Abstract
Double membrane structure, autophagosome, is formed de novo in the process of autophagy in the yeast Saccharomyces cerevisiae, and many Apg proteins participate in this process. To further understand autophagy, we analyzed the involvement of factors engaged in the secretory pathway. First, we showed that Sec18p (N-ethylmaleimide-sensitive fusion protein, NSF) and Vti1p (soluble N-ethylmaleimide-sensitive fusion protein attachment protein, SNARE), and soluble N-ethylmaleimide-sensitive fusion protein receptor are required for fusion of the autophagosome to the vacuole but are not involved in autophagosome formation. Second, Sec12p was shown to be essential for autophagy but not for the cytoplasm to vacuole-targeting (Cvt) (pathway, which shares mostly the same machinery with autophagy. Subcellular fractionation and electron microscopic analyses showed that Cvt vesicles, but not autophagosomes, can be formed in sec12 cells. Three other coatmer protein (COPII) mutants, sec16, sec23, and sec24, were also defective in autophagy. The blockage of autophagy in these mutants was not dependent on transport from endoplasmic reticulum-to-Golgi, because mutations in two other COPII genes, SEC13 and SEC31, did not affect autophagy. These results demonstrate the requirement for subgroup of COPII proteins in autophagy. This evidence demonstrating the involvement of Sec proteins in the mechanism of autophagosome formation is crucial for understanding membrane flow during the process.
INTRODUCTION
Organelles carry out many cellular functions in eucaryotic cells. Membranes consisting of the endomembrane system, such as the endoplasmic reticulum (ER), Golgi apparatus, lysosome/vacuole, endosome, and plasma membrane, are maintained by dynamic membrane flow between them (Palade, 1975). Autophagy displays unique membrane dynamics, distinct from classical membrane trafficking (Klionsky and Ohsumi, 1999). Under nutrient starvation conditions, an isolation membrane extends to enclose the cytosol and organelles, resulting in a double-membrane structure called an autophagosome. The yeast vacuolar enzyme aminopeptidase I (API) is synthesized in the cytosol and transported to the vacuole, a process known as the cytoplasm to vacuole targeting (Cvt) pathway (Klionsky and Ohsumi, 1999). In a growing cell, the precursor of API (proAPI) in the cytoplasm is selectively sequestered to the Cvt vesicle (130–150 nm in diameter), which is smaller than the autophagosome (300–900 nm), and transported to the vacuole (Scott et al., 1996; Baba et al., 1997). Under starvation conditions, proAPI is transported to the vacuole via the autophagosome (Baba et al., 1997). The outer membrane of the autophagosome fuses to the lysosome/vacuole, and its contents are degraded (Dunn, 1990; Baba et al., 1994). Vam3p (t-soluble N-ethylmaleimide [NEM]-sensitive fusion protein [NSF] attachment protein [SNAP] receptor [SNARE]) and Vps18p have been identified as the fusion factors of the vacuole (Darsow et al., 1997; Rieder and Emr, 1997). However, the complete set of components making up the fusion machinery has not yet been determined.
Several groups, including ours, have isolated yeast mutants defective in autophagy or the Cvt pathway (apg, aut, and cvt) to elucidate their molecular mechanisms (Tsukada and Ohsumi, 1993; Thumm et al., 1994; Harding et al., 1995). These gene products contain novel characteristic proteins such as components of a ubiquitin-like protein conjugation system, a protein lipidation system, protein kinase complexes, and an autophagy-specific phosphatidyl inositol 3 kinase complex (Mizushima et al., 1998; Ichimura et al., 2000; Kamada et al., 2000; Kihara et al., 2001). Most autophagy mutants are also defective in the Cvt pathway, indicating that the molecular machinery required for these two pathways overlaps significantly (Harding et al., 1996; Scott et al., 1996). On the other hand, some proteins function exclusively in one pathway or the other. Tlg2p and Vps45p were shown to be essential for the Cvt pathway but not for autophagy (Abeliovich et al., 1999). Recently, Cvt9p and Vac8p were found to be required only for the Cvt pathway, and Apg17p is necessary only for autophagy (Kamada et al., 2000; Scott et al., 2000).
In mammalian cells, some studies have suggested that the autophagosome is derived from the ER, but the results of other studies have not been consistent with this conclusion (Dunn, 1990; Yamamoto et al., 1990; Ueno et al., 1991; Stromhaug et al., 1998). In yeast, we proposed through morphological studies that preexisting organelles do not directly form the isolation membrane (Baba et al., 1994); instead, certain intermediate structures were found at the extending sites of the isolation membrane (Kirisako et al., 1999). Until now, the relationship between the autophagosomal membrane and the secretory pathway has not been analyzed in yeast.
We have screened autophagy-defective mutants, excluding those mutants with growth defects or aberrant vacuolar morphology. Therefore, all apg mutants show specific defects in autophagy and the Cvt pathway (Klionsky and Ohsumi, 1999). Here, we investigated a series of temperature-sensitive mutants in the secretory pathway to evaluate their roles in autophagy. The fusion apparatus, NSF/SNARE, was revealed to mediate fusion between the autophagosome and the vacuole. In addition, a specific subgroup of COPII vesicle-budding factors is necessary for autophagosome formation but not for Cvt vesicle formation, implying a close relation between autophagy and the secretory pathway.
MATERIALS AND METHODS
Strains, Media, and Materials
Media used in this study were prepared as described previously (Adams et al., 1997; Kirisako et al., 1999). Yeast strains in this study were constructed with the use of standard yeast genetic methods for sporulation, tetrad analysis, and gene disruption (Adams et al., 1997). TN125 has been described previously (Noda and Ohsumi, 1998). CKY496 (sec24-1; Kurihara et al., 2000) and RSY1004 (sec31-1; Salama et al., 1997) were gifts from Dr. R. Schekman (University of California, Berkeley, CA). The other original sec mutants were provided by Dr. A. Nakano (The Institute of Physical and Chemical Research (RIKEN, Wako); Novick et al., 1980). FvMY7 (vti1-1), FvMY24 (vti1-2), and FvMY21 (vti1-11; Fischer von Mollard and Stevens, 1999) were gifts from Dr. T. Stevens (University of Oregon, Eugene, OR). RSY1181 (cdc48-3; Latterich et al., 1995) was a gift from Dr. M. Latterich (Salk Institute, La Jolla, CA). YNM104 (MAT a leu2 ura3 trp1 his3 lys2 ade2 pho8::PHO8Δ60 Δypt7::HIS3), YNI02 (MATa leu2 ura3 trp1 his3 lys2 ade2 pho8::PHO8Δ60 Δypt7::URA3 Δapg7::HIS3), and NINY1 (MATa leu2 ura3 trp1 his3 lys2 ade2 pho8::PHO8Δ60 Δnyv1::LEU2) were constructed from TN125. KVY55 (MATα leu2 ura3 trp1 his3 lys2 suc2 pho8::PHO8Δ60) was constructed from SEY6210. NIS4 (MATa leu2 ura3 trp1 his3 lys2 pho8::PHO8Δ60 sec4-2), NIS12 (MATa leu2 ura3 trp1 his3 lys2 ade2 pho8::PHO8Δ60 sec12-4), NIS13 (MATa leu2 ura3 trp1 his3 lys2 pho8::PHO8Δ60 sec13-1), MHY100 (MATa leu2 ura3 trp1 his3 lys2 pho8::PHO8Δ60 sec15-1), NIS16 (MATα his3 pho8::PHO8Δ60 sec16-2), NIS17 (MATa leu2 ura3 trp1 his3 lys2 pho8::PHO8Δ60 sec17-1), NIS18 (MATα leu2 ura3 trp1 his3 lys2 ade2 pho8::PHO8Δ60 sec18-1), NIS23 (MATa trp1 pho8::PHO8Δ60 sec23-1), MHY24 (MATα leu2 ura3 trp1 his3 ade2 pho8::PHO8Δ60 sec24-1), MHY22 (MATa leu2 ura3 trp1 his3 ade2 pho8::PHO8Δ60 sec31-1), NIV111 (MATα leu2 ura3 trp1 his3 pho8::PHO8Δ60 vti1-11), and NIC48 (MATa leu2 ura3 trp1 pho8::PHO8Δ60 cdc48-3) were obtained by mating original mutants with KVY55 or TN125 and by tetrad dissection. YNI07 (MATa leu2 trp1 his3 lys2 ade2 pho8::PHO8Δ60 Δypt7::URA3 sec12-4) and YNI08 (MATa ura3 trp1 his3 lys2 ade2 pho8::PHO8Δ60 Δcvt9::LEU2 sec12-4) were constructed from NIS12.
Plasmids pSHY6-4 and pANY2-7 (Nakano and Muramatsu, 1989) were gifts from Dr. A. Nakano (RIKEN), antiserum against API was provided by Dr. D. J. Klionsky (University of Michigan, Ann Arbor, MI), and antiserum against Bmh1p was a gift from Drs. M. Sakaguchi and K. Mihara (Kyushu University, Fukuoka, Japan).
OptiPrep (Nycomed Pharma, Oslo, Norway), Zymolyase 100T (Seikagaku Kogyo, Tokyo, Japan), protease inhibitor cocktail Complete EDTA-free (Boehringer Mannheim, Mannheim, Germany), EXPRESS protein-labeling mixture, 35S (NEN, Boston, MA), and protein A-Sepharose CL-4B (Amersham Pharmacia, Uppsala, Sweden) were each purchased.
Subcellular Fractionation and Proteinase K Treatment
Cell lysate was prepared as previously described (Harding et al., 1995), with minor modifications. Cells (200 OD600 U) cultured in YPD medium (OD600 = 1) or SD(-N) medium (OD600 = 2) were harvested, washed with 100 mM Tris-HCl, pH 9.0, 40 mM 2-mercaptoethanol, and resuspended in 10 ml of YPD supplemented with 1 M sorbitol and 20 mM Tris-HCl, pH 7.5, for growing cells or SD(-N) supplemented with 1 M sorbitol and 20 mM Tris-HCl, pH 7.5, for starved cells. After the addition of 1 mg of Zymolyase 100T, the cell suspensions were shaken gently for 30 min. The spheroplasts were harvested, washed with 1 M sorbitol, resuspended at 30 OD600/ml in a lysis buffer (20 mM 1,4-piperazinediethanesulfonic acid (PIPES)-KOH, pH 6.8, 200 mM sorbitol, 1 mM phenylmethylsulfonyl fluoride [PMSF], and the protease inhibitor cocktail), and incubated on ice for 5 min. Cleared lysate (total) was generated by two consecutive centrifugations at 500 × g for 5 min. The lysate was spun at 13,000 × g for 15 min to separate the pellet (LSP), and the supernatant was centrifuged again at 100,000 × g for 1 h to generate a pellet (HSP) and supernatant (HSS). LSP and HSP were each resuspended in a volume of lysis buffer equal to the original lysate. For milder cell lysis (Figure 2B), cells were converted to spheroplasts in 1.4 M sorbitol, 20 mM Tris-HCl, pH 7.5, with SD(-N) and resuspended in lysis buffer with 1 M sorbitol, 0.5% Ficoll 400, and 1 mM MgCl2. Under these conditions, most of the cells were not lysed. The spheroplast suspension was passed through a polycarbonate filter with 3-μm pores (Nucleopore, Whatman, Maidstone, United Kingdom), centrifuged at 500 × g for 5 min to remove cell debris, and fractionated as described above.
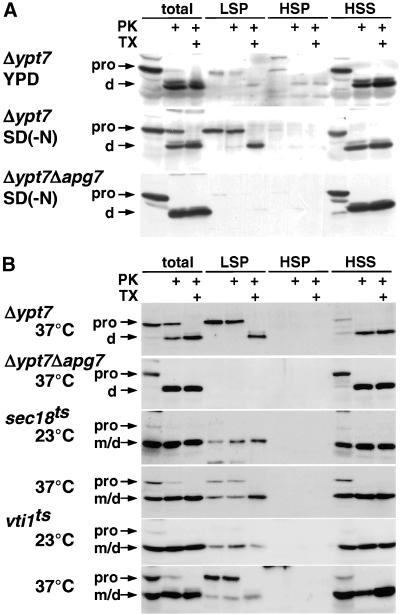
Figure 2.
Accumulation of autophagosomes in sec18 and vti1 mutants. (A) Fractionation of autophagosomes and Cvt vesicles. Δypt7 cells growing in YPD or Δypt7 cells and Δypt7Δapg7 cells starved in SD(-N) were converted to spheroplasts and lysed. Cleared lysates were obtained by centrifugation at 500 × g for 5 min and were then centrifuged at 13,000 × g for 15 min to generate the LSP. The supernatants were further centrifuged at 100,000 × g for 1 h to separate the HSP and HSS. Each fraction was treated with 100 μg/ml proteinase K (PK) with or without 1% Triton X-100 (TX) and then analyzed by immunoblotting with antiserum to API. (B) Biochemical detection of autophagosomes in sec18 and vti1-11. Δypt7 and Δypt7Δapg7 cells were cultured at 37°C, and sec18 and vti1-11 were cultured at 23 or 37°C in SD(-N) for 3 h. Cell lysis under milder conditions, subcellular fractionation, and protease treatment were performed as described in MATERIALS AND METHODS. Each sample was subjected to SDS-PAGE and immunoblotting with anti-API serum. Precursor (pro) and mature forms (m) of API are indicated. d, the proteinase K digestion products of proAPI.
To examine proteinase K sensitivity, each fraction was diluted twofold in the lysis buffer without protease inhibitors and treated with 100 μg/ml proteinase K on ice for 30 min with or without 1% Triton X-100. The samples were precipitated with 10% trichloroacetic acid, washed once with ice-cold acetone, resuspended in SDS-PAGE sample buffer, and analyzed by SDS-PAGE.
Density Gradient Centrifugation
OptiPrep solutions were prepared in lysis buffer supplemented with 1 mM MgCl2. A density gradient was prepared from the bottom to the top, as follows: 0.5 ml of 50%, 1 ml of 40%, 1 ml of 30%, 1.5 ml of 25%, 2 ml of 20%, 2 ml of 15%, and 1.5 ml of 10% (wt/vol.). One milliliter of the LSP fraction was layered on top of the gradient and centrifuged for 16 h at 174,000 × g at 4°C in a P40ST rotor (Hitachi, Tokyo, Japan). Twenty-eight drops each were collected from the top of the gradient, resulting in 14 fractions. Each fraction was solubilized with SDS-PAGE sample buffer and analyzed by SDS-PAGE.
Alkaline Phosphatase Assay
Alkaline phosphatase (ALP) assay was performed as previously described (Noda and Ohsumi, 1998).
Phloxine B Staining
Staining of the cells with Phloxine B was carried out as previously described (Tsukada and Ohsumi, 1993).
Cell Labeling and Immunoprecipitation
Cells precultured in SC(-Met) at 23°C were suspended at 5 OD600/ml in 0.6 ml of SC(-Met) and cultured at 37°C for 10 or 60 min. The cells were pulse-labeled by adding 100 μCi of [35S]methionine at 37°C for 20 min. The cell culture was chased by fivefold dilution with SC(-Met) containing 8 mM methionine and 4 mM cysteine and incubated at 37°C. For the starved conditions, the cell culture was chased by dilution with SD(-N) containing the same concentrations of methionine and cysteine. The cells were harvested, washed once, and disrupted with glass beads (425–600 μm; Sigma, St. Louis, MO) in 200 μl of 1% SDS in TEN (50 mM Tris-HCl, pH 7.5, 5 mM EDTA, and 150 mM NaCl). The crude lysate was incubated for 5 min at 95°C, diluted with 800 μl of 2% Triton X-100 in TEN, and centrifuged at 15,000 × g for 10 min to remove insoluble materials. After addition of anti-API serum and 10 μl of protein A-Sepharose beads, the sample was incubated for 2 h at room temperature. The beads were washed sequentially twice with IP buffer (0.2% SDS and 1% Triton X-100 in TEN), twice with urea buffer (2 M urea in IP buffer), once with high-salt buffer (500 mM NaCl in IP buffer), and once with TEN. Proteins were eluted with SDS-PAGE sample buffer and analyzed by SDS-PAGE and a Bioimage analyzer (BAS2000, Fuji Photo Film, Tokyo, Japan).
To assay protein secretion to the medium, cells were labeled with [35S]methionine in SC(-Met) containing 100 μg/ml α2-macroglobulin and 300 μg/ml bovine serum albumin. Proteins in the medium were precipitated with a final concentration of 10% trichloroacetic acid, washed twice in 10% trichloroacetic acid, and washed once in ice-cold acetone. The incorporated radioactivity into the proteins was measured in liquid scintillation counter.
Electron Microscopy
Wild-type, sec12, and sec18 cells grown in YPD at 23°C were collected, transferred to SD(-N) at 2 OD600/ml, and incubated at 23°C for 1 h. After a 30-min incubation at 37°C, cells were treated with PMSF to prevent autophagic bodies from degrading and further incubated at 37°C for 2.5 h. The cells were harvested, sandwiched between two aluminum disks, and quickly frozen by a high-pressure freezing machine (HPM010S, BAL-TEC, Principality of Liechtenstein). The cells were fixed in acetone containing 2% osmium tetroxide, kept below -80°C for 3 d, and then gradually warmed at room temperature. The cells were placed in propylene oxide for 20 min and then embedded in Spurr resin. Thin sections were cut with a diamond knife in a Ultracut R (Leica, Japan), stained with 4% uranyl acetate for 1 h and with lead citrate for 5 min, and examined in a Hitachi H-7500 electron microscope at an acceleration voltage of 80 kV.
RESULTS
Sec18p and Vti1p Are Essential for Autophagy
First, we investigated the membrane fusion machinery involved in autophagy. Most of the fusion processes in the endomembrane system are promoted by NSF, SNAP, and SNARE (Beckers et al., 1989; Graham and Emr, 1991; Mayer et al., 1996; Yoshimori et al., 1996).
Recently, the multispecific v-SNARE Vti1p was shown to be essential for the Cvt pathway (Fischer von Mollard and Stevens, 1999); however, its requirement for autophagy has not been elucidated. We therefore examined the autophagic activity of vti1ts mutants with the use of the ALP assay system, in which a truncated form of Pho8p (Pho8Δ60p) expressed in the cytosol is transported to the vacuole via autophagy and processed to an active form (Noda et al., 1995; Noda and Ohsumi, 1998).
ALP activity did not increase when the vti1-11ts mutant was cultured in starvation medium SD(-N) at a nonpermissive temperature, 34°C, whereas it increased at a permissive temperature, 23°C (Figure 1A). The mutant cell was confirmed in this and all subsequent experiments to express similar amounts of Pho8Δ60p as seen in the wild-type cells. Other allelic mutants, vti1-1ts and vti1-2ts, were also defective at a nonpermissive temperature. Thus, Vti1p appears to be essential for autophagy.
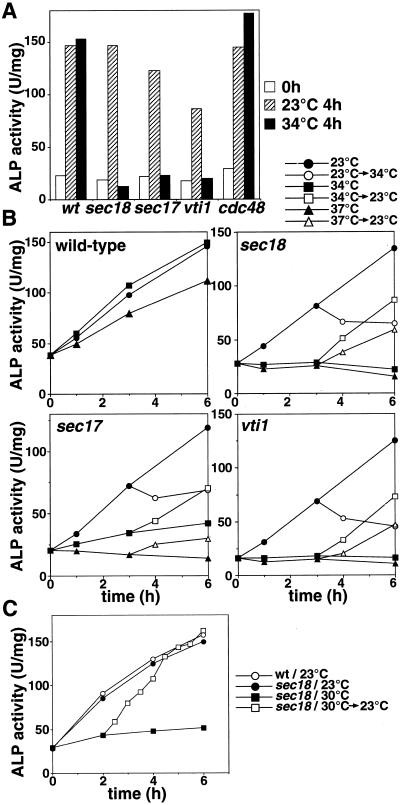
Figure 1.
Sec18p and Vti1p are essential for the autophagy. (A) Cells expressing Pho8Δ60p (wild-type [wt], sec18, sec17, vti1-11, and cdc48-3) cultured in YPD at 23°C (open bar) were shifted to SD(-N) and cultured for 4 h at 23°C (shaded bar) or 34°C (filled bar). The ALP activity of the lysate was measured to estimate autophagic activity. (B) Time course of autophagy. Wild-type, sec18, sec17, and vti1-11 cultured in YPD at 23°C were shifted to SD(-N) at 23°C (circle), 34°C (square), or 37°C (triangle). Cells cultured in SD(-N) for 3 h were shifted from 23 to 34°C (open circle), from 34 to 23°C (open square), or from 37 to 23°C (open triangle). (C) Recovery of autophagy in sec18. Wild-type (wt) and sec18 cells cultured in YPD at 23°C were shifted to SD(-N) at 23°C (wt, open circle; sec18, filled circle) or 30°C (sec18, filled square). sec18 cells cultured in SD(-N) at 30°C for 2 h were shifted to 23°C (open square).
Next, we analyzed an effect of a temperature-sensitive mutation in SEC18 to examine the involvement of NSF in autophagy (Figure 1A). In the sec18 mutant, autophagy, monitored by ALP activity, proceeded normally at a permissive temperature under starvation conditions but did not occur at a nonpermissive temperature (34°C), implying that Sec18p is also required for the process. This mutant accumulated the p1 form of the vacuolar enzyme carboxypeptidase Y at nonpermissive temperatures, indicating that ER-to-Golgi transport was blocked. The temperature-sensitive mutant of SEC17, encoding α-SNAP, was also defective in autophagy at a nonpermissive temperature (Figure 1A), supporting the notion that NSF is crucial to the process. Cdc48p, which has significant homology with Sec18p, is known to be required for ER homotypic fusion (Latterich et al., 1995). The cdc48-3ts mutant cultured in SD(-N) at 34°C showed normal autophagic activity, as seen in wild-type cells (Figure 1A), although >70% of the mutant cells were arrested at the G2 stage in the cell cycle and ER fusion has been reported to be defective at this temperature (Latterich et al., 1995). Thus, we concluded that Cdc48p is not required for autophagy.
When the sec18 cells cultured in SD(-N) at 23°C for 3 h were shifted to 34°C, the increase in ALP activity stopped immediately (Figure 1B). This illustrates the direct effect of the sec18 mutation on autophagy. Blockage of secretion may cause cell death by loss of integrity of the plasma membrane. However, ALP activity increased in cells subsequently shifted down to 23°C after 3 h of incubation at the restrictive temperature (Figure 1B). In addition, after 3 h incubation at 34 or 37°C, >70% of the sec18 mutant cells were still viable, judging from the staining with phloxine B, a specific dye against dead cells. Similarly, in vti1 and sec17 cells, ALP activity was recovered by shifting from 34 to 23°C, and ∼70% (vti1) and 80% (sec17) of the cells were viable. From these results, we reasoned that the defect was not due to a loss of viability but a direct effect of the inactivation of functional proteins.
Next, the processing of API was examined by pulse-chase analysis (Figure 5A). In the sec18 mutant, proAPI failed to mature at the nonpermissive temperature in a rich medium, indicating that the Cvt pathway was also defective. Even under starvation conditions, the mature form of API did not appear. From these results, we concluded that Sec18p is essential for both autophagy and the Cvt pathway.
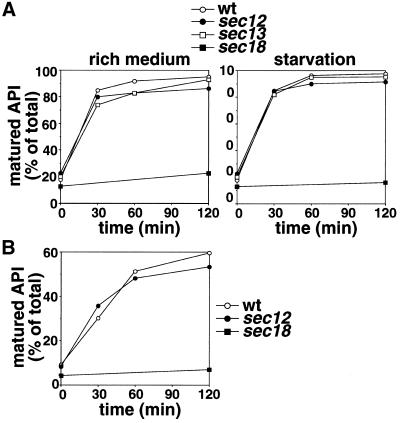
Figure 5.
API transport in early secretory mutants. (A) Wild-type (wt, open circle), sec12 (filled circle), sec13 (open square), or sec18 (filled square) cultured to the midlog phase in SC(-Met) at 23°C were shifted to 37°C for 10 min, labeled with [35S]methionine for 20 min at 37°C, and then chased after addition of SC(-Met) (rich medium) or SD(-N) (starvation) containing methionine/cysteine at 37°C for the times indicated. Immunoprecipitation with anti-API serum was carried out as described in MATERIALS AND METHODS and analyzed by SDS-PAGE and a Bioimage analyzer, BAS2000. (B) Wild-type, sec12, and sec18 cells cultured in SC(-Met) at 23°C were heated to 37°C for 1 h, labeled for 20 min at that temperature, and then chased in a rich medium for the time indicated. Immunoprecipitation was carried out as described in MATERIALS AND METHODS.
Increased ALP activity was observed for >6 h at 23°C in sec18 cells but not at 30°C (Figure 1C). The cells of the sec18 mutant were first cultured in SD(-N) at 30°C for 2 h and then shifted down to 23°C. ALP activity immediately increased without any time lag, and the rate of autophagy was accelerated after shifting down to 23°C, becoming equal to the ALP activity level of the control culture 3 h after the shift. At the nonpermissive temperature, sec18 cells should arrest at a certain stage of autophagy, causing the accumulation of some potent intermediates.
Biochemical Detection of Autophagosome
In autophagy, membrane fusion event may be required for several steps, such as isolation of membrane extension, sealing of the isolation membrane, and fusion of the autophagosome to the vacuole. Therefore, we tried to identify which fusion step is affected in the above mutants. To detect the accumulated autophagosomes, we attempted to separate the autophagosomes by subcellular fractionation (Figure 2A). Autophagosomes are transient structures that immediately fuse to the vacuole, but in Δypt7 cells they accumulate under starvation conditions (Kirisako et al., 1999). We had reported by electron microscopy that under starvation conditions proAPI is preferentially sequestered in the autophagosome (Baba et al., 1997), allowing the use of API as a marker of that organelle.
Δypt7 cell lysate was fractionated by differential centrifugation. ProAPI was recovered in the LSP at 13,000 × g and in the HSS at 100,000 × g. The amount of proAPI in the LSP increased approximately fivefold upon starvation. ProAPI in the LSP from cells cultured in both YPD and SD(-N) media was completely resistant to proteinase K but was digested in the presence of Triton X-100, resulting in a partial digestion product, indicating that it is enclosed within membrane-bound structures. It has been reported that proAPI peripherally bound to the membrane was released when cell fractionation is performed in the absence of Mg2+, although it was recovered in the LSP in a buffer containing Mg2+ (Kim et al., 1999). Consistent with this, proAPI recovered in the LSP from Δypt7 cells was resistant to proteinase K, whereas that in the HSS was digested in Mg2+-deficient buffer. Similar results were obtained with the use of Δslp1/vps33 cells, which also accumulate autophagosomes (Baba et al., 1994). Formation of an autophagosome/Cvt vesicle is defective in Δypt7Δapg7 cells (Kim et al., 1999; Tanida et al., 1999), from which no proAPI could be recovered from the LSP. From these results, we conclude that the amount of proAPI in the LSP from starved cells reflects the presence of accumulated autophagosomes.
Sec18p and Vti1p Mediate Fusion of the Autophagosome to the Vacuole
We identified the step in autophagy that requires Sec18p by subcellular fractionation (Figure 2B). When the cells were cultured at 37°C under starvation conditions, protease-resistant proAPI was recovered in the LSP from Δypt7 cells, whereas no proAPI was recovered in LSP from Δypt7Δapg7 cells. In sec18 cells cultured at 23°C in starvation medium, almost all of the API was found in its mature form. Mature API was recovered in the LSP, indicating that API was targeted to the vacuole, although some mature protein was recovered in the HSS by rupture of the vacuolar membrane as previously described (Vida and Gerhardt, 1999). When the cells were cultured at 37°C under starvation conditions, proteinase K-resistant proAPI was detectable and was recovered in the LSP fraction, indicating that autophagosomes accumulate in the sec18 mutant cells at a nonpermissive temperature. This demonstrated that Sec18p functions during autophagosome-vacuole fusion but not during the formation of the autophagosome.
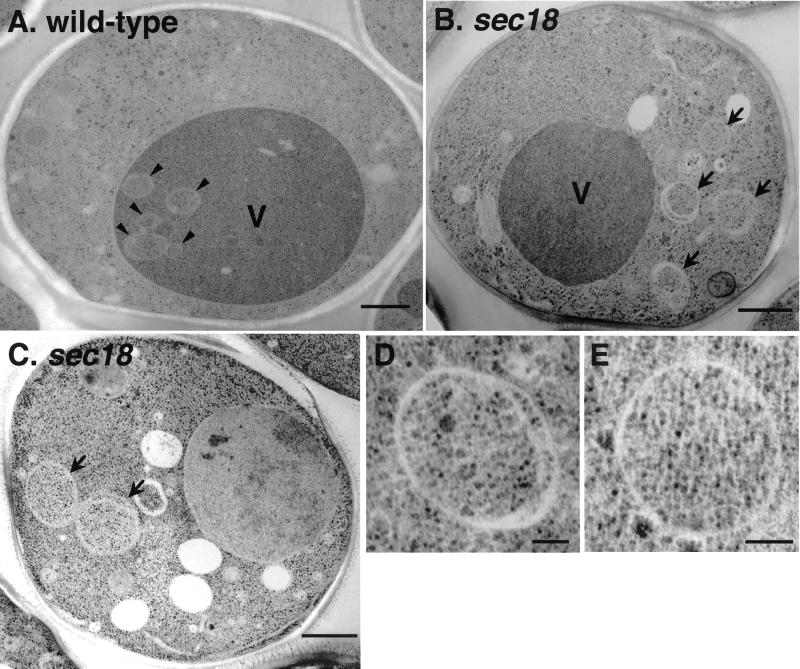
Electron microscopy of wild-type cells cultured under starvation conditions in the presence of PMSF at 37°C revealed the presence of autophagic bodies in the vacuole (Figure 3A, arrowhead). In sec18 cells, no autophagic bodies were found in the vacuole, and many autophagosomes with normal morphology were detected in the cytoplasm (Figure 3, B and C, arrow, and D and E).
Figure 3.
Electron micrographs of autophagosomes in the sec18 mutant. Wild-type (A) and sec18 (B and C) cells were cultured in SD(-N) containing PMSF at 37°C for 2.5 h and fixed by the freeze substitution technique as described in MATERIALS AND METHODS. Higher magnification images of the autophagosomes in sec18 are shown in D and E. Arrows, autophagosome; arrowheads, autophagic body; V, vacuole. Bars, 500 nm (A–C); 100 nm (D and E).
We also explored a defective step in the vti1 mutant. After cell fractionation of vti1-11ts mutant cells cultured in SD(-N) at a nonpermissive temperature, protease-resistant proAPI was recovered in the LSP (Figure 2B). Therefore, Vti1p is another essential factor for autophagosome-vacuole fusion. Vacuolar t-SNARE Vam3p was also shown to be required for this step (Darsow et al., 1997). Together with these results, Vti1p, functioning as the v-SNARE, and Vam3p, as the t-SNARE, may make up the SNARE factors that function together with Sec18p in the fusion of the autophagosome to the vacuole.
Requirement of the COPII Subunits in Autophagy
The block in the secretory pathway is known to bring about several cellular defects such as endocytosis, ribosome biogenesis, and nuclear transport directly or indirectly (Mizuta and Warner, 1994; Hicke et al., 1997; Nanduri et al., 1999). In the secretory pathway, Sec18p plays an essential role in mediating the fusion of an ER-derived vesicle to the Golgi complex and of the secretory vesicle to the plasma membrane (Beckers et al., 1989; Kaiser and Schekman, 1990; Graham and Emr, 1991). Here, we showed that autophagosome formation could occur in the sec18 mutant (Figures 2 and 3) and that blocking of transport from ER to Golgi or from Golgi to plasma membrane may not affect autophagosome formation. We next investigated the autophagic activity in late secretory mutants. The autophagy was not induced in late sec mutants, sec4 and sec15 cells, at 37°C; however, the ALP activity was not recovered when shifted to permissive temperature in contrast to other sec mutants (Figures 1B and 4B). The restrictive temperature, 34°C, at which a colony formation does not take place and the general secretion was reduced to 20% (in sec4) and 30% (in sec15) of wild-type cells, was selected to study their autophagic activities. As a result, the defect was not found in these late sec mutants at this condition (Figure 4D); thus, autophagy does not necessarily require the late Sec proteins.
Figure 4.
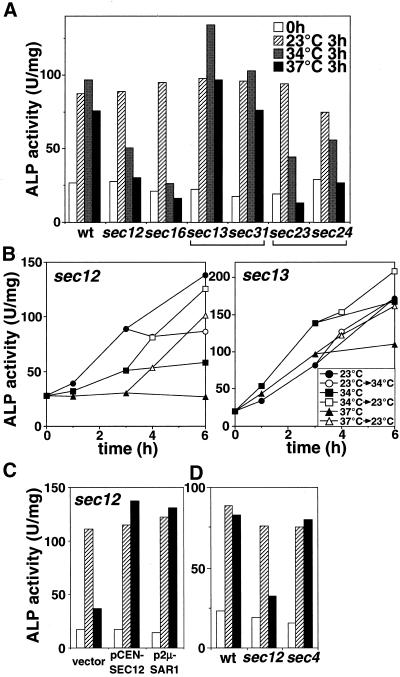
Autophagic activity in the COPII mutants. (A) Cells expressing Pho8Δ60p (wild-type [wt], sec12, sec16, sec13, sec31, sec23, or sec24) were cultured in YPD at 23°C (open bar), transferred to SD(-N) at 23°C (shaded bar) or 34°C (gray bar) or 37°C for 3 h (filled bar), lysed, and assessed for ALP activity. (B) Time course of autophagy in sec12 and sec13. sec12 and sec13 cells grown in YPD at 23°C were cultured in SD(-N) at 23°C (circle), 34°C (square), or 37°C (triangle). The cells cultured in SD(-N) for 3 h were shifted from 23 to 34°C (open circle), from 34 to 23°C (open square), and from 37 to 23°C (open triangle). (C) sec12 cells carrying pRS316 (vector), pSHY6-4 (pCEN-SEC12), or pANY2-7 (p2 μ-SAR1) grown in SC(-Ura) at 23°C were cultured in YPD for 4 h at 23°C (open bar) and then transferred to SD(-N) for 4 h at 23°C (shaded bar) or 34°C (filled bar). (D) Wild-type (wt), sec12, or sec4 cells grown in YPD at 23°C (open bar) were cultured in SD(-N) at 23°C (shaded bar) or 34°C (filled bar) for 2 h. The ALP activities of the lysate were measured as described in MATERIALS AND METHODS.
The requirement of early Sec proteins in autophagy was then studied. Formation of the COPII vesicle from the ER is essential for vesicular transport to the Golgi complex (Kaiser and Schekman, 1990; Barlowe et al., 1994). In the temperature-sensitive sec13 or sec31 mutants (Salama et al., 1997), autophagy proceeded normally at a nonpermissive temperature (Figure 4A), although transport of a vacuolar enzyme, carboxypeptidase Y, from the ER was severely impaired. The secretory membrane flow from the ER to the Golgi via the COPII vesicle apparently is not involved in autophagy.
Unexpectedly, another temperature-sensitive COPII mutant, sec12, exhibited defective autophagy at nonpermissive temperatures, 34 and 37°C (Figure 4A). Shifting from a permissive temperature to 34°C stopped the increase in ALP activity (Figure 4C). Approximately 80% (at 34°C) or 70% (at 37°C) of sec12 cells were viable after incubation in SD(-N) for 3 h. Subsequent shift down to 23 from 34 or 37°C resulted in immediate recovery of autophagic activity. These results indicate that the defect in autophagy must be a direct effect of the SEC12 mutation. Sec12p is a GDP/GTP exchange factor, functioning in conjunction with the Sar1p GTPase (Nakano and Muramatsu, 1989; Barlowe et al., 1994). Sec12p seems to function through Sar1p function during autophagy, similar to its actions in the ER-to-Golgi transport step (Nakano and Muramatsu, 1989), because the defect in the sec12 mutant at 34°C was recovered by overproduction of Sar1p via a multicopy plasmid (Figure 4B).
Furthermore, sec16 (Espenshade et al., 1995), sec23, and sec24 (Barlowe et al., 1994) also showed temperature-sensitive autophagic defects (Figure 4A). Sec13p and Sec31p form a subcomplex in the COPII coat, whereas Sec23p and Sec24p make up another subcomplex (Barlowe et al., 1994; Salama et al., 1997). These results suggest that certain factors in the COPII apparatus are required for autophagy.
Sec12p Is Essential for Autophagy but Not for the Cvt Pathway
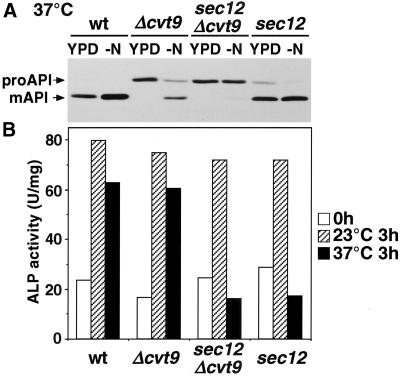
The Cvt pathway has been reported to be normal in the sec12 mutant (Klionsky et al., 1992), a finding that we confirmed in the present study (Figure 5A, left). In the sec12 mutant at 37°C, the rate of API maturation in a rich medium was indistinguishable from that in wild-type cells. Even after preincubation for 1 h at 37°C, the rate of proAPI maturation in sec12 was similar to that seen in wild-type cells (Figure 5B). These results indicate that autophagy requires Sec12p, whereas the Cvt pathway does not.
Furthermore, even under starvation conditions at 37°C, proAPI matured at the same rate in sec12 mutant and wild-type cells (Figure 5A, right). To determine whether the Cvt pathway proceeds in starved sec12 cells, we constructed a double mutant, sec12Δcvt9 (Figure 6). Cvt9p is essential for the Cvt pathway but not for autophagy (Harding et al., 1996; Kamada et al., 2000; Kim et al., 2001). When sec12 cells were cultured in either YPD or SD(-N) at a nonpermissive temperature, the mature form of API was detected, which is consistent with the results in Figure 5. However, in sec12Δcvt9 mutant cells, API failed to mature under either nutrient condition at a nonpermissive temperature. Therefore, the maturation of API in starved sec12 cells depended on the function of Cvt9p. These results raise the possibility that the Cvt pathway proceeds even under starvation conditions in sec12 mutant cells.
Figure 6.
API transport was impaired in sec12Δcvt9 cells. (A) Wild-type (wt), Δcvt9, sec12Δcvt9, and sec12 cells grown in YPD at 23°C were cultured at 37°C in YPD or SD(-N) for 3 h, lysed, and analyzed by immunoblotting with anti-API serum. (B) The mutant cells in A were cultured in YPD at 23°C (open bar), transferred to SD(-N) at 23°C (shaded bar) or 34°C (filled bar) for 3 h, lysed, and assessed for ALP activity.
Autophagosomes Can Be Separated from Cvt Vesicles by Density Gradient Centrifugation
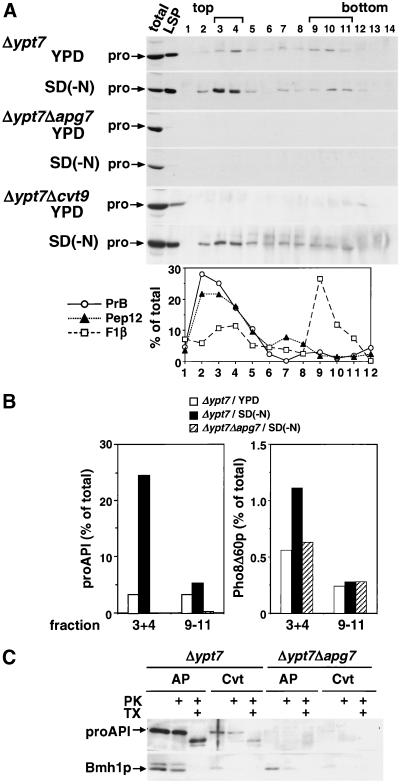
Both autophagosomes and Cvt vesicles were recovered in the LSP fraction after the above fractionation protocol (Figure 2). For further analysis, we established a method to separate autophagosomes from Cvt vesicles by equilibrium density gradient centrifugation (Figure 7). The LSP fraction from Δypt7 cells cultured in YPD or SD(-N) was layered on top of a 10–50% OptiPrep gradient and centrifuged at 174,000 × g for 16 h (Figure 7A, Δypt7). For starved cells, proAPI was detected most strongly in fractions 3 and 4, with a small portion also detected in heavier fractions. For growing cells, proAPI was detected in fractions 9–11 in addition to fractions 3 and 4. The appearance of proAPI in these fractions completely depended on the APG gene (Figure 7A, Δypt7Δapg7). ProAPI in all of these fractions must be contained within a membrane-bound structure, because it was resistant to proteinase K treatment (Figure 7C). We compared the amount of proAPI in these two peaks at different nutrient conditions. More than sevenfold increase was observed in the light fractions under starvation conditions, whereas no significant change was detected in the heavy fractions (Figure 7B, proAPI). In Δypt7Δcvt9 cells, no proAPI was detected in either fraction under growing conditions, whereas it was detected in the light fractions under starvation. Then, we examined the colocalization of cytosolic marker proteins in these fractions (Figure 7B). Autophagosome sequesters cytoplasmic components nonselectively; on the other hand, the Cvt vesicle selectively encloses the Cvt complex and excludes cytosol (Baba et al., 1997). Under starvation conditions, the amount of Pho8Δ60p in the light fractions increased in Δypt7 cells but did not in Δypt7Δapg7 cells. Conversely, no such increase was detected in the heavy fractions of these cells after starvation. Another cytosolic marker protein, Bmh1p, was also recovered in the light fractions under starvation conditions in the Δypt7 cells (Figure 7C), and was mostly resistant to proteinase K. Based on these observations, we concluded that the light fractions (3 and 4) are enriched in autophagosomes, whereas the heavy fractions 9–11 contain Cvt vesicles. This density gradient centrifugation then allows the separation of autophagosomes from Cvt vesicles.
Figure 7.
Fractionation of autophagosomes and Cvt vesicles by density gradient centrifugation. (A) Δypt7, Δypt7Δapg7, and Δypt7Δcvt9 cells were cultured in YPD or SD(-N) at 30°C. The LSP fractions were prepared as described in Figure 2 and were loaded on top of a 10–50% OptiPrep gradient and centrifuged for 16 h at 174,000 × g. Fourteen fractions were collected from the top of the tubes. An equal volume of each fraction was subjected to immunoblotting with anti-API serum. As a control, one-sixth of the cell lysate (total) and half of the LSP were used. Organelle markers of the vacuole (proteinase B, PrB), endosomes (Pep12p), and mitochondria (F1β) were detected by immunoblotting of starved Δypt7 cells and estimated by densitometry. (B) The relative amounts of proAPI and Pho8Δ60p in fractions 3 and 4 or fractions 9–11 in the density gradients in A were determined by immunoblotting with their respective antisera. (C) Fractions 3 and 4 from starved cells (AP) or fractions 9–11 from YPD-grown cells (Cvt) in A were pooled, treated with proteinase K (PK) with or without Triton X-100 (TX), and then subjected to immunoblotting with anti-API or anti-Bmh1p antiserum.
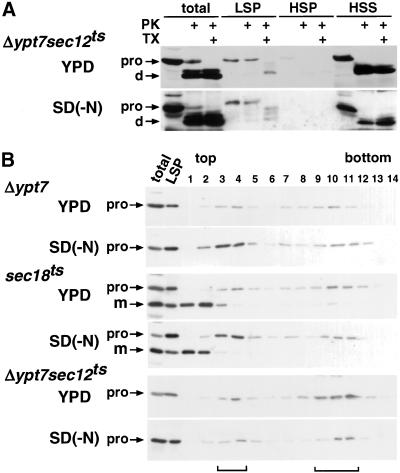
The sec18 mutant was analyzed by density gradient centrifugation (Figure 8B). When sec18 cells were cultured in SD(-N) at 37°C, proAPI was efficiently recovered in the light fractions, as in Δypt7 cells. This also confirms that sec18 mutant cells accumulate autophagosomes under starvation conditions.
Figure 8.
Autophagosome formation in sec12 and sec18 cells. (A) Δypt7 sec12 cells were cultured in YPD or SD(-N) at 37°C for 3 h. Fractionation and protease treatment were performed as in Figure 2. (B) Δypt7, sec18, and Δypt7 sec12 cells precultured in YPD at 23°C were cultured in YPD or SD(-N) at 37°C for 3 h. Density gradient centrifugation and immunoblotting were carried out as described in Figure 7. As a control, one-tenth of the total cell lysate and half of the LSP were also analyzed.
Sec12p Functions in the Formation of Autophagosomes but Not in the Formation of Cvt Vesicles
We then examined the specific step in the autophagy that is affected in the sec12 mutant (Figure 8). Because proAPI is transported to the vacuole in sec12 mutant cells even at nonpermissive temperatures (Figures 5 and 6), we tried to evaluate the formation of autophagosomes or Cvt vesicles by with the use of a double mutant, Δypt7 sec12. When the double mutant was cultured in YPD or SD(-N) at 37°C, the protease-resistant form of proAPI was detected in the LSP (Figure 8A), which was then further fractionated by density gradient centrifugation (Figure 8B). When cultured in a rich medium, proAPI accumulated mostly in the heavy fractions of the density gradient. Even when incubated in starvation medium, the amount of proAPI in the light fractions did not increase, and most proAPI was recovered in the heavy fractions. Thus, in the sec12 mutant, autophagosomes are not formed under starvation conditions, whereas Cvt vesicles are formed under both nutrient-rich and starvation conditions.
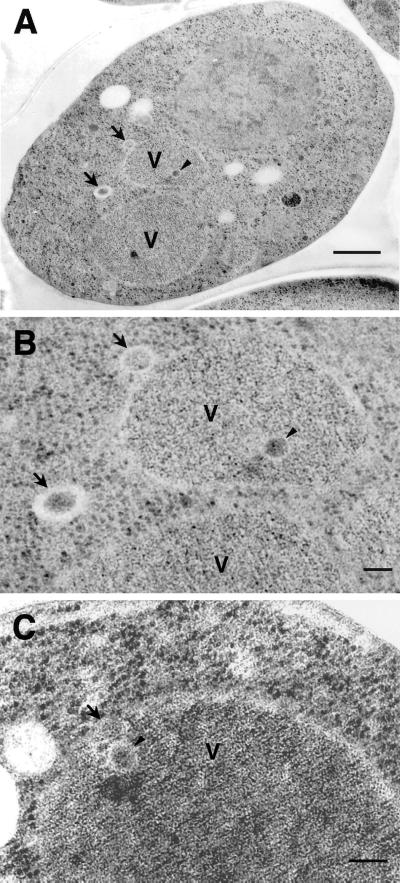
Finally, we observed sec12 mutant cells by electron microscopy (Figure 9). Autophagic bodies were never detected in the vacuole under starvation conditions in the presence of PMSF at 37°C (Figure 9A). Instead, vesicular structures were detected in the cytoplasm (arrow) and the vacuole (arrowhead). They enclosed electron-dense materials, excluding cytosolic components such as ribosomes (Figure 9B). Many of the vesicles in the cytoplasm were localized near the vacuole, and some vesicles attached to the vacuolar membrane (Figure 9, B and C), causing them to be designated Cvt vesicles and Cvt bodies, respectively. This indicates that the sec12 mutant forms Cvt vesicles under starvation conditions at nonpermissive temperatures.
Figure 9.
Electron micrographs of sec12 under starvation conditions. sec12 mutant cells were cultured in SD(-N) containing PMSF at 37°C for 2.5 h and prepared for electron microscopy as described in MATERIALS AND METHODS. Higher magnification images are shown in B and C. Arrows, Cvt vesicle; arrowheads, Cvt body; V, vacuole. Bars, 500 nm (A); 100 nm (B and C).
These data from subcellular fractionation and EM analysis are consistent with results obtained from the enzymatic assays (Figures 4–6), that Sec12p is required for autophagosome but not Cvt vesicle formation.
DISCUSSION
We investigated the requirement of various factors in the classical vesicular transport pathway for autophagy, a topic of long-standing interest in mammals that has become amenable to analysis because of the recent establishment of markers in yeast, such as API and Pho8Δ60p. Sec18p and Vti1p, known to function in membrane fusion, are necessary for the fusion of the autophagosome to the vacuole, and Sec12p, which has been implicated in vesicle budding from the ER, is required for autophagosome formation but not for the Cvt pathway. Autophagy requires a special set of COPII factors, which includes Sec12p, Sec16p, Sec23p, and Sec24p but not Sec13p and Sec31p.
Separation of Autophagosomes from Cvt Vesicles
We established a method to isolate autophagosomes from yeast cells for the first time. Density gradient centrifugation gives rise to two peaks of membrane structures enclosing proAPI. The light peak appears to contain the autophagosome fraction for the following reasons: 1) the amount of proAPI in this peak is increased by starvation, 2) other cytosolic proteins resistant to proteinase K are also found in this peak, and 3) the recovery of proAPI and cytosolic proteins from this peak is dependent on the presence of active Apg protein. Aut7p/Apg8p was shown to associate with the autophagosome and its intermediate structures (Kirisako et al., 1999). As expected, a part of Apg8p was detected in the light peak fractions (Ishihara and Ohsumi, unpublished results). The heavy peak appears to contain Cvt vesicles, because it is detectable in growing cells and shows no association with cytosolic proteins. The difference in the densities of these two structures may reflect their contents; the autophagosome contains cytoplasm and the Cvt vesicle contains a dense Cvt complex (Baba et al., 1997). We believe that our method will be useful for further biochemical characterization of the yeast autophagosome.
NSF/SNAREs Implicated in Autophagosome Formation and Vacuolar Fusion
According to electron microscopy, the autophagosome does not form from preexisting organelles (Baba et al., 1994). Recently, we showed that autophagosome formation can be traced by detecting Apg8p as a specific marker that localizes on intermediate membrane structures and autophagosomes. Such Apg8p-containing structures were found at a region next to the isolation membrane, suggesting that autophagosomes are assembled from some small membrane structures (Kirisako et al., 1999). Here, we clearly showed that Sec18p (NSF) is not required for events occurring during autophagosome formation, such as extension of the isolation membrane or sealing of the autophagosome (Figures 2 and 3). To date, several NSF-independent membrane fusion events have been reported, such as apical transport in polarized cells (Low et al., 1998), a cognate pathway in nonpolarized cells (Yoshimori et al., 1996), Golgi cisternal growth in mitotic mammalian cells (Rabouille et al., 1995) and homotypic fusion of ER membranes in yeast (Latterich et al., 1995). Cdc48p is related to NSF and functions in ER membrane fusion, but a temperature-sensitive cdc48 mutant was normal with respect to autophagy (Figure 1). Some specific fusion factor(s), distinct from the general fusion machinery, may play a role in the formation of autophagosomes.
We showed in this study that Sec18p and Vti1p are necessary for autophagosome-vacuole fusion (Figures 1 and 2). Here, we summarize our present knowledge with regard to SNARE molecules in both autophagy and the Cvt pathway. Vacuolar t-SNAREs, including Vam3p and Vam7p, function in vacuolar fusion steps in various pathways, including autophagy and the Cvt pathway (Darsow et al., 1997; Sato et al., 1998); however, the endosomal t-SNARE Pep12p was dispensable in both pathways (Abeliovich et al., 1999). The vacuolar v-SNARE Nyv1p was shown to be nonessential for the Cvt pathway (Fischer von Mollard and Stevens, 1999); and we confirmed that Δnyv1 cells undergo autophagy normally. Recently, the t-SNARE Tlg2p was found to be essential for Cvt vesicle formation but not for autophagy (Abeliovich et al., 1999). The v-SNARE Vti1p is essential for the Cvt pathway (Fischer von Mollard and Stevens, 1999) and autophagy (Figure 1). Vti1p was shown to bind with Vam3p (Holthuis et al., 1998), suggesting that these two SNAREs form a complex during the fusion step between the autophagosome and the vacuole. Sec18p, Vti1p, and Vam3p would promote heterotypic fusion of the outer autophagosomal membrane to the vacuole, possibly together with the Rab protein Ypt7p and a class C vacuolar protein sorting (Vps) protein complex containing Vps18p and Slp1p/Vps33p. Vti1p most likely resides on the autophagosomal membrane, although clear demonstration is difficult because of its multiple sites of localization (Fischer von Mollard et al., 1997). The manner in which Vti1p is transported to the autophagosome and/or Cvt vesicle is the next issue that must be addressed.
Subgroup of COPII Factors Functions in Autophagy
We showed that autophagosome formation proceeds normally in the early secretory mutants, sec13, sec31, and sec18 (Figures 2 and 4), suggesting that membrane flow from the ER to the Golgi is not involved in autophagosome formation. Furthermore, the late sec mutants, sec4 and sec15, did not exhibit defects in autophagy at 34°C, although secretion was severely affected, supporting the idea that secretory membrane flow is not directly required for autophagosome formation.
We also demonstrated in this study that Sec12p is required for autophagosome formation (Figures 4, 8, and 9). The defect in sec12 is recovered by overexpression of Sar1p, suggesting that Sar1p also functions with Sec12p in autophagy. Sec12p is a regulatory factor, modulating the activity of Sar1 GTPase, which is required for COPII vesicle budding from the ER (Nakano and Muramatsu, 1989). Some factors that are required for ER-to-Golgi trafficking also play a role in autophagosome formation.
Interestingly, although six of the early Sec proteins, Sec12p, Sec13p, Sec16p, Sec23p, Sec24p, and Sec31p, participate in COPII vesicle formation, these mutants showed distinct effects on autophagy (Figure 3). Recent studies showed that nonclassical COPII-like factors were involved in nonessential membrane organization processes. Lst8p, a homologue of Sec13p, was shown to play a role in the sorting step of amino acid permeases from the Golgi to the plasma membrane (Roberg et al., 1997a, b). Iss1p, which bears a striking resemblence to Sec24p, functions interchangeably with it in vesicle formation (Kurihara et al., 2000). Lst1p, which also has homology with Sec24p, forms a complex with Sec23p and plays a role in the formation of specialized vesicles from the ER for transporting Pma1p (Roberg et al., 1999). We suppose that Sec12p, Sec16p, and a coat subcomplex composed of Sec23p and Sec24p function in the formation of a specialized vesicle essential for autophagosome formation. Further studies of the function of COPII factors in autophagy may further elucidate the origin of the autophagosomal membrane.
Differences between the Formation of Autophagosomes and Cvt Vesicles
The difference between the Cvt pathway and autophagy is unclear, because most apg mutants have defects in both pathways (Scott et al., 1996; Kamada et al., 2000). Here, we uncovered a key distinction, that the requirement of Sec12p in the two pathways is different (Figures 5, 6, 8, and 9). There are two possible explanations for this phenomenon. One is that both pathways utilize the same machinery, but the Cvt vesicle requires lesser amounts of membrane because of its smaller size compared with the autophagosome. In this case, the autophagic pathway may be more strongly dependent on membrane supply regulated by Sec12p than the Cvt pathway. However, even after a long preincubation at a nonpermissive temperature, the efficiency of the Cvt pathway was not affected in sec12 mutants (Figure 5B). The alternative and more plausible explanation is that both pathways utilize overlapped but substantially different mechanisms. The lack of a deficit in the Cvt pathway after severe blockage of Sec12p can be explained better by this idea. We found that Cvt vesicles form even under starvation conditions in the sec12 mutant and that this process depends completely on Cvt9 function (Figures 8 and 9). Thus, there is a clear distinction between autophagy and the Cvt pathway in their requirements. Other observations also support the latter explanation. Δtlg2 and vps45ts mutants were reported to be defective in Cvt vesicle formation but not in autophagy (Abeliovich et al., 1999). Formation of the autophagosome and Cvt vesicle may use different processes even though they share many Apg proteins.
We propose the working hypothesis of the membrane trafficking factors for autophagosomal and Cvt vesicle formation as follows. Under starvation conditions, a subset of membrane is destined to form a double membrane of the autophagosome and is dependent on a special set of COPII proteins, including Sec12p, but independent of NSF and Cdc48p function. Other Sec12p-independent membranes are constitutively supplied to form the Cvt vesicles, a process that is dependent on Tlg2p and Vps45p. We emphasize that autophagosomes are possibly built up from multiple sources of membrane, and further studies will uncover the entire set of membrane sources. Autophagosomes undergo fusion to the vacuolar membrane in a process mediated by a vacuolar fusion apparatus consisting of Vti1p, Vam3p, and NSF. Compared with the Cvt vesicles, the formation of autophagosomes requires a large amount of membrane when nutrient supply is limited in the environment, and organelles in the secretory pathway may be suitable as a membrane source for the autophagosome.
In this report, we demonstrated that several factors involved in classical membrane trafficking are required for at least two steps in autophagy. Further studies of endomembrane-trafficking factors and specific Apg proteins will uncover the details of membrane dynamics during the autophagy.
ACKNOWLEDGMENTS
We thank Dr. A. Nakano (RIKEN), Dr. R. Schekman (University of California, Berekley), Dr. T. Stevens (University of Oregon), Dr. M. Latterich (Salk Institute), Dr. D. J. Klionsky (University of Michigan), Drs. M. Sakaguchi and K. Mihara (Kyushu University), and Drs. N. Mizushima and T. Kirisako (NIBB), for providing strains, plasmids, or antibodies and Dr. Y. Hayashi, M. Kondo, and T. Notomi (NIBB) for technical support for EM analysis. This work was supported in part by Grants-in-Aids from the Ministry of Education, Science, Sports and Culture of Japan.
Abbreviations used:
- ALP
alkaline phosphatase: API, aminopeptidase I
- COP
coatmer protein
- Cvt
cytoplasm to vacuole targeting
- ER
endoplasmic reticulum
- HSP
high-speed pellet
- HSS
high-speed supernatant
- LSP
low-speed pellet
- NSF
N-ethylmaleimide-sensitive fusion protein
- PMSF
phenylmethylsulfonyl fluoride
- proAPI
precursor form of aminopeptidase I
- ProK
proteinase K
- SNAP
soluble NSF attachment protein
- SNARE
SNAP receptor
REFERENCES
- Abeliovich H, Darsow T, Emr SD. Cytoplasm to vacuole trafficking of aminopeptidase I requires a t-SNARE-Sec1p complex composed of Tlg2p and Vps45p. EMBO J. 1999;18:6005–6016. doi: 10.1093/emboj/18.21.6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams A, Gottschling DE, Kaiser CA, Stearns T. Methods in Yeast Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997. [Google Scholar]
- Baba M, Osumi M, Scot SV, Klionsky DJ, Ohsumi Y. Two distinct pathways for targeting proteins from the cytoplasm to the vacuole/lysosome. J Cell Biol. 1997;139:1687–1695. doi: 10.1083/jcb.139.7.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba M, Takeshige K, Baba N, Ohsumi Y. Ultrastructural analysis of the autophagic process in yeast: detection of autophagosomes and their characterization. J Cell Biol. 1994;124:903–913. doi: 10.1083/jcb.124.6.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlowe C, Orc L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, Rexach MF, Ravazzola M, Amherdt M, Scheckman R. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. doi: 10.1016/0092-8674(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Beckers CJM, Block MR, Glick BS, Rothman JE, Balch WE. Vesicular transport between the endoplasmic reticulum and Golgi stack requires the NEM sensitive fusion protein. Nature. 1989;339:397–398. doi: 10.1038/339397a0. [DOI] [PubMed] [Google Scholar]
- Darsow T, Rieder SE, Emr SD. A multispecificity syntaxin homologue, Vam3p, essential for autophagic and biosynthetic protein transport to the vacuole. J Cell Biol. 1997;138:517–529. doi: 10.1083/jcb.138.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn WA. Studies on the mechanisms of autophagy: formation of the autophagic vacuole. J Cell Biol. 1990;110:1923–1933. doi: 10.1083/jcb.110.6.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espenshade P, Gimeno RE, Holzmacher E, Teung P, Kaiser CA. Yeast SEC16 gene encodes a multidomain vesicle coat protein that interact with Sec23p. J Cell Biol. 1995;131:311–324. doi: 10.1083/jcb.131.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer von Mollard G, Nothwehr SF, Stevens TH. The yeast v-SNARE Vti1p mediates two vesicle transport pathways through interaction with t-SNAREs Sed5p and Pep12p. J Cell Biol. 1997;137:1511–1524. doi: 10.1083/jcb.137.7.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer von Mollard G, Stevens TH. The Sccharomyces cerevisiae v-SNARE Vti1p is required for multiple membrane transport pathways to the vacuole. Mol Biol Cell. 1999;10:1719–1732. doi: 10.1091/mbc.10.6.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham TR, Emr SD. Compartmental organization of Golgi-specific protein modification and vacuolar protein sorting event defined in a yeast sec18 (NSF) mutant. J Cell Biol. 1991;114:207–218. doi: 10.1083/jcb.114.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding TM, Hefner-Gravink A, Thumm M, Klionsky DJ. Genetic and phenotypic overlap between autophagy and the cytoplasm to vacuole targeting pathway. J Biol Chem. 1996;271:17621–17624. doi: 10.1074/jbc.271.30.17621. [DOI] [PubMed] [Google Scholar]
- Harding TM, Morano KA, Scott SV, Klionsky DJ. Isolation and characterization of yeast mutants in cytoplasm to vacuole protein targeting pathway. J Cell Biol. 1995;131:591–602. doi: 10.1083/jcb.131.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicke L, Zanolari B, Pypaert M, Rohrer J, Riezman H. Transport through the yeast endocytic pathway occurs through morphologically distinct compartments and requires an active secretory pathway and Sec18p/N-ethylmaleimide-sensitive fusion protein. Mol Biol Cell. 1997;8:13–31. doi: 10.1091/mbc.8.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthuis JCM, Nichols BJ, Dhruvakumar S, Pelham HRB. Two syntaxin homologues in the TGN/endosomal system of yeast. EMBO J. 1998;17:113–126. doi: 10.1093/emboj/17.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura Y, Kirisako T, Takao T, Satomi Y, Shimonishi Y, Ishihara N, Mizushima N, Tanida I, Kominami E, Ohsumi M, Noda T, Ohsumi Y. A ubiquitin-like system mediates protein lipidation. Nature. 2000;408:488–492. doi: 10.1038/35044114. [DOI] [PubMed] [Google Scholar]
- Kaiser CA, Schekman R. Distinct sets of SEC genes govern transport vesicle formation and fusion early in the secretory pathway. Cell. 1990;61:723–733. doi: 10.1016/0092-8674(90)90483-u. [DOI] [PubMed] [Google Scholar]
- Kamada Y, Funakoshi T, Shintani T, Nagano K, Ohsumi M, Ohsumi Y. Tor-mediated induction of autophagy via an Apg1 protein kinase complex. J Cell Biol. 2000;150:1507–1513. doi: 10.1083/jcb.150.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara A, Noda T, Ishihara N, Ohsumi Y. Two distinct Vps34 phosphatidylinositol 3-kinase complexes function in autophagy and carboxypeptidase Y sorting in Saccharomyces cerevisiae. J Cell Biol. 2001;152:519–530. doi: 10.1083/jcb.152.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Dalton VM, Eggerton KP, Scott SV, Klionsky DJ. Apg7p/Cvt2p is required for the cytoplasm-to-vacuole targeting, macroautophagy, and peroxisome degradation pathways. Mol Biol Cell. 1999;10:1337–1351. doi: 10.1091/mbc.10.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kamada Y, Stromhaug PE, Guan J, Hefner-Gravink A, Baba M, Scott SV, Ohsumi Y, Dunn WA, Jr, Klionsky DJ. Cvt9/Gsa9 functions in sequestering selective cytosolic cargo destined for the vacuole. J Cell Biol. 2001;153:381–396. doi: 10.1083/jcb.153.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirisako T, Baba M, Ishihara N, Miyazawa K, Ohsumi M, Yoshimori T, Noda T, Ohsumi Y. Formation process of autophagosome is traced with Apg8p/Aut7p in yeast. J Cell Biol. 1999;147:435–446. doi: 10.1083/jcb.147.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ, Cueva R, Yaver DS. Aminopeptidase I of Sccharomyces cerevisiae is localized to the vacuole independent of the secretory pathway. J Cell Biol. 1992;119:287–299. doi: 10.1083/jcb.119.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ, Ohsumi Y. Vacuolar import of proteins and organelles from the cytoplasm. Annu Rev Cell Dev Biol. 1999;15:1–32. doi: 10.1146/annurev.cellbio.15.1.1. [DOI] [PubMed] [Google Scholar]
- Kurihara T, Hamamoto S, Gimeno RE, Kaiser CA, Schekman R, Yoshihisa T. Sec24p and Iss1p function interchangeably in transport vesicle formation from the endoplasmic reticulum in Saccharomyces cerevisiae. Mol Biol Cell. 2000;11:983–998. doi: 10.1091/mbc.11.3.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latterich M, Frohlich KU, Scheckman R. Membrane fusion and the cell cycle: Cdc48p participates in the fusion of ER membranes. Cell. 1995;82:885–893. doi: 10.1016/0092-8674(95)90268-6. [DOI] [PubMed] [Google Scholar]
- Low SH, Chapin SJ, Wimmer C, Whiteheart SW, Komuves LG, Mostov KE, Weimbs T. The SNARE machinery is involved in apical plasma membrane trafficking in MDCK cells. J Cell Biol. 1998;141:1503–1513. doi: 10.1083/jcb.141.7.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A, Wickner W, Haas A. Sec18p (NSF)-driven release of Sec17p (α-SNAP) can precede docking and fusion of yeast vacuoles. Cell. 1996;85:83–94. doi: 10.1016/s0092-8674(00)81084-3. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Noda T, Yoshinori T, Tanaka Y, Ishii T, George MD, Klionsky DJ, Ohsumi M, Ohsumi Y. A protein conjugation system essential for autophagy. Nature. 1998;395:395–398. doi: 10.1038/26506. [DOI] [PubMed] [Google Scholar]
- Mizuta K, Warner JR. Continued functioning of the secretory pathway is essential for ribosome synthesis. Mol Cell Biol. 1994;14:2493–2502. doi: 10.1128/mcb.14.4.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano A, Muramatsu M. A novel GTP-binding protein, Sar1p, is involved in transport from the endoplasmic reticulum to the Golgi apparatus. J Cell Biol. 1989;109:2677–2691. doi: 10.1083/jcb.109.6.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanduri J, Mitra S, Andrei C, Liu Y, Yu Y, Hitomi M, Tartakoff AM. An unexpected link between the secretory path and the organization of the nucleus. J Biol Chem. 1999;274:33785–33789. doi: 10.1074/jbc.274.47.33785. [DOI] [PubMed] [Google Scholar]
- Noda T, Matsuura A, Wada Y, Ohsumi Y. Novel system for monitoring autophagy in the yeast Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1995;210:126–132. doi: 10.1006/bbrc.1995.1636. [DOI] [PubMed] [Google Scholar]
- Noda T, Ohsumi Y. Tor, a phosphatidylinositol kinase homologue, controls autophagy in yeast. J Biol Chem. 1998;273:3963–3966. doi: 10.1074/jbc.273.7.3963. [DOI] [PubMed] [Google Scholar]
- Novick P, Field C, Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980;21:205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975;189:347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Rabouille C, Levine TP, Peters JM, Warren G. An NSF-like ATPase, p97, and NSF mediate cisternal regrowth from mitotic Golgi fragments. Cell. 1995;82:905–914. doi: 10.1016/0092-8674(95)90270-8. [DOI] [PubMed] [Google Scholar]
- Rieder SE, Emr SD. A novel RING finger protein complex essential for a late step in protein transport to the yeast vacuole. Mol Biol Cell. 1997;8:2307–2327. doi: 10.1091/mbc.8.11.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberg KJ, Bickel S, Rowley N, Kaiser CA. Control of amino acid permease sorting in the late secretory pathway of Saccharomyces cerevisiae by SEC13, LST4, LST7 and. LST8. Genetics. 1997a;147:1569–1584. doi: 10.1093/genetics/147.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberg KJ, Crotwell M, Espenshade P, Gimeno R, Kaiser CA. LST1 is a SEC24 homologue used for selective export of the plasma membrane ATPase from the endoplasmic reticulum. J Cell Biol. 1999;145:659–672. doi: 10.1083/jcb.145.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberg KJ, Rowley N, Kaiser CA. Physiological regulation of membrane protein sorting late in the secretory pathway of Saccharomyces cerevisiae. J Cell Biol. 1997b;137:1469–1482. doi: 10.1083/jcb.137.7.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama NR, Chuang JS, Schekman RW. Sec31 encodes an essential component of the COPII coat required for transport vesicle budding from the endoplasmic reticulum. Mol Biol Cell. 1997;8:205–217. doi: 10.1091/mbc.8.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato TK, Darsow T, Emr SD. Vam7p, a SNAP-25-like molecule, and Vam3p, a syntaxin homolog, function together in yeast vacuolar protein trafficking. Mol Cell Biol. 1998;18:5308–5319. doi: 10.1128/mcb.18.9.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SV, Hefner-Gravink A, Morano KA, Noda T, Ohsumi Y, Klionsky DJ. Cytoplasm-to-vacuole targeting and autophagy employ the same machinery to deliver proteins to the yeast vacuole. Proc Natl Acad Sci USA. 1996;93:12304–12308. doi: 10.1073/pnas.93.22.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SV, Nice DC, Nau JJ, Weisman LS, Kamada Y, Keizer-Gunnink I, Funakoshi T, Veenhuis M, Ohsumi Y, Klionsky DJ. Apg13p and Vac8p are part of a complex of phosphoproteins that are required for cytoplasm to vacuole targeting. J Biol Chem. 2000;275:25840–25849. doi: 10.1074/jbc.M002813200. [DOI] [PubMed] [Google Scholar]
- Stromhaug PE, Berg TO, Fengsrud M, Seglen PO. Purification and characterization of autophagosomes from rat hepatocytes. Biochem J. 1998;335:217–224. doi: 10.1042/bj3350217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanida I, Mizushima N, Kiyooka M, Ohsumi M, Ueno T, Ohsumi Y, Kominami E. Apg7/Cvt2p: a novel protein-activating enzyme essential for autophagy. Mol Biol Cell. 1999;10:1367–1379. doi: 10.1091/mbc.10.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thumm M, Egner R, Koch B, Schlumpberger M, Straub M, Veenhuis M, Wolf DH. Isolation of autophagocytosis mutants of Saccharomyces cerevisiae. FEBS Lett. 1994;349:275–280. doi: 10.1016/0014-5793(94)00672-5. [DOI] [PubMed] [Google Scholar]
- Tsukada M, Ohsumi Y. Isolation and characterization of autophagy-defective mutant of Saccharomyces cerevisiae. FEBS Lett. 1993;333:169–174. doi: 10.1016/0014-5793(93)80398-e. [DOI] [PubMed] [Google Scholar]
- Ueno T, Muno D, Kominami E. Membrane markers of endoplasmic reticulum preserved in autophagic vacuolar membranes isolated from leupeptin-administered rat liver. J Biol Chem. 1991;266:18995–18999. [PubMed] [Google Scholar]
- Vida T, Gerhardt B. A cell-free assay allows reconstitution of Vps33p-dependent transport to the yeast vacuole/lysosome. J Cell Biol. 1999;146:85–98. doi: 10.1083/jcb.146.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A, Masaki R, Fukui Y, Tashiro Y. Absence of cytochrome P-450 and presence of autophagosomal membrane antigens on the isolation membranes and autophagosomal membranes in rat hepatocytes. J Histochem Cytochem. 1990;38:1571–1581. doi: 10.1177/38.11.2212617. [DOI] [PubMed] [Google Scholar]
- Yoshimori T, Keller P, Roth MG, Simons K. Different biosynthetic transport routes to the plasma membrane in BHK and CHO cells. J Cell Biol. 1996;133:247–256. doi: 10.1083/jcb.133.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]