumps insulin into the arterial circulation to be delivered to its target tissues (e.g., skeletal muscle, liver, adipose tissue, and the brain). It is important to note that insulin returns to the liver, this time via the hepatic artery, which pours again into the hepatic sinusoid, where the hormone is subject to a second round of insulin degradation (second pass) within hepatocytes.
Beyond first- and second-pass insulin clearance, the hepatocytes are essential metabolic responders to insulin, where one of the major actions of the hormone is to suppress gluconeogenesis and glycogenolysis (Lin and Accili, 2011). This ensures that a portion of dietary glucose is effectively stored in the liver and is only released to the rest of the body upon cessation of insulin action (between meals) or upon metabolic demand enacted by other “counterregulatory” hormones (Samuel and Shulman, 2018). This is a vast area of study that is however not further discussed here.
Insulin interaction with the vasculature
Hemodynamic insulin action on arteries and arterioles
The peripheral actions of insulin begin inside the vessels of the systemic circulation, where the hormone exerts its hemodynamic effects on endothelial cells to promote blood flow and ensure its delivery to peripheral tissues (Barrett et al., 2009). Endothelial cells line each blood vessel and constitute a crucial interface between the circulation and the tissue parenchyma. In large blood vessels such as the aorta and large arteries, insulin acts on the IR of endothelial cells, causing phosphorylation of the major endothelial IR substrate, IRS2. This leads to activation of class I phosphatidylinositol 3-kinase (PI3K), which signals downstream to the serine and threonine kinase Akt/PKB. In turn, Akt activates endothelial NO synthase to catalyze the conversion of l-arginine to NO (Palmer et al., 1988; Zeng et al., 2000). NO is a potent vasodilator that rapidly diffuses to the vessels’ outer layer of smooth muscle cells, where it activates intracellular guanylate cyclase to increase cyclic guanosine monophosphate production (Arnold et al., 1977). Cyclic guanosine monophosphate–dependent reductions in intracellular Ca2+ concentration (Carvajal et al., 2000) prevent phosphorylation of myosin light chain required for cytoskeletal cross-bridge formation and contraction (Lee et al., 1997; Mizuno et al., 2008), thereby resulting in vessel relaxation (Fig. 4 A).
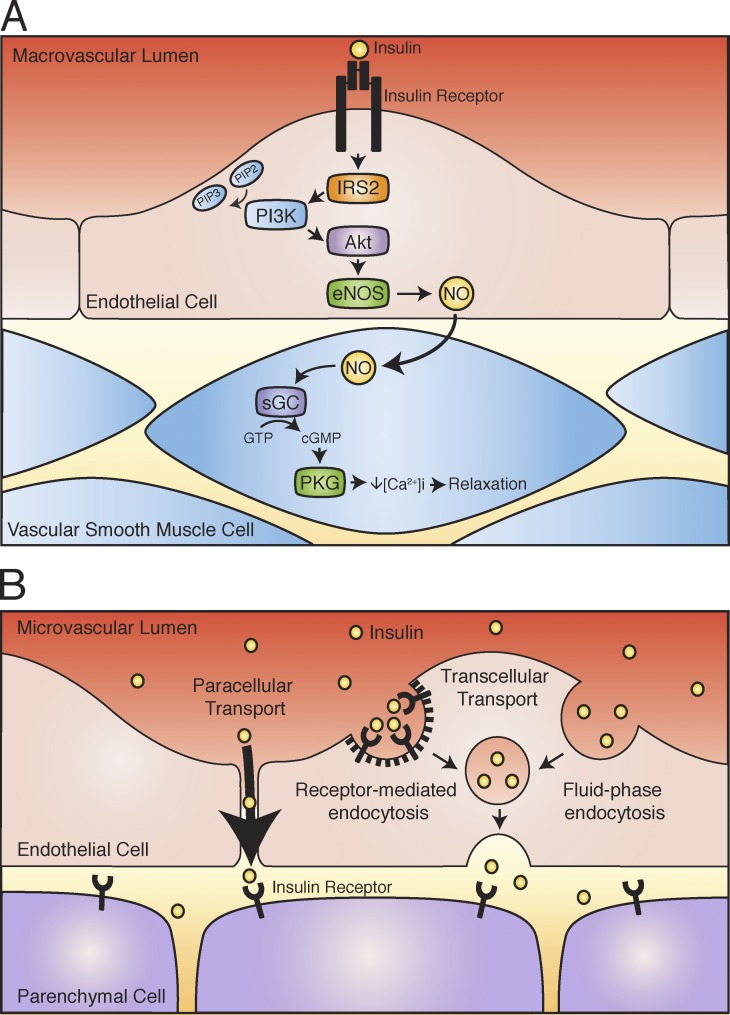
Figure 4.
Insulin interactions with the vasculature. (A) Endothelial insulin signaling leading to vasodilation in the macrovasculature. The endothelial cell IR engages its major substrate in these cells, IRS2, leading downstream to activation of Akt. Akt phosphorylates endothelial NO synthase (eNOS), which catalyzes the production of NO from l-arginine. NO freely diffuses to the underlying vascular smooth muscle layer, where it leads to cyclic guanosine monophosphate production to induce vasorelaxation. (B) Possible routes for insulin exit across microvascular endothelial cells toward the interstitial space in muscle and fat tissue. Insulin may cross the microvascular capillary endothelium either paracellularly (between adjacent endothelial cells) or transcellularly (through individual endothelial cells). For the transcellular route, both receptor-mediated and fluid-phase mechanisms of transport have been proposed.
As a consequence of endothelial NO production, insulin stimulates dilation of arteries and arterioles (Steinberg et al., 1994; Vincent et al., 2002). Within minutes, vasodilation of precapillary arterioles irrigates previously collapsed capillaries with blood carrying insulin (capillary recruitment), thereby promoting insulin delivery to the tissue (Vincent et al., 2002). With continued insulin circulation (∼30 min), the hormone induces relaxation of larger, upstream resistance vessels to further promote limb blood flow (Baron et al., 1996). Insulin action in target tissues is temporally linked to these vascular effects (Barrett et al., 2009); in particular, the full stimulation of skeletal muscle glucose uptake in vivo is contingent on prior NO-mediated vasodilation (Vincent et al., 2003; Bradley et al., 2013).
Insulin transit across the microvascular endothelium
Once insulin arrives at the capillaries of skeletal muscle and adipose tissue, it must exit the circulation to reach the parenchymal cells (muscle fibers and adipocytes). Unlike the fenestrated capillaries of the liver, the capillary endothelium in skeletal and adipose tissues is continuous, functioning as a stringent barrier between the circulation and the interstitial space. Each capillary is constituted by a single layer of endothelial cells, supported by interendothelial junctions that selectively restrict the passage of contents between the blood and the tissue. Bergman et al. first reported a delay in muscle insulin action relative to the rise in insulin in the circulation (Kolka and Bergman, 2012), and several studies have documented that the capillary endothelium is a barrier to insulin delivery to muscle in vivo, maintaining a disequilibrium between circulating and interstitial insulin levels (Jansson et al., 1993; Herkner et al., 2003).
Insulin may cross the tight capillary endothelia by two potential routes: transcellular (through individual cells) or paracellular (between neighboring endothelial cells; Fig. 4 B). Although there is evidence that expression of the IR or vascular insulin signaling is required for overall insulin egress from the circulation toward tissues (Kubota et al., 2011; Majumdar et al., 2012; Meijer et al., 2016; Konishi et al., 2017), opposing studies challenge a receptor-mediated (Vicent et al., 2003; Duncan et al., 2008; Williams et al., 2018) or saturable process (Steil et al., 1996). To this day, the exact route (intracellular vs. paracellular) and supporting mechanisms remain a matter of debate (Lee and Klip, 2016). The impasse in discerning this route lies in the limitation to differentiate in vivo between a distinctly local role of the endothelial IR in the cellular transport of insulin across the endothelium from its complementary role in capillary recruitment. Recent studies that have bypassed the hemodynamic concerns have also yielded opposite results. Thus, even with chemically induced vasodilation, a new endothelial cell-specific IR knockout mouse model (Konishi et al., 2017) shows defective insulin delivery and action. In contrast, sophisticated imaging of the muscle distribution of somewhat high doses of fluorescent insulin injected into the circulation was best fitted to a model of distribution that does not obey saturation kinetics, suggesting that the IR may not be a major conduit under these conditions (Williams et al., 2018).
Controversy about the mechanism of insulin transit across the microvasculature also arises upon scrutiny in vitro, as cell culture studies have rendered inconsistent results regarding the precise role of the endothelial IR in the uptake of fluorescently conjugated insulin, potentially dependent on their niche origin: microvascular (Azizi et al., 2015) or macrovascular (Wang et al., 2008). Moreover, imaging the internalized insulin, needed to establish the hormone’s intracellular route, has required the use of supraphysiological doses of insulin to achieve detectable levels (Wang et al., 2008; Azizi et al., 2015), confounding the identification of the physiological mechanism. On the other hand, the uptake of physiological levels of 125I-insulin into microvascular endothelial cells has uniformly revealed participation of the IR (Jialal et al., 1984; Gray et al., 2017; Jaldin-Fincati et al., 2018). This includes the transfer of insulin across cells of the blood–brain barrier (Jialal et al., 1984; Gray et al., 2017), an important conduit for the now-recognized neuronal actions of the hormone evinced by the neuron-specific IR gene depletion (Brüning et al., 2000). How internalized insulin is spared from degradation in endothelial cells, as opposed to its fate in hepatocytes (described in Insulin clearance by the liver: Its receptor-mediated endocytosis and degradation), remains unsolved. Potentially, this may involve routing of insulin into sorting tubules akin to those recently described for transferrin receptor-mediated transcytosis through blood–brain endothelia (Villaseñor et al., 2017).
Central insulin action: Brief focus on the brain
Emerging from the circulation, insulin begins its multifaceted action on central and peripheral tissues. As outlined above, insulin crosses the blood–brain barrier through a receptor-mediated process (Woods et al., 2003). Thought to be insulin unresponsive in the past, the central nervous system is well recognized to be exquisitely responsive to the incoming hormone (Porte et al., 2005). The concentration of insulin in the cerebrospinal fluid is one third that in the circulation, but it nonetheless fluctuates according to the latter and acts on IR on neurons and glial cells. Notable among the evoked central functions is the regulation of appetite and energy expenditure (Filippi et al., 2013; García-Cáceres et al., 2016). Insulin regulates appetite by reducing expression of neuropeptide Y and Agouti-related peptide (orexigenic) and, conversely, elevating expression of pro-opiomelanocortin (anorexigenic; Schwartz et al., 2000). Insulin also exerts trophic and developmental actions on neurons and glial cells, and new evidence suggests it modulates cognition, memory, and mood (Lee et al., 2016). Conversely, central defects in insulin action are emerging as a potential contributor to the development of Alzheimer’s disease (Griffith et al., 2018), possibly as a result of abnormal phosphorylation of tau protein (Kleinridders et al., 2014). Insulin acting centrally also evokes efferent inputs into peripheral tissue metabolism (Ferris and Kahn, 2016), contributing to the suppression of gluconeogenesis in the liver and the counterregulatory response to hypoglycemia (Diggs-Andrews et al., 2010). Acting centrally on IR, insulin contributes to thermoregulation by activating heat-liberating mechanisms in brown adipose tissue (Kleinridders et al., 2014).
The cellular mechanisms underlying each of these complex, integrated responses are still to be elucidated, especially in so far as identification of the specific intra- and intercellular neuronal responses that are likely to be carefully decoded through spatial, temporal, and amplitude parameters. Although rich information is being gathered through electrophysiological approaches (van der Heide et al., 2005; Könner et al., 2007; Korol et al., 2018), there is a rich opportunity to explore additional mechanisms through the advent of real-time intravital imaging of the central nervous system (Forli et al., 2018).
Insulin in action: Stimulation of glucose uptake in muscle and fat cells
The actions of insulin on the parenchyma of peripheral tissues are diverse, and paramount among them is the regulation of glucose metabolism. The major function of insulin in muscle and adipose tissues is to increase their uptake of carbon sources and store them for the energetic needs of tissue. With glucose transport into these tissues being rate limiting for its storage (as glycogen and triglycerides, respectively), it is no surprise that insulin regulates glucose uptake. This is brought about by an exquisite series of signals that cooperate in bringing glucose transporters (GLUT4 isoform) to the cell surface. This process is generically known as GLUT4 translocation, and 30 years of research has revealed regulation at a number of stages in this intracellular process (Bryant and Gould, 2011; Kandror and Pilch, 2011; Stöckli et al., 2011; Bogan, 2012; Leto and Saltiel, 2012; Klip et al., 2014; Jaldin-Fincati et al., 2017).
GLUT4 translocation takes places within minutes of insulin binding to its receptors at the surface of myocytes and adipocytes and does not involve internalization of the hormone. Major aspects of GLUT4 translocation are illustrated in Fig. 5.
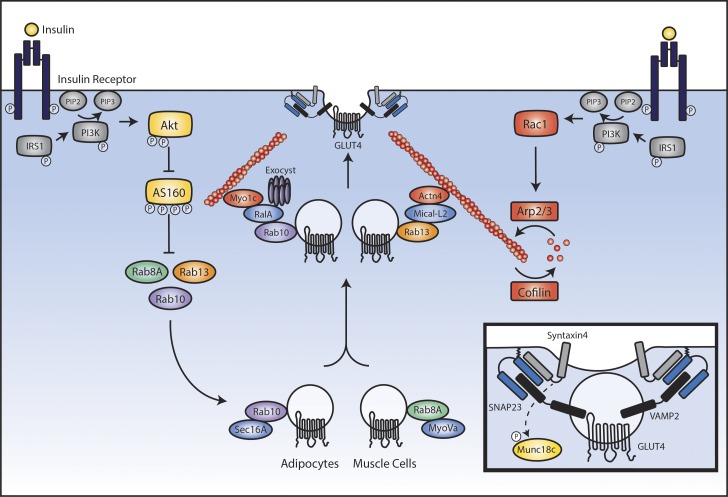
Figure 5.
Insulin signaling in muscle and adipose cells leading to recruitment of GLUT4 to the plasma membrane. Insulin binds to its receptor on the surface of muscle or fat cells and activates the canonical insulin-signaling cascade to PI3K and Akt. Downstream of Akt, phosphorylation of AS160 allows for the full activation of Rab8A and Rab13 (in muscle cells) and Rab10 (in adipocytes). In the perinuclear region, Rab8A engages with its effector, MyoVa, and Rab10 with its effector, Sec16A, to promote outward vesicle traffic. Near the plasma membrane, Rab13 engages with MICAL-L2 and Actinin-4, whereas Rab10 engages with RalA, Myo1c, and Exocyst components. Simultaneously, downstream of PI3K, insulin leads to activation of Rac1 that promotes a dynamic cycle of cortical actin remodeling. Together, these actions tether GLUT4 vesicles to the actin cytoskeleton near the plasma membrane. Inset: Docked GLUT4 vesicle ready to fuse with the plasma membrane. Immobilized GLUT4 vesicles fuse with the membrane through formation of a SNARE complex between vesicular VAMP2 and syntaxin4 and SNAP23 on the plasma membrane.
The unique GLUT4 compartment
The molecular signature of GLUT4 allows it to be diverted away from the continuously recycling pathway (a ubiquitous intracellular route that constantly removes and returns membrane proteins by internalization toward endosomes and reexternalization) to constitute a functionally defined “organelle” called GLUT4 storage vesicles. Several elements contribute to the genesis and maintenance of this storage compartment, including sortilin (Huang et al., 2013), the Rab GTPase-activating protein (GAP) AS160/TBC1D4, syntaxin 6/16 (Bryant and Gould, 2011; Klip et al., 2014), the cleavable tether protein TUG (Belman et al., 2015), and, in human muscle, clathrin heavy chain 22 (Vassilopoulos et al., 2009). The storage compartment is in dynamic communication with recycling endosomes (Coster et al., 2004; Karylowski et al., 2004; Kandror and Pilch, 2011). This dynamic sorting generates a steady state whereby the majority (∼90–95%) of GLUT4 resides intracellularly at any point in time in both muscle and adipose cells. This effective removal from the plasma membrane at any point in time is rather unique for GLUT4, as is its intracellular sorting to a compartment that is only slowly or ineffectively available for recycling.
Insulin signals quickly mobilize GLUT4-containing vesicles of ∼70 nm in diameter from perinuclear/cytosolic depots toward the cell periphery. These emanate directly from the storage compartment; however, insulin also appears to redirect vesicles from this compartment toward the general recycling endosomes, from whence they reach the cell periphery in the form of somewhat larger vesicles (Xu et al., 2011). A current model proposes that the initial gain in surface GLUT4 emanates from the storage compartment, whereas maintenance of the steady state involves the endosomal route (Bryant and Gould, 2011; Kandror and Pilch, 2011; Stöckli et al., 2011; Bogan, 2012; Leto and Saltiel, 2012; Klip et al., 2014; Jaldin-Fincati et al., 2017). However, another prevailing model proposes that the majority of vesicles furnishing the cell membrane with GLUT4 contain the fusogenic protein VAMP2, which segregates away with the storage compartment and is largely absent from recycling endosomes (Randhawa et al., 2000, 2004; Török et al., 2004). Once at the cell periphery, insulin signals further promote vesicle fusion with the plasma membrane. Within minutes, this concerted action brings about a new steady state with double the number of GLUT4 units at the plasma membrane in muscle cells. Although this gain represents only ∼20% of the total GLUT4, given the large mass of muscle in vivo, this gain sustains the vastly preferential deposition of diet-ingested glucose into skeletal muscles. In adipocytes, the insulin-dependent gain in surface-exposed GLUT4 ranges from twofold (human) to 10-fold or higher (rodents) and is typically calculated that this gain involves 30–50% of the total GLUT4 complement in these cells. In both muscle and fat cells, the new steady state lasts for as long as insulin is present.
The GLUT4 polypeptide has a very long lifetime (∼40 h); hence, its continuous removal from the membrane allows for multiple rounds of endocytosis, sorting, and translocation. It is understood that insulin promotes GLUT4 exit from retention in the storage compartment (Xu and Kandror, 2002; Coster et al., 2004; Martin et al., 2006; Bogan et al., 2012) and also regulates GLUT4 vesicle tethering, docking, and fusion with the plasma membrane through mechanisms that borrow principles from those of synaptic vesicle and insulin granule fusion.
Insulin signals involved in GLUT4 translocation
The connection between IR-derived signals (Klip et al., 2014) and the elements that mobilize GLUT4 vesicles and enact their fusion with the membrane is beginning to unravel. Insulin activates the IR tyrosine kinase activity toward autophosphorylation by inducing structural rearrangement of the transmembrane domains to bring them into close proximity with each other (Gutmann et al., 2018), and the consequent activation of the IR tyrosine kinase toward phosphorylation of its major substrates IRS1,2 (Copps and White, 2012). Phosphorylation sites on IRS1,2 constitute entropic information to attract class I PI3K, which rapidly generates membrane domains enriched in PI(3,4,5)P3 (PIP3) within minutes (Ruderman et al., 1990). Two major consequences of the PIP3 burst relevant for GLUT4 translocation are activation of the kinases Akt1, 2 (Brozinick and Birnbaum, 1998; Wang et al., 1999) and of the Rho-family GTPase Rac1 (Chiu et al., 2011).
For the first signal, PIP3 attracts the PH domain of Akt, which makes the protein available for phosphorylation by two kinases, PDK-1, and mTORC2. Activated Akt1,2 migrates to the cytosol and intracellular membranes (Zheng and Cartee, 2016), where it phosphorylates AS160, a substrate of 160 kD more appropriately named TBC1D4 (Sano et al., 2003; Lansey et al., 2012). The TBC domain of AS160/TBC1D4 defines its GAP activity toward Rab family small GTPases. Phosphorylation of AS160/TBC1D4 inhibits its GAP activity; hence, insulin signaling leads to inactivation of an inhibitor of Rab GTPases. This realization constituted the first involvement of elements capable of specifically regulating vesicle traffic in the pathway, as Rab GTPases regulate vesicle fission, destination, and fusion. AS160/TBC1D4 targets a cluster of Rabs, particularly the phylogenetically related Rabs 8A, 10, and 13. In addition, these three GTPases are stabilized by the holdase chaperone RABIF/MSS4 (Gulbranson et al., 2017). As a result of AS160/TBC1D4 inactivation, these Rab GTPases prevail in their active, GTP-loaded state; hence, their regulation is largely via inhibition of their GAP (whereas their currently incompletely identified guanine nucleotide exchange factors (GEFs) might be constitutively active, as in the case of the Sec10, the GEF for Rab10 (Sano et al., 2011; Fig. 5).
In parallel to activation of this “Akt cascade,” the burst in plasma membrane–associated PIP3 leads to activation of GEFs (and possibly inhibition of GAPs) for Rac1 (Takenaka et al., 2014, 2016). The resulting Rac1 activation leads to a dynamic remodeling of cortical actin filaments via cycles of actin filament branching enacted by Arp2/3 and actin severing enacted by cofilin, which is best mapped in muscle cells (Chiu et al., 2010) and tissue (Sylow et al., 2013; Fig. 5).
From signals to effectors: Mechanical elements of GLUT4 vesicle translocation
Rab GTPases lie at the crux of signal transmission to mechanical transduction, as several Rab GTPase effectors collude with actin filaments (whether those remodeling at the cell cortex and/or other filamentous configurations) to de facto mobilize GLUT4 to the plasma membrane. In adipocytes, Rab10 is the preferred GTPase in GLUT4 translocation (Sano et al., 2008), whereas in muscle cells, it is Rab8A and Rab13 (Ishikura et al., 2007; Sun et al., 2010, 2014, 2016). Although Rab10, Rab8A, and Rab13 have been studied the most, other Rab family GTPases contribute to the overall GLUT4 traffic, such as Rab28, which is also a substrate of AS160/TBC1D4; Rab14, involved in intracellular GLUT4 sorting; Rabs 4 and 11, involved in constitutive GLUT4 cycling; and Rab5, involved in early GLUT4 endocytosis (Jaldin-Fincati et al., 2017).
In adipocytes, Rab10 promotes GLUT4 mobilization from the perinuclear region toward the plasma membrane (Sano et al., 2007; Bruno et al., 2016), specifically by interacting with Sec16A (Sano et al., 2007; Bruno et al., 2016). In addition, a function for Rab10 at the cell periphery was also proposed (Chen and Lippincott-Schwartz, 2013), as will be discussed.
In muscle cells, the perinuclear Rab8A engages its effector Myosin Va thereby promoting GLUT4 exit from the storage compartment (Sun et al., 2014). This processive molecular motor allows migration of GLUT4 vesicles along actin filaments toward the cell periphery. Rab13 is more peripherally located, and its effector is the cortically located protein MICAL-L2, which in turn binds the cortical cytoskeleton protein α-actinin4. In response to insulin, these three proteins can be visualized near the cell surface along with GLUT4 and cortical actin (Sun et al., 2016). In this way, Rab8A and Rab13 ensure GLUT4 vesicle mobilization toward the periphery and tethering to cytoskeletal elements in this region, respectively.
In addition to the Rab13–MICAL-L2–α-actinin4 connection, GLUT4 vesicles tether to actin filaments via Myosin 1c (Bose et al., 2002; Boguslavsky et al., 2012). This restricts GLUT4 mobility beneath the membrane, a phenomenon nicely documented through total internal reflection fluorescence microscopy of muscle and adipose cells (Bai et al., 2007; Xiong et al., 2010; Boguslavsky et al., 2012; Lizunov et al., 2012). GLUT4 vesicle retention near the membrane also involves the exocyst subunit Exo70 (Lizunov et al., 2012). Tethering may be regulated by insulin, as stimulation leads to phosphorylation of Exo84 (Uhm et al., 2017). In addition, active Rab10 binds to Exoc6/6b (Sano et al., 2015), and the Rab10 effector RalA and its GEF, Rlf, interact with exocyst components (Karunanithi et al., 2014).
The GLUT4 vesicle fusion machinery
GLUT4 vesicles immobilized at the cell periphery rapidly fuse with the membrane. This is brought about through formation of a SNARE complex between VAMP2 on the vesicles and synatxin4 and SNAP23 on the plasma membrane (Cheatham et al., 1996; Foster and Klip, 2000; Thurmond and Pessin, 2001). The formation of the SNARE complex is regulated by a fine balance of a number of proteins such as Munc18c, Synip, and Doc2b, which receive input emanating from Akt and the phosphatase PTP-1B (Yamada et al., 2005; Fukuda et al., 2009; Bakke et al., 2013; Garrido-Sanchez et al., 2013; Fig. 5).
The kinetics, stoichiometry, and upstream regulation of the fusion step still need to be fully investigated. Intriguingly, there are studies of additional participation of Ca2+-regulated proteins such as Doc2b, Tctex1d2, and E-Syt1 (Lalioti et al., 2009; Friedrich et al., 2010; Shimoda et al., 2015), and insulin-dependent Ca2+-spikes have been recorded in muscle cells (Contreras-Ferrat et al., 2014), suggesting that the ion may impart some fine-tuning to the fidelity and timeliness of GLUT4 vesicle fusion. Lastly, and importantly, the fusion event requires insulin-induced actin polymerization, evincing the contribution of the actin cytoskeleton at different steps in the process of GLUT4 translocation (Lopez et al., 2009).
The end: Insulin degradation in the kidney
Insulin is no longer detectable in the circulation 30 min after its release from the pancreas, and its half-life once in the circulation is ∼6 min (Robbins et al., 1985; Marino, 2009). In addition to its clearance by the liver (50% in first and another 25% in second pass), the hormone is also slowly internalized by most cells, including myoblasts and adipocytes, where it is routed to the lysosome for degradation. This is a mechanism to end insulin action, but it accounts for the destruction of only a fraction of the circulating insulin. The brunt of the degradation of the circulating hormone remaining after second pass through the liver occurs when it reaches the kidney. Here, its fate is threesome. Upon filtration at the level of the glomeruli, insulin enters the luminal space and reaches the proximal tubule, from whence it is rapidly reabsorbed by the renal epithelial cells. This reabsorption involves saturable binding to low-affinity, high-capacity sites at the brush border membrane, which are demonstrated to be not the IR (Meezan et al., 1988; Sato et al., 1991; Nielsen, 1993, 1994) but possibly scavenger receptors such as megalin (member of the low-density lipoprotein receptor family; Christensen et al., 1998; Kolman et al., 2009) and cubilin, proteins that recover a number of proteins by endocytosis. Insulin thus internalized enters the retroendocytic pathway, where it dissociates from its binding sites to proceed to lysosomes for degradation.
Second, about an equal amount of insulin also enters renal tubular cells from the contraluminal side facing the renal peritubular capillaries, especially in the convoluted tubule (Rabkin et al., 1984). Here, IRs on the epithelial cells bind insulin and transport it intracellularly for degradation (Nielsen et al., 1987). In addition, these IR are important sites sensing the hormone to stimulate important functions such as reabsorption of sodium, phosphate, and glucose (Rabkin et al., 1984; Hale and Coward, 2013). It has been proposed that these two renal mechanisms of insulin internalization are responsible for clearing up to 6–8 U insulin per day (Palmer and Henrich, 2017) amounting to up to 25% of the insulin secreted by the pancreas, or ∼50% of the circulating insulin, although this might be an overestimation. Nonetheless, renal insulin clearance may explain the curious fact that type 1 diabetic patients with onset renal failure can end up reducing their requirement for injected insulin (Rubenstein and Spitz, 1968; Rabkin et al., 1984).
Third, though most of the internalized insulin is degraded by the above pathways, a small fraction is reabsorbed back to the renal circulation through retroendocytosis (Dahl et al., 1989). Notably, alterations in insulin renal clearance prolong the permanence of insulin in the blood (Dahl et al., 1989), evincing the importance of this process to insulin’s half-life in the circulation.
Concluding remarks
We have analyzed the fundamental physiological journey of insulin in the body by alternating a bird’s-eye view of the integrative phenomenon with close-ups into the key cellular processes of the hormone’s secretion, partial clearance in the liver, distribution to the circulation and exit to target tissues, its action to promote glucose uptake in muscle and fat, and ultimately its degradation in the kidney. In spite of the depth of knowledge available to us on each of these cellular stages in the journey, there are many mechanistic and integrated aspects that remain unknown. However, the current knowledge already allows us to understand how each stage is in communication with the other. The temporal periodicity of insulin secretion out of the pancreas is sensed by the hepatocytes, which synchronously clear a portion of the secreted insulin; insulin action on the macrovasculature allows recruitment of the microcirculation for full enactment of insulin delivery to tissues; and insulin action in the liver, muscle, and fat cells results in a lowering of blood glucose, thus terminating the prime stimulus for insulin secretion. In pace with insulin action, the kidney engages in its subsequent degradation, putting an end to the hormone’s action with just the right time delay to ensure optimal metabolic homeostasis.
Acknowledgments
We thank profusely the input received for the analysis presented herein from Drs. Sonia M. Najjar, Alan D. Cherrington, and Philip J. Bilan.
Work in P.E. MacDonald’s laboratory on insulin secretion is funded by the Canadian Institutes of Health Research (foundation grant FRN 148451). Work in A. Klip’s laboratory on insulin delivery and action is funded by the Canadian Institutes of Health Research (foundation grant FRN: FND-143203). A. Klip is the recipient of the Tier I Canada Research Chair “Cell Biology of Insulin Action.” V. Tokarz was supported by the University of Toronto (Ontario Graduate Scholarship) and The Hospital for Sick Children (Restracomp Scholarship).
The authors declare no competing financial interests.
A. Klip conceived the subject and format of this article; V.L. Tokarz, P.E. MacDonald, and A. Klip analyzed the literature, discussed the material, wrote the article, and conceived the figures. V.L. Tokarz produced the majority of the figures.
References
- Abdul-Hay S.O., Kang D., McBride M., Li L., Zhao J., and Leissring M.A.. 2011. Deletion of insulin-degrading enzyme elicits antipodal, age-dependent effects on glucose and insulin tolerance. PLoS One. 6:e20818 10.1371/journal.pone.0020818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ader M., Stefanovski D., Kim S.P., Richey J.M., Ionut V., Catalano K.J., Hucking K., Ellmerer M., Van Citters G., Hsu I.R., et al. 2014. Hepatic insulin clearance is the primary determinant of insulin sensitivity in the normal dog. Obesity (Silver Spring). 22:1238–1245. 10.1002/oby.20625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alenkvist I., Gandasi N.R., Barg S., and Tengholm A.. 2017. Recruitment of Epac2A to Insulin Granule Docking Sites Regulates Priming for Exocytosis. Diabetes. 66:2610–2622. 10.2337/db17-0050 [DOI] [PubMed] [Google Scholar]
- Andrali S.S., Sampley M.L., Vanderford N.L., and Ozcan S.. 2008. Glucose regulation of insulin gene expression in pancreatic beta-cells. Biochem. J. 415:1–10. 10.1042/BJ20081029 [DOI] [PubMed] [Google Scholar]
- Arnold W.P., Mittal C.K., Katsuki S., and Murad F.. 1977. Nitric oxide activates guanylate cyclase and increases guanosine 3′:5′-cyclic monophosphate levels in various tissue preparations. Proc. Natl. Acad. Sci. USA. 74:3203–3207. 10.1073/pnas.74.8.3203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artner I., and Stein R.. 2008. Transcriptional regulation of insulin gene expression. In Pancreatic Beta Cell in Health and Disease. Springer, Tokyo. 13–30. [Google Scholar]
- Aslamy A., and Thurmond D.C.. 2017. Exocytosis proteins as novel targets for diabetes prevention and/or remediation? Am. J. Physiol. Regul. Integr. Comp. Physiol. 312:R739–R752. 10.1152/ajpregu.00002.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizi P.M., Zyla R.E., Guan S., Wang C., Liu J., Bolz S.-S., Heit B., Klip A., and Lee W.L.. 2015. Clathrin-dependent entry and vesicle-mediated exocytosis define insulin transcytosis across microvascular endothelial cells. Mol. Biol. Cell. 26:740–750. 10.1091/mbc.E14-08-1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L., Wang Y., Fan J., Chen Y., Ji W., Qu A., Xu P., James D.E., and Xu T.. 2007. Dissecting multiple steps of GLUT4 trafficking and identifying the sites of insulin action. Cell Metab. 5:47–57. 10.1016/j.cmet.2006.11.013 [DOI] [PubMed] [Google Scholar]
- Bakke J., Bettaieb A., Nagata N., Matsuo K., and Haj F.G.. 2013. Regulation of the SNARE-interacting protein Munc18c tyrosine phosphorylation in adipocytes by protein-tyrosine phosphatase 1B. Cell Commun. Signal. 11:57 10.1186/1478-811X-11-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron A.D., Brechtel-Hook G., Johnson A., Cronin J., Leaming R., and Steinberg H.O.. 1996. Effect of perfusion rate on the time course of insulin-mediated skeletal muscle glucose uptake. Am. J. Physiol. 271:E1067–E1072. [DOI] [PubMed] [Google Scholar]
- Baron V., Alengrin F., and Van Obberghen E.. 1998. Dynamin associates with Src-Homology Collagen (Shc) and becomes tyrosine phosphorylated in response to insulin. Endocrinology. 139:3034–3037. 10.1210/endo.139.6.6131 [DOI] [PubMed] [Google Scholar]
- Barrett E.J., Eggleston E.M., Inyard A.C., Wang H., Li G., Chai W., and Liu Z.. 2009. The vascular actions of insulin control its delivery to muscle and regulate the rate-limiting step in skeletal muscle insulin action. Diabetologia. 52:752–764. 10.1007/s00125-009-1313-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belman J.P., Bian R.R., Habtemichael E.N., Li D.T., Jurczak M.J., Alcázar-Román A., McNally L.J., Shulman G.I., and Bogan J.S.. 2015. Acetylation of TUG protein promotes the accumulation of GLUT4 glucose transporters in an insulin-responsive intracellular compartment. J. Biol. Chem. 290:4447–4463. 10.1074/jbc.M114.603977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron J.J.M., Di Guglielmo G.M., Dahan S., Dominguez M., and Posner B.I.. 2016. Spatial and Temporal Regulation of Receptor Tyrosine Kinase Activation and Intracellular Signal Transduction. Annu. Rev. Biochem. 85:573–597. 10.1146/annurev-biochem-060815-014659 [DOI] [PubMed] [Google Scholar]
- Bergsten P., Grapengiesser E., Gylfe E., Tengholm A., and Hellman B.. 1994. Synchronous oscillations of cytoplasmic Ca2+ and insulin release in glucose-stimulated pancreatic islets. J. Biol. Chem. 269:8749–8753. [PubMed] [Google Scholar]
- Bertram R., Satin L.S., and Sherman A.S.. 2018. Closing in on the Mechanisms of Pulsatile Insulin Secretion. Diabetes. 67:351–359. 10.2337/dbi17-0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan A.P., Burgess J.W., Drake P.G., Shaver A., Bergeron J.J., and Posner B.I.. 1995. Selective activation of the rat hepatic endosomal insulin receptor kinase. Role for the endosome in insulin signaling. J. Biol. Chem. 270:10784–10791. 10.1074/jbc.270.18.10784 [DOI] [PubMed] [Google Scholar]
- Bogan J.S. 2012. Regulation of glucose transporter translocation in health and diabetes. Annu. Rev. Biochem. 81:507–532. 10.1146/annurev-biochem-060109-094246 [DOI] [PubMed] [Google Scholar]
- Bogan J.S., Rubin B.R., Yu C., Löffler M.G., Orme C.M., Belman J.P., McNally L.J., Hao M., and Cresswell J.A.. 2012. Endoproteolytic cleavage of TUG protein regulates GLUT4 glucose transporter translocation. J. Biol. Chem. 287:23932–23947. 10.1074/jbc.M112.339457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boguslavsky S., Chiu T., Foley K.P., Osorio-Fuentealba C., Antonescu C.N., Bayer K.U., Bilan P.J., and Klip A.. 2012. Myo1c binding to submembrane actin mediates insulin-induced tethering of GLUT4 vesicles. Mol. Biol. Cell. 23:4065–4078. 10.1091/mbc.E12-04-0263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose A., Guilherme A., Robida S.I., Nicoloro S.M.C., Zhou Q.L., Jiang Z.Y., Pomerleau D.P., and Czech M.P.. 2002. Glucose transporter recycling in response to insulin is facilitated by myosin Myo1c. Nature. 420:821–824. 10.1038/nature01246 [DOI] [PubMed] [Google Scholar]
- Boucher J., Kleinridders A., and Kahn C.R.. 2014. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb. Perspect. Biol. 6:a009191 10.1101/cshperspect.a009191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley E.A., Richards S.M., Keske M.A., and Rattigan S.. 2013. Local NOS inhibition impairs vascular and metabolic actions of insulin in rat hindleg muscle in vivo. Am. J. Physiol. Endocrinol. Metab. 305:E745–E750. 10.1152/ajpendo.00289.2013 [DOI] [PubMed] [Google Scholar]
- Braet F., De Zanger R., Baekeland M., Crabbé E., Van Der Smissen P., and Wisse E.. 1995. Structure and dynamics of the fenestrae-associated cytoskeleton of rat liver sinusoidal endothelial cells. Hepatology. 21:180–189. [PubMed] [Google Scholar]
- Broussard J.L., Castro A.V.B., Iyer M., Paszkiewicz R.L., Bediako I.A., Szczepaniak L.S., Szczepaniak E.W., Bergman R.N., and Kolka C.M.. 2016. Insulin access to skeletal muscle is impaired during the early stages of diet-induced obesity. Obesity (Silver Spring). 24:1922–1928. 10.1002/oby.21562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozinick J.T. Jr., and Birnbaum M.J.. 1998. Insulin, but not contraction, activates Akt/PKB in isolated rat skeletal muscle. J. Biol. Chem. 273:14679–14682. 10.1074/jbc.273.24.14679 [DOI] [PubMed] [Google Scholar]
- Brüning J.C., Gautam D., Burks D.J., Gillette J., Schubert M., Orban P.C., Klein R., Krone W., Müller-Wieland D., and Kahn C.R.. 2000. Role of brain insulin receptor in control of body weight and reproduction. Science. 289:2122–2125. 10.1126/science.289.5487.2122 [DOI] [PubMed] [Google Scholar]
- Bruno J., Brumfield A., Chaudhary N., Iaea D., and McGraw T.E.. 2016. SEC16A is a RAB10 effector required for insulin-stimulated GLUT4 trafficking in adipocytes. J. Cell Biol. 214:61–76. 10.1083/jcb.201509052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant N.J., and Gould G.W.. 2011. SNARE proteins underpin insulin-regulated GLUT4 traffic. Traffic. 12:657–664. 10.1111/j.1600-0854.2011.01163.x [DOI] [PubMed] [Google Scholar]
- Carpentier J.L., Fehlmann M., Van Obberghen E., Gorden P., and Orci L.. 1985. Redistribution of 125I-insulin on the surface of rat hepatocytes as a function of dissociation time. Diabetes. 34:1002–1007. 10.2337/diabetes.34.10.1002 [DOI] [PubMed] [Google Scholar]
- Carpentier J.L., Paccaud J.P., Gorden P., Rutter W.J., and Orci L.. 1992. Insulin-induced surface redistribution regulates internalization of the insulin receptor and requires its autophosphorylation. Proc. Natl. Acad. Sci. USA. 89:162–166. 10.1073/pnas.89.1.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvajal J.A., Germain A.M., Huidobro-Toro J.P., and Weiner C.P.. 2000. Molecular mechanism of cGMP-mediated smooth muscle relaxation. J. Cell. Physiol. 184:409–420. [DOI] [PubMed] [Google Scholar]
- Cheatham B., Volchuk A., Kahn C.R., Wang L., Rhodes C.J., and Klip A.. 1996. Insulin-stimulated translocation of GLUT4 glucose transporters requires SNARE-complex proteins. Proc. Natl. Acad. Sci. USA. 93:15169–15173. 10.1073/pnas.93.26.15169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., and Lippincott-Schwartz J.. 2013. Insulin triggers surface-directed trafficking of sequestered GLUT4 storage vesicles marked by Rab10. Small GTPases. 4:193–197. 10.4161/sgtp.26471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu T.T., Patel N., Shaw A.E., Bamburg J.R., and Klip A.. 2010. Arp2/3- and cofilin-coordinated actin dynamics is required for insulin-mediated GLUT4 translocation to the surface of muscle cells. Mol. Biol. Cell. 21:3529–3539. 10.1091/mbc.E10-04-0316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu T.T., Jensen T.E., Sylow L., Richter E.A., and Klip A.. 2011. Rac1 signalling towards GLUT4/glucose uptake in skeletal muscle. Cell. Signal. 23:1546–1554. 10.1016/j.cellsig.2011.05.022 [DOI] [PubMed] [Google Scholar]
- Christensen E.I., Birn H., Verroust P., and Moestrup S.K.. 1998. Membrane receptors for endocytosis in the renal proximal tubule. Int. Rev. Cytol. 180:237–284. 10.1016/S0074-7696(08)61772-6 [DOI] [PubMed] [Google Scholar]
- Clerk L.H., Vincent M.A., Jahn L.A., Liu Z., Lindner J.R., and Barrett E.J.. 2006. Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes. 55:1436–1442. 10.2337/db05-1373 [DOI] [PubMed] [Google Scholar]
- Cohen S.E., Kokkotou E., Biddinger S.B., Kondo T., Gebhardt R., Kratzsch J., Mantzoros C.S., and Kahn C.R.. 2007. High circulating leptin receptors with normal leptin sensitivity in liver-specific insulin receptor knock-out (LIRKO) mice. J. Biol. Chem. 282:23672–23678. 10.1074/jbc.M704053200 [DOI] [PubMed] [Google Scholar]
- Contreras-Ferrat A., Lavandero S., Jaimovich E., and Klip A.. 2014. Calcium signaling in insulin action on striated muscle. Cell Calcium. 56:390–396. 10.1016/j.ceca.2014.08.012 [DOI] [PubMed] [Google Scholar]
- Copps K.D., and White M.F.. 2012. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia. 55:2565–2582. 10.1007/s00125-012-2644-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coster A.C.F., Govers R., and James D.E.. 2004. Insulin stimulates the entry of GLUT4 into the endosomal recycling pathway by a quantal mechanism. Traffic. 5:763–771. 10.1111/j.1600-0854.2004.00218.x [DOI] [PubMed] [Google Scholar]
- Czech M.P. 2017. Insulin action and resistance in obesity and type 2 diabetes. Nat. Med. 23:804–814. 10.1038/nm.4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl D.C., Tsao T., Duckworth W.C., Mahoney M.J., and Rabkin R.. 1989. Retroendocytosis of insulin in a cultured kidney epithelial cell line. Am. J. Physiol. 257:C190–C196. 10.1152/ajpcell.1989.257.2.C190 [DOI] [PubMed] [Google Scholar]
- Dean P.M., and Matthews E.K.. 1970. Glucose-induced electrical activity in pancreatic islet cells. J. Physiol. 210:255–264. 10.1113/jphysiol.1970.sp009207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R.A., Ferrannini E., Groop L., Henry R.R., Herman W.H., Holst J.J., Hu F.B., Kahn C.R., Raz I., Shulman G.I., et al. 2015. Type 2 diabetes mellitus. Nat. Rev. Dis. Primers. 1:15019 10.1038/nrdp.2015.19 [DOI] [PubMed] [Google Scholar]
- de Jongh R.T., Serné E.H., IJzerman R.G., de Vries G., and Stehouwer C.D.A.. 2004. Impaired microvascular function in obesity: implications for obesity-associated microangiopathy, hypertension, and insulin resistance. Circulation. 109:2529–2535. 10.1161/01.CIR.0000129772.26647.6F [DOI] [PubMed] [Google Scholar]
- Diggs-Andrews K.A., Zhang X., Song Z., Daphna-Iken D., Routh V.H., and Fisher S.J.. 2010. Brain insulin action regulates hypothalamic glucose sensing and the counterregulatory response to hypoglycemia. Diabetes. 59:2271–2280. 10.2337/db10-0401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X.C., Copps K.D., Guo S., Li Y., Kollipara R., DePinho R.A., and White M.F.. 2008. Inactivation of hepatic Foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulation. Cell Metab. 8:65–76. 10.1016/j.cmet.2008.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker D.J., Habener J.F., and Holst J.J.. 2017. Discovery, characterization, and clinical development of the glucagon-like peptides. J. Clin. Invest. 127:4217–4227. 10.1172/JCI97233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W., Zhou M., Zhao W., Cheng D., Wang L., Lu J., Song E., Feng W., Xue Y., Xu P., and Xu T.. 2016. HID-1 is required for homotypic fusion of immature secretory granules during maturation. eLife. 5:241 10.7554/eLife.18134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth W.C. 1988. Insulin degradation: mechanisms, products, and significance. Endocr. Rev. 9:319–345. 10.1210/edrv-9-3-319 [DOI] [PubMed] [Google Scholar]
- Duckworth W.C., Runyan K.R., Wright R.K., Halban P.A., and Solomon S.S.. 1981. Insulin degradation by hepatocytes in primary culture. Endocrinology. 108:1142–1147. 10.1210/endo-108-4-1142 [DOI] [PubMed] [Google Scholar]
- Duncan E.R., Crossey P.A., Walker S., Anilkumar N., Poston L., Douglas G., Ezzat V.A., Wheatcroft S.B., Shah A.M., Kearney M.T., and Kearney M.I.. 2008. Effect of endothelium-specific insulin resistance on endothelial function in vivo. Diabetes. 57:3307–3314. 10.2337/db07-1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris W., Mansourian S., Chang Y., Lindsley L., Eckman E.A., Frosch M.P., Eckman C.B., Tanzi R.E., Selkoe D.J., and Guenette S.. 2003. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc. Natl. Acad. Sci. USA. 100:4162–4167. 10.1073/pnas.0230450100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehlmann M., Carpentier J.L., Le Cam A., Thamm P., Saunders D., Brandenburg D., Orci L., and Freychet P.. 1982. Biochemical and morphological evidence that the insulin receptor is internalized with insulin in hepatocytes. J. Cell Biol. 93:82–87. 10.1083/jcb.93.1.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdaoussi M., and MacDonald P.E.. 2017. Toward Connecting Metabolism to the Exocytotic Site. Trends Cell Biol. 27:163–171. 10.1016/j.tcb.2016.10.003 [DOI] [PubMed] [Google Scholar]
- Ferris H.A., and Kahn C.R.. 2016. Unraveling the Paradox of Selective Insulin Resistance in the Liver: the Brain-Liver Connection. Diabetes. 65:1481–1483. 10.2337/dbi16-0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi B.M., Abraham M.A., Yue J.T.Y., and Lam T.K.T.. 2013. Insulin and glucagon signaling in the central nervous system. Rev. Endocr. Metab. Disord. 14:365–375. 10.1007/s11154-013-9258-4 [DOI] [PubMed] [Google Scholar]
- Foley K., Boguslavsky S., and Klip A.. 2011. Endocytosis, recycling, and regulated exocytosis of glucose transporter 4. Biochemistry. 50:3048–3061. 10.1021/bi2000356 [DOI] [PubMed] [Google Scholar]
- Forli A., Vecchia D., Binini N., Succol F., Bovetti S., Moretti C., Nespoli F., Mahn M., Baker C.A., Bolton M.M., et al. 2018. Two-Photon Bidirectional Control and Imaging of Neuronal Excitability with High Spatial Resolution In Vivo. Cell Reports. 22:3087–3098. 10.1016/j.celrep.2018.02.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster L.J., and Klip A.. 2000. Mechanism and regulation of GLUT-4 vesicle fusion in muscle and fat cells. Am. J. Physiol. Cell Physiol. 279:C877–C890. 10.1152/ajpcell.2000.279.4.C877 [DOI] [PubMed] [Google Scholar]
- Friedrich R., Yeheskel A., and Ashery U.. 2010. DOC2B, C2 domains, and calcium: A tale of intricate interactions. Mol. Neurobiol. 41:42–51. 10.1007/s12035-009-8094-8 [DOI] [PubMed] [Google Scholar]
- Fukuda N., Emoto M., Nakamori Y., Taguchi A., Miyamoto S., Uraki S., Oka Y., and Tanizawa Y.. 2009. DOC2B: a novel syntaxin-4 binding protein mediating insulin-regulated GLUT4 vesicle fusion in adipocytes. Diabetes. 58:377–384. 10.2337/db08-0303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaisano H.Y. 2017. Recent new insights into the role of SNARE and associated proteins in insulin granule exocytosis. Diabetes Obes. Metab. 19(Suppl 1):115–123. 10.1111/dom.13001 [DOI] [PubMed] [Google Scholar]
- Gandasi N.R., and Barg S.. 2014. Contact-induced clustering of syntaxin and munc18 docks secretory granules at the exocytosis site. Nat. Commun. 5:3914 10.1038/ncomms4914 [DOI] [PubMed] [Google Scholar]
- Gandasi N.R., Yin P., Riz M., Chibalina M.V., Cortese G., Lund P.-E., Matveev V., Rorsman P., Sherman A., Pedersen M.G., and Barg S.. 2017. Ca2+ channel clustering with insulin-containing granules is disturbed in type 2 diabetes. J. Clin. Invest. 127:2353–2364. 10.1172/JCI88491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao T., McKenna B., Li C., Reichert M., Nguyen J., Singh T., Yang C., Pannikar A., Doliba N., Zhang T., et al. 2014. Pdx1 maintains β cell identity and function by repressing an α cell program. Cell Metab. 19:259–271. 10.1016/j.cmet.2013.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Cáceres C., Quarta C., Varela L., Gao Y., Gruber T., Legutko B., Jastroch M., Johansson P., Ninkovic J., Yi C.-X., et al. 2016. Astrocytic Insulin Signaling Couples Brain Glucose Uptake with Nutrient Availability. Cell. 166:867–880. 10.1016/j.cell.2016.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido-Sanchez L., Escote X., Coin-Aragüez L., Fernandez-Garcia J.C., El Bekay R., Vendrell J., Garcia-Fuentes E., and Tinahones F.J.. 2013. Munc18c in adipose tissue is downregulated in obesity and is associated with insulin. PLoS One. 8:e63937 10.1371/journal.pone.0063937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey W.T., Maianu L., Zhu J.H., Brechtel-Hook G., Wallace P., and Baron A.D.. 1998. Evidence for defects in the trafficking and translocation of GLUT4 glucose transporters in skeletal muscle as a cause of human insulin resistance. J. Clin. Invest. 101:2377–2386. 10.1172/JCI1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloyn A.L., Noordam K., Willemsen M.A.A.P., Ellard S., Lam W.W.K., Campbell I.W., Midgley P., Shiota C., Buettger C., Magnuson M.A., et al. 2003. Insights into the biochemical and genetic basis of glucokinase activation from naturally occurring hypoglycemia mutations. Diabetes. 52:2433–2440. 10.2337/diabetes.52.9.2433 [DOI] [PubMed] [Google Scholar]
- Goodner C.J., Sweet I.R., and Harrison H.C. Jr. 1988. Rapid reduction and return of surface insulin receptors after exposure to brief pulses of insulin in perifused rat hepatocytes. Diabetes. 37:1316–1323. 10.2337/diab.37.10.1316 [DOI] [PubMed] [Google Scholar]
- Gray S.M., Aylor K.W., and Barrett E.J.. 2017. Unravelling the regulation of insulin transport across the brain endothelial cell. Diabetologia. 60:1512–1521. 10.1007/s00125-017-4285-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith C.M., Eid T., Rose G.M., and Patrylo P.R.. 2018. Evidence for altered insulin receptor signaling in Alzheimer’s disease. Neuropharmacology.:S0028-3908(18)30008-X 10.1016/j.neuropharm.2018.01.008 [DOI] [PubMed] [Google Scholar]
- Gulbranson D.R., Davis E.M., Demmitt B.A., Ouyang Y., Ye Y., Yu H., and Shen J.. 2017. RABIF/MSS4 is a Rab-stabilizing holdase chaperone required for GLUT4 exocytosis. Proc. Natl. Acad. Sci. USA. 114:E8224–E8233. 10.1073/pnas.1712176114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann T., Kim K.H., Grzybek M., Walz T., and Coskun Ü.. 2018. Visualization of ligand-induced transmembrane signaling in the full-length human insulin receptor. J. Cell Biol.:jcb.201711047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gylfe E., Grapengiesser E., Dansk H., and Hellman B.. 2012. The neurotransmitter ATP triggers Ca2+ responses promoting coordination of pancreatic islet oscillations. Pancreas. 41:258–263. 10.1097/MPA.0b013e3182240586 [DOI] [PubMed] [Google Scholar]
- Haeusler R.A., McGraw T.E., and Accili D.. 2018. Biochemical and cellular properties of insulin receptor signalling. Nat. Rev. Mol. Cell Biol. 19:31–44. 10.1038/nrm.2017.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale L.J., and Coward R.J.M.. 2013. Insulin signalling to the kidney in health and disease. Clin. Sci. (Lond.). 124:351–370. 10.1042/CS20120378 [DOI] [PubMed] [Google Scholar]
- Hamel F.G., Mahoney M.J., and Duckworth W.C.. 1991. Degradation of intraendosomal insulin by insulin-degrading enzyme without acidification. Diabetes. 40:436–443. 10.2337/diab.40.4.436 [DOI] [PubMed] [Google Scholar]
- Henquin J.C. 2000. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes. 49:1751–1760. 10.2337/diabetes.49.11.1751 [DOI] [PubMed] [Google Scholar]
- Henquin J.C., Meissner H.P., and Schmeer W.. 1982. Cyclic variations of glucose-induced electrical activity in pancreatic B cells. Pflugers Arch. 393:322–327. 10.1007/BF00581418 [DOI] [PubMed] [Google Scholar]
- Herkner H., Klein N., Joukhadar C., Lackner E., Langenberger H., Frossard M., Bieglmayer C., Wagner O., Roden M., and Müller M.. 2003. Transcapillary insulin transfer in human skeletal muscle. Eur. J. Clin. Invest. 33:141–146. 10.1046/j.1365-2362.2003.01106.x [DOI] [PubMed] [Google Scholar]
- Hoehn K.L., Hohnen-Behrens C., Cederberg A., Wu L.E., Turner N., Yuasa T., Ebina Y., and James D.E.. 2008. IRS1-independent defects define major nodes of insulin resistance. Cell Metab. 7:421–433. 10.1016/j.cmet.2008.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingdal M., Juhl C.B., Pincus S.M., Sturis J., Veldhuis J.D., Polonsky K.S., Pørksen N., and Schmitz O.. 2000. Failure of physiological plasma glucose excursions to entrain high-frequency pulsatile insulin secretion in type 2 diabetes. Diabetes. 49:1334–1340. 10.2337/diabetes.49.8.1334 [DOI] [PubMed] [Google Scholar]
- Horwitz D.L., Starr J.I., Mako M.E., Blackard W.G., and Rubenstein A.H.. 1975. Proinsulin, insulin, and C-peptide concentrations in human portal and peripheral blood. J. Clin. Invest. 55:1278–1283. 10.1172/JCI108047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G., Buckler-Pena D., Nauta T., Singh M., Asmar A., Shi J., Kim J.Y., and Kandror K.V.. 2013. Insulin responsiveness of glucose transporter 4 in 3T3-L1 cells depends on the presence of sortilin. Mol. Biol. Cell. 24:3115–3122. 10.1091/mbc.E12-10-0765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton J.C. 1994. Insulin secretory granule biogenesis and the proinsulin-processing endopeptidases. Diabetologia. 37(Suppl 2):S48–S56. [DOI] [PubMed] [Google Scholar]
- Ishikura S., Bilan P.J., and Klip A.. 2007. Rabs 8A and 14 are targets of the insulin-regulated Rab-GAP AS160 regulating GLUT4 traffic in muscle cells. Biochem. Biophys. Res. Commun. 353:1074–1079. 10.1016/j.bbrc.2006.12.140 [DOI] [PubMed] [Google Scholar]
- Jaldin-Fincati J.R., Pavarotti M., Frendo-Cumbo S., Bilan P.J., and Klip A.. 2017. Update on GLUT4 Vesicle Traffic: A Cornerstone of Insulin Action. Trends Endocrinol. Metab. 28:597–611. 10.1016/j.tem.2017.05.002 [DOI] [PubMed] [Google Scholar]
- Jaldin-Fincati J.R., Pereira R.V.S., Bilan P.J., and Klip A.. 2018. Insulin uptake and action in microvascular endothelial cells of lymphatic and blood origin. Am. J. Physiol. Endocrinol. Metab.:ajpendo.00008.2018 10.1152/ajpendo.00008.2018 [DOI] [PubMed] [Google Scholar]
- Jansson P.-A.E., Fowelin J.P., von Schenck H.P., Smith U.P., and Lönnroth P.N.. 1993. Measurement by microdialysis of the insulin concentration in subcutaneous interstitial fluid. Importance of the endothelial barrier for insulin. Diabetes. 42:1469–1473. 10.2337/diab.42.10.1469 [DOI] [PubMed] [Google Scholar]
- Jialal I., King G.L., Buchwald S., Kahn C.R., and Crettaz M.. 1984. Processing of insulin by bovine endothelial cells in culture. Internalization without degradation. Diabetes. 33:794–800. 10.2337/diab.33.8.794 [DOI] [PubMed] [Google Scholar]
- Johnston N.R., Mitchell R.K., Haythorne E., Pessoa M.P., Semplici F., Ferrer J., Piemonti L., Marchetti P., Bugliani M., Bosco D., et al. 2016. Beta Cell Hubs Dictate Pancreatic Islet Responses to Glucose. Cell Metab. 24:389–401. 10.1016/j.cmet.2016.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S.-H., Jung C.-H., Reaven G.M., and Kim S.H.. 2018. Adapting to insulin resistance in obesity: role of insulin secretion and clearance. Diabetologia. 61:681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalwat M.A., and Thurmond D.C.. 2013. Signaling mechanisms of glucose-induced F-actin remodeling in pancreatic islet β cells. Exp. Mol. Med. 45:e37 10.1038/emm.2013.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanasaki K., Kitada M., Kanasaki M., and Koya D.. 2013. The biological consequence of obesity on the kidney. Nephrol. Dial. Transplant. 28(Suppl 4):iv1–iv7. 10.1093/ndt/gft098 [DOI] [PubMed] [Google Scholar]
- Kandror K.V., and Pilch P.F.. 2011. The sugar is sIRVed: sorting Glut4 and its fellow travelers. Traffic. 12:665–671. 10.1111/j.1600-0854.2011.01175.x [DOI] [PubMed] [Google Scholar]
- Karamitsos D.T. 2011. The story of insulin discovery. Diabetes Res. Clin. Pract. 93(Suppl 1):S2–S8. 10.1016/S0168-8227(11)70007-9 [DOI] [PubMed] [Google Scholar]
- Karunanithi S., Xiong T., Uhm M., Leto D., Sun J., Chen X.-W., and Saltiel A.R.. 2014. A Rab10:RalA G protein cascade regulates insulin-stimulated glucose uptake in adipocytes. Mol. Biol. Cell. 25:3059–3069. 10.1091/mbc.E14-06-1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karylowski O., Zeigerer A., Cohen A., and McGraw T.E.. 2004. GLUT4 is retained by an intracellular cycle of vesicle formation and fusion with endosomes. Mol. Biol. Cell. 15:870–882. 10.1091/mbc.E03-07-0517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebede M.A., Oler A.T., Gregg T., Balloon A.J., Johnson A., Mitok K., Rabaglia M., Schueler K., Stapleton D., Thorstenson C., et al. 2014. SORCS1 is necessary for normal insulin secretory granule biogenesis in metabolically stressed β cells. J. Clin. Invest. 124:4240–4256. 10.1172/JCI74072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keske M.A., Clerk L.H., Price W.J., Jahn L.A., and Barrett E.J.. 2009. Obesity blunts microvascular recruitment in human forearm muscle after a mixed meal. Diabetes Care. 32:1672–1677. 10.2337/dc09-0206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinridders A., Ferris H.A., Cai W., and Kahn C.R.. 2014. Insulin action in brain regulates systemic metabolism and brain function. Diabetes. 63:2232–2243. 10.2337/db14-0568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klip A., Ramlal T., Bilan P.J., Cartee G.D., Gulve E.A., and Holloszy J.O.. 1990. Recruitment of GLUT-4 glucose transporters by insulin in diabetic rat skeletal muscle. Biochem. Biophys. Res. Commun. 172:728–736. 10.1016/0006-291X(90)90735-6 [DOI] [PubMed] [Google Scholar]
- Klip A., Sun Y., Chiu T.T., and Foley K.P.. 2014. Signal transduction meets vesicle traffic: the software and hardware of GLUT4 translocation. Am. J. Physiol. Cell Physiol. 306:C879–C886. 10.1152/ajpcell.00069.2014 [DOI] [PubMed] [Google Scholar]
- Kolic J., and MacDonald P.E.. 2015. cAMP-independent effects of GLP-1 on β cells. J. Clin. Invest. 125:4327–4330. 10.1172/JCI85004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolka C.M., and Bergman R.N.. 2012. The barrier within: endothelial transport of hormones. Physiology (Bethesda). 27:237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolman P., Pica A., Carvou N., Boyde A., Cockcroft S., Loesch A., Pizzey A., Simeoni M., Capasso G., and Unwin R.J.. 2009. Insulin uptake across the luminal membrane of the rat proximal tubule in vivo and in vitro. Am. J. Physiol. Renal Physiol. 296:F1227–F1237. 10.1152/ajprenal.90351.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M., Sakaguchi M., Lockhart S.M., Cai W., Li M.E., Homan E.P., Rask-Madsen C., and Kahn C.R.. 2017. Endothelial insulin receptors differentially control insulin signaling kinetics in peripheral tissues and brain of mice. Proc. Natl. Acad. Sci. USA. 114:E8478–E8487. 10.1073/pnas.1710625114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Könner A.C., Janoschek R., Plum L., Jordan S.D., Rother E., Ma X., Xu C., Enriori P., Hampel B., Barsh G.S., et al. 2007. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 5:438–449. 10.1016/j.cmet.2007.05.004 [DOI] [PubMed] [Google Scholar]
- Korol S.V., Tafreshiha A., Bhandage A.K., Birnir B., and Jin Z.. 2018. Insulin enhances GABAAreceptor-mediated inhibitory currents in rat central amygdala neurons. Neurosci. Lett. 671:76–81. 10.1016/j.neulet.2018.02.022 [DOI] [PubMed] [Google Scholar]
- Kubota T., Kubota N., Kumagai H., Yamaguchi S., Kozono H., Takahashi T., Inoue M., Itoh S., Takamoto I., Sasako T., et al. 2011. Impaired insulin signaling in endothelial cells reduces insulin-induced glucose uptake by skeletal muscle. Cell Metab. 13:294–307. 10.1016/j.cmet.2011.01.018 [DOI] [PubMed] [Google Scholar]
- Laedtke T., Kjems L., Pørksen N., Schmitz O., Veldhuis J., Kao P.C., and Butler P.C.. 2000. Overnight inhibition of insulin secretion restores pulsatility and proinsulin/insulin ratio in type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 279:E520–E528. 10.1152/ajpendo.2000.279.3.E520 [DOI] [PubMed] [Google Scholar]
- Lalioti V., Muruais G., Dinarina A., van Damme J., Vandekerckhove J., and Sandoval I.V.. 2009. The atypical kinase Cdk5 is activated by insulin, regulates the association between GLUT4 and E-Syt1, and modulates glucose transport in 3T3-L1 adipocytes. Proc. Natl. Acad. Sci. USA. 106:4249–4253. 10.1073/pnas.0900218106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang D.A., Matthews D.R., Burnett M., and Turner R.C.. 1981. Brief, irregular oscillations of basal plasma insulin and glucose concentrations in diabetic man. Diabetes. 30:435–439. 10.2337/diab.30.5.435 [DOI] [PubMed] [Google Scholar]
- Lansey M.N., Walker N.N., Hargett S.R., Stevens J.R., and Keller S.R.. 2012. Deletion of Rab GAP AS160 modifies glucose uptake and GLUT4 translocation in primary skeletal muscles and adipocytes and impairs glucose homeostasis. Am. J. Physiol. Endocrinol. Metab. 303:E1273–E1286. 10.1152/ajpendo.00316.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. 2011. The CEACAM1 expression is decreased in the liver of severely obese patients with or without diabetes. Diagn. Pathol. 6:40 10.1186/1746-1596-6-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W.L., and Klip A.. 2016. Endothelial Transcytosis of Insulin: Does It Contribute to Insulin Resistance? Physiology (Bethesda). 31:336–345. [DOI] [PubMed] [Google Scholar]
- Lee M.R., Li L., and Kitazawa T.. 1997. Cyclic GMP causes Ca2+ desensitization in vascular smooth muscle by activating the myosin light chain phosphatase. J. Biol. Chem. 272:5063–5068. 10.1074/jbc.272.8.5063 [DOI] [PubMed] [Google Scholar]
- Lee S.-H., Zabolotny J.M., Huang H., Lee H., and Kim Y.-B.. 2016. Insulin in the nervous system and the mind: Functions in metabolism, memory, and mood. Mol. Metab. 5:589–601. 10.1016/j.molmet.2016.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees J.A., Messa M., Sun E.W., Wheeler H., Torta F., Wenk M.R., De Camilli P., and Reinisch K.M.. 2017. Lipid transport by TMEM24 at ER-plasma membrane contacts regulates pulsatile insulin secretion. Science. 355:6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire K., Ravier M.A., Schraenen A., Creemers J.W.M., Van de Plas R., Granvik M., Van Lommel L., Waelkens E., Chimienti F., Rutter G.A., et al. 2009. Insulin crystallization depends on zinc transporter ZnT8 expression, but is not required for normal glucose homeostasis in mice. Proc. Natl. Acad. Sci. USA. 106:14872–14877. 10.1073/pnas.0906587106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leto D., and Saltiel A.R.. 2012. Regulation of glucose transport by insulin: traffic control of GLUT4. Nat. Rev. Mol. Cell Biol. 13:383–396. 10.1038/nrm3351 [DOI] [PubMed] [Google Scholar]
- Li G., Rungger-Brändle E., Just I., Jonas J.C., Aktories K., and Wollheim C.B.. 1994. Effect of disruption of actin filaments by Clostridium botulinum C2 toxin on insulin secretion in HIT-T15 cells and pancreatic islets. Mol. Biol. Cell. 5:1199–1213. 10.1091/mbc.5.11.1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang T., Qin T., Xie L., Dolai S., Zhu D., Prentice K.J., Wheeler M., Kang Y., Osborne L., and Gaisano H.Y.. 2017. New Roles of Syntaxin-1A in Insulin Granule Exocytosis and Replenishment. J. Biol. Chem. 292:2203–2216. 10.1074/jbc.M116.769885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H.V., and Accili D.. 2011. Hormonal regulation of hepatic glucose production in health and disease. Cell Metab. 14:9–19. 10.1016/j.cmet.2011.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizunov V.A., Stenkula K.G., Lisinski I., Gavrilova O., Yver D.R., Chadt A., Al-Hasani H., Zimmerberg J., and Cushman S.W.. 2012. Insulin stimulates fusion, but not tethering, of GLUT4 vesicles in skeletal muscle of HA-GLUT4-GFP transgenic mice. Am. J. Physiol. Endocrinol. Metab. 302:E950–E960. 10.1152/ajpendo.00466.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez J.A., Burchfield J.G., Blair D.H., Mele K., Ng Y., Vallotton P., James D.E., and Hughes W.E.. 2009. Identification of a distal GLUT4 trafficking event controlled by actin polymerization. Mol. Biol. Cell. 20:3918–3929. 10.1091/mbc.E09-03-0187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar S., Genders A.J., Inyard A.C., Frison V., and Barrett E.J.. 2012. Insulin entry into muscle involves a saturable process in the vascular endothelium. Diabetologia. 55:450–456. 10.1007/s00125-011-2343-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino M.2009. Diabetes drugs: Insulin. https://www.diabetesselfmanagement.com/blog/diabetes-drugs-insulin/ (accessed March 15, 2018).
- Martin O.J., Lee A., and McGraw T.E.. 2006. GLUT4 distribution between the plasma membrane and the intracellular compartments is maintained by an insulin-modulated bipartite dynamic mechanism. J. Biol. Chem. 281:484–490. 10.1074/jbc.M505944200 [DOI] [PubMed] [Google Scholar]
- Matveyenko A.V., Liuwantara D., Gurlo T., Kirakossian D., Dalla Man C., Cobelli C., White M.F., Copps K.D., Volpi E., Fujita S., and Butler P.C.. 2012. Pulsatile portal vein insulin delivery enhances hepatic insulin action and signaling. Diabetes. 61:2269–2279. 10.2337/db11-1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch L.J., van de Bunt M., Braun M., Frayn K.N., Clark A., and Gloyn A.L.. 2011. GLUT2 (SLC2A2) is not the principal glucose transporter in human pancreatic beta cells: implications for understanding genetic association signals at this locus. Mol. Genet. Metab. 104:648–653. 10.1016/j.ymgme.2011.08.026 [DOI] [PubMed] [Google Scholar]
- McDonald A., Fogarty S., Leclerc I., Hill E.V., Hardie D.G., and Rutter G.A.. 2009. Control of insulin granule dynamics by AMPK dependent KLC1 phosphorylation. Islets. 1:198–209. 10.4161/isl.1.3.9608 [DOI] [PubMed] [Google Scholar]
- Meezan E., Pillion D.J., and Elgavish A.. 1988. Binding and degradation of 125I-insulin by isolated rat renal brush border membranes: evidence for low affinity, high capacity insulin recognition sites. J. Membr. Biol. 105:113–129. 10.1007/BF02009165 [DOI] [PubMed] [Google Scholar]
- Meglasson M.D., Burch P.T., Berner D.K., Najafi H., Vogin A.P., and Matschinsky F.M.. 1983. Chromatographic resolution and kinetic characterization of glucokinase from islets of Langerhans. Proc. Natl. Acad. Sci. USA. 80:85–89. 10.1073/pnas.80.1.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier J.J., Veldhuis J.D., and Butler P.C.. 2005. Pulsatile insulin secretion dictates systemic insulin delivery by regulating hepatic insulin extraction in humans. Diabetes. 54:1649–1656. 10.2337/diabetes.54.6.1649 [DOI] [PubMed] [Google Scholar]
- Meijer R.I., Gray S.M., Aylor K.W., and Barrett E.J.. 2016. Pathways for insulin access to the brain: the role of the microvascular endothelial cell. Am. J. Physiol. Heart Circ. Physiol. 311:H1132–H1138. 10.1152/ajpheart.00081.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael M.D., Kulkarni R.N., Postic C., Previs S.F., Shulman G.I., Magnuson M.A., and Kahn C.R.. 2000. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol. Cell. 6:87–97. 10.1016/S1097-2765(05)00015-8 [DOI] [PubMed] [Google Scholar]
- Mizuno Y., Isotani E., Huang J., Ding H., Stull J.T., and Kamm K.E.. 2008. Myosin light chain kinase activation and calcium sensitization in smooth muscle in vivo. Am. J. Physiol. Cell Physiol. 295:C358–C364. 10.1152/ajpcell.90645.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy R.F., Powers S., and Cantor C.R.. 1984. Endosome pH measured in single cells by dual fluorescence flow cytometry: rapid acidification of insulin to pH 6. J. Cell Biol. 98:1757–1762. 10.1083/jcb.98.5.1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najjar S.M. 2002. Regulation of insulin action by CEACAM1. Trends Endocrinol. Metab. 13:240–245. 10.1016/S1043-2760(02)00608-2 [DOI] [PubMed] [Google Scholar]
- Najjar S.M., Philippe N., Suzuki Y., Ignacio G.A., Formisano P., Accili D., and Taylor S.I.. 1995. Insulin-stimulated phosphorylation of recombinant pp120/HA4, an endogenous substrate of the insulin receptor tyrosine kinase. Biochemistry. 34:9341–9349. 10.1021/bi00029a009 [DOI] [PubMed] [Google Scholar]
- Nielsen S. 1993. Sorting and recycling efficiency of apical insulin binding sites during endocytosis in proximal tubule cells. Am. J. Physiol. 264:C810–C822. 10.1152/ajpcell.1993.264.4.C810 [DOI] [PubMed] [Google Scholar]
- Nielsen S. 1994. Endocytosis in renal proximal tubules. Experimental electron microscopical studies of protein absorption and membrane traffic in isolated, in vitro perfused proximal tubules. Dan. Med. Bull. 41:243–263. [PubMed] [Google Scholar]
- Nielsen S., Nielsen J.T., and Christensen E.I.. 1987. Luminal and basolateral uptake of insulin in isolated, perfused, proximal tubules. Am. J. Physiol. 253:F857–F867. [DOI] [PubMed] [Google Scholar]
- Odegaard J.I., and Chawla A.. 2013. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science. 339:172–177. 10.1126/science.1230721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer B.F., and Henrich W.L.. 2017. Carbohydrate and insulin metabolism in chronic kidney disease. https://www.uptodate.com/contents/carbohydrate-and-insulin-metabolism-in-chronic-kidney-disease (accessed March 15, 2018).
- Palmer R.M.J., Ashton D.S., and Moncada S.. 1988. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 333:664–666. 10.1038/333664a0 [DOI] [PubMed] [Google Scholar]
- Patterson J.N., Cousteils K., Lou J.W., Manning Fox J.E., MacDonald P.E., and Joseph J.W.. 2014. Mitochondrial metabolism of pyruvate is essential for regulating glucose-stimulated insulin secretion. J. Biol. Chem. 289:13335–13346. 10.1074/jbc.M113.521666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilch P.F., Shia M.A., Benson R.J., and Fine R.E.. 1983. Coated vesicles participate in the receptor-mediated endocytosis of insulin. J. Cell Biol. 96:133–138. 10.1083/jcb.96.1.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pørksen N., Munn S., Steers J., Vore S., Veldhuis J., and Butler P.. 1995. Pulsatile insulin secretion accounts for 70% of total insulin secretion during fasting. Am. J. Physiol. 269:E478–E488. [DOI] [PubMed] [Google Scholar]
- Porte D. Jr., Baskin D.G., and Schwartz M.W.. 2005. Insulin signaling in the central nervous system: a critical role in metabolic homeostasis and disease from C. elegans to humans. Diabetes. 54:1264–1276. 10.2337/diabetes.54.5.1264 [DOI] [PubMed] [Google Scholar]
- Poy M.N., Yang Y., Rezaei K., Fernström M.A., Lee A.D., Kido Y., Erickson S.K., and Najjar S.M.. 2002. CEACAM1 regulates insulin clearance in liver. Nat. Genet. 30:270–276. 10.1038/ng840 [DOI] [PubMed] [Google Scholar]
- Rabkin R., Ryan M.P., and Duckworth W.C.. 1984. The renal metabolism of insulin. Diabetologia. 27:351–357. 10.1007/BF00304849 [DOI] [PubMed] [Google Scholar]
- Randhawa V.K., Bilan P.J., Khayat Z.A., Daneman N., Liu Z., Ramlal T., Volchuk A., Peng X.R., Coppola T., Regazzi R., et al. 2000. VAMP2, but not VAMP3/cellubrevin, mediates insulin-dependent incorporation of GLUT4 into the plasma membrane of L6 myoblasts. Mol. Biol. Cell. 11:2403–2417. 10.1091/mbc.11.7.2403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randhawa V.K., Thong F.S.L., Lim D.Y., Li D., Garg R.R., Rudge R., Galli T., Rudich A., and Klip A.. 2004. Insulin and hypertonicity recruit GLUT4 to the plasma membrane of muscle cells by using N-ethylmaleimide-sensitive factor-dependent SNARE mechanisms but different v-SNAREs: role of TI-VAMP. Mol. Biol. Cell. 15:5565–5573. 10.1091/mbc.E04-03-0266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravier M.A., Güldenagel M., Charollais A., Gjinovci A., Caille D., Söhl G., Wollheim C.B., Willecke K., Henquin J.-C., and Meda P.. 2005. Loss of connexin36 channels alters beta-cell coupling, islet synchronization of glucose-induced Ca2+ and insulin oscillations, and basal insulin release. Diabetes. 54:1798–1807. 10.2337/diabetes.54.6.1798 [DOI] [PubMed] [Google Scholar]
- Robbins D.C., Shoelson S.E., Tager H.S., Mead P.M., and Gaynor D.H.. 1985. Products of therapeutic insulins in the blood of insulin-dependent (type I) diabetic patients. Diabetes. 34:510–519. 10.2337/diab.34.5.510 [DOI] [PubMed] [Google Scholar]
- Rorsman P., and Renström E.. 2003. Insulin granule dynamics in pancreatic beta cells. Diabetologia. 46:1029–1045. 10.1007/s00125-003-1153-1 [DOI] [PubMed] [Google Scholar]
- Rorsman P., Braun M., and Zhang Q.. 2012. Regulation of calcium in pancreatic α- and β-cells in health and disease. Cell Calcium. 51:300–308. 10.1016/j.ceca.2011.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein A.H., and Spitz I.. 1968. Role of the kidney in insulin metabolism and excretion. Diabetes. 17:161–169. 10.2337/diab.17.3.161 [DOI] [PubMed] [Google Scholar]
- Ruderman N.B., Kapeller R., White M.F., and Cantley L.C.. 1990. Activation of phosphatidylinositol 3-kinase by insulin. Proc. Natl. Acad. Sci. USA. 87:1411–1415. 10.1073/pnas.87.4.1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo L., Muturi H.T., Ghadieh H.E., Ghanem S.S., Bowman T.A., Noh H.L., Dagdeviren S., Dogbey G.Y., Kim J.K., Heinrich G., and Najjar S.M.. 2017. Liver-specific reconstitution of CEACAM1 reverses the metabolic abnormalities caused by its global deletion in male mice. Diabetologia. 60:2463–2474. 10.1007/s00125-017-4432-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel V.T., and Shulman G.I.. 2012. Mechanisms for insulin resistance: common threads and missing links. Cell. 148:852–871. 10.1016/j.cell.2012.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel V.T., and Shulman G.I.. 2016. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J. Clin. Invest. 126:12–22. 10.1172/JCI77812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel V.T., and Shulman G.I.. 2018. Nonalcoholic Fatty Liver Disease as a Nexus of Metabolic and Hepatic Diseases. Cell Metab. 27:22–41. 10.1016/j.cmet.2017.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano H., Kane S., Sano E., Mîinea C.P., Asara J.M., Lane W.S., Garner C.W., and Lienhard G.E.. 2003. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J. Biol. Chem. 278:14599–14602. 10.1074/jbc.C300063200 [DOI] [PubMed] [Google Scholar]
- Sano H., Eguez L., Teruel M.N., Fukuda M., Chuang T.D., Chavez J.A., Lienhard G.E., and McGraw T.E.. 2007. Rab10, a target of the AS160 Rab GAP, is required for insulin-stimulated translocation of GLUT4 to the adipocyte plasma membrane. Cell Metab. 5:293–303. 10.1016/j.cmet.2007.03.001 [DOI] [PubMed] [Google Scholar]
- Sano H., Roach W.G., Peck G.R., Fukuda M., and Lienhard G.E.. 2008. Rab10 in insulin-stimulated GLUT4 translocation. Biochem. J. 411:89–95. 10.1042/BJ20071318 [DOI] [PubMed] [Google Scholar]
- Sano H., Peck G.R., Kettenbach A.N., Gerber S.A., and Lienhard G.E.. 2011. Insulin-stimulated GLUT4 protein translocation in adipocytes requires the Rab10 guanine nucleotide exchange factor Dennd4C. J. Biol. Chem. 286:16541–16545. 10.1074/jbc.C111.228908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano H., Peck G.R., Blachon S., and Lienhard G.E.. 2015. A potential link between insulin signaling and GLUT4 translocation: Association of Rab10-GTP with the exocyst subunit Exoc6/6b. Biochem. Biophys. Res. Commun. 465:601–605. 10.1016/j.bbrc.2015.08.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satin L.S., Butler P.C., Ha J., and Sherman A.S.. 2015. Pulsatile insulin secretion, impaired glucose tolerance and type 2 diabetes. Mol. Aspects Med. 42:61–77. 10.1016/j.mam.2015.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Yoshioka K., Terasaki T., and Tsuji A.. 1991. Receptor-mediated endocytosis of A14-125I-insulin by the nonfiltering perfused rat kidney. Biochim. Biophys. Acta. 1073:442–450. 10.1016/0304-4165(91)90213-Z [DOI] [PubMed] [Google Scholar]
- Schwartz M.W., Woods S.C., Porte D. Jr., Seeley R.J., and Baskin D.G.. 2000. Central nervous system control of food intake. Nature. 404:661–671. 10.1038/35007534 [DOI] [PubMed] [Google Scholar]
- Shimoda Y., Okada S., Yamada E., Pessin J.E., and Yamada M.. 2015. Tctex1d2 Is a Negative Regulator of GLUT4 Translocation and Glucose Uptake. Endocrinology. 156:3548–3558. 10.1210/en.2015-1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song E.S., Jang H., Guo H.-F., Juliano M.A., Juliano L., Morris A.J., Galperin E., Rodgers D.W., and Hersh L.B.. 2017. Inositol phosphates and phosphoinositides activate insulin-degrading enzyme, while phosphoinositides also mediate binding to endosomes. Proc. Natl. Acad. Sci. USA. 114:E2826–E2835. 10.1073/pnas.1613447114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S.H., McIntyre S.S., Shah H., Veldhuis J.D., Hayes P.C., and Butler P.C.. 2000. Direct measurement of pulsatile insulin secretion from the portal vein in human subjects. J. Clin. Endocrinol. Metab. 85:4491–4499. [DOI] [PubMed] [Google Scholar]
- Spoto B., Pisano A., and Zoccali C.. 2016. Insulin resistance in chronic kidney disease: a systematic review. Am. J. Physiol. Renal Physiol. 311:F1087–F1108. 10.1152/ajprenal.00340.2016 [DOI] [PubMed] [Google Scholar]
- Steil G.M., Ader M., Moore D.M., Rebrin K., and Bergman R.N.. 1996. Transendothelial insulin transport is not saturable in vivo. No evidence for a receptor-mediated process. J. Clin. Invest. 97:1497–1503. 10.1172/JCI118572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg H.O., Brechtel G., Johnson A., Fineberg N., and Baron A.D.. 1994. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J. Clin. Invest. 94:1172–1179. 10.1172/JCI117433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steneberg P., Bernardo L., Edfalk S., Lundberg L., Backlund F., Ostenson C.-G., and Edlund H.. 2013. The type 2 diabetes-associated gene ide is required for insulin secretion and suppression of α-synuclein levels in β-cells. Diabetes. 62:2004–2014. 10.2337/db12-1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson R.W., Cherrington A.D., and Steiner K.E.. 1985. The relationship between plasma concentration and disappearance rate of immunoreactive insulin in the conscious dog. Horm. Metab. Res. 17:551–553. 10.1055/s-2007-1013604 [DOI] [PubMed] [Google Scholar]
- Stöckli J., Fazakerley D.J., and James D.E.. 2011. GLUT4 exocytosis. J. Cell Sci. 124:4147–4159. 10.1242/jcs.097063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Bilan P.J., Liu Z., and Klip A.. 2010. Rab8A and Rab13 are activated by insulin and regulate GLUT4 translocation in muscle cells. Proc. Natl. Acad. Sci. USA. 107:19909–19914. 10.1073/pnas.1009523107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Chiu T.T., Foley K.P., Bilan P.J., and Klip A.. 2014. Myosin Va mediates Rab8A-regulated GLUT4 vesicle exocytosis in insulin-stimulated muscle cells. Mol. Biol. Cell. 25:1159–1170. 10.1091/mbc.E13-08-0493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Jaldin-Fincati J., Liu Z., Bilan P.J., and Klip A.. 2016. A complex of Rab13 with MICAL-L2 and α-actinin-4 is essential for insulin-dependent GLUT4 exocytosis. Mol. Biol. Cell. 27:75–89. 10.1091/mbc.E15-05-0319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylow L., Jensen T.E., Kleinert M., Højlund K., Kiens B., Wojtaszewski J., Prats C., Schjerling P., and Richter E.A.. 2013. Rac1 signaling is required for insulin-stimulated glucose uptake and is dysregulated in insulin-resistant murine and human skeletal muscle. Diabetes. 62:1865–1875. 10.2337/db12-1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaka N., Yasuda N., Nihata Y., Hosooka T., Noguchi T., Aiba A., and Satoh T.. 2014. Role of the guanine nucleotide exchange factor in Akt2-mediated plasma membrane translocation of GLUT4 in insulin-stimulated skeletal muscle. Cell. Signal. 26:2460–2469. 10.1016/j.cellsig.2014.07.002 [DOI] [PubMed] [Google Scholar]
- Takenaka N., Nihata Y., and Satoh T.. 2016. Rac1 Activation Caused by Membrane Translocation of a Guanine Nucleotide Exchange Factor in Akt2-Mediated Insulin Signaling in Mouse Skeletal Muscle. PLoS One. 11:e0155292 10.1371/journal.pone.0155292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S.-C., Baeyens L., Shen C.-N., Peng S.-J., Chien H.-J., Scheel D.W., Chamberlain C.E., and German M.S.. 2018b Human pancreatic neuro-insular network in health and fatty infiltration. Diabetologia. 61:168–181. 10.1007/s00125-017-4409-x [DOI] [PubMed] [Google Scholar]
- Tang S.-C., Shen C.-N., Lin P.-Y., Peng S.-J., Chien H.-J., Chou Y.-H., Chamberlain C.E., and Pasricha P.J.. 2018a Pancreatic neuro-insular network in young mice revealed by 3D panoramic histology. Diabetologia. 61:158–167. 10.1007/s00125-017-4408-y [DOI] [PubMed] [Google Scholar]
- Taniguchi C.M., Emanuelli B., and Kahn C.R.. 2006. Critical nodes in signalling pathways: insights into insulin action. Nat. Rev. Mol. Cell Biol. 7:85–96. 10.1038/nrm1837 [DOI] [PubMed] [Google Scholar]
- Tarasov A.I., Griffiths E.J., and Rutter G.A.. 2012. Regulation of ATP production by mitochondrial Ca(2+). Cell Calcium. 52:28–35. 10.1016/j.ceca.2012.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarasov A.I., Semplici F., Li D., Rizzuto R., Ravier M.A., Gilon P., and Rutter G.A.. 2013. Frequency-dependent mitochondrial Ca(2+) accumulation regulates ATP synthesis in pancreatic β cells. Pflugers Arch. 465:543–554. 10.1007/s00424-012-1177-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurmond D.C., and Pessin J.E.. 2001. Molecular machinery involved in the insulin-regulated fusion of GLUT4-containing vesicles with the plasma membrane (review). Mol. Membr. Biol. 18:237–245. 10.1080/09687680110082400 [DOI] [PubMed] [Google Scholar]
- Török D., Patel N., Jebailey L., Thong F.S., Randhawa V.K., Klip A., and Rudich A.. 2004. Insulin but not PDGF relies on actin remodeling and on VAMP2 for GLUT4 translocation in myoblasts. J. Cell Sci. 117:5447–5455. 10.1242/jcs.01421 [DOI] [PubMed] [Google Scholar]
- Uhm M., Bazuine M., Zhao P., Chiang S.-H., Xiong T., Karunanithi S., Chang L., and Saltiel A.R.. 2017. Phosphorylation of the exocyst protein Exo84 by TBK1 promotes insulin-stimulated GLUT4 trafficking. Sci. Signal. 10:5085. [DOI] [PubMed] [Google Scholar]
- van der Heide L.P., Kamal A., Artola A., Gispen W.H., and Ramakers G.M.J.. 2005. Insulin modulates hippocampal activity-dependent synaptic plasticity in a N-methyl-d-aspartate receptor and phosphatidyl-inositol-3-kinase-dependent manner. J. Neurochem. 94:1158–1166. 10.1111/j.1471-4159.2005.03269.x [DOI] [PubMed] [Google Scholar]
- Vassilopoulos S., Esk C., Hoshino S., Funke B.H., Chen C.-Y., Plocik A.M., Wright W.E., Kucherlapati R., and Brodsky F.M.. 2009. A role for the CHC22 clathrin heavy-chain isoform in human glucose metabolism. Science. 324:1192–1196. 10.1126/science.1171529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicent D., Ilany J., Kondo T., Naruse K., Fisher S.J., Kisanuki Y.Y., Bursell S., Yanagisawa M., King G.L., and Kahn C.R.. 2003. The role of endothelial insulin signaling in the regulation of vascular tone and insulin resistance. J. Clin. Invest. 111:1373–1380. 10.1172/JCI15211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villaseñor R., Schilling M., Sundaresan J., Lutz Y., and Collin L.. 2017. Sorting Tubules Regulate Blood-Brain Barrier Transcytosis. Cell Reports. 21:3256–3270. 10.1016/j.celrep.2017.11.055 [DOI] [PubMed] [Google Scholar]
- Vincent M.A., Dawson D., Clark A.D.H., Lindner J.R., Rattigan S., Clark M.G., and Barrett E.J.. 2002. Skeletal muscle microvascular recruitment by physiological hyperinsulinemia precedes increases in total blood flow. Diabetes. 51:42–48. 10.2337/diabetes.51.1.42 [DOI] [PubMed] [Google Scholar]
- Vincent M.A., Barrett E.J., Lindner J.R., Clark M.G., and Rattigan S.. 2003. Inhibiting NOS blocks microvascular recruitment and blunts muscle glucose uptake in response to insulin. Am. J. Physiol. Endocrinol. Metab. 285:E123–E129. 10.1152/ajpendo.00021.2003 [DOI] [PubMed] [Google Scholar]
- Wang Z., and Thurmond D.C.. 2009. Mechanisms of biphasic insulin-granule exocytosis - roles of the cytoskeleton, small GTPases and SNARE proteins. J. Cell Sci. 122:893–903. 10.1242/jcs.034355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Wang A.X., Liu Z., and Barrett E.J.. 2008. Insulin signaling stimulates insulin transport by bovine aortic endothelial cells. Diabetes. 57:540–547. 10.2337/db07-0967 [DOI] [PubMed] [Google Scholar]
- Wang Q., Somwar R., Bilan P.J., Liu Z., Jin J., Woodgett J.R., and Klip A.. 1999. Protein kinase B/Akt participates in GLUT4 translocation by insulin in L6 myoblasts. Mol. Cell. Biol. 19:4008–4018. 10.1128/MCB.19.6.4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams I.M., Valenzuela F.A., Kahl S.D., Ramkrishna D., Mezo A.R., Young J.D., Wells K.S., and Wasserman D.H.. 2018. Insulin exits skeletal muscle capillaries by fluid-phase transport. J. Clin. Invest. 128:699–714. 10.1172/JCI94053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisse E. 1970. An electron microscopic study of the fenestrated endothelial lining of rat liver sinusoids. J. Ultrastruct. Res. 31:125–150. 10.1016/S0022-5320(70)90150-4 [DOI] [PubMed] [Google Scholar]
- Woods S.C., Seeley R.J., Baskin D.G., and Schwartz M.W.. 2003. Insulin and the blood-brain barrier. Curr. Pharm. Des. 9:795–800. 10.2174/1381612033455323 [DOI] [PubMed] [Google Scholar]
- Xiong W., Jordens I., Gonzalez E., and McGraw T.E.. 2010. GLUT4 is sorted to vesicles whose accumulation beneath and insertion into the plasma membrane are differentially regulated by insulin and selectively affected by insulin resistance. Mol. Biol. Cell. 21:1375–1386. 10.1091/mbc.E09-08-0751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., and Kandror K.V.. 2002. Translocation of small preformed vesicles is responsible for the insulin activation of glucose transport in adipose cells. Evidence from the in vitro reconstitution assay. J. Biol. Chem. 277:47972–47975. 10.1074/jbc.C200486200 [DOI] [PubMed] [Google Scholar]
- Xu Y., Rubin B.R., Orme C.M., Karpikov A., Yu C., Bogan J.S., and Toomre D.K.. 2011. Dual-mode of insulin action controls GLUT4 vesicle exocytosis. J. Cell Biol. 193:643–653. 10.1083/jcb.201008135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada E., Okada S., Saito T., Ohshima K., Sato M., Tsuchiya T., Uehara Y., Shimizu H., and Mori M.. 2005. Akt2 phosphorylates Synip to regulate docking and fusion of GLUT4-containing vesicles. J. Cell Biol. 168:921–928. 10.1083/jcb.200408182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokono K., Roth R.A., and Baba S.. 1982. Identification of insulin-degrading enzyme on the surface of cultured human lymphocytes, rat hepatoma cells, and primary cultures of rat hepatocytes. Endocrinology. 111:1102–1108. 10.1210/endo-111-4-1102 [DOI] [PubMed] [Google Scholar]
- Yonezawa K., Yokono K., Shii K., Hari J., Yaso S., Amano K., Sakamoto T., Kawase Y., Akiyama H., Nagata M., et al. 1988. Insulin-degrading enzyme is capable of degrading receptor-bound insulin. Biochem. Biophys. Res. Commun. 150:605–614. 10.1016/0006-291X(88)90436-6 [DOI] [PubMed] [Google Scholar]
- Zarkovic M., and Henquin J.-C.. 2004. Synchronization and entrainment of cytoplasmic Ca2+ oscillations in cell clusters prepared from single or multiple mouse pancreatic islets. Am. J. Physiol. Endocrinol. Metab. 287:E340–E347. 10.1152/ajpendo.00069.2004 [DOI] [PubMed] [Google Scholar]
- Zeng G., Nystrom F.H., Ravichandran L.V., Cong L.N., Kirby M., Mostowski H., and Quon M.J.. 2000. Roles for insulin receptor, PI3-kinase, and Akt in insulin-signaling pathways related to production of nitric oxide in human vascular endothelial cells. Circulation. 101:1539–1545. 10.1161/01.CIR.101.13.1539 [DOI] [PubMed] [Google Scholar]
- Zheng X., and Cartee G.D.. 2016. Insulin-induced Effects on the Subcellular Localization of AKT1, AKT2 and AS160 in Rat Skeletal Muscle. Sci. Rep. 6:39230 10.1038/srep39230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D., Xie L., Karimian N., Liang T., Kang Y., Huang Y.-C., and Gaisano H.Y.. 2015. Munc18c mediates exocytosis of pre-docked and newcomer insulin granules underlying biphasic glucose stimulated insulin secretion in human pancreatic beta-cells. Mol. Metab. 4:418–426. 10.1016/j.molmet.2015.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierath J.R., He L., Gumà A., Odegoard Wahlström E., Klip A., and Wallberg-Henriksson H.. 1996. Insulin action on glucose transport and plasma membrane GLUT4 content in skeletal muscle from patients with NIDDM. Diabetologia. 39:1180–1189. 10.1007/BF02658504 [DOI] [PubMed] [Google Scholar]