Abstract
Cerebrotendinous xanthomatosis (CTX) is a rare, autosomal recessive, inborn disruption in bile acid synthesis characterized by severe systemic xanthomas, cataracts and neurological injuries occurring before adolescence without elevation of the serum cholesterol or triglyceride levels. CTX is caused by a deficiency of the mitochondrial enzyme sterol 27-hydroxylase, which is encoded by the CYP27A1 gene. We herein report a 50-year-old Japanese woman with late-onset CTX who had no relevant symptoms before the development of bilateral Achilles tendon xanthomas in middle age. A genetic analysis revealed a compound heterozygous mutation in the CYP27A1 gene with a previously known missense mutation (NM_000784.3:c.1421 G>A) and a novel frame shift mutation of NM_000784.3:c.1342_1343insCACC.
Keywords: cerebrotendinous xanthomatosis (CTX), sterol 27-hydroxylase (CYP27A1), cholestanol, norcholic acid, chenodeoxycholic acid (CDCA), cholesterol efflux
Introduction
Cerebrotendinous xanthomatosis (CTX) is an autosomal recessive lipid storage disease caused by disruption in bile acid synthesis induced by mutations in the CYP27A1 gene on chromosome 2q33-qter (1). Fifty-four different mutations in CYP27A1 have been reported thus far (2). The first case of CTX was described in 1937 by van Bogaert (3). Since then, more than 300 cases have been reported in countries around the world. There is no sex predominance. CTX is an extremely rare disease; its prevalence was estimated as 1.9 per 100,000 people among Caucasians of European ancestry (2). The estimated incidence of CTX is higher in East Asia, at approximately 15.5 per 100,000 people (4). However, precise epidemiologic data from Japan are lacking.
The CYP27A1 gene encodes the mitochondrial enzyme sterol 27-hydroxylase, which hydroxylates the steroid side chain at C-27 (5). Deficiency of this enzyme leads to the accumulation of cholestanol and bile alcohols in connective tissues and neuronal cells and results in systemic symptoms, such as neurological disorders, atherosclerosis, tendon xanthomas and cataracts. Patients with CTX typically develop cataracts before their 10th birthday and develop systemic xanthomas in the second or third decade of life. Although most CTX patients have normal intelligence until puberty, intellectual functioning worsens with age, and over 50% of CTX patients show an intellectual decline in their 20s. Slowly progressive neurologic symptoms, such as pyramidal tract signs, cerebellar ataxia and peripheral neuropathy, are typically observed in their 20s and 30s (2). There is significant clinical heterogeneity, even among siblings with the same mutation in the CYP27A1 gene; as such, the clinical phenotypes do not seem to be strictly correlated with the genotypes in CTX (2).
Chenodeoxycholic acid (CDCA) has been demonstrated to decrease the levels of serum cholestanol and urinary bile alcohols in CTX patients (6). Treatment with CDCA can stabilize or even reverse some of the symptoms in most patients with CTX (7). Starting CDCA during childhood or earlier is believed to be more beneficial than starting treatment in adulthood (8).
We herein report a Japanese woman diagnosed with CTX at age 50 who had no related symptoms of CTX before the development of bilateral Achilles tendon xanthomas in middle age.
Case Report
Case presentation
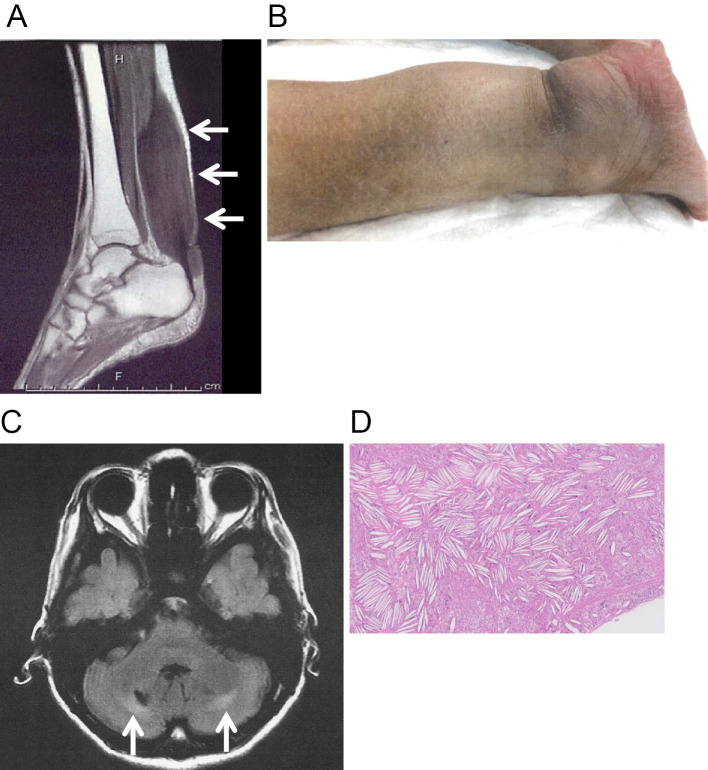
The patient was a 50-year-old Japanese woman from non-consanguineous parents. She has no siblings but had a child. There was no apparent family history (parents, child and parent’s siblings) of neurological disorders, cardiovascular diseases or any lipid storage diseases inducing xanthomas, including CTX. She had no history of neonatal jaundice or chronic infantile diarrhea. She had been diagnosed with idiopathic epilepsy and medicated from 7 to 10 years of age. Thereafter, she had no remarkable medical developments until middle age. She insidiously developed painless tendon thickness of the bilateral Achilles tendons and right triceps muscle at 47 years of age. The gradual enlargement of the Achilles tendons caused gait disturbance when she was 49 years of age. T1- and T2-weighted magnetic resonance imaging (MRI) revealed low-intensity lesions in the thickened bilateral Achilles tendons (Fig. 1A) and right triceps muscle, suggestive of xanthomas. She was admitted to Nagasaki University Hospital for further investigation at 50 years of age.
Figure 1.
A: Xanthoma of the Achilles tendon on T2-weighed MRI. B: Extremely thickened Achilles tendon. C: FLAIR MRI of the brain. Arrows indicate bilateral high-intensity areas in the dorsal lesion behind the cerebellar dentate nucleus. D: Hematoxylin and Eosin staining of xanthoma in the Achilles tendon. Foamy cells admixed with inflammatory giant cells infiltrated the tendinous tissue, and cholesterol clefts were visible.
Clinical, laboratory and imaging examinations
The patient was lean (height 149.0 cm, weight 39.2 kg, BMI 17.7 kg/m2), and the findings of a physical examination were unremarkable except for confirmation of xanthomas on the bilateral Achilles tendons (Fig. 1B) and right triceps muscle. She had age-appropriate slight cataracts in the bilateral eyes and did not have dementia based on the Revised Hasegawa Dementia Scale (HSD-R; 26/30), Mini-Mental State Examination (MMSE; 27/30), and Frontal Assessment Battery (FAB; 16/18) scores. On a neurological examination, her gait appeared somewhat spastic, and all of her deep tendon reflexes were mildly enhanced bilaterally with positive Babinski and Chaddock reflexes, indicating impairment in the bilateral pyramidal tracts. There were no neurological findings suggesting cerebellar impairment.
Regarding the laboratory findings (Table 1), there was no elevation in the levels of serum cholesterol or triglycerides or deficiencies in apolipoproteins or thyroid hormones. As the clinical findings suggested CTX, we studied the bile acids and sterols in her serum and urine (Table 2). The primary bile acids were extremely low in the serum and urine when measured by both high-performance liquid chromatography (HPLC) and gas chromatography/mass spectrometer (GC/MS). The urinary levels of bile alcohols, especially 5β-cholestane-3α,7α,12α,24,25-pentol, were markedly elevated. Norcholic acid, which is mainly derived from 5β-cholestane-3α,7α,12α,23,25-pentol, was highly elevated and accounted for 78.4% of all bile acids in the urine. The serum level of cholestanol was extremely elevated, while those of sitosterol and campesterol were not elevated.
Table 1.
Serum Lipids and Associated Laboratory Findings on Admission.
| Case | Reference | Case | Reference | |||
|---|---|---|---|---|---|---|
| Total cholesterol (mg/dL) | 203 | 142-248 | Total bilirubin (mg/dL) | 1.9 | 0.4-1.5 | |
| Triglyceride (mg/dL) | 123 | 30-117 | Direct bilirubin (mg/dL) | 0.2 | ≤ 0.4 | |
| HDL-C (mg/dL) | 75 | 48-103 | AST (IU/L) | 16 | 13-30 | |
| LDL-C (mg/dL) | 103 | 65-163 | ALT (IU/L) | 9 | 7-23 | |
| Apo A-I (mg/dL) | 167 | 119-155 | γ-GTP (IU/L) | 8 | 9-32 | |
| Apo A-II (mg/dL) | 28.1 | 25.9-35.7 | BUN (mg/dL) | 9.0 | 8-20 | |
| Apo B (mg/dL) | 80 | 73-109 | Creatinine (mg/dL) | 0.52 | 0.46-0.79 | |
| Apo C-II (mg/dL) | 7.0 | 1.8-4.6 | Calcium (mg/dL) | 9.1 | 8.8-10.1 | |
| Apo C-III (mg/dL) | 11.2 | 5.8-10.0 | Phosphate (mg/dL) | 3.0 | 2.7-4.6 | |
| Apo E (mg/dL) | 6.3 | 2.7-4.3 | Free T4 (ng/dL) | 1.27 | 0.95-1.57 | |
| Free cholesterol (mg/dL) | 56 | 34-66 | TSH (μIU/mL) | 0.43 | 0.48-5.08 | |
| Free fatty acid (mEq/L) | 0.59 | 0.10-0.90 | Intact PTH (pg/mL) | 69.0 | 10.3-65.9 | |
| Phospholipid (mg/dL) | 243 | 150-280 | 25(OH)VitD (ng/mL) | 14 | 30-60 | |
| Lipoprotein(a) (mg/dL) | 11.4 | ≤ 40 | 1,25(OH)2VitD (pg/mL) | 48 | 20-60 | |
| MDA-LDL (U/L) | 119 | 46-105 | TRACP-5b (mU/dL) | 385 | 120-420 | |
| SAA-LDL (µg/dL) | 5.8 | <20 | Intact P1NP (µg/L) | 66.2 | 17.1-64.7 | |
| Glucose (mg/dL) | 80 | 73-109 | LH (IU/L) | 37.5 | 1.4-15 | |
| Insulin (mIU/L) | 2.3 | 0.87-10.7 | FSH (IU/L) | 39.1 | 3.0-24.0 | |
| HbA1c (%) | 5.3 | 4.9-6.0 | Estradiol (pg/mL) | 360.1 | 28.8-491.9 |
HDL-C: high-density lipoprotein cholesterol, LDL-C: low-density lipoprotein cholesterol, Apo: apoprotein, MDA-LDL: malondialdehyde-modified LDL, SAA-LDL: serum amyloid A-LDL, AST: aspartate aminotransferase, ALT: alanine aminotransferase, γ-GTP: gamma-glutamyltransferase, BUN: blood urea nitrogen, TSH: thyrotropin, PTH: parathyroid hormone, TRACP-5b: tartrate resistant acid phosoatase-5b, P1NP: procollagen type 1 N-terminal propeptide, LH: luteinzing hormone, FSH: fertilizing hormone
Table 2.
Serum Levels of Bile Acids and Associated Biosynthetic Products before and 6 Months after Administration of CDCA.
| Before | After | Before | After | |||
|---|---|---|---|---|---|---|
| Serum bile acid (HPLC; μmol/L) | Serum bile acid (GC/MS; μmol/L) | |||||
| Glycoursodeoxycholic acid | N.D. | 2.1 | Total bile acid | 0.10 | 6.82 | |
| Tauroursodeoxycholic acid | N.D. | N.D. | Cholic acid | N.D. | N.D. | |
| Ursodeoxycholic acid | N.D. | 1.0 | Chenodeoxycholic acid | N.D. | 3.80 | |
| Glycocholic acid | N.D. | N.D. | Ursodeoxycholic acid | N.D. | 2.10 | |
| Taurocholic acid | N.D. | N.D. | Deoxycholic acid | N.D. | N.D. | |
| Cholic acid | N.D. | N.D. | Lithocholic acid | N.D. | N.D. | |
| Glycochenodeoxycholic acid | N.D. | 3.4 | Norcholic acid | 0.10 | N.D. | |
| Taurochenodeoxycholic acid | N.D. | N.D. | Others | N.D. | 0.92 | |
| Chenodeoxycholic acid | N.D. | 1.2 | ||||
| Glycodeoxycholic acid | N.D. | N.D. | Urinary bile acid (GC/MS; mmol/mol·Cr) | |||
| Taurodeoxycholic acid | N.D. | N.D. | Total bile acid | 0.50 | 1.09 | |
| Deoxycholic acid | N.D. | N.D. | Cholic acid | 0.03 | N.D. | |
| Glycolithocholic acid | N.D. | N.D. | Chenodeoxycholic acid | 0.01 | 0.12 | |
| Taurolithocholic acid | N.D. | 0.3 | Ursodeoxycholic acid | N.D. | 0.64 | |
| Lithocholic acid | N.D. | N.D. | Deoxycholic acid | N.D. | N.D. | |
| Lithocholic acid | N.D. | 0.06 | ||||
| Norcholic acid | 0.39 | 0.04 | ||||
| Others | 0.07 | 0.23 | ||||
| Serum sterol (GC; μg/mL) | Urinary bile alcohol (GC/MS; mmol/mol·Cr) | |||||
| Cholestanol | 25.2 | 7.8 | Total bile alcohol | 3.44 | 1.62 | |
| Sitosterol | 4.0 | 3.0 | 5β-cholestane-3α,7α,12α, 24,25-pentol | 2.02 | 1.11 | |
| Campesterol | 10.1 | 5.1 | ||||
| Others | 1.42 | 0.51 | ||||
CDCA: chenodeoxycholic acid, HPLC: high performance liquid chromatography, GC: gas chromatography, GC/MS: gas chromatography mass spectrometry, N.D.: not detectable, Cr: creatinine
T2-weighted and fluid-attenuated inversion recovery (FLAIR) MRI of the brain showed bilateral low-intensity areas in the dentate nuclei and high-intensity areas in the peripheral white matter the cerebellum (Fig. 1C). We also found bilateral high-intensity areas along the pyramidal tracts from the pons to the cerebral crus. Brain atrophy was not remarkable. Based on these clinical, laboratory and radiological findings, the patient was diagnosed with CTX.
Ultrasound of the carotid arteries showed a slightly thickened intima-media complex at the left common carotid artery (maximum intima-media thickness, 1.2 mm). However, there was no evidence of cardiovascular disease on electrocardiogram, echocardiography and MR angiography of the brain and lower extremities.
Despite being premenopausal (Table 1), she had osteoporosis as determined by dual-energy X-ray absorptiometry, which showed a bone density of 61% and 75% the young-adult-mean in the lumbar vertebra and femoral neck, respectively.
Genetic diagnosis
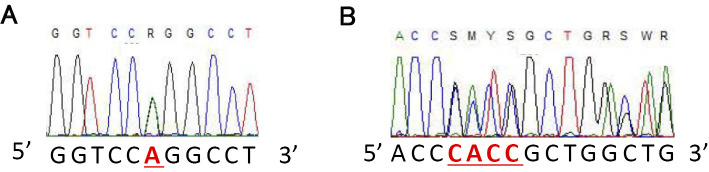
Genomic DNA was isolated from peripheral blood leukocytes. All 9 exons of the CYP27A1 gene were amplified by polymerase chain reaction (PCR) and sequenced. Two mutations were identified. One was a known missense mutation of NM_000784.3:c.1421GA in exon 8 (Fig. 2A), resulting in an amino acid substitution of arginine to glutamine at codon 474 (R474Q), as reported previously (9, 10). The other was a novel frame-shift mutation of NM_000784.3:c.1342_1343insCACC in exon 8 (Fig. 2B). As we did not perform genetic analyses of these two mutations in the patient's parents, we were unable to determine whether the patient was a compound heterozygote of two mutated alleles or had double mutations in one allele. Therefore, we assumed the patient to be a compound heterozygote of these two mutations based on the clinical and laboratory findings.
Figure 2.
A direct nucleotide sequence analysis of the exon 8 of CYP27A1 gene. A: The patient was heterozygous for the missense mutation of NM_000784.3: c.1421 G>A, resulting in the amino acid substitution of arginine to glutamine at codon 474 (NP_000775.1: pArg474Gln), as previously reported (Ref. 9). B: The patient was also heterozygous for a frame-shift mutation of NM_000784.3: c.1342_1343insCACC (NP_000775.1: p.Arg448ProfsTer521), which has never been reported before.
Cholesterol efflux assay
A cholesterol efflux assay was carried out as described previously (11). In brief, ATP-binding cassette transporter A1 (ABCA1)-transfected cell were incubated with [3H]-cholesterol. After incubation, the radioactive counts in media and cell lysates were measured by liquid scintillation counting. The ABCA1-mediated cholesterol efflux capacity, expressed as the percentage of labeled cholesterol moved from the cells to the medium, was 11.0% in the patient (reference level: > 7.0%).
Treatments
After CTX was diagnosed, the patient was treated with CDCA at 500 mg per day, divided into 2 doses. Alendronate at 35 mg per week and alfacalcidol at 0.5 µg per day were prescribed to treat her osteoporosis. These treatments are ongoing. She underwent bilateral Achilles-tendon plastic surgery, and the gait disturbance was improved dramatically after surgery. A pathological examination of the excised tissue revealed foamy cells admixed with inflammatory giant cells and cholesterol clefts surrounding tendinous tissues (Fig. 1D).
The serum levels of several bile acids were increased to detectable ranges, and the serum cholestanol and urinary norcholic acid levels were dramatically decreased six months after the administration of CDCA was started (Table 2). The serum cholestanol levels remained substantially low for at least 18 months after the initiation of CDCA treatment (4.5 and 5.4 µg/mL at 12 and 18 months, respectively). The patient has shown no regrowth of the tendon xanthomas or any other complications in the post-treatment period.
Discussion
We here present a case of late-onset CTX with compound heterozygous mutations with a known missense and a novel frame shift mutation in the CYP27A1 gene.
The pathogenesis of neuronal injury and other involvements in CTX has not been fully clarified. The leading hypothesis is that accumulation of cholestanol and cholesterol in the plasma membrane induces apoptosis of the neurons, and accumulation in other tissues causes xanthomas, juvenile-onset atherosclerosis and cataracts (12, 13). However, our patient was almost lacking in neurological and atherosclerotic findings other than the slight pyramidal-tract impairment and senile cataract. It is impossible to exclude that she had spinal cord involvements because we did not evaluate the spinal cord by MRI before starting CDCA treatment. The idiopathic epilepsy medicated in her childhood might be a symptom of CTX. It is unclear why she mainly developed xanthomas without other symptoms seen in CTX until middle age.
It was reported that sterol 27-hydroxylase expressed in macrophages enhances the cholesterol efflux capacity (14, 15). 27-hydroxycholesterol is a ligand of liver X receptor α (LXRα) (16) and plays a role in up-regulating the cholesterol efflux via the ABCA1 pathway (17). In this context, it would be of interest to know whether or not the cholesterol efflux is impaired for atherosclerotic formation in patients with sterol 27-hydroxylase deficiency, i.e. CTX. Although we performed an ABCA1-mediated cholesterol efflux assay in the present CTX patient, the efflux capacity was found to be relatively normal, which may partially account for the absence of apparent atherosclerotic disease in our case. More data are needed to clarify the relationship between sterol 27-hydroxylase and the cholesterol efflux in patients with CTX.
It has been reported that patients with CTX tend to develop osteoporosis (18). Our patient developed osteoporosis before menopause, and we found that her serum level of 25(OH)VitD was markedly decreased. Sterol 27-hydroxylase is one of several enzymes with vitamin D 25-hydroxylase activity (19), which may be a molecular mechanism underlying the development of osteoporosis in CTX. A previous report in a small group of CTX patients suggested an association of low serum 25(OH)VitD levels with osteoporosis (20), although other studies have reported relatively normal levels of 25(OH)VitD (18, 21). Therefore, the role of 25(OH)VitD in osteoporosis in CTX remains controversial (22).
Although a fundamental treatment for CTX has not yet been established, CDCA was shown to stabilize and even reverse some of the associated symptoms of CTX (2, 8). This is because the supplementation of CDCA, a primary bile acid, induces negative feedback in the catabolism of the various sterol intermediates (6). The abnormally high production of cholestanol thereby decreases, relieving its accumulation in the plasma membranes of neural cells. Indeed, it was reported that CDCA treatment improves the neurological manifestations and abnormal findings of brain MRI in CTX patients (8, 23). Our CTX patient mainly developed xanthoma of the tendons; as such, her neurological, neuropsychiatric, and cardiovascular manifestations might have been slowly progressive compared to other reported cases (8, 24, 25). As the serum levels of cholestanol decreased after starting CDCA, we remain hopeful that further complications of CTX can be prevented in this case.
Conclusion
We reported a middle-aged patient with late-onset CTX harboring a novel mutation in the CYP27A1 gene. It is important to begin CDCA treatment in patients with CTX before the onset of neurological dysfunction in order to prevent irreversible neuronal injury. Clinicians should consider CTX as a differential diagnosis when encountering patients with xanthomas of unknown etiology.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Cali JJ, Hsieh CL, Francke U, Russell DW. Mutations in the bile acid biosynthetic enzyme sterol 27-hydroxylase underlie cerebrotendinous xanthomatosis. J Biol Chem 266: 7779-7783, 1991. [PMC free article] [PubMed] [Google Scholar]
- 2. Waldman AT. Percy AK. In: Cetebrotendinous xanthomatosis. Up To Date. Wolter Kluwer, Alphen aan den Rijn, Netherlands, 2016. [Google Scholar]
- 3. van Bogaert L, Scherer HJ, Epstein E In: Une forme cérébrale de la cholestérinose généralisée. Masson et Cie, Paris, 1987. (in French). [Google Scholar]
- 4. Appadurai V, DeBarber A, Chiang PW, et al. Apparent underdiagnosis of Cerebrotendinous Xanthomatosis revealed by analysis of ~60,000 human exomes. Mol Genet Metab 116: 298-304, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leitersdorf E, Reshef A, Meiner V, et al. Frameshift and splice-junction mutations in the sterol 27-hydroxylase gene cause cerebrotendinous xanthomatosis in Jews or Moroccan origin. J Clin Invest 91: 2488-2496, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Salen G, Meriwether TW, Nicolau G. Chenodeoxycholic acid inhibits increased cholesterol and cholestanol synthesis in patients with cerebrotendinous xanthomatosis. Biochem Med 14: 57-74, 1975. [DOI] [PubMed] [Google Scholar]
- 7. vanHeijst AF, Verrips A, Wevers RA, Cruysberg JR, Renier WO, Tolboom JJ. Treatment and follow-up of children with cerebrotendinous xanthomatosis. Eur J Pediatr 157: 313-316, 1998. [DOI] [PubMed] [Google Scholar]
- 8. Yahalom G, Tsabari R, Molshatzki N, Ephraty L, Cohen H, Hassin-Baer S. Neurological outcome in cerebrotendinous xanthomatosis treated with chenodeoxycholic acid: early versus late diagnosis. Clin Neuropharmacol 36: 78-83, 2013. [DOI] [PubMed] [Google Scholar]
- 9. Kim KS, Kubota S, Kuriyama M, et al. Identification of new mutations in sterol 27-hydroxylase gene in Japanese patients with cerebrotendinous xanthomatosis (CTX). J Lipid Res 35: 1031-1039, 1994. [PubMed] [Google Scholar]
- 10. Gallus GN, Dotti MT, Federico A. Clinical and molecular diagnosis of cerebrotendinous xanthomatosis with a review of the mutations in the CYP27A1 gene. Neurol Sci 27: 143-149, 2006. [DOI] [PubMed] [Google Scholar]
- 11. Remaley AT, Thomas F, Stonik JA, et al. Synthetic amphipathic helical peptides promote lipid efflux from cells by an ABCA1-dependent and an ABCA1-independent pathway. J Lipid Res 44: 828-836, 2003. [DOI] [PubMed] [Google Scholar]
- 12. Bjorkhem I, Meaney S. Brain cholesterol: long secret life behind a barrier. Arterioscler Thromb Vasc Biol 24: 806-815, 2004. [DOI] [PubMed] [Google Scholar]
- 13. Bjorkhem I, Hansson M. Cerebrotendinous xanthomatosis: an inborn error in bile acid synthesis with defined mutations but still a challenge. Biochem Biophys Res Commun 396: 46-49, 2010. [DOI] [PubMed] [Google Scholar]
- 14. Babiker A, Andersson O, Lund E, et al. Elimination of cholesterol in macrophages and endothelial cells by the sterol 27-hydroxylase mechanism. Comparison with high density lipoprotein-mediated reverse cholesterol transport. J Biol Chem 272: 26253-26261, 1997. [DOI] [PubMed] [Google Scholar]
- 15. Bjorkhem I, Andersson O, Diczfalusy U, et al. Atherosclerosis and sterol 27-hydroxylase: evidence for a role of this enzyme in elimination of cholesterol from human macrophages. Proc Natl Acad Sci U S A 91: 8592-8596, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fu X, Menke JG, Chen Y, et al. 27-hydroxycholesterol is an endogenous ligand for liver X receptor in cholesterol-loaded cells. J Biol Chem 276: 38378-38387, 2001. [DOI] [PubMed] [Google Scholar]
- 17. Javitt NB. 25R,26-hydroxycholesterol revisited: synthesis, metabolism, and biologic roles. J Lipid Res 43: 665-670, 2002. [PubMed] [Google Scholar]
- 18. Federico A, Dotti MT, Lore F, Nuti R. Cerebrotendinous xanthomatosis: pathophysiological study on bone metabolism. J Neurol Sci 115: 67-70, 1993. [DOI] [PubMed] [Google Scholar]
- 19. Zhu J, DeLuca HF. Vitamin D 25-hydroxylase - four decades of searching, are we there yet? Arch Biochem Biophys 523: 30-36, 2012. [DOI] [PubMed] [Google Scholar]
- 20. Berginer VM, Shany S, Alkalay D, et al. Osteoporosis and increased bone fractures in cerebrotendinous xanthomatosis. Metabolism 42: 69-74, 1993. [DOI] [PubMed] [Google Scholar]
- 21. Kuriyama M, Fujiyama J, Kubota R, Nakagawa M, Osame M. Osteoporosis and increased bone fractures in cerebrotendinous xanthomatosis. Metabolism 42: 1497-1498, 1993. [DOI] [PubMed] [Google Scholar]
- 22. Martini G, Mignarri A, Ruvio M, et al. Long-term bone density evaluation in cerebrotendinous xanthomatosis: evidence of improvement after chenodeoxycholic acid treatment. Calcif Tissue Int 92: 282-286, 2013. [DOI] [PubMed] [Google Scholar]
- 23. Berginer VM, Salen G, Shefer S. Long-term treatment of cerebrotendinous xanthomatosis with chenodeoxycholic acid. N Engl J Med 311: 1649-1652, 1984. [DOI] [PubMed] [Google Scholar]
- 24. Clayton PT, Verrips A, Sistermans E, Mann A, Mieli-Vergani G, Wevers R. Mutations in the sterol 27-hydroxylase gene (CYP27A) cause hepatitis of infancy as well as cerebrotendinous xanthomatosis. J Inherit Metab Dis 25: 501-513, 2002. [DOI] [PubMed] [Google Scholar]
- 25. Cruysberg JR, Wevers RA, van Engelen BG, Pinckers A, van Spreeken A, Tolboom JJ. Ocular and systemic manifestations of cerebrotendinous xanthomatosis. Am J Ophthalmol 120: 597-604, 1995. [DOI] [PubMed] [Google Scholar]