Abstract
Pregnancy in women with systemic lupus erythematosus (SLE)-associated pulmonary arterial hypertension (PAH) remains a high risk. We successfully managed a pregnancy in a patient with SLE-PAH. A 31-year-old pregnant woman with SLE-PAH had worsening PAH and SLE flare-up during pregnancy and a sudden increase in pulmonary arterial pressure after delivery. SLE-PAH was controlled by continuous intravenous epoprostenol and inhaled nitric oxide therapy combined with high-dose corticosteroids under close hemodynamic monitoring. Women with SLE-PAH should avoid pregnancy. However, in case of a similar event, we recommend our case as a good reference for improving the outcome of pregnancy with SLE-PAH.
Keywords: systemic lupus erythematosus, pulmonary arterial hypertension, pregnancy
Introduction
When women of childbearing age develop systemic lupus erythematosus (SLE), it results in many difficulties during pregnancy. A high mortality rate of 66% has been reported in SLE-associated pulmonary arterial hypertension (SLE-PAH) (1). As such, patients with SLE-PAH are recommended to avoid pregnancy. Nevertheless, some patients with SLE-PAH become pregnant and wish to continue the pregnancy.
In the management of PAH during pregnancy, only conventional therapies for PAH are recommended (2), and the therapeutic strategies are unclear. Similarly, specific evidence-based therapies for SLE-PAH during pregnancy are still unknown.
We herein report the successful management of pregnancy in a woman with SLE-PAH by continuous intravenous epoprostenol therapy, high-dose corticosteroids (CS), and inhaled nitric oxide (iNO).
Case Report
A 31-year-old pregnant woman with SLE-PAH was admitted to our university hospital for the management of pregnancy and delivery. The patient had been diagnosed with SLE 16 years earlier based on the American College of Rheumatology criteria, including malar rash, photosensitivity, leukopenia, positive anti-double-stranded (ds) DNA antibody, positive antinuclear antibody (ANA), and lupus hepatitis. The patient had become pregnant nine years earlier but selected artificial abortion because of SLE flare.
Approximately two years earlier, she presented with lupus flare with serositis, PAH, and 2003 International Society of Nephrology/Renal Pathology Society classification IV-S (A) lupus nephritis [systemic lupus erythematosus disease activity index (SLEDAI) score: 21]. Transthoracic echocardiography (TTE) revealed an estimated systolic pulmonary arterial pressure (sPAP) of 98 mmHg; no left-sided heart failure was noted. Lung perfusion scintigraphy revealed no definite evidence of thromboembolism. The patient was classified as World Health Organization (WHO) functional class (FC) III.
Right heart catheterization (RHC) after the initiation of therapy revealed a cardiac output (CO) of 5.56 L/min, cardiac index (CI) of 4.08 L/min/m2, pulmonary arterial pressure (PAP) of 53/22 mmHg, mean PAP (mPAP) of 35 mmHg, pulmonary capillary wedge pressure of 11 mmHg, pulmonary vascular resistance (PVR) of 345 dyne·s·cm-5, and right ventricular systolic pressure of 55 mmHg; the results confirmed the diagnosis of PAH. Based on the above findings, we initiated high-dose CS combined with biweekly intravenous cyclophosphamide pulse therapy (IVCY: 15 mg/kg), bosentan (250 mg/day), sildenafil (60 mg/day), and beraprost (360 μg/day). After six courses of IVCY (integrated quantity: 3.66 g), the patient achieved clinical remission (SLEDAI score: 0), and the estimated sPAP decreased to 54.9 mmHg. Subsequently, mizoribine (150 mg/day) was used as maintenance therapy, and the administration of CS was gradually reduced to a lower dose for approximately 2 years.
Seven months before this admission, her SLEDAI score was 0, and PAH was suspected based on an estimated sPAP of 43 mmHg and WHO FC I. Five months before this admission, the patient was found to be pregnant, at 6 weeks of gestation. The patient and her family were informed of maternal, fetal, and neonatal risks due to lupus flare, exacerbated PAH, medicines, and positive anti-SS-A/Ro antibody; they decided to continue her pregnancy. Because pregnancy is a contraindication for mizoribine, bosentan, and beraprost administration, CS and sildenafil were continued. Betamethasone [BMS (0.75 mg/day)] was switched to prednisolone (PSL), and the dose was increased to 25 mg/day with lupus flare (SLEDAI score: 7). The patient had dyspnea following ordinary exertion (WHO FC II) a few weeks before admission, and TTE revealed an estimated sPAP of 61.8 mmHg. Accordingly, continuing her pregnancy was difficult, and the patient was admitted to our hospital at 26 weeks and 1 day of gestation.
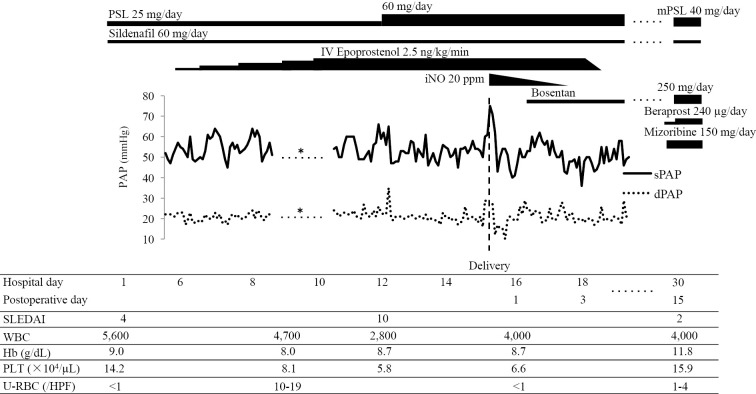
On admission, the pulse, blood pressure (BP), body temperature, respiratory rate, and oxygen saturation at room air were 87 beats/min, 121/64 mmHg, 37.3°C, 20 breaths/min, and 98%, respectively. A physical examination showed malar rash, erythema of the palms and proximal nail folds, and oral ulcers; cardiac auscultation findings were normal. A laboratory examination revealed microcytic anemia [hemoglobin (Hb), 9.0 g/dL], inflammatory response (erythrocyte sedimentation rate, 73 mm/h), increased N-terminal prohormone of brain natriuretic peptide level (163 pg/mL), positive anti-SS-A/Ro and SS-B/La antibody, and positive ANA (×2,560); anti-dsDNA and anti-phospholipid antibodies were negative, and the complement level, urinalysis and renal function tests, and arterial blood gas findings were normal. Chest X-ray (CXR) showed a marked increase in the size of the heart shadow [cardiothoracic ratio (CTR), 53%]. An electrocardiogram showed a normal tracing. TTE revealed a dilated right ventricle, an ejection fraction of 55-60%, estimated sPAP of 61.8 mmHg, and mPAP of 34.1 mmHg. Taken together, these findings confirmed an SLEDAI score of 4, and the disease activity was considered low. However, these cardiologic findings showed worsening PAH. Accordingly, we decided to initiate epoprostenol to prevent further worsening PAH after childbirth and resolved to perform Caesarean delivery (Figure).
Figure.
Clinical course. *not available due to overdampening of pulmonary artery pressure waveforms. PSL: prednisolone, IV: intravenous, mPSL: methylprednisolone, iNO: inhaled nitric oxide, PAP: pulmonary arterial pressure, sPAP: systolic pulmonary arterial pressure, dPAP: dilated pulmonary arterial pressure, SLEDAI: systemic lupus erythematosus disease activity index, WBC: white blood cells, Hb: hemoglobin, PLT: platelets, U-RBC: urinary sediment of red blood cells, HPF: high-power field
Supportive therapy (such as oxygen inhalation and iron administration) was initiated. On the first 2 hospital days, BMS (12 mg/day) was administered intramuscularly to accelerate fetal lung maturation. On the fifth hospital day, a Swan-Ganz catheter was placed, and continuous epoprostenol infusion was started. RHC before the administration revealed CO of 7.5 L/min, CI of 5.5 L/min/m2, PAP of 52/22 mmHg, and mPAP of 34 mmHg. For deep vein thrombosis prevention, continuous intravenous heparin was administered until Caesarean section. On the ninth hospital day, the epoprostenol dose was increased to 2.5 ng/kg/min, and PAP was 45-64/17-24 mmHg (mPAP, 30-41 mmHg). Thereafter, the platelet (PLT) count gradually decreased to 58,000/μL, suggesting an adverse reaction of epoprostenol. However, the patient developed leukopenia (2,800/μL), hemolytic anemia (Hb, 8.0 g/dL; reticulocyte count, 157,320/μL; lactate dehydrogenase, 288 U/L; and haptoglobin, 6 mg/dL), and hematuria (urinary sediment of red blood cells, 10-19/high-power field). Hence, SLE flare (SLEDAI score: 10) was diagnosed, and the PSL dose was increased to 60 mg on the twelfth hospital day.
We decided to perform Caesarean delivery at 28 weeks and 1 day (the fifteenth hospital day) in light of the worsening maternal condition, stable PAP (PAP, 45-66/16-35 mmHg; mPAP, 29-49 mmHg) and the fetus being appropriate for gestational age. The PAP was 60/29 mmHg before the surgery. Caesarean section was performed under general anesthesia with continuing administration of epoprostenol; the baby weighed 1,286 g. Apgar scores were 1 and 4 at 1 and 5 minutes, respectively, and the baby received intensive care. Because the sPAP suddenly spiked (75 mmHg) and the systolic BP (SBP) dropped (90 mmHg) immediately after delivery and local injection of oxytocin (10 units) to the uterus, iNO at 20 ppm was initiated. The final PAP was 62/12 mmHg. iNO under intubation and mechanical ventilation was continued after the surgery, and PAP was maintained without worsening, with a PAP value of 40-62/10-29 mmHg and an mPAP value of 28-39 mmHg. iNO was therefore discontinued simultaneously with extubation on the second postoperative day. Bosentan was resumed, and epoprostenol was discontinued on the third postoperative day. PAH, thereafter, was stabilized (PAP, 46-58/17-29 mmHg; mPAP, 31-37 mmHg). Subsequently, beraprost and mizoribine were resumed, and the CS dose was switched to 40 mg/day of methylprednisolone (mPSL). Laboratory findings revealed an elevated blood cell count (White blood cell, 4,000/μL; Hb, 11.8 g/dL; PLT, 159,000/μL) and normal urinalysis results. The SLEDAI score and WHO FC decreased to 2 and I, respectively. CXR showed a marked decrease in the CTR (from 62% before delivery to 48% after). TTE revealed an estimated sPAP of 53.6 mmHg and mPAP of 31.7 mmHg. On the twelfth postoperative day, the mPSL dose was reduced to 36 mg/day, and the patient was discharged. Her baby was discharged on day 118 of life.
Discussion
The present case highlighted two important clinical issues: SLE-PAH during pregnancy can be controlled by intensive combination therapy, and continuous intravenous epoprostenol therapy, high-dose corticosteroids, and iNO are useful for the management of this condition.
First, SLE-PAH during pregnancy can be controlled by intensive combination therapy. A systemic review reported the pregnancy outcome of PAH, which included connective tissue disease (CTD)-associated PAH (3). However, the treatment of SLE-PAH during pregnancy is unclear. Case reports have described in detail five pregnant patients with PAH associated with SLE (Table). Three of the patients died after delivery, with right-sided heart failure due to PAH deemed the cause of death in two of these patients (4-6). In the two patients who survived, one had been administered long-term intravenous epoprostenol therapy, and SLE and PAH had not been exacerbated during pregnancy and delivery (7). The other had been treated with prednisone, hydroxychloroquine, azathioprine, sildenafil, and inhaled iloprost, and while SLE flared up at delivery, PAH was stable (8).
Table.
Review of Published Cases.
| Reference | Age | Gravity Parity | SLE activity | PAH severity | CS, Immunosapressants | Vasodilators | Delivery | Maternal outcome | Fetal outcome |
|---|---|---|---|---|---|---|---|---|---|
| (4) | 19 | NR | NR | PAP 90/30 mmHg after delivery | small dose of PSL | None | Caesarean | Death on day 6 postpartum | NR |
| (5) | 25 | G3P0 | Absence of lupus features | before delivery, RVSP 79 mmHg with moderate to severe TR, mRAP 10 mmHg, RV and RA dilation with RV hypokinesis, mild to moderate sized pericardial effusion | PSL 10 mg every two days → PSL 15 mg/day | None | Caesarean | Death on day 4 postpartum | Alive |
| (6) | 30 | G2P1 | No features of active SLE | at delivery, mPAP 70-80 mmHg | NR | during operation, iNO | Caesarean | Death less than 24 hours after delivery | Alive |
| (7) | 23 | NR | Good condition | No evidence of RHF, PAP 52/26 mmHg, PVR 143 dyn.sec.cm-5 | NR | IV Epoprostenol | Caesarean | Alive | Alive |
| (8) | 29 | NR | SLEDAI 2 → 14 at delivery | at delivery, NYHA III, sPAP 45 mmHg, mPAP 21 mmHg, PVR 250 dyn.sec.cm-5 | prednisone 25 → 50 mg/day at delivery+ HCQ 200 mg/day, AZ 100 mg/day | Sildenafil 150 mg/day, Inhaled Iloprost 50 → 100 μg/day at delivery | Caesarean | Alive | Alive |
| Present case | 31 | G1P0 | before pregnancy, SLEDAI 0; during pregnancy SLEDAI 7 → 4 → 10 | before pregnancy, WHO FC I, estimated sPAP 43 mmHg; during pregnancy, WHO FC II, PAP 45-66/16-35 mmHg; right after delivery, sPAP 75 mmHg | during pregnancy, PSL 25 → 60 mg/day; after delivery, mPSL 40 → 36 mg/day, MZR 150 mg/day | during pregnancy, Sildenafil 60 mg/day, IV Epoprostenol 2.5 ng/kg/min+ after delivery, iNO 20 ppm | Caesarean | Alive | Alive |
SLE: systemic lupus erythematosus, PAH: pulmonary arterial hypertension, CS: corticosteroids, NR: not reported, PAP: pulmonary arterial pressure, PSL: prednisolone, RVSP: right ventricular systolic pressure, TR: tricuspid regurgitation, mRAP: mean right atrial pressure, RV: right ventricular, RA: right atrial, iNO: inhaled nitric oxide, RHF: Right heart failure, PVR: pulmonary vascular resistance, IV: intravenous, SLEDAI: systemic lupus erythematosus disease activity index, NYHA: New York Heart Association, sPAP: systolic pulmonary arterial pressure, HCQ: hydroxychloroquine, AZ: azathioprine, WHO FC: World Health Organization functional class, MZR: mizoribine
In the present case, SLE flared up during pregnancy, and PAH was exacerbated during pregnancy and immediately after delivery. However, intensive combination therapy inhibited further exacerbation and led to the successful management of pregnancy. To our knowledge, no patients with worsening SLE and PAH who successfully gave birth have yet been reported, and our report is the first case. We therefore feel that our case will be a good reference for similar cases.
Second, high-dose corticosteroids, iNO, and intravenous epoprostenol therapy are useful for the management of this condition. Intravenous epoprostenol inhibited the further exacerbation of PAH due to pregnancy. Generally, the blood volume and cardiac output are increased, whereas systemic vascular resistance are decreased during pregnancy, causing worsening PAH (2). Our patient continued sildenafil after pregnancy, but she experienced a rapid progression of PAH symptoms (WHO FC I to II, estimated sPAP 43 to 61.8 mmHg). Pregnancy in women with SLE-PAH has been reported to carry a high maternal mortality rate (1). Advanced therapy has been recommended to be initiated early, before cardiopulmonary decompensation (3). Epoprostenol have been used during pregnancy to improve PAH (3). The efficacy of intravenous epoprostenol has been shown in SLE-PAH patients (9). For this reason, we selected the induction of continuous intravenous epoprostenol during pregnancy.
High-dose corticosteroid therapy is useful for the management of active SLE-PAH. Our patient had SLE flare (SLEDAI score: 10) during pregnancy that was accompanied by worsening PAH. Accordingly, high-dose corticosteroid therapy was initiated. Corticosteroid therapy has been shown to be effective for SLE-PAH (10).
iNO is useful for the management of PAH right after delivery. The blood volume and cardiac output are further increased after delivery; these changes cause worsening of PAH, resulting in right-sided heart failure (2). iNO is a selective pulmonary vasodilator and decreases the PVR without affecting the systemic BP (11). In our patient, the sPAP suddenly spiked (60 to 75 mmHg) and the SBP dropped immediately after delivery. Accordingly, iNO was initiated, decreasing the sPAP (62 mmHg) without a further drop in the systemic BP. iNO is often used as a last resort when PAH is unstable (3).
We must also note that controlling the SLE activity and PAH severity before pregnancy and worsening PAH with lupus flare were important to achieving a good outcome in the present patient. SLE can cause adverse maternal and fetal outcomes. The risk factors include SLE activity/flares in the last 6-12 months or at conception, the use of CS (≥10-20 mg/day of PSL equivalent), and active nephritis (12). In addition, PAH probably results in a high rate of maternal mortality. One of the major risk factors is the severity of PAH, and the risk probably increases with an increase in the PAP (2). Furthermore, as described above, SLE-PAH is typically improved with therapy for SLE, ameliorating its symptoms.
In the present case, before pregnancy, the SLE activity was “clinical remission” (SLEDAI score: 0) under low-dose CS, and the severity of PAH was also mild (WHO FC I; estimated sPAP, 43 mmHg) with appropriate therapy. SLE flare was accompanied by worsening PAH during pregnancy. Such conditions may have had a major positive effect on the good outcome in our patient.
However, we must bear in mind that women of childbearing age with SLE-PAH should be counseled about pregnancy risks and encouraged to avoid pregnancy (12), and the termination of a pregnancy should be considered at conception given the high maternal mortality risk (2). Our patient had no cognitive dysfunction due to neuropsychiatric SLE; she decided to become pregnant and continue the pregnancy of her own will while understanding the risks as we had explained them. We must recognize that the risks should be discussed from the first physician-patient encounter, and not only patients but also their family and partner should be fully counseled (12).
In conclusion, SLE-PAH during pregnancy can be controlled by immunosuppression and vasodilators, and continuous intravenous epoprostenol therapy, high-dose corticosteroids, and iNO are effective for treating SLE-PAH. Women with SLE-PAH should avoid pregnancy. However, some will still wish to become pregnant and continue the pregnancy despite the risks. More cases, therefore, should be documented in order to improve the outcome of pregnancy with SLE-PAH.
Author's disclosure of potential Conflicts of Interest (COI).
Yoshiya Tanaka: Honoraria, Abbvie, Chugai, Astellas, Takeda, Santen, Mitsubishi-Tanabe, Pfizer, Janssen, Eisai, Daiichi-Sankyo, UCB, GlaxoSmithKline and Bristol-Myers; Research funding, Mitsubishi-Tanabe, Chugai, MSD, Astellas and Novartis.
References
- 1. Martin WL, Gordon C, Kilby MD. Systemic lupus erythematosus. Lancet 358: 586, 2001. [DOI] [PubMed] [Google Scholar]
- 2. Regitz-Zagrosek V, Blomstrom Lundqvist C, Borghi C, et al. ESC Guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). European Society of Gynecology (ESG); Association for European Paediatric Cardiology (AEPC); German Society for Gender Medicine (DGesGM). Eur Heart J 32: 3147-3197, 2011. [DOI] [PubMed] [Google Scholar]
- 3. Bédard E, Dimopoulos K, Gatzoulis MA. Has there been any progress made on pregnancy outcomes among women with pulmonary arterial hypertension? Eur Heart J 30: 256-265, 2009. [DOI] [PubMed] [Google Scholar]
- 4. Greenstone MA. Delayed diagnosis of systemic lupus erythematosus associated pulmonary hypertension. Br J Rheumatol 30: 391, 1991. [DOI] [PubMed] [Google Scholar]
- 5. Ray J, Sermer M. Systemic lupus erythematosus and pulmonary hypertension during pregnancy: report of a case fatality. Can J Cardiol 12: 753-756, 1996. [PubMed] [Google Scholar]
- 6. McMillan E, Martin WL, Waugh J, et al. Management of pregnancy in women with pulmonary hypertension secondary to SLE and anti-phospholipid syndrome. Lupus 11: 392-398, 2002. [DOI] [PubMed] [Google Scholar]
- 7. Bendayan D, Hod M, Oron G, et al. Pregnancy outcome in patients with pulmonary arterial hypertension receiving prostacyclin therapy. Obstet Gynecol 106: 1206-1210, 2005. [DOI] [PubMed] [Google Scholar]
- 8. Streit M, Speich R, Fischler M, Ulrich S. Successful pregnancy in pulmonary arterial hypertension associated with systemic lupus erythematosus: a case report. J Med Case Rep 3: 7255, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shirai Y, Yasuoka H, Takeuchi T, Satoh T, Kuwana M. Intravenous epoprostenol treatment of patients with connective tissue disease and pulmonary arterial hypertension at a single center. Mod Rheumatol 23: 1211-1220, 2013. [DOI] [PubMed] [Google Scholar]
- 10. Kato M, Kataoka H, Odani T, et al. The short-term role of corticosteroid therapy for pulmonary arterial hypertension associated with connective tissue diseases: report of five cases and a literature review. Lupus 20: 1047-1056, 2011. [DOI] [PubMed] [Google Scholar]
- 11. Griffiths MJ, Evans TW. Inhaled nitric oxide therapy in adults. N Engl J Med 353: 2683-2695, 2005. [DOI] [PubMed] [Google Scholar]
- 12. Andreoli L, Bertsias GK, Agmon-Levin N, et al. EULAR recommendations for women's health and the management of family planning, assisted reproduction, pregnancy and menopause in patients with systemic lupus erythematosus and/or antiphospholipid syndrome. Ann Rheum Dis 76: 476-485, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]