Abstract
Objective
Obstructive sleep apnea (OSA) is assumed to influence the circadian blood pressure (BP) fluctuation, particularly causing nocturnal hypertension and changing the dipping pattern of nocturnal BP. This study aimed to clarify the triggers of the non-dipper pattern in nocturnal BP in Japanese patients with severe OSA (the apnea-hypopnea index ≥30/h).
Methods
Of 541 patients with OSA diagnosed using polysomnography (PSG) and ambulatory BP monitoring (ABPM), 163 patients <60 years of age (Younger group) and 101 patients ≥60 years of age (Older group) were stratified into the dipper or non-dipper pattern groups.
Results
A logistic regression analysis was performed using a non-dipper pattern as a dependent variable. A multivariate analysis demonstrated that the cumulative percentage of time at saturation below 90% was the only independent risk factor for the non-dipper and riser patterns in the Younger group (odds ratio, 1.022; 95% confidence interval, 1.001-1.044; p=0.035), whereas slow-wave sleep (odds ratio, 0.941; 95% confidence interval, 0.891-0.990; p=0.019) and the use of angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers (odds ratio, 2.589; 95% confidence interval, 1.051-6.848; p=0.039) were risk factors in the Older group.
Conclusion
These findings suggested that the degree of desaturation in young OSA patients and sleep quality in old OSA patients might influence the dipping patterns in nocturnal BP.
Keywords: ambulatory blood pressure monitoring, circadian rhythm, nocturnal blood pressure, polysomnography, sleep-disordered breathing
Introduction
Obstructive sleep apnea (OSA) is assumed to influence the development of hypertension (1) and circadian blood pressure (BP) fluctuation patterns (1-3). OSA is well known to elevate the nocturnal BP, which is characterized as non-dipper and riser patterns, and to cause organ damage (4, 5).
This study aimed to clarify the triggers of non-dipper and riser patterns in nocturnal BP in Japanese patients with severe OSA.
Materials and Methods
Subjects
A total of 541 Japanese patients who visited the Sleep Apnea Syndrome Outpatient Department in the St. Marianna University School of Medicine Hospital between February 2004 and March 2013 and underwent polysomnography for suspected sleep-disordered breathing (SDB) and ambulatory BP monitoring (ABPM) for investigating abnormal circadian BP rhythm within 2 months of polysomnography (PSG) were selected (Figure). Of these, 304 patients whose apnea hypopnea index (AHI) was ≥30/h were investigated for their gender, age, height, weight, body mass index (BMI), and the use or non-use of antihypertensive agents, diabetes drugs, including insulin, and lipid metabolism disorder drugs at the first visit. Daytime sleepiness was also assessed using the Epworth Sleepiness Scale (ESS), Japanese version (6).
Figure.
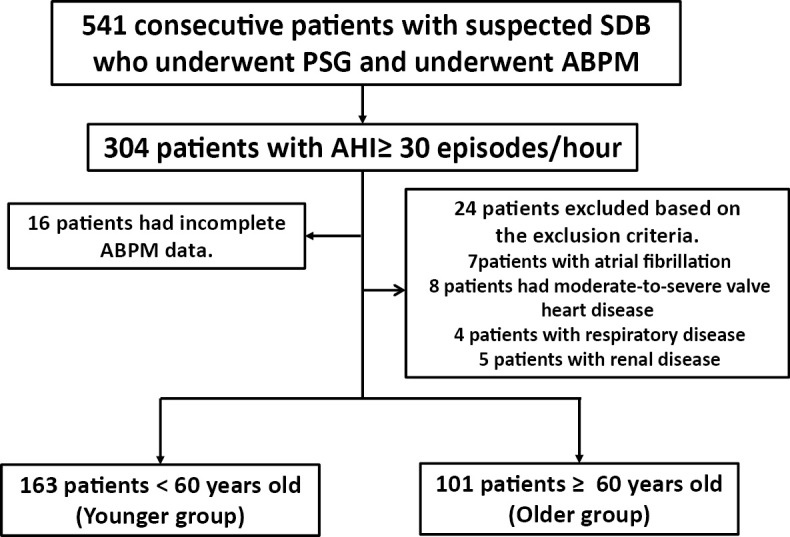
Patients inclusion flowchart.
The patients with atrial fibrillation, those with moderate-to-severe heart valve disease, those with respiratory disease, those with renal disease, those <18 years of age and those with insufficient data were excluded from this study (n=40). Ultimately, 264 Japanese patients were enrolled into this study.
All patients diagnosed with SDB had OSA and had not previously received OSA treatment. Based on the results of an earlier study demonstrating the association of SDB with hypertension in patients <60 years of age (7), our study patients were stratified into the following groups: patients <60 years of age (Younger group, n=163) and those ≥60 years of age (Older group, n=101).
The present study was performed in accordance with the ethical principles set forth in the Declaration of Helsinki. The study protocol was reviewed and approved by the Institutional Review Board of the St. Marianna University School of Medicine and implemented in compliance with the Personal Information Protection Law (Ethical Committee Approval Nos. 1142 and 1603).
Blood parameters
Blood samples were collected for measuring the serum concentration of fasting plasma glucose, glycosylated hemoglobin, triglyceride, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol and creatinine in the fasting state at the first examination. Diabetes mellitus was defined as glucose ≥126 mg/dL in the fasting state or glycosylated hemoglobin ≥6.5% according to the National Glycohemoglobin Standardization Program. Patients with low-density lipoprotein cholesterol ≥140 mg/dL, high-density lipoprotein cholesterol <40 mg/dL or triglyceride ≥150 mg/dL and those who were receiving lipid metabolism disorder treatment were defined as having dyslipidemia. According to the recommendation of the Japanese Society of Nephrology, the estimated glomerular filtration rate (eGFR) was calculated as follows: eGFR (mL/min/1.73 m2) = 194× Cr-1.094 × age-0.287 (×0.739 for women) (8).
Evaluation of sleep-disordered breathing
Polysomnography (SleepWatcherⓇ, Compumedics, Abbotsford, Australia; or PolymateⓇ, Miyuki Giken, Tokyo, Japan) was employed to evaluate SDB. A nasal cannula was placed at the nares to measure the respiratory airflow using a disposable airflow sensor; a strain gauge sensor monitored respiratory movements of the chest and abdominal walls. Arterial oxygen saturation (SpO2) was measured with a pulse oximeter detecting the percentage of oxygenated hemoglobin in arterial blood by two wavelengths of light transmitted to the finger to extract the pulse wave component according to the heart beat (9, 10). The results obtained during sleep were manually analyzed by laboratory technician specialists using the Rechtschaffen and Kales criteria (11); breathing events and cessation of airflow for 10 seconds or longer were recognized as apnea. Hypopnea was defined as an obvious reduction in airflow (less than 50%) for 10 seconds or longer compared with stable breathing before and after an event and decrease in oxygen saturation of 3% from the baseline or when the event is associated with arousal (12). AHI is defined as the frequency of apnea/hypopnea per hour. Low-oxygen exposure was defined as SpO2 <90% and the rate of SpO2 <90% (cumulative percentage of time at saturation below 90%, CT90%). The non-REM sleep stages 3 and 4 was defined as slow-wave sleep (SWS) in the present study.
Blood pressure measurement in a doctor's office
Each patient sat in a backed chair in the doctor's office without crossing their legs for a few minutes. BP was then measured using the brachial artery by physicians with electric automated manometers (Omron Healthcare, Kyoto, Japan; or Terumo, Tokyo, Japan); its accuracy was comparable to the BP measured with the auscultatory method using a mercury manometer. BP was measured during the usual outpatient consultation time between 1 PM and 5 PM after adjusting the height of the measuring table in order to keep the position of the cuff at heart level; the upper arm was then wrapped with a tourniquet (width, 13 cm; length, 22 cm). Two stable measurements were used for the average BP calculation. The BP at the first visit was evaluated.
24-h ABPM
Non-invasive ABPM was performed for 24 hours using an FM-800 (Fukuda Denshi, Tokyo, Japan) at 30-minute intervals (2). ABPM and PSG were assessed on two different days. The BP was measured by the oscilloscopic method with an automated BP cuff or by the Korotkoff method. The ABPM data were analyzed based on the method described by Kario et al. (13). The following systolic BP (SBP) and diastolic BP (DBP) were measured: 24-hour BP, the average BP during the recording day; awake BP, the average BP during the rest of the day; and sleep BP, the average BP during sleep at night. The non-dipping BP pattern (ND pattern) was defined when the reduction in the awake SBP was <10% of the sleep SBP, whereas the dipping BP pattern (D pattern) was defined when the reduction in the awake SBP was ≥10% of the sleep SBP (14, 15).
Statistical analyses
All measurements were indicated as mean ± standard deviation. The Mann-Whitney's U-test and Pearson's χ2 test were used for comparisons between the D and ND patterns in the Younger and Older groups. Univariate and multiple logistic regression analyses were performed to determine the factors related to the ND pattern. Since there were significant differences between the D and ND patterns in the Younger and Older groups, the determinant factors for the ND pattern were analyzed in the BMI and CT90% in the Younger group and the use or non-use of angiotensin-converting enzyme inhibitors (ACE-I) or angiotensin II receptor blockers (ARB) and the rate of SWS and REM in the Older group. Statistical analyses were conducted using the JMPⓇ software program for WindowsⓇ (version 10.0; SAS Institute, Cary, USA). Significant difference was set at a p value of <0.05 for the hazard ratio.
Results
Patients' characteristics (Table 1-3)
Table 1.
Patient Background Data.
| Group | All patients | Younger group | Older group | p value |
|---|---|---|---|---|
| Number of patients | 264 | 163 | 101 | / |
| Male sex no. (%) | 219 (83) | 151 (93) | 68 (67) | <0.001 |
| Age (years) | 54.8±12.9 | 46.7±9.0 | 67.9±5.1 | <0.001 |
| BMI (kg/m2) | 28.1±5.5 | 29.1±5.8 | 26.6±4.7 | <0.001 |
| ESS | 8.9±5.9 | 10.0±5.9 | 7.3±5.4 | <0.001 |
| Heart diseses and/or cerebrovascular diseases no. (%) | 43 (16) | 19 (12) | 24 (24) | 0.010 |
| Antihypertensive agents use no. (%) | 93 (35) | 43 (26) | 50 (50) | <0.001 |
| Calcium-channel blocker | 62 (23) | 32 (20) | 30 (30) | 0.060 |
| ACE-I/ARB | 66 (25) | 30 (18) | 36 (36) | 0.002 |
| Diuretic | 23 (9) | 7 (4) | 16 (16) | 0.001 |
| β blocker | 15 (6) | 9 (6) | 6 (6) | 0.886 |
| α blocker | 6 (2) | 3 (2) | 3 (3) | 0.549 |
| Aldosterone blocker | 5 (2) | 0 (0) | 5(5) | 0.004 |
| Diabetes mellitus no. (%) | 57 (22) | 28 (17) | 29 (28) | 0.027 |
| Dyslipidemia no. (%) | 152 (58) | 96 (59) | 56 (55) | 0.581 |
| Creatinine (mg/dL) | 0.93±0.63 | 0.86±0.19 | 1.04±0.98 | 0.022 |
| eGFR (mL/min/1.73m2) | 71.8±19.5 | 78.2±17.3 | 61.4±18.4 | <0.001 |
| 24-h Systolic blood pressure (mmHg) | 133.2±11.7 | 132.9±11.4 | 133.6±12.3 | 0.653 |
| AHI (/h) | 54.1±17.4 | 55.7±17.3 | 51.5±17.2 | 0.054 |
BMI: body mass index, ESS: Epworth sleepiness scale, ACE-I: angiotensin-converting enzyme inhibitor, ARB: angiotensin II receptor blocker, eGFR: estimated glomerular filtration rate, AHI: apnea-hypopnea index
The values are presented as the mean±s.d.
Table 2.
Patient Background Data.
| Group | Younger group (n=163) | Older group (n=101) | |||||
|---|---|---|---|---|---|---|---|
| D pattern | ND pattern | p value | D pattern | ND pattern | p value | ||
| Number of patients | 85 | 78 | / | 39 | 62 | / | |
| Dipping status (%) | +10.2 ~ +33.5 | -9.2 ~ +9.9 | / | +10.4 ~ +22.0 | -26.1 ~ +9.9 | / | |
| Male sex no. (%) | 77 (91) | 74 (95) | 0.296 | 27 (69) | 41 (66) | 0.746 | |
| Age (years) | 46.4±9.9 | 47.1±7.9 | 0.595 | 67.3±4.8 | 68.3±5.3 | 0.309 | |
| BMI (kg/m2) | 28.1±5.3 | 30.1±6.2 | 0.026 | 26.6±3.3 | 26.7±5.4 | 0.911 | |
| ESS | 10.1±5.5 | 9.8±6.4 | 0.770 | 7.3±6.1 | 7.2±4.9 | 0.923 | |
| Heart diseses and/or cerebrovascular diseases no. (%) | 11 (13) | 8 (10) | 0.594 | 8 (21) | 16 (26) | 0.543 | |
| Antihypertensive agents use no. (%) | 24 (28) | 19 (24) | 0.575 | 17 (44) | 33 (53) | 0.346 | |
| Calcium-channel blocker | 18 (21) | 14 (18) | 0.604 | 9 (23) | 21 (34) | 0.248 | |
| ACE-I/ARB | 19 (22) | 11 (14) | 0.175 | 9 (23) | 27 (44) | 0.034 | |
| Diuretic | 4 (5) | 3 (4) | 0.775 | 6 (15) | 10 (16) | 0.821 | |
| β blocker | 5 (6) | 4 (5) | 0.833 | 1 (3) | 5 (8) | 0.254 | |
| α blocker | 2 (2) | 1 (1) | 0.611 | 0 (0) | 3 (5) | 0.163 | |
| Aldosterone blocker | 0 (0) | 0 (0) | / | 2 (5) | 3 (5) | 0.948 | |
| Diabetes mellitus no. (%) | 16 (19) | 12 (15) | 0.561 | 18 (29) | 11 (28) | 0.929 | |
| Dyslipidemia no. (%) | 45 (53) | 51 (65) | 0.107 | 23 (59) | 33 (53) | 0.572 | |
| Creatinine (mg/dL) | 0.86±0.21 | 0.86±0.17 | 0.957 | 1.19±1.51 | 0.95±0.38 | 0.240 | |
| eGFR (mL/min/1.73m2) | 78.5±17.5 | 78.0±17.1 | 0.860 | 60.7±19.1 | 61.8±19.1 | 0.771 | |
BMI: body mass index, ESS: Epworth sleepiness scale, ACE-I: angiotensin-converting enzyme inhibitor, ARB: angiotensin II receptor blocker, eGFR: estimated glomerular filtration rate
The values are presented as the mean±s.d.
Table 3.
Sleep and Blood Pressure Data.
| Younger group | Older group | |||||||||
| ABPM |
All patients (n=163) |
D pattern (n=85) |
ND pattern (n=78) |
p value |
All patients (n=101) |
D pattern (n=39) |
ND pattern (n=62) |
p value | ||
| Period |
Blood pressure (mmHg) |
|||||||||
| 24-h | Systole | 133±11 | 132±11 | 135±12 | 0.105 | 134±12 | 133±11 | 134±13 | 0.457 | |
| Diastole | 88±9 | 87±9 | 89±9 | 0.071 | 82±9 | 82±9 | 82±9 | 0.817 | ||
| Awake | Systole | 138±11 | 138±11 | 137±12 | 0.359 | 137±13 | 140±11 | 136±13 | 0.118 | |
| Diastole | 91±9 | 91±9 | 91±9 | 0.929 | 84±9 | 87±9 | 83±9 | 0.057 | ||
| Sleep | Systole | 123±14 | 117±11 | 130±12 | <0.001 | 127±14 | 118±12 | 130±13 | <0.001 | |
| Diastole | 81±10 | 77±9 | 85±9 | <0.001 | 77±10 | 73±10 | 79±10 | <0.001 | ||
| Dipping status (%) | 11.0±7 | 15.7±4.5 | 4.9±4.6 | <0.010 | 7.3±8 | 14.6±3.3 | 2.7±5.5 | <0.001 | ||
| Polysmonography | All patients | D pattern | ND pattern | p value | All patients | D pattern | ND pattern | p value | ||
| AHI (/h) | 55.7±17.3 | 53.7±16.9 | 58.0±17.6 | 0.110 | 51.5±17.2 | 48.1±15.1 | 53.7±18.2 | 0.115 | ||
| AI (/h) | 30.3±23.2 | 30.1±22.5 | 30.6±24.0 | 0.875 | 24.2±16.2 | 23.7±16.6 | 24.5±16.1 | 0.806 | ||
| OA (/h) | 27.5±22.5 | 26.9±22.1 | 28.2±23.1 | 0.715 | 18.8±15.6 | 18.4±17.0 | 19.1±14.8 | 0.831 | ||
| CA (/h) | 1.1±1.9 | 1.3±2.3 | 0.8±1.2 | 0.628 | 3.1±5.9 | 2.7±4.8 | 3.3±6.6 | 0.626 | ||
| CT90% (%) | 15.6±19.3 | 11.4±12.5 | 20.1±24.0 | 0.004 | 10.9±14.2 | 8.8±12.1 | 12.3±15.4 | 0.230 | ||
| Lowest oxygen saturation (%) | 73.7±8.6 | 74.9±11.0 | 72.3±8.7 | 0.058 | 76.0±9.2 | 76.6±10.0 | 75.5±8.7 | 0.572 | ||
| SWS (%) | 10.3±8.8 | 9.5±7.9 | 11.1±9.7 | 0.237 | 11.2±8.5 | 13.7±8.9 | 9.6±7.8 | 0.017 | ||
| Arousal index (/h) | 50.4±20.2 | 49.4±19.8 | 51.1±20.8 | 0.636 | 46.9±19.3 | 43.9±14.3 | 48.7±21.8 | 0.217 | ||
ABPM: ambulatory blood pressure monitoring, AHI: apnea-hypopnea index, AI: apnea index, OA: obstructive apnea, CA: central apnea, ODI: oxygen desaturation index, CT90%: cumulative percentages of time at saturation below 90%, SWS: slow wave sleep
The values are presented as the mean±s.d.
The patients' background characteristics are summarized in Tables 1 and 2. Significant differences in the BMI in the Younger group (p=0.026) and the use of ACE-I/ARB in the Older group (p=0.034) were found between the D and ND patterns (Table 2). Table 3 shows the obtained sleep and BP data in the two groups.
Evaluation of the ND pattern for each factor Table 4
Table 4.
Results of Logistic Regression Analyses to Determine Factors Affecting the Non-dipping Blood Pressure Pattern.
| Younger group | ND pattern | Younger group | ND pattern | ||||
|---|---|---|---|---|---|---|---|
| Univariate analysis | Odds ratio | 95%CI | p value | Multiple analysis | Odds ratio | 95% CI | p value |
| BMI (kg/m2) | 1.065 | 1.008-1.131 | 0.024 | BMI | 1.033 | 0.969-1.104 | 0.318 |
| CT90% (%) | 1.026 | 1.008-1.047 | 0.004 | CT90% | 1.022 | 1.001-1.044 | 0.035 |
| Older group | ND pattern | Older group | ND pattern | ||||
| Univariate analysis | Odds ratio | 95%CI | p value | Multiple analysis | Odds ratio | 95%CI | p value |
| ACE-I/ARB (0=no, 1=yes) | 2.570 | 1.075-6.576 | 0.034 | ACE-I/ARB | 2.589 | 1.051-6.848 | 0.039 |
| SWS (%) | 0.943 | 0.895-0.990 | 0.017 | SWS | 0.941 | 0.891-0.990 | 0.019 |
95% CI: 95% confidence interval, BMI: body mass index, CT90%: cumulative percentages of time at saturation below 90%, ACE-I: angiotensin-converting enzyme inhibitor, ARB: angiotensin II receptor blocker, SWS: slow wave sleep
A logistic regression analysis was performed using the ND pattern as a dependent variable (Table 4). The parameters demonstrating significant differences between the D and ND patterns in the Younger and Older groups were evaluated. The results of a univariate analysis indicated a significant relationship between the occurrence of the ND pattern, the BMI and the CT90% in the Younger group and between the use of ACE-I/ARB and SWS in the Older group. The multivariate analysis demonstrated that CT90% was the only independent risk factor in the Younger group [odds ratio, 1.022; 95% confidence interval (CI), 1.001-1.044; p=0.035]; whereas SWS (odds ratio, 0.941; 95% CI, 0.891-0.990; p=0.019) and the use of ACE-I or ARB (odds ratio, 2.589; 95% CI, 1.051-6.848; p=0.039) were risk factors in the Older group.
Discussion
OSA is a known cause of nocturnal hypertension and a non-dipper or riser pattern (1-3). To our knowledge, only a handful of study reports have discussed the factors determining nocturnal BP fluctuation patterns in patients with OSA (16). Analyzing the factors affecting nocturnal BP fluctuation patterns and providing appropriate therapeutic intervention will probably improve nocturnal BP fluctuation and prevent subsequent organ damage in patients with OSA. Several epidemiological studies have reported that the factors of OSA associated with the onset of hypertension vary among age groups (7, 17). Therefore, in the present study, we divided OSA patients into two groups (patients <60 and ≥60 years of age) and then divided those two groups into two subgroups among patients in whom the mean 24-hour BP was nearly equal (no significant differences observed in the mean value) but the nocturnal BP fluctuation patterns were different (D pattern vs. ND pattern). We then analyzed the factors influencing nocturnal BP dipping patterns. In particular, we selected those patients with severe OSA to clarify the possible influence of OSA.
Differences between young and old OSA patients
The BMI and the degree of sleepiness were greater in the Younger group than in the Older group even though the 24-hour SBP and AHI were similar (Table 1). Significant differences were found in the morbidity of cardiovascular diseases, diabetes mellitus and renal dysfunction and in the use of antihypertensive agents. These findings are often seen in older SDB patients in the clinical setting (18). In the younger generation, OSA is considered an independent factor for developing hypertension. However, some reports have suggested that OSA may independently trigger hypertension in individuals ≥60 years of age (7, 17). The possible reasons behind this difference are as follows: 1) the factors associated with the onset of OSA vary by age; and 2) the influence of OSA on human bodies also differs by age (7). There are some factors associated with the onset of OSA due to age, such as the degree of obesity, the structure and function of the upper respiratory tract and the respiratory control function (7). In general, older patients with OSA tend to have less obesity and a lower prevalence of hypoxemia than younger patients, even if their AHIs are similar (19). Indeed, the patient background in the present study showed that the older patients with severe OSA tended to have less obesity and lower oxygen desaturation (CT90% and lowest oxygen saturation) than the younger patients with severe OSA.
Factors associated with the non-dipper pattern in severe OSA patients
• Younger group
Our multivariate analysis demonstrated that CT90% was the only independent factor determining the non-dipper and riser patterns (ND group). Previous studies on OSA patients have shown that CT90% was associated with the onset of left ventricular hypertrophy (20) and CT90%, a parameter of SDB, was the only factor associated with brain natriuretic peptide in patients with chronic heart failure (21). Other studies have also reported that OSA patients with a higher degree of obesity had a higher level of oxygen desaturation (22, 23). Similarly, a correlation was confirmed between the BMI and CT90% in the present study (Younger group, R=0.502 and p<0.001; Older group, R=0.513 and p<0.001). OSA causes negative intrathoracic pressure, hypersensitivity of the carotid body chemoreflex, hypoxemia and microarousal; these changes induced OSA-related nocturnal hypertension (24). Accordingly, younger OSA patients who tend to have a higher degree of obesity (higher BMI) may be affected more profoundly by oxygen desaturation due to OSA than older OSA patients. The data obtained from the Younger group suggested that the depth and length of oxygen desaturation, represented by CT90%, might trigger a fall in nocturnal BP and lower nocturnal BP variability.
• Older group
The rate of SWS is an independent factor influencing the ND pattern. During non-rapid eye movement (NREM) sleep, sympathetic nervous activity decreases, BP declines, and BP drops as the sleep stages deepen (25). The results of a multivariate analysis in the present study showed that a decrease in the rate of SWS during NREM sleep was an independent trigger of the ND pattern. In one study conducted in healthy subjects, the deprivation of SWS resulted in a lesser extent of nocturnal reduction (26). Decreased SWS might influence nocturnal BP fluctuations. In patients ≥65 years of age, decreased SWS was identified as a predictor for the onset of hypertension during the average follow-up period of 3.4 years (27). The results of our study also support those of the earlier studies and suggest that a reduction in SWS may affect nocturnal BP fluctuations in older OSA patients.
The use of ACE-I or ARB was an independent risk factor in the occurrence of the ND pattern. In the present study, many older patients received ACE-I/ARB; of these, some additionally took other antihypertensive agents. These antihypertensive effects might have persisted in the daytime and ceased at night. Consequently, the 24-hour BP and awake BP in these OSA patients using ACE-I/ARB were relatively well-controlled, whereas the sleep BP was not well-controlled (28).
Clinical implications of the results of this study
In severe OSA patients, the impact of OSA on the nocturnal BP fluctuation may differ by age. That is, even if the severity of OSA based on AHI is similar between younger and older patients, the degree of obesity and the level of hypoxemia differ between the two groups. This difference affects the nocturnal BP in OSA differently.
In clinical practice, continuous positive airway pressure (CPAP) therapy should be actively promoted in younger patients with severe OSA in order to improve desaturation caused by OSA. CPAP therapy improves the nocturnal BP fluctuation patterns and eventually contributes to the prevention of cardiovascular disease. However, whether or not OSA is a trigger for reducing SWS and shortening the duration of SWS in older OSA patients with an ND pattern remains unclear. Physiologically, SWS is known to decrease by age (29); however, one study indicated that CPAP therapy increased SWS (30). Accordingly, CPAP therapy should be considered as a therapeutic option for older patients with severe OSA.
Study limitations
This study was a small, retrospective, single-center and cross-sectional. As such, a prospective study is needed. The disease duration of OSA was not considered in this study. The prognosis of the patients in this study was not investigated. ABPM has poor reproducibility, so whether or not ABPM data accurately reflect the original BP is debatable (31).
The authors state that they have no Conflict of Interest (COI).
References
- 1. Kario K. Obstructive sleep apnea syndrome and hypertension: ambulatory blood pressure. Hypertens Res 32: 428-432, 2009. [DOI] [PubMed] [Google Scholar]
- 2. Sekizuka H, Kida K, Akashi YJ, et al. . Relationship between sleep apnea syndrome and sleep blood pressure in patients without hypertension. J Cardiol 55: 92-98, 2010. [DOI] [PubMed] [Google Scholar]
- 3. Sekizuka H, Osada N, Kida K, Yoneyama K, Eguchi Y, Miyake F. Relationship between chronic kidney disease and sleep blood pressure in patients with sleep apnea syndrome. Hypertens Res 33: 1278-1282, 2010. [DOI] [PubMed] [Google Scholar]
- 4. Kario K, Pickering TG, Matsuo T, Hoshide S, Schwartz JE, Shimada K. Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension 38: 852-857, 2001. [DOI] [PubMed] [Google Scholar]
- 5. Ohkubo T, Hozawa A, Yamaguchi J, et al. . Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens 20: 2183-2189, 2002. [DOI] [PubMed] [Google Scholar]
- 6. John MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 14: 540-545, 1991. [DOI] [PubMed] [Google Scholar]
- 7. Haas DC, Foster GL, Nieto FJ, et al. . Age-dependent associations between sleep-disordered breathing and hypertension: importance of discriminating between systolic/diastolic hypertension and isolated systolic hypertension in the Sleep Heart Health Study. Circulation 11: 614-621, 2005. [DOI] [PubMed] [Google Scholar]
- 8. Matsuo S, Imai E, Horio M, et al. . Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53: 932-935, 2009. [DOI] [PubMed] [Google Scholar]
- 9. Chesson AL Jr, Berry RB, Pack A. Practice parameters for the use of portable monitoring devices in the investigation of suspected obstructive sleep apnea in adults. Sleep 26: 907-913, 2003. [DOI] [PubMed] [Google Scholar]
- 10. Flemons WW, Littner MR, Rowley JA, et al. . Home diagnosis of sleep apnea: a systematic review of the literature. Chest 124: 1543-1579, 2003. [DOI] [PubMed] [Google Scholar]
- 11. Rechtschaffen A, Kales A In: Manual of standardized technology, techniques and scoring system for sleep stage of human subjects. Brain Information Service, Brain Research Institute, UCLA, Los Angeles, 1968. [Google Scholar]
- 12. American Academy of Sleep Medicine Task Force Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep 22: 667-689, 1999. [PubMed] [Google Scholar]
- 13. Kario K, Pickering TG, Umeda Y, et al. . Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation 107: 1401-1406, 2003. [DOI] [PubMed] [Google Scholar]
- 14. Shimamoto K, Ando K, Fujita T, et al. . The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens Res 37: 253-392, 2014. [DOI] [PubMed] [Google Scholar]
- 15. Hoshide S, Kario K, Hoshide Y, et al. . Associations between nondipping of nocturnal blood pressure decrease and cardiovascular target organ damage in strictly selected community-dwelling normotensives. Am J Hypertens 16: 434-438, 2003. [DOI] [PubMed] [Google Scholar]
- 16. Sasaki N, Ozono R, Yamauchi R, et al. . Age-related differences in the mechanism of nondipping among patients with obstructive sleep apnea syndrome. Clin Exp Hypertens 34: 270-277, 2012. [DOI] [PubMed] [Google Scholar]
- 17. Nieto FJ, Young TB, Lind BK, et al. . Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 283: 1829-1836, 2000. [DOI] [PubMed] [Google Scholar]
- 18. Teramoto S, Inoue Y, Ouchi Y. Clinical significance of geriatric sleep apnea syndrome. Geriatr Gerontol Int 2: 163-171, 2002. [Google Scholar]
- 19. Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med 157: 144-148, 1998. [DOI] [PubMed] [Google Scholar]
- 20. Chami HA, Devereux RB, Gottdiener JS, et al. . Left ventricular morphology and systolic function in sleepdisordered breathing: the Sleep Heart Health Study. Circulation 117: 2599-2607, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gottlieb JD, Schwartz AR, Marshall J, et al. . Hypoxia, not the frequency of sleep apnea, induces acute hemodynamic stress in patients with chronic heart failure. J Am Coll Cardiol 54: 1706-1712, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ernst G, Bosio M, Salvado A, Dibur E, Nigro C, Borsini E. Difference between apnea-hypopnea index (AHI) and oxygen desaturation index (ODI): proportional increase associated with degree of obesity. Sleep Breath 20: 1175-1183, 2016. [DOI] [PubMed] [Google Scholar]
- 23. Kendzerska T, Leung RS, Gershon AS, Tomlinson G, Ayas N. The interaction of obesity and nocturnal hypoxemia on cardiovascular consequences in adults with suspected obstructive sleep apnea. A historical observational study. Ann Am Thorac Soc 13: 2234-2341, 2016. [DOI] [PubMed] [Google Scholar]
- 24. Kario K. Obst ruct ive sleep apnea syndrome and hypertension: mechanism of the linkage and 24-h blood pressure control. Hypertens Res 32: 537-541, 2009. [DOI] [PubMed] [Google Scholar]
- 25. Snyder F, Hobson JA, Morrison DF, Goldfrank F. Changes in respiration heart rate and systolic blood pressure in human sleep. J Appl Physiol 19: 417-422, 1964. [DOI] [PubMed] [Google Scholar]
- 26. Sayk F, Teckentrup C, Becker C, et al. . Effects of selective slow-wave sleep deprivation on nocturnal blood pressure dipping and daytime blood pressure regulation. Am J Physiol Regul Integr Comp Physiol 298: R191-R197, 2010. [DOI] [PubMed] [Google Scholar]
- 27. Fung MM, Peters K, Redline S, et al. . Decreased slow wave sleep increases risk of developing hypertension in elderly men.; Osteoporotic Fractures in Men Research Group. Hypertension 58: 596-603, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pelttari LH, Hietanen EK, Salo TT, Kataja MJ, Kantola IM. Little effect of ordinary antihypertensive therapyon nocturnal high blood pressure in patients with sleep disordered breathing. Am J Hypertens 11: 272-279, 1998. [DOI] [PubMed] [Google Scholar]
- 29. Wolkove N, Elkholy O, Baltzan M, Palayew M. Sleep and aging: 1. Sleep disorders commonly found in older people. CMAJ 176: 1299-1304, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Heinzer R, Gaudreau H, Décary A, et al. . Slow-wave activity in sleep apnea patients before and after continuous positive airway pressure treatment: contribution to daytime sleepiness. Chest 119: 1807-1813, 2001. [DOI] [PubMed] [Google Scholar]
- 31. Mochizuki Y, Okutani M, Donfeng Y, et al. . Limited reproducibility of circadian variation in blood pressure dippers and non-dippers. Am J Hypertens 11: 403-409, 1998. [DOI] [PubMed] [Google Scholar]


