Abstract
Sodium polystyrene sulfonate (SPS: KayexalateⓇ) is an ion-exchange resin used to treat hyperkalemia in patients with chronic kidney disease. It is known that this resin sometimes causes colonic necrosis and perforation, but there are few reports about small bowel necrosis associated with SPS. We herein report the case of a patient who developed SPS-induced small bowel necrosis, which was diagnosed based on the examination of a small bowel endoscopic biopsy specimen. The SPS-induced small bowel necrosis was resistant to conservative treatment including the cessation of SPS, and finally required surgical bowel resection.
Keywords: sodium polystyrene sulfonate, chronic kidney disease, small bowel necrosis
Introduction
Sodium polystyrene sulfonate (SPS; KayexalateⓇ) is reported to cause colonic necrosis and perforation with hard stool, especially in patients with chronic kidney disease (CKD). However, SPS rarely induces small intestinal necrosis, and few reports contain endoscopic images and the preoperative pathological diagnosis of biopsy specimens showing small intestinal necrosis caused by SPS. A case of SPS-induced small intestinal necrosis is described, followed by a review of the relevant literature.
Case Report
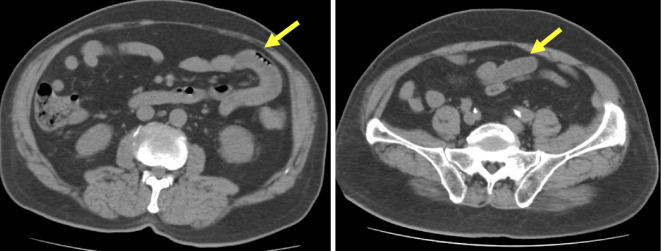
A 79-year-old man presented to the Department of Gastroenterology at our hospital with abdominal pain, diarrhea, and vomiting. The patient had been found to have CKD due to diabetic nephropathy, hypertension, and chronic heart failure. His medications included olmesartan,rosuvastatin, pregabalin, teneligliptin, and long-acting insulin. He had also been started on SPS for hyperkalemia eight months previously. His temperature was 38.2°C, his pulse rate was 88 beats per minute, and his blood pressure was 197/82 mmHg. On examination, his abdomen was soft with diffuse tenderness, but his bowel sounds were normal. A digital rectal examination showed dark red stool. The significant laboratory values included: leucocytes, 19,100/μL; C-reactive protein, 1.45 mg/dL; blood urea nitrogen, 35.7 mg/dL; creatinine, 1.42 mg/dL; and potassium, 4.3 mEq/L. Computed tomography (CT) showed thickening of the wall of the small intestine, especially the terminal ileum (Fig. 1). The patient was diagnosed with infectious colitis and was admitted to our hospital for conservative treatment. His symptoms and laboratory data failed to improve, despite conservative treatment, including antibiotics, and CT imaging performed 7 days later showed both small intestinal and colonic wall edema. Stool and blood cultures were also negative. Colonoscopy was performed, and dirty geographic ulceration was observed in the cecum (Fig. 2). A pathological examination revealed erosion, lymphocyte infiltration, and dark violet SPS crystal deposition within the colonic mucosa. According to the drug information, the patient's diarrhea might have been caused by olmesartan, rosuvastatin and pregabalin, but none of the drugs he was prescribed have been reported to be associated with enteritis. Thus, a diagnosis of bowel edema induced by SPS was made, and SPS was stopped on day 7.
Figure 1.
On CT examination of the abdomen, diffuse wall thickening and liquid retention were observed in the small intestine.
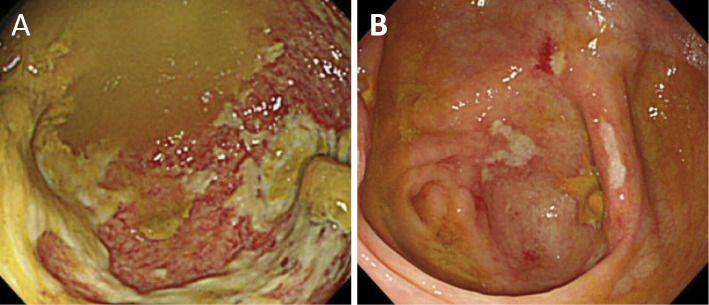
Figure 2.
On colonoscopy, dirty geographic ulceration was found in the cecum.
Despite the cessation of SPS, the diarrhea continued, and the abdominal pain and fever relapsed after eating. CT was performed again, and small intestinal edema was found to have extended throughout the ileum, while the colonic edema had improved. Small intestinal endoscopy showed dirty white-coated longitudinal ulceration with a bleeding tendency on the ileum, and small intestinal radiography showed segmental edematous lesions in the ileum (Fig. 3). A pathological examination revealed nonspecific all-layer necrosis and SPS crystal deposition in the deep layer of the submucosa, and the infiltration of lymphocyte cells around the SPS (Fig. 4). It was therefore determined that surgical treatment would be preferable, and after obtaining informed consent, small intestinal resection was performed on day 64.
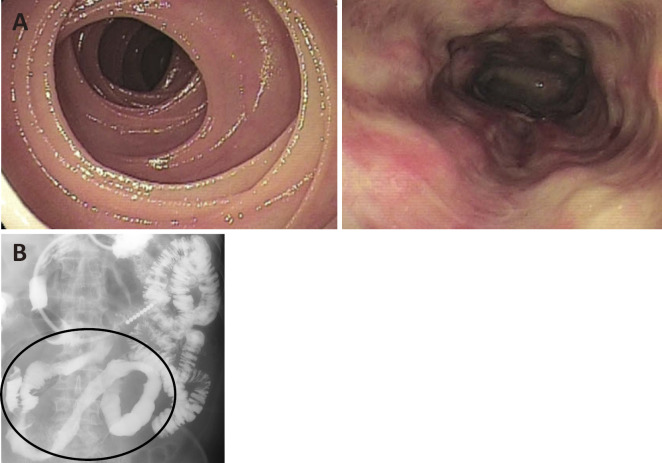
Figure 3.
A: Small bowel endoscopy showed dirty white-coated longitudinal ulceration. B: On small bowel barium X-ray examination, Kerckring’s folds only disappeared in the segmental ileum.
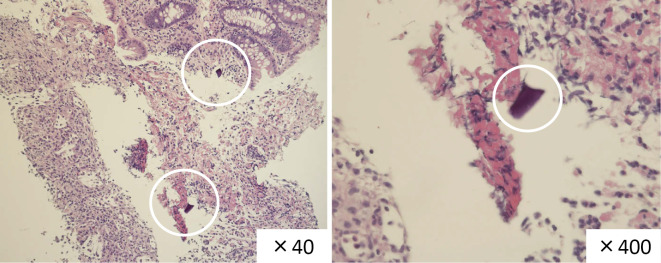
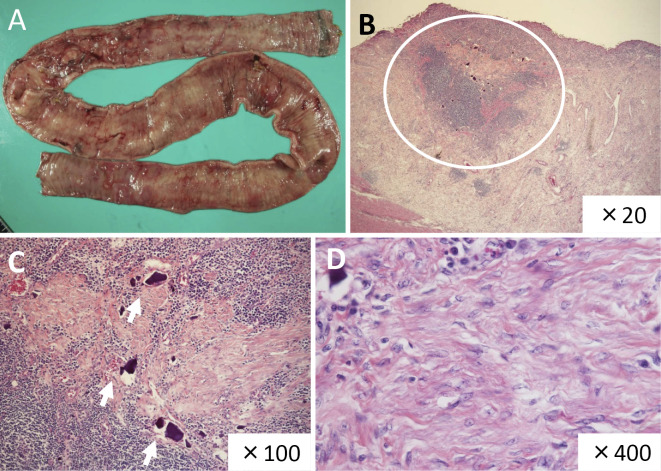
Figure 4.
The pathological findings showed nonspecific all-layer necrosis and SPS crystal deposition in the deep layer of the submucosa and the infiltration of lymphocytes around the SPS.
Edema and redness were observed over approximately 130 cm of the small intestine from a point 20 cm on the oral side of the ileocecal area. The ileum was resected. In the resected specimens, SPS crystals were observed in the submucosal layer of the lesion, and inflammatory cells were observed around the crystals. Cytomegalovirus (CMV) was not detected and the pathological examination of tissue specimens did not reveal any granulomas suggestive of tuberculosis or Crohn's disease. Furthermore, there were no typical ischemic changes because of the nonspecific all-layer necrosis. SPS was not present in the normal intestinal tract of the resected margin (Fig. 5). The patient's postoperative course was good, and he was discharged 81 days after hospitalization. This was a rare case of SPS-induced enteritis requiring surgical treatment because of all-layer necrosis after the cessation of SPS.
Figure 5.
A: Edema and redness of the small intestine were observed in an area of approximately 130 cm from a point 20 cm on the oral side of the ileocecal area. The ileum was resected. B: Diffuse mucosal absence, necrosis and erosion were observed in the lesion area. Cytomegalovirus (CMV) was not detected and a pathological examination of a tissue specimen revealed no evidence of granulomas suggestive of tuberculosis or Crohn’s disease. Furthermore, no typical ischemic changes were observed because of the nonspecific all-layer necrosis. C: The crystals were located in the submucosal layer. Inflammatory cells, mainly lymphocytes, infiltrated around the crystal. D: All-layer necrosis was found.
Discussion
SPS is commonly used to treat hyperkalemia of various causes (1). It is an ion-exchange resin that can be administered orally or rectally. Its mechanism is to exchange its bound sodium with potassium in the colon, prompting the overall excretion of potassium (2). Its administration with sorbitol has a well-documented association with bowel necrosis and ulceration (3, 4). Sorbitol is believed to directly damage the intestinal mucosa, leading to vasospasm, the exacerbation of inflammation through elevated prostaglandin levels, and ultimately, vascular injury (5). Lillemoe et al. reported on colonic necrosis due to SPS-sorbitol enema, along with experimental evidence suggesting that the necrosis was due to sorbitol rather than the SPS in the presence of uremia (5). Thus, the US Food and Drug Administration (FDA) removed 70% sorbitol-containing SPS from the market in 2006. Since that time case reports linking the administration of SPS without sorbitol to colonic ulceration, necrosis, and ischemia, have continued to be published (6). In the present case, the SPS was given without sorbitol. Recently, a systematic review of gastrointestinal adverse events associated with SPS was published by Harel et al. (1). According to their report, there were 58 cases of adverse events (41 occurred in patients receiving preparations containing sorbitol and 17 occurred in patients receiving preparations without sorbitol). The colon was the most common site of injury (n=44; 74%), with histopathological examinations revealing transmural necrosis in 62% (n=36), ulceration in 48% (n=28), and perforation in 9% (n=5). Forty-one patients (71%) had a history of CKD or end-stage renal disease.
In another report, 91% of patients with colon perforation after the administration of SPS had a history of acute kidney injury, CKD, or end-stage renal disease (1). One proposed mechanism of injury was the elevated renin level seen in patients with renal failure. Renin's activation of angiotensin II and subsequent splanchnic vasoconstriction can lead to non-occlusive mesenteric ischemia, which predisposes the colonic mucosa to injury following dramatic electrolyte and, in turn, fluid shifts (7). Otherwise, there have been few reports on cases in which SPS alone induced all-layer necrosis or perforation. In the present case, the patient had a high risk of arteriosclerosis due to a history of CKD, hypertension, and diabetes mellitus. In the gastrointestinal tract of susceptible individuals, such as those with CKD, the elaboration of inflammatory cytokines and prostaglandins may lead to the further impairment of local hemodynamic mechanisms, leading to vascular injury and subsequent mucosal injury (5). In addition, endoscopic and radiological examinations showed segmental and longitudinal ulceration. Thus, bowel ischemia would have been present first, and then SPS would have invaded the submucosal layer of the ischemic mucosa.
Although various pathological features have been described in SPS-induced gastrointestinal tract injury, including mucosal ulceration, erosion, and transluminal necrosis, the identification of SPS crystals is considered to be the histological hallmark of SPS toxicity (2, 8, 9). These crystals have a characteristic polygonal, refractile, and basophilic appearance, with a typical “fish scale”/mosaic pattern that displays a red color on PAS and acid fast staining (2). Thus, SPS-induced enteritis was diagnosed based on the findings of dark violet SPS crystal deposition and the infiltration of lymphoid cells around the SPS crystals. Conservative treatment, including the cessation of SPS is the standard therapy; however, if the SPS invades the deep layers, the clearance of SPS becomes difficult, and conservative treatment would not be successful, as in the present case.
In the present case, the mucosal injury that was observed on admission improved in the colon and terminal ileum after conservative treatment, which included the cessation of SPS, while the damage on the oral side of the ileal mucosa did not, despite the cessation of SPS. The ileal mucosal injury, which was refractory to conservative treatment and segmental, finally required surgical resection. The resected pathological specimen showed all-layer necrosis, the presence of SPS crystals in the submucosa and muscularis propria, and the infiltration of inflammatory cells. SPS crystals were not observed outside of the necrotic lesions on both sides, and the mucosa was normal. We considered that the invasion of SPS into the deep layer is the reason why this lesion was refractory to the cessation of SPS. Thus, this case might be explained by a two-hit theory, in which intestinal bowel damage occurred first, and then SPS crystals entered the submucosa or muscularis propria, causing all-layer necrosis. We hypothesized that the initial intestinal bowel damage might have been ischemic because the lesion of all-layer necrosis was segmental, and because the patient had CKD and endoscopy-confirmed mucosal damage with longitudinal ulceration.
The strength of the present report is that it was a rare case that was diagnosed before surgery based on the examination of a biopsy specimen from the area of SPS-induced mucosal damage. Furthermore, endoscopic images were obtained by balloon endoscopy before surgery. In addition, it was possible to follow the conservative clinical course for one month after the cessation of SPS and then finally successfully treat the patient by surgical resection.
The present study is associated with some limitations. First, the typical findings of SPS-induced enteritis have not been fully clarified because SPS enteritis is rare entity. We considered that the SPS-induced enteritis might have developed through a two-hit mechanism, as mentioned above. Thus, the findings of endoscopy and small intestinal radiography may not have been caused by SPS alone. However, SPS was not present in the normal intestinal tract of the resected margin. This fact also indicated that SPS was an important cause of this enteritis. Second, in general, ischemic enterocolitis is most likely to develop in the left side of the colon. However, in the present case, this enteritis occurred in the small intestine. The reason for this was not clear. However, we considered that the intestinal bowel damage might have initially been ischemic change because the lesion of all-layer necrosis was segmental; furthermore the patient had CKD and the endoscopy-confirmed mucosal damage was longitudinal ulceration. The reason why typical pathological ischemic change was not found in the resected specimen might be that long-term inflammation due to the deposition of SPS crystals in the deep layer of the submucosa caused mucosal shedding and the nonspecific all-layer necrosis.
The use of SPS in the treatment of hyperkalemia may be associated with serious gastrointestinal adverse events. Physicians and nephrologists in particular should be cognizant of the potential side effects of SPS in CKD patients. When abdominal symptoms (i.e., pain or hematochezia) appear in CKD patients who are prescribed SPS, the possibility of an SPS-induced adverse event should be considered, and appropriate examinations should be performed, especially in CKD patients.
Conclusion
SPS is commonly used in the treatment of hyperkalemia. The frequency of gastrointestinal adverse events is not high. However, in addition to gastrointestinal perforation, all-layer necrosis may occur and physicians should keep this possibility in mind.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Harel Z, Harel S, Shah PS, et al. Gastrointestinal adverse events with sodium polystyrene sulfonate (Kayexalate) use: a systematic review. Am J Med 126: 264 e9-e24, 2013. [DOI] [PubMed] [Google Scholar]
- 2. Abraham SC, Bhagavan BS, Lee LA, et al. Upper gastrointestinal tract injury in patients receiving kayexalate (sodium polystyrene sulfonate) in sorbitol: clinical, endoscopic, and histopathologic findings. Am J Surg Pathol 25: 637-644, 2001. [DOI] [PubMed] [Google Scholar]
- 3. Watson MA, Baker TP, Nguyen A, et al. Association of prescription of oral sodium polystyrene sulfonate with sorbitol in an inpatient setting with colonic necrosis: a retrospective cohort study. Am J Kidney Dis 60: 409-416, 2012. [DOI] [PubMed] [Google Scholar]
- 4. Sterns RH, Rojas M, Bernstein P, et al. Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective? J Am Soc Nephrol 21: 733-735, 2010. [DOI] [PubMed] [Google Scholar]
- 5. Lillemoe KD, Romolo JL, Hamilton SR, et al. Intestinal necrosis due to sodium polystyrene (Kayexalate) in sorbitol enemas: clinical and experimental support for the hypothesis. Surgery 101: 267-272, 1987. [PubMed] [Google Scholar]
- 6. Goutorbe P, Montcriol A, Lacroix G, et al. Intestinal necrosis associated with orally administered calcium polystyrene sulfonate without sorbitol. Ann Pharmacother 45: e13, 2011. [DOI] [PubMed] [Google Scholar]
- 7. Rogers FB, Li SC. Acute colonic necrosis associated with sodium polystyrene sulfonate (Kayexalate) enemas in a critically ill patient: case report and review of the literature. J Trauma 51: 395-397, 2001. [DOI] [PubMed] [Google Scholar]
- 8. Romano RC, Thirumala S, Cushman WH, et al. Inflammatory pseudotumor containing kayexalate crystals: a case report and review of the literature. Int J Surg Pathol 22: 464-469, 2014. [DOI] [PubMed] [Google Scholar]
- 9. McGowan CE, Saha S, Chu G, et al. Intestinal necrosis due to sodium polystyrene sulfonate (Kayexalate) in sorbitol. South Med J 102: 493-497, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]