Abstract
Animals in groups can benefit from synchronising their behaviour, where multiple individuals conduct similar activities at the same moment in time. Previous studies have demonstrated that some species show synchronisation of vigilance behaviour, but have not explored the mechanism driving this behaviour. Synchronisation could be driven by animals copying their closest neighbours, which would mean that close proximity should lead to increased synchronisation. We simultaneously observed the behaviour of multiple individual black-headed gulls (Chroicocephalus ridibundus) within resting groups, and compared the activity of a focal individual with its two closest neighbours and a randomly selected control individual. Focal individuals were more likely to be synchronised with their closest neighbour. Synchronisation became less likely if individuals were not the closest neighbour. This suggests that synchronisation seen within groups is dependent upon the spatial positions of its members, and black-headed gulls pay more attention to their closest neighbours.
Introduction
Spending time in groups gives animals many benefits1, such as enhancing foraging success2,3, or reducing predation risk4–6 or energetic expenditure7. Group members will show specific behaviours that enhance the advantages of being together, such as coordinating their activity to efficiently utilise a resource or achieve a goal. Mechanisms involving individuals paying attention to the actions of a small number of close individuals can make it possible to coordinate a larger group8,9. This is particularly apparent in large flocks of birds10,11 or shoals of fish12,13, which can self-organise if individuals within the group coordinate through paying attention to the actions of their nearest neighbours. Coordinated behaviour can also reduce the individual predation risk experienced by group members, when the vigilance behaviour of multiple individuals allows for the early detection of predators5,14,15. Although earlier theoretical predictions suggested that it is simplest for group members to scan for predators independently of each other5,16, recent work has shown that coordination of vigilance behaviour can sometimes be beneficial. This beneficial coordination can either have individuals spreading their vigilance behaviour so that they are not scanning for the predators at the same time as each other17, or synchronising their behaviour to scan at the same time17,18.
Some species of gull rest in loafing groups during the non-breeding season. In these groups, gulls show synchronisation of non-vigilant sleeping behaviour with their neighbours19, with focal individuals tending to be asleep when the nearest two neighbours are also sleeping. This behaviour leads to temporal waves of behaviour, where the group shows cyclical patterns of vigilance or sleep20,21. Although this temporal group synchronisation could be driven by individuals monitoring the behaviour of the entire group, it is perhaps more likely that they are paying more attention to their immediate neighbours, given that other group behaviours like flocking may only involve paying attention to the actions of close neighbours10,11. We would therefore expect to see a spatial relationship between individuals and their behaviour, with closer neighbours being more likely to be vigilant or non-vigilant at the same time22. Here, we test whether black-headed gulls (Chroicocephalus ridibundus) show this spatial synchronisation in their vigilance behaviour, by comparing the behaviours of individuals with neighbours and individuals that are further away.
Materials and Methods
The Severn Estuary is home to a large wintering population of black-headed gulls23, consisting of both local breeders and winter migrants from the UK and northern Europe24. Between October and December 2017, we observed loafing groups of these gulls at a daytime aggregation site along the tidal River Avon, Bristol (looking at groups of birds collecting in the 2 km stretch between the Clifton Suspension Bridge and Gaol Ferry Bridge, with effort concentrated around the tidally-exposed mud banks around Brunel Way Bridge, 51° 26′ 55′′ N 2° 37′ 30′′ W). Observations were conducted within two hours of low tide. Most aggregations of birds seen (and all the groups considered here) consisted solely of black-headed gulls, although there were occasional visits from herring gulls (Larus argentatus), lesser black-backed gulls (Larus fuscus), and mallards (Anas platyrhynchos).
Groups of gulls were defined as all the birds associated no more than five body lengths away from each other, and we only considered groups that had a minimum of four individuals, and where there were no visual obstructions present25, such that all individuals had a clear line-of-sight to every other member of the group. The distance of five body-lengths was chosen following pilot testing, and was used because estimating individual separations beyond this length was logistically difficult for distant gulls. Choosing an arbitrary cut-off like this can have implications for generating larger association matrices26, but was sufficient for the simple relationships we consider here. Having identified a group, two observers waited at a maximum of 30 m from the birds for five minutes, to allow for habituation. At the end of this period, one individual in the group was randomly selected as a focal individual using the randomisation techniques described by Rands et al.22. After starting a timer, one observer recorded the behaviour of this focal gull after thirty seconds and then at thirty second intervals, collecting eleven consecutive observations. The gull was recorded as being vigilant if it had its head up and was moving it laterally. If its head was either down or facing forwards without moving, or its eyes were closed, or it was preening, we recorded its behaviour as non-vigilant. Wing and leg-stretches were also counted as non-vigilant behaviour. Each individual was observed for five seconds, and if it displayed any vigilance during this time, its behaviour was classified as vigilant for the period. At the same time as these observations, the other observer identified and recorded the behaviour of the closest and second-closest neighbour, along with a randomly selected control individual in the group that was neither the focal or one of its two closest neighbours. The distance of this random individual from the focal individual could therefore be anything between the distance from the focal bird to its third-closest neighbour and the distance from the focal to the furthest individual within the defined group. Because gulls could move during a series of observations, the identity of the neighbours and random gulls selected from observation to observation could potentially change, but the identity of the control bird was only changed if it either left the group or became one of the closer neighbours. After collecting a set of eleven datapoints on a group, the observers stopped recording and moved to identify a new group, and alternated tasks so that the observer following the focal individual changed. If the focal bird left the group, or if the group were disturbed and left before all eleven observations had been made, the observation attempt was abandoned. In total, 55 full sets of observations were made, and an additional 22 incomplete sets were abandoned for the reasons described (full data are available in Supplementary Data S1). The local population of gulls was estimated to be in the hundreds, and so we assumed that it was unlikely that we sampled previously observed focal individuals for each set of observations, minimising any effect of pseudoreplication through repeated sampling of the same individual.
Data were processed to calculate the proportion of observations that the nearest neighbour, second nearest neighbour, and control birds were conducting the same behaviour (using the ‘vigilant’ or ‘non-vigilant’ dichotomisation defined above) over the eleven observations. Because the focal individual was the unit of replication in this study, we compared synchrony proportions with a repeated-measures analysis of variance using a GLM in SPSS 23, after checking test requirements (the residuals were not skewed enough to impact on the results, and sphericity assumptions were met). Post hoc pairwise comparisons were conducted with a Bonferroni adjustment.
Data availability
The dataset supporting this article is available as Supplementary Data S1.
Ethical Statement
This observational study was carried out with the approval of the University of Bristol Animal Welfare and Ethics Review Body (UIN/17/064), and conducted in accordance with UK legislation.
Results
Gulls differed in their behavioural synchrony dependent upon proximity to the focal bird (F2,108 = 7.334, p = 0.001, Fig. 1), where the focal bird was vigilant for 37.4 ± 26.7% of observations (mean ± SD). The amount of synchronisation fell as the social proximity to the focal individual was reduced, with the randomly picked control birds being much less synchronised than the closest neighbour to the focal (p = 0.001, Fig. 1).
Figure 1.
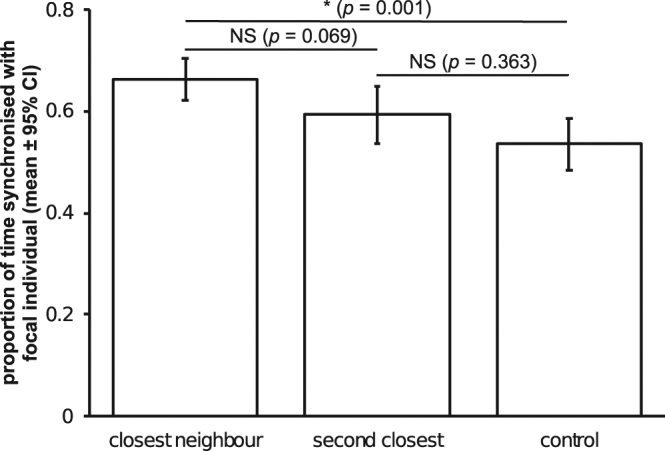
Mean proportion of time that the focal bird was synchronised with individuals of differing social proximity to it. Post hoc pairwise comparisons are reported above the bars.
Discussion
This study demonstrates that resting black-headed gulls tend to synchronise their vigilance behaviour with conspecifics who are close to them. This synchronisation is strongest with the closest neighbour, and there is a trend towards synchronisation reducing as the topological distance between individuals increases (Fig. 1), echoing similar patterns seen in red deer (Cervus elaphus)22 and in cows (Bos taurus)27. This means that as well as potentially showing temporal waves of synchronisation (as seen in other species of gull20), gulls may also show spatial patterns of synchronised behaviour.
More work would be required to identify the mechanism for the synchronisation that we observed. Synchrony within groups can emerge through social facilitation28–30, where an individual is more likely to conduct a behaviour when in the presence of another individual that is already engaged in conducting the behaviour. Alternatively, synchrony can result from some external local influence or zeitgeber31,32 causing similar responses to occur in multiple individuals at the same time. Studies that have suggested that synchronisation is induced by local stimuli usually consider behaviours that require individuals to be aggregated at a local resource, such as foraging behaviour seen at a feeder (e.g.32–34). The loafing behaviour we recorded here is less likely to be either clustered or influenced by local stimuli than feeding behaviours, but could conceivably be driven by local habitat differences in predation risk that affect vigilance behaviour, such as exposure and proximity to cover35–39, as well as position within the group and proximity to other individuals4,6,40–43. Both the influence of other individuals or environmental factors could have caused the synchronisation we observed here. We could attempt to pick apart these mechanisms by tracking the spread of a behaviour within the group using continuous sampling techniques, and we could record the spatial position and orientation of individuals along with their proximity of potentially influencing environmental factors. However, without intentional experimental manipulation, it is unclear whether there are predictable differences that separate the possible mechanisms causing the synchronisation behaviour that we observed, and we call for more sophisticated experiments and analytical techniques (such as those described in44–49) that would allow us to tease apart the mechanisms causing spatial synchronisation in groups. Any experimental manipulation of either wild resting groups or their environment is not a trivial task: the effects of social composition could be investigated using managed groups where individuals are identifiable and can be removed, whilst the effects of perceived predation risk and disturbance could be investigated by manipulating cover or cues that indicate predator presence.
We assume that individuals are simply synchronising their behaviour with whichever individual is closest, but other factors may be important for driving how individuals interact with each other. As well as the physical constraints experienced by the gulls in monitoring each other such as orientation50 and the visual field51 and the exact behavioural rules they are following52, individuals may mediate their behaviour dependent upon their sex, age or social status53, social affiliation54, energetic state55,56, personality57 or some other benefit to be gained from synchronising with a neighbour’s behaviour58. Both modelling59 and empirical evidence17,19,60 also suggest that the likelihood of synchronisation occurring will be influenced by group size, distance between individuals, and the level of disturbance and predation risk, where these different factors are likely to interact in a complex manner61,62. We also only took point-samples of behaviour at defined moments in time, and may have missed subtler, fast behaviours, such as briefly scanning to check on the behaviour of their neighbours19.
Although much work has looked at how individual behaviours can lead to complex group behaviours, surprisingly little has been done to explore how behaviours can spread within groups of animals that are known to synchronise with each other22. Using a simple observational technique like the one discussed here can allow us to explore how proximity affects behaviour, and could easily be applied to other species that collect in observable static groups. Copying the vigilance of neighbours could lead to spatial waves of vigilance in groups, and there is scope for more sophisticated theoretical work and observations that allow us to explore how collective behaviours start and spread within groups.
Electronic supplementary material
Acknowledgements
We thank the handling editorial board member and two reviewers, whose comments greatly enhanced the manuscript. SAR was supported by a NERC standard grant (NE/P012639/1).
Author Contributions
M.E. and K.L. conducted the observations and processed the data as part of their final-year BSc undergraduate project, after designing the experiment with S.A.R. S.A.R. analysed the data and wrote the initial draft of the manuscript, which was then finalised by all the authors.
Competing Interests
The authors declare no competing interests.
Footnotes
Madeleine H. R. Evans and Katie L. Lihou contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-28378-x.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Krause, J. & Ruxton, G. D. Living in groups (Oxford University Press, 2002).
- 2.Dall SRX. Can information sharing explain recruitment to food from communal roosts? Behav. Ecol. 2002;13:42–51. doi: 10.1093/beheco/13.1.42. [DOI] [Google Scholar]
- 3.Zahavi A. The function of pre-roost gatherings and communal roosts. Ibis. 1971;113:106–109. doi: 10.1111/j.1474-919X.1971.tb05131.x. [DOI] [Google Scholar]
- 4.Hamilton WD. Geometry for the selfish herd. J. Theor. Biol. 1971;31:295–311. doi: 10.1016/0022-5193(71)90189-5. [DOI] [PubMed] [Google Scholar]
- 5.Pulliam HR. On the advantages of flocking. J. Theor. Biol. 1973;38:419–422. doi: 10.1016/0022-5193(73)90184-7. [DOI] [PubMed] [Google Scholar]
- 6.Rands SA, Pettifor RA, Rowcliffe JM, Cowlishaw G. State-dependent foraging rules for social animals in selfish herds. Proc. R. Soc. B. 2004;271:2613–2620. doi: 10.1098/rspb.2004.2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marras S, et al. Fish swimming in schools save energy regardless of their spatial position. Behav. Ecol. Sociobiol. 2015;69:219–226. doi: 10.1007/s00265-014-1834-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Couzin ID, Krause J. Self-organization and collective behavior in vertebrates. Adv. Stud. Behav. 2003;32:1–75. doi: 10.1016/S0065-3454(03)01001-5. [DOI] [Google Scholar]
- 9.Jackson AL, Ruxton GD. Toward an individual-level understanding of vigilance: the role of social information. Behav. Ecol. 2006;17:532–538. doi: 10.1093/beheco/arj060. [DOI] [Google Scholar]
- 10.Cavagna A, et al. Scale-free correlations in starling flocks. Proc. Natl. Acad. Sci. USA. 2010;107:11865–11870. doi: 10.1073/pnas.1005766107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evangelista DJ, Ray DD, Raja SK, Hedrick TL. Three-dimensional trajectories and network analyses of group behaviour within chimney swift flocks during approaches to the roost. Proc. R. Soc. B. 2017;284:20162602. doi: 10.1098/rspb.2016.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herbert-Read JE, et al. Inferring the rules of interaction of shoaling fish. Proc. Natl. Acad. Sci. USA. 2011;108:18726–18731. doi: 10.1073/pnas.1109355108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang L, et al. Identifying influential neighbors in animal flocking. PLoS Comput. Biol. 2017;13:e1005902. doi: 10.1371/journal.pcbi.1005822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elgar MA. Predator vigilance and group size in mammals and birds: a critical review of the empirical evidence. Biol. Rev. 1989;64:13–33. doi: 10.1111/j.1469-185X.1989.tb00636.x. [DOI] [PubMed] [Google Scholar]
- 15.Roberts G. Why individual vigilance declines as group size increases. Anim. Behav. 1996;51:1077–1086. doi: 10.1006/anbe.1996.0109. [DOI] [Google Scholar]
- 16.Ward PI. Why birds in flocks do not coordinate their vigilance periods. J. Theor. Biol. 1985;114:383–385. doi: 10.1016/S0022-5193(85)80173-9. [DOI] [Google Scholar]
- 17.Ge C, Beauchamp G, Li Z. Coordination and synchronisation of anti-predator vigilance in two crane species. PLoS One. 2011;6:e26447. doi: 10.1371/journal.pone.0026447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pays O, et al. Prey synchronize their vigilant behaviour with other group members. Proc. R. Soc. B. 2007;274:1287–1291. doi: 10.1098/rspb.2006.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beauchamp G. Sleeping gulls monitor the vigilance behaviour of their neighbours. Biol. Lett. 2009;5:9–11. doi: 10.1098/rsbl.2008.0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beauchamp G. Collective waves of sleep in gulls (Larus spp.) Ethology. 2011;117:326–331. doi: 10.1111/j.1439-0310.2011.01875.x. [DOI] [Google Scholar]
- 21.Beauchamp G, Alexander P, Jovani R. Consistent waves of collective vigilance in groups using public information about predation risk. Behav. Ecol. 2012;23:368–374. doi: 10.1093/beheco/arr194. [DOI] [Google Scholar]
- 22.Rands SA, Muir H, Terry NL. Red deer synchronise their activity with close neighbours. PeerJ. 2014;2:e344. doi: 10.7717/peerj.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burton NHK, Musgrove AJ, Rehfisch MM, Clark NA. Birds of the Severn Estuary and Bristol Channel: their current status and key environmental issues. Marine Poll. Bull. 2010;61:115–123. doi: 10.1016/j.marpolbul.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 24.MacKinnon GE, Coulson JC. The temporal and geographical distribution of Continental black-headed gulls Larus ridibundus in the British Isles. Bird Study. 1987;34:1–9. doi: 10.1080/00063658709476927. [DOI] [Google Scholar]
- 25.Beauchamp G. Difficulties in monitoring conspecifics mediate the effects of visual obstruction on the level and synchronization of vigilance. Front. Ecol. Environ. 2017;5:12. [Google Scholar]
- 26.Rands SA. Nearest-neighbour clusters as a novel technique for assessing group associations. R. Soc. Open Sci. 2015;2:140232. doi: 10.1098/rsos.140232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stoye S, Porter MA, Dawkins MS. Synchronized lying in cattle in relation to time of day. Livest. Sci. 2012;149:70–73. doi: 10.1016/j.livsci.2012.06.028. [DOI] [Google Scholar]
- 28.Clayton DA. Socially facilitated behavior. Q. Rev. Biol. 1978;53:373–392. doi: 10.1086/410789. [DOI] [Google Scholar]
- 29.Crawford MP. The social psychology of the vertebrates. Psychol. Bull. 1939;36:407–446. doi: 10.1037/h0056268. [DOI] [Google Scholar]
- 30.Deneubourg JL, Goss S. Collective patterns and decision-making. Ethol. Ecol. Evol. 1989;1:295–311. doi: 10.1080/08927014.1989.9525500. [DOI] [Google Scholar]
- 31.Flury R, Gygax L. Daily patterns of synchrony in lying and feeding of cows: quasi-natural state and (anti-) synchrony factors. Behav. Process. 2016;133:56–61. doi: 10.1016/j.beproc.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Keeling LJ, Newberry RC, Estevez I. Flock size during rearing affects pullet behavioural synchrony and spatial clustering. Appl. Anim. Behav. Sci. 2017;194:36–41. doi: 10.1016/j.applanim.2017.04.002. [DOI] [Google Scholar]
- 33.Collins LM, Sumpter DJT. The feeding dynamics of broiler chickens. J. R. Soc. Interface. 2007;4:65–72. doi: 10.1098/rsif.2006.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collins LM, Asher L, Pfeiffer DU, Browne WJ, Nicol CJ. Clustering and synchrony in laying hens: the effect of environmental resources on social dynamics. Appl. Anim. Behav. Sci. 2011;129:43–53. doi: 10.1016/j.applanim.2010.10.007. [DOI] [Google Scholar]
- 35.Lima SL, Dill LM. Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 1990;68:619–640. doi: 10.1139/z90-092. [DOI] [Google Scholar]
- 36.Nonacs, P. & Blumstein, D. T. in Evolutionarybehavioral ecology (eds D. F. Westneat & C. W. Fox) 207–221 (Oxford University Press, 2010).
- 37.Blanchard P, Lauzeral C, Chamaillé-Jammes S, Yoccoz NG, Pontier D. Analyzing the proximity to cover in a landscape of fear: a new approach to fine-scale habitat use by rabbits facing feral cat predation on Kerguelen archipelago. PeerJ. 2016;4:e1769. doi: 10.7717/peerj.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rands SA. Leaving safety to visit a feeding site: is it optimal to hesitate while exposed? R. Soc. Open Sci. 2017;4:160910. doi: 10.1098/rsos.160910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown JS. Vigilance, patch use and habitat selection: foraging under predation risk. Evol. Ecol. Res. 1999;1:49–71. [Google Scholar]
- 40.Morton TL, Haefner JW, Nugala V, Decimo RD, Mendes L. The selfish herd revisited: do simple movement rules reduce relative predation risk? J. Theor. Biol. 1994;167:73–79. doi: 10.1006/jtbi.1994.1051. [DOI] [Google Scholar]
- 41.Viscido SV, Miller M, Wethey DS. The response of a selfish herd to an attack from outside the group perimeter. J. Theor. Biol. 2001;208:315–328. doi: 10.1006/jtbi.2000.2221. [DOI] [PubMed] [Google Scholar]
- 42.Morrell LJ, Romey WL. Optimal individual positions within animal groups. Behav. Ecol. 2008;19:909–919. doi: 10.1093/beheco/arn050. [DOI] [Google Scholar]
- 43.Morrell LJ, Ruxton GD, James R. Spatial positioning in the selfish herd. Behav. Ecol. 2011;22:16–22. doi: 10.1093/beheco/arq157. [DOI] [Google Scholar]
- 44.Zwicker B, Weber R, Wechsler B, Gygax L. Degree of synchrony based on individual observations underlines the importance of concurrent access to enrichment materials in finishing pigs. Appl. Anim. Behav. Sci. 2015;172:26–32. doi: 10.1016/j.applanim.2015.08.037. [DOI] [Google Scholar]
- 45.Engel J, Lamprecht J. Doing what everybody does? A procedure for investigating behavioural synchronisation. J. Theor. Biol. 1997;185:255–262. doi: 10.1006/jtbi.1996.0359. [DOI] [PubMed] [Google Scholar]
- 46.Ruckstuhl KE. To synchronise or not to synchronise: a dilemma for young bighorn males? Behaviour. 1999;136:805–818. doi: 10.1163/156853999501577. [DOI] [Google Scholar]
- 47.Asher L, Collins LM. Assessing synchrony in groups: are you measuring what you think you are measuring? Appl. Anim. Behav. Sci. 2012;138:162–169. doi: 10.1016/j.applanim.2012.02.004. [DOI] [Google Scholar]
- 48.Rook AJ, Penning RD. Synchronisation of eating, ruminating and idling activity by grazing sheep. Appl. Anim. Behav. Sci. 1991;32:157–166. doi: 10.1016/S0168-1591(05)80039-5. [DOI] [Google Scholar]
- 49.Raussi S, et al. A note on overdispersion as an index of behavioural synchrony: a pilot study in dairy cows. Animal. 2011;5:428–432. doi: 10.1017/S1751731110001928. [DOI] [PubMed] [Google Scholar]
- 50.McDougall PL, Ruckstuhl KE. Doing what your neighbour does: neighbour proximity, familiarity and postural alignment increase behavioural mimicry. Anim. Behav. 2018;135:177–185. doi: 10.1016/j.anbehav.2017.11.009. [DOI] [Google Scholar]
- 51.Butler SR, Hosinski EC, Lucas JR, Fernández-Juricic E. Social birds copy each other’s lateral scans while monitoring group mates with low-acuity vision. Anim. Behav. 2016;121:21–31. doi: 10.1016/j.anbehav.2016.08.002. [DOI] [Google Scholar]
- 52.Rands SA. Approximating optimal behavioural strategies down to rules-of-thumb: energy reserve changes in pairs of social foragers. PLoS One. 2011;6:e22104. doi: 10.1371/journal.pone.0022104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rands SA. The effects of dominance on leadership and energetic gain: a dynamic game between pairs of social foragers. PLoS Comput. Biol. 2011;7:e1002252. doi: 10.1371/journal.pcbi.1002252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Connor RC, Smolker R, Bejder L. Synchrony, social behaviour and alliance affiliation in Indian Ocean bottlenose dolphins. Tursiops aduncus. Anim. Behav. 2006;72:1371–1378. doi: 10.1016/j.anbehav.2006.03.014. [DOI] [Google Scholar]
- 55.Rands SA, Cowlishaw G, Pettifor RA, Rowcliffe JM, Johnstone RA. The spontaneous emergence of leaders and followers in a foraging pair. Nature. 2003;423:432–434. doi: 10.1038/nature01630. [DOI] [PubMed] [Google Scholar]
- 56.Rands SA, Cowlishaw G, Pettifor RA, Rowcliffe JM, Johnstone RA. The emergence of leaders and followers in foraging pairs when the qualities of individuals differ. BMC Evol. Biol. 2008;8:51. doi: 10.1186/1471-2148-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McDonald ND, Rands SA, Hill F, Elder C, Ioannou CC. Consensus and experience trump leadership, suppressing individual personality during social foraging. Sci. Adv. 2016;2:e1600892. doi: 10.1126/sciadv.1600892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duranton C, Gaunet F. Behavioural synchronization from an ethological perspective: overview of its adaptive value. Adapt. Behav. 2016;24:181–191. doi: 10.1177/1059712316644966. [DOI] [Google Scholar]
- 59.Fernández-Juricic E, Siller S, Kacelnik A. Flock density, social foraging, and scanning: an experiment with starlings. Behav. Ecol. 2004;15:371–379. doi: 10.1093/beheco/arh017. [DOI] [Google Scholar]
- 60.Öst M, Tierala T. Synchronized vigilance while feeding in common eider brood-rearing coalitions. Behav. Ecol. 2011;22:378–384. doi: 10.1093/beheco/arq223. [DOI] [Google Scholar]
- 61.Pays O, Dubot A-L, Jarman PJ, Loisel P, Goldizen AW. Vigilance and its complex synchrony in the red-necked pademelon. Thylogale thetis. Behav. Ecol. 2009;20:22–29. doi: 10.1093/beheco/arn110. [DOI] [Google Scholar]
- 62.Li C, Beauchamp G, Wang Z, Cui P. Collective vigilance in the wintering hooded crane: the role of flock size and anthropogenic disturbances in a human-dominated landscape. Ethology. 2016;122:999–1008. doi: 10.1111/eth.12570. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset supporting this article is available as Supplementary Data S1.


