Abstract
We herein report a case of a 31-year-old Japanese man who simultaneously had a positive influenza A virus antigen test result and Vogt-Koyanagi-Harada disease (VKHD), demonstrated by both diffuse multiple early hyperfluorescent points on fluorescein fundus photography and serous retinal detachments on optical coherence tomography. He had meningitis. It was difficult to determine whether the main cause of meningitis was influenza A or VKHD. After initial treatment with peramivir for influenza A and then methylprednisolone pulse with subsequent corticosteroid therapy for VKHD, his symptoms improved gradually. These findings suggest that influenza A virus infection contributes to the onset or exacerbation of VKHD.
Keywords: Vogt-Koyanagi-Harada disease, influenza A virus, meningitis, peramivir, corticosteroids therapy
Introduction
Vogt-Koyanagi-Harada disease (VKHD), also known as uveomeningoencephalitic syndrome, is a systemic granulomatous autoimmune disorder that causes inflammation of melanocyte-rich tissues, such as the eye, inner ear, meninges, skin, and hair (1). Diffuse choroiditis with serous retinal detachment and optic disc hyperemia and edema is a feature of VKHD (2). In order to achieve better visual outcomes, this condition needs to be diagnosed promptly, followed by early, aggressive, and long-term treatment with high-dose corticosteroids (1, 2). The most accepted mechanism of VKHD involves an autoimmune reaction to antigens associated with melanocytes in a genetically susceptible individual after infection triggers the involved viruses, such as Epstein-Barr virus (3) and cytomegalovirus (4), especially in the presence of the human leukocyte antigen (HLA)-DR4 allele (1, 2). VKHD often involves the acute onset of bilateral blurred vision with hyperemia that is preceded by flu-like symptoms (5).
To our knowledge, no previous report has indicated that influenza A virus infection may be related to the onset or exacerbation of VKHD. We herein report a patient with HLA-DR4 allele positivity who simultaneously developed influenza A virus infection and VKHD, suggesting a relationship between these two clinical conditions.
Case Report
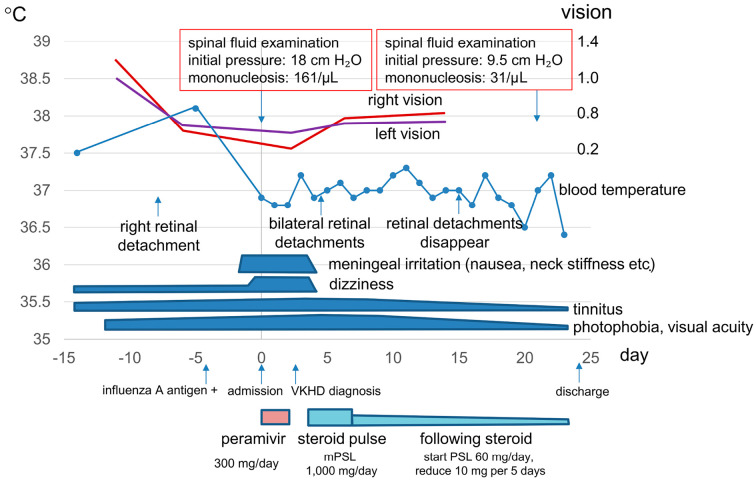
A 31-year-old Japanese non-smoking man was admitted to our medical center with a fever, posterior neck pain, nausea, visual field distortion, and photophobia. The clinical course is shown in Fig. 1. Since 14 days before admission, he had experienced dizziness, had difficulty hearing, and had common cold symptoms. He had experienced left visual field distortion and visited an ophthalmologist 12 days before admission. At that visit, optical coherence tomography indicated left serous retinal detachment. Then, 8 days before admission, he visited the same ophthalmologist because of right visual field distortion, and right serous retinal detachment was noted. He again visited the same clinic because of metamorphopsia and dizziness worsening despite treatment with eye drops, and he had a fever (temperature 38℃) 5 days before admission. The ophthalmologist referred him to our ophthalmology department under suspicion of VKHD. On visiting our emergency department 4 days before admission, influenza A antigen positivity was found by the rapid influenza diagnostic test using a nasal swab, and oseltamivir was prescribed. The patient had not received the influenza vaccine that season. On the day of admission, his fever, dizziness, nausea, vomiting, and posterior neck pain worsened. He was prone to vomiting on body movement, and he consulted another clinic. As meningitis or VKHD was suspected, he was transported to our emergency department in an ambulance.
Figure 1.
Clinical course before and during hospitalization. VKHD: Vogt-Koyanagi-Harada disease, mPSL: methylprednisolone, PSL: prednisolone
At this admission, his body temperature was 36.8℃, blood pressure was 134/60 mmHg, pulse rate was 78 beats/min (regular), and respiratory rate was 30 breaths/min. He reported tinnitus, stiffness, and severe posterior neck pain. Vomiting could be easily induced on neck rotation. The cerebrospinal fluid examination results were as follows: initial pressure, 18 cmH2O; color, clear; glucose level, 50 mg/dL; protein level, 69 mg/dL; and mononucleosis, 161 /μL. Laboratory test results were as follows: white blood cell count, 6,160 /μL; C-reactive protein level, 0.23 mg/dL; aspartate aminotransferase level, 23 U/L; alanine aminotransferase level, 18 U/L; and lactate dehydrogenase level, 204 U/L. Additionally, he was positive for HLA-DR4. We believed that his condition was complicated with influenza A meningitis. He was treated with peramivir for 2 days for influenza meningitis. Thereafter, the results of influenza tests of the cerebrospinal fluid, including tests for influenza A/B virus antigen by reverse transcriptase-polymerase chain reaction (RT-PCR), H1N1 and H3N2 antibody by hemagglutination inhibition reaction (HI), and influenza virus A antibody using complement fixation (CF) reaction, were all negative (Table).
Table.
Series of Influenza Virus Tests.
| Testing Material | Influenza virus Laboratory tests | 4 days before admission | Hospital day 1 | Hospital day 4 | Hospital day 22 |
|---|---|---|---|---|---|
| Nasal swab | Rapid influenza diagnostic test* | A (+), B (-) | |||
| Serum | Influenza virus A antibody (CF titers) | 8 | 16 | ||
| Spinal fluid | Influenza virus A RNA (RT-PCR) | Negative | |||
| Influenza virus B RNA (RT-PCR) | Negative | ||||
| Influenza virus A (H1N1) antibody (HI titers) | <10 | <10 | |||
| Influenza virus A (H3N2) antibody (HI titers) | <10 | <10 | |||
| Influenza virus A antibody (CF titers) | <1 | <1 |
CF: complement fixation reaction, RT-PCR: reverse transcriptase-polymerase chain reaction, HI: hemagglutination inhibition reaction
Rapid influenza diagnostic test*was performed by immunochromatography test using enzyme immunoassay for rapid detection of influenza A and B viruses (Espline® Influenza A&B–N).
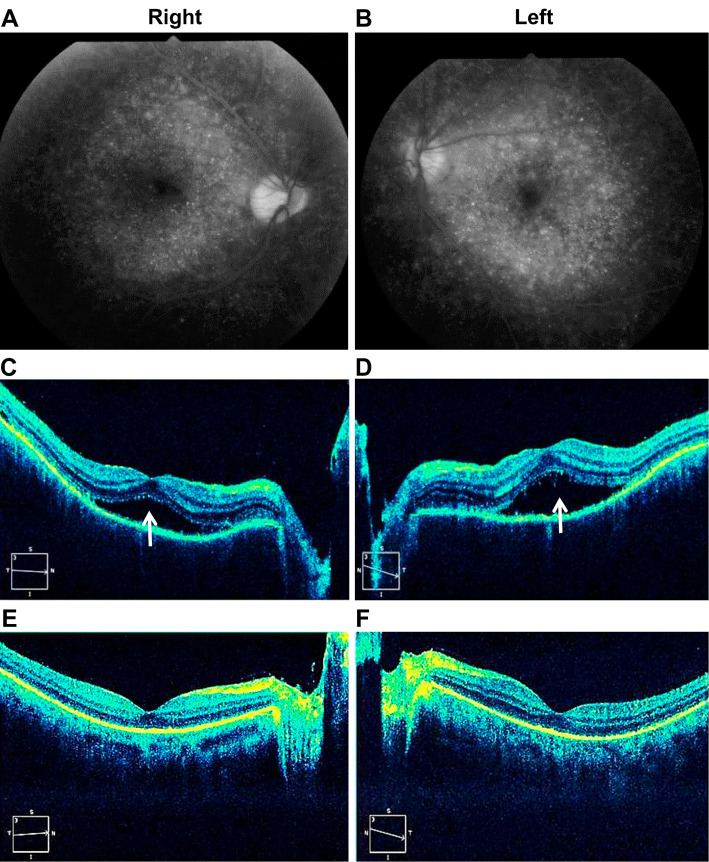
The ophthalmological findings, including bilateral diffuse multiple early hyperfluorescent points on fluorescein fundus photography (Fig. 2A and B) and serous retinal detachments on optical coherence tomography (Fig. 2C and D), clinical history (no eye trauma or intraocular surgery), meningitis symptoms, tinnitus, and mononucleosis of the cerebellar fluid met the diagnostic criteria for VKHD (6). Soon after the initiation of methylprednisolone pulse therapy (1,000 mg/day for 3 days) on hospital day 4, the patient’s neck stiffness, jolt accentuation, dizziness, nausea, and vomiting resolved. Subsequently, prednisolone was started (1 mg/kg/day) on hospital day 7, and the dose was reduced by 10 mg every 5 days. His photophobia, tinnitus, and visual acuity gradually improved. On hospital day 16, bilateral serous retinal detachment disappeared (Fig. 2E and F). On hospital day 22, cerebrospinal fluid examination results showed improvement and were as follows: initial pressure, 9.5 cmH2O and mononuclear cell count, 31 /μL. H1N1 and H3N2 antibody by HI and influenza virus A antibody in the cerebrospinal fluid by CF were negative. In contrast, the serum CF titers of influenza A virus were positive and increased from 8 times at hospital day 4 to 16 times at hospital day 22 (Table). On hospital day 24, he was discharged home.
Figure 2.
Fluorescein fundus photography and optical coherence tomography. A, B: Fluorescein fundus photography shows diffuse multiple early hyperfluorescent points on hospital day 4. C, D: Optical coherence tomography shows serous retinal detachments (arrows) on hospital day 4. E, F: Optical coherence tomography shows that the bilateral serous retinal detachments diminished on hospital day 16.
Discussion
Our patient, who developed symptoms and findings of meningitis originally considered to be caused by influenza A virus infection or VKHD, successfully recovered with initial anti-influenza treatment involving peramivir and subsequent corticoid therapy.
Two important clinical issues arise from the clinical course of the present patient. The first is how to manage a patient with the symptoms and signs of meningitis simultaneously complicated with influenza A virus infection and VKHD. The second is whether or not influenza A virus infection can contribute to the onset or exacerbation of VKHD.
A few reports have described cases of influenza meningitis (7), and the frequency of meningitis caused by VKHD has been reported to be 80% in Japan (8). In the present patient, it was important to determine whether the meningeal symptoms and findings were caused by influenza virus infection or VKHD. Viral meningitis and VKHD-associated meningitis cannot be distinguished with a regular cerebrospinal fluid examination, as the examination shows only a dominant increase in mononuclear cells. To confirm the pathogenesis of meningeal symptoms and findings, we tried to identify influenza A virus RNA of cerebrospinal fluid directly using RT-PCR; however, the result was negative. In addition, there was no increase in the pair serum antibody titers of influenza A virus using HI or CF in the cerebrospinal fluid. These findings confirmed that this patient was not complicated with influenza meningitis. Influenza virus antigen and antibody tests in the cerebrospinal fluid may be useful in similar situations in order to obtain a correct diagnosis, although there can be a delay in obtaining test results.
The treatment of VKHD involves the prompt administration of high-dose corticosteroids in order to reduce the chance of recurrence (1, 2, 9). The present patient’s condition was complicated with influenza A virus infection, and corticosteroid administration can exacerbate the inflammation caused by the influenza virus (10). We practiced caution, being aware of the possibility of severe complications, such as influenza pneumonia, influenza encephalopathy, and Reye’s syndrome, although they have been reported to occur at a very low frequency of about <1% (11). Influenza infection generally requires 3-4 days for the alleviation of a fever and an average of 7 days for healing (12). When VKHD patients show flu-like symptoms as prodromal symptoms, it is difficult to judge the timing of healing from the acute phase of influenza infection. Usually, however, recovery from the acute phase of influenza infection does not take a long time. Therefore, corticosteroid therapy should be started as soon as the acute phase of influenza infection is complete. The present patient immediately received treatment with high-dose corticosteroid for meningeal irritation symptoms caused by VKHD three days after the confirmation of the alleviation of influenza A infection.
The false-positive rate for the EsplineⓇ rapid influenza diagnostic test a using nasal swab was determined to be 2.4%, with a reported sensitivity of 96.8% and specificity of 97.6% (13). A diagnosis of influenza A virus infection was made in this patient because not only was the rapid influenza diagnostic test positive, but the pair serum CF antibody titers of influenza A virus had increased from 8 times at hospital day 4 to 16 times at hospital day 22. These points are critical, as we now suggest an as-yet-unreported association between VKHD and influenza A virus infection.
The influenza virus may be associated with the onset or exacerbation of VKHD. Several viruses have been considered as triggers for the onset of VKHD (1, 3, 4) The genome of viruses from the herpes family (Epstein-Barr virus) was detected by PCR in the vitreous fluid from VKHD patients (3). Sugita et al. mentioned that T cells from the peripheral blood and intraocular fluid in patients with VKHD cross-reacted with tyrosinase protein as well as with highly homologous cytomegalovirus-specific sequences (4). Several studies have shown that HLA-DR4 is strongly associated with VKHD patients, as in the present case (1, 2). Antigen-presenting cells may be involved in the onset and progression of VKHD through antigen presentation to T cells via HLA-DR4, owing to some viral infections (1, 14, 15). One report described a case of VKHD following influenza vaccination (16). Minakawa et al. observed seasonality in Hokkaido, Japan, with the onset of VKHD tending to be high in the early spring and autumn, with peaks in November (17). This report suggests the possibility of a VKHD epidemic.
In conclusion, we encourage physicians and ophthalmologists to ask new patients with VKHD about their recent history of flu-like symptoms and vaccinations, including influenza. Such questions may therefore be important for clarifying the association between VKHD and viruses.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Lavezzo MM, Sakata VM, Morita C, et al. Vogt-Koyanagi-Harada disease: review of a rare autoimmune disease targeting antigens of melanocytes. Orphanet J Rare Dis 11: 29, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baltmr A, Lightman S, Tomkins-Netzer O. Vogt- Koyanagi- Harada syndrome-current perspectives. Clin Opthalmol 10: 2345-2361, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bassili SS, Peyman GA, Gebhardt BM, Daun M, Ganiban GJ RA. Detection of Epstein-Barr virus DNA by polymerase chain reaction in the vitreous from a patient with Vogt-Koyanagi-Harada syndrome. Retina 16: 160-161, 1996. [DOI] [PubMed] [Google Scholar]
- 4. Sugita S, Takase H, Kawaguchi T, Taguchi C, Mochizuki M. Cross-reaction between tyrosinase peptides and cytomegalovirus antigen by T cells from patients with Vogt-Koyanagi-Harada disease. Int Ophthalmol 27: 87-95, 2007. [DOI] [PubMed] [Google Scholar]
- 5. Moorthy R, Inomata H, Rao N. Vogt-Koyanagi-Harada syndrome. Surv Ophthalmol 39: 265-292, 1995. [DOI] [PubMed] [Google Scholar]
- 6. da Silva FTBGC, Damico FM, Marin ML, et al. Revised diagnostic criteria for Vogt-Koyanagi-Harada disease: considerations on the different disease categories. Am J Ophthalmol 147: 339-345. e5, 2009. [DOI] [PubMed] [Google Scholar]
- 7. Newland JG, Laurich VM, Rosenquist AW, et al. Neurologic complications in children hospitalized with influenza: characteristics, incidence, and risk factors. J Pediatr 150: 306-310, 2007. [DOI] [PubMed] [Google Scholar]
- 8. Horie Y, Kitaichi N, Takemoto Y, et al. Polymorphism of IFNgamma gene and Vogt-Koyanagi-Harada disease. Mol Vis 13: 2334-2338, 2007. [PubMed] [Google Scholar]
- 9. Sakata VM, da Silva FT, Hirata CE, et al. High rate of clinical recurrence in patients with Vogt-Koyanagi-Harada disease treated with early high-dose corticosteroids. Graefe's Arch Clin Exp Ophthalmol 253: 785-790, 2015. [DOI] [PubMed] [Google Scholar]
- 10. Rodrigo C, Leonardi-Bee J, Nguyen-Van-Tam J, Lim W. Corticosteroids as adjunctive therapy in the treatment of influenza ( Review ). Cochrane Database Syst Rev (in press). [DOI] [PubMed] [Google Scholar]
- 11. Thompson MG, Shay DK, Zhou H, et al. Estimates of deaths associated with seasonal influenza-United States, 1976-2007. MMWR Morb Mortal Wkly Rep 59: 1057-1062, 2010. [PubMed] [Google Scholar]
- 12. Brankston G, Gitterman L, Hirji Z, Lemieux C, Gardam M. Transmission of influenza A in human beings. Lancet Infect Dis 7: 257-265, 2007. [DOI] [PubMed] [Google Scholar]
- 13. Mitamura K, Yamazaki M, Ichikawa M, et al. Evaluation of an immunochromatography test using enzyme immunoassay for rapid detection of influenza A and B viruses. Kansenshogaku Zasshi (J Jpn Assoc Infect Dis) 78: 597-603, 2004(in Japanese, Abstract in English). [DOI] [PubMed] [Google Scholar]
- 14. Sugita S, Takase H, Taguchi C, et al. Ocular infiltrating CD4+ T cells from patients with Vogt-Koyanagi-Harada disease recognize human melanocyte antigens. Investig Ophthalmol Vis Sci 47: 2547-2554, 2006. [DOI] [PubMed] [Google Scholar]
- 15. Damico FM, Cunha-Neto E, Goldberg AC, et al. T-cell recognition and cytokine profile induced by melanocyte epitopes in patients with HLA-DRB1*0405-positive and -negative Vogt-Koyanagi-Harada uveitis. Investig Ophthalmol Vis Sci 46: 2465-2471, 2005. [DOI] [PubMed] [Google Scholar]
- 16. Kim M. Vogt-Koyanagi-Harada Syndrome following influenza vaccination. Indian J Ophthalmol 64: 98, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Minakawa R, Ohno S, Hirose S, et al. Clinical manifestation of Vogt-Koyanagi-Harada's disease. Jpn J Clin Ophthalmol 39: 1249-1253, 1985(in Japanese). [Google Scholar]