Abstract
A 58-year-old man consulted our hospital due to a 2-year history of dysarthria and a 1-month history of blepharospasm. In addition to the ataxic dysarthria and blepharospasm, a neurological examination demonstrated slight ataxia of the trunk and lower limbs. Brain MRI demonstrated atrophy of the upper portion of the cerebellar vermis. Gene analysis established a diagnosis of spinocerebellar ataxia type 31 (SCA31). Single photon emission computed tomography (SPECT) with the three-dimensional stereotaxic ROI template (3DSRT) software program demonstrated hyperperfusion in the lenticular nucleus and thalamus. Although the association between SCA31 and blepharospasm in our patient remains unclear, we considered that this combination might be more than coincidental.
Keywords: blepharospasm, SPECT, spinocerebellar ataxia type 31 (SCA31), three-dimensional stereotaxic ROI template (3DSRT)
Introduction
Spinocerebellar ataxias (SCAs) are a large group of inherited disorders presenting with cerebellar ataxia. Some patients may also have associated hyperkinetic movements, including dystonia, chorea, myoclonus, and tremor. SCA type 31 (SCA31) is an adult-onset autosomal-dominant neurodegenerative disorder showing a late-onset, relatively pure cerebellar form of ataxia and the degeneration of Purkinje cells (1, 2). Recently, Nakamura et al. reported that one of 44 patients with SCA31 presented with “facial dystonia”, but the details were not described (3).
Blepharospasm is a form of focal dystonia characterized by involuntary orbicularis oculi muscle spasms (4, 5). Studies of patients with blepharospasm have demonstrated hyperactivity of various brain regions, including the lenticular nucleus and thalamus (4). Recent studies also demonstrated cerebellar involvement in blepharospasm patients (4, 6).
Thus far, blepharospasm has been reported in patients with several neurodegenerative diseases, including multiple system atrophy (7) and SCA types 1 (8, 9) and 3 (10). Although Cardoso et al. stated that blepharospasm in SCA3 patients may be associated with lesions of the basal ganglia (10), the pathologic mechanisms of blepharospasm in SCA patients remain unclear. We herein report the case of an SCA31 patient presenting with blepharospasm following the occurrence of ataxic dysarthria, in whom hyperperfusion in the lenticular nucleus and thalamus was demonstrated on Technetium-99m ethyl cysteinate dimer (99mTc-ECD) single photon emission computed tomography (SPECT).
Case Report
A 58-year-old man consulted our hospital due to an approximately 2-year history of slowly progressive dysarthria. One month prior to the consultation, the patient noticed photophobia and blepharoptosis, and consulted an ophthalmology clinic. Based on a diagnosis of blepharospasm, botulinum toxin was injected into the patient's orbicularis oculi muscle, which relieved the symptom. His past medical history included hypertension and dyslipidemia, for which he had been taking losartan potassium, amlodipine, and pitavastatin. His brother had been diagnosed with SCA31(which has not been accompanied by blepharospasm) at another hospital. In addition to ataxic dysarthria and blepharospasm, a neurological examination of our patient demonstrated slight ataxia of the trunk and lower limbs; however, there was no complaint of gait imbalance. There was no apparent nystagmus, hearing impairment, cervical or limb dystonia, tremor, akinesia, or muscle rigidity. Brain MRI demonstrated an arachnoid cyst in the posterior fossa and atrophy of the upper portion of the cerebellar vermis (Fig. 1). Brain MR angiography demonstrated no stenosis of the intracranial major arteries. Genetic tests were performed after obtaining the patient's informed consent. Genomic DNA was purified from peripheral blood leukocytes by phenol-chloroform extraction. The genomic region spanning the SCA31 mutation site was amplified by a PCR, and the presence of a (TGGAA)n insertion was confirmed, as previously described (1). The blepharospasm at 60 years of age is shown in a video (Supplementary material). He could transiently open his bilateral eyelids by touching the lateral portion of the unilateral eyebrow (Supplementary material). This finding was considered to be a sensory trick associated with blepharospasm (5). Botulinum toxin injections had been conducted every 3 months.
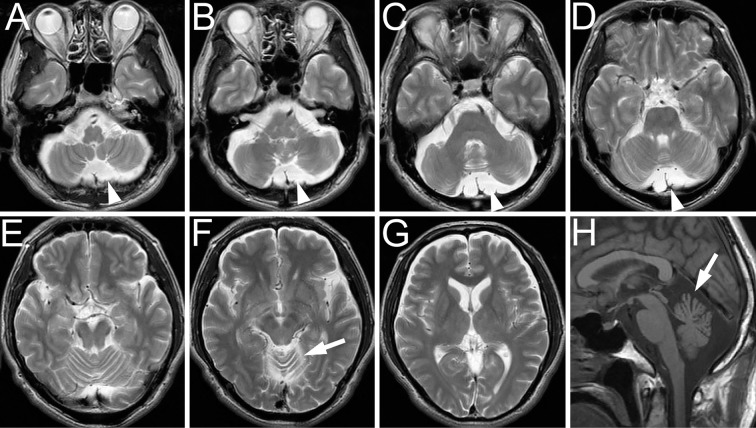
Figure 1.
Axial images of T2-weighted MRI (A-G) demonstrated an arachnoid cyst showing a slight mass effect in the posterior fossa (A-D, arrowheads) and atrophy of the upper portion of the cerebellar vermis (F, arrow). There were no abnormal signal intensities in the cerebellum, lenticular nucleus, or thalamus. A midsagittal image of T1-weighted MRI (H) showed atrophy of the upper portion of the cerebellar vermis (arrow).
99mTc-ECD SPECT of the brain was performed to detect functional abnormalities associated with the blepharospasm. While SPECT showed no apparent hypoperfusion in the cerebellum on visual inspection (Fig. 2), hyperperfusion was suspected in the bilateral lenticular nuclei and right temporal lobe (Fig. 2). The regional cerebral blood flow (rCBF) was compared with the age-matched database of eZIS (FUJIFILM RI Pharma, Tokyo, Japan), which included patients of 60-69 years of age (11). The two-tailed view in eZIS demonstrated regions with a Z-score of ≤-2 in the bilateral cerebellar hemisphere and the upper portion of the cerebellar vermis (Fig. 2). The hypoperfusion of the bilateral cerebellar hemisphere may be associated with the arachnoid cyst in the posterior fossa. In contrast, there were regions showing a Z-score of ≥2 in the midbrain, right lenticular nucleus, bilateral thalamus, and bilateral frontotemporal lobes (Fig. 2).
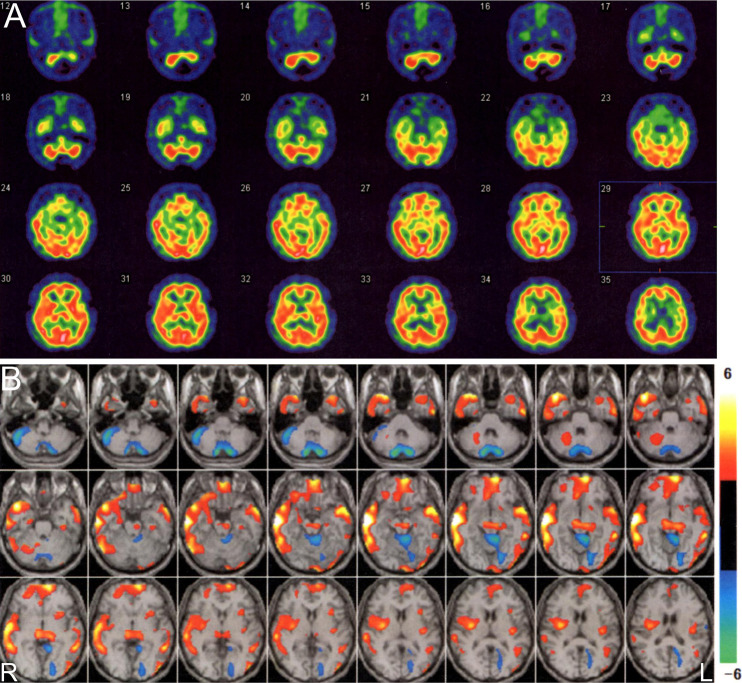
Figure 2.
99mTc-ECD SPECT of the brain (A) showed no apparent hypoperfusion in the cerebellum. Hyperperfusion was suspected in the bilateral lenticular nuclei and right temporal lobe. The two-tailed view display in eZIS (B) demonstrated regions showing a Z-score of ≤-2 in the bilateral cerebellar hemisphere and the upper portion of the cerebellar vermis. The hypoperfusion of the bilateral cerebellar hemisphere might have been associated with the arachnoid cyst in the posterior fossa. In contrast, there were regions showing a Z-score of ≥2 in the midbrain, right lenticular nucleus, bilateral thalamus, and bilateral frontotemporal lobes.
The rCBF was measured using a Patlak plot method and the three-dimensional stereotaxic ROI template software program (3DSRT, FUJIFILM RI Pharma) (12, 13), in which the SPECT images were spatially normalized to a standard template using SPM2 followed by the quantification of 318 constant ROIs, grouped into 12 segments (Table). The table shows the results of rCBF measurements (mL/100 g/min) using the software 3DSRT. The results for the regions showing abnormalities are indicated in bold typeface. The reference ranges of Japanese normal controls are expressed as the mean±SD. As a result, the total CBF was 45.1 mL/100 g/min, which was within the reference range of the total CBF of the age-matched normal controls (Table). The rCBF of the left cerebellum was found to decreased (Table); this may have been associated with the arachnoid cyst in the posterior fossa. In contrast, the rCBFs of the right temporal regions, right lenticular nucleus, left thalamus, and right hippocampus were increased in comparison to the reference ranges of the rCBFs of the controls (Table). When the patient was 61 years of age, his total Scale for the Assessment and Rating of Ataxia (SARA) score was 7.5.
Table.
Results of the rCBF Measurements (mL/100 g/min), which were Performed Using the 3DSRT Software Program.
| Patient | Controls | |||||
|---|---|---|---|---|---|---|
| Age | 60 years | 60-87 years | 40-59 years | |||
| n=22 | n=21 | |||||
| Total brain | 45.1 | 46.6±4.6 | 46.9±3.6 | |||
| Right side Patient | Controls | Left side Patient | Controls | |||
| Age | 60 years | 60-87 years | 40-59 years | 60 years | 60-87 years | 40-59 years |
| Callosomarginal | 42.4 | 43.1±4.7 | 43.8±4.3 | 42.6 | 42.9±4.9 | 43.7±4.4 |
| Precentral | 45.3 | 44.0±4.7 | 44.1±4.3 | 44.1 | 43.7±4.4 | 43.8±4.6 |
| Central | 40.1 | 42.7±5.2 | 41.0±3.8 | 41.3 | 42.4±5.7 | 40.8±4.4 |
| Parietal | 43.7 | 41.9±5.3 | 40.8±4.1 | 41.8 | 41.6±5.6 | 40.3±4.5 |
| Angular | 49.3 | 44.3±5.9 | 43.6±4.6 | 49.3 | 43.8±5.7 | 42.8±4.8 |
| Temporal | 46.6 | 40.6±4.4 | 41.3±3.1 | 43.6 | 40.0±4.6 | 40.9±3.6 |
| Posterior | 49.8 | 47.9±5.3 | 47.0±3.4 | 48.4 | 48.0±5.3 | 46.9±3.5 |
| Pericallosal | 45.5 | 46.1±5.7 | 46.2±4.8 | 45.4 | 45.5±5.6 | 45.3±4.5 |
| Lenticular nucleus | 52.2 | 46.7±4.9 | 47.2±4.6 | 48.9 | 45.5±4.5 | 47.1±4.9 |
| Thalamus | 47.7 | 44.9±6.3 | 47.3±5.2 | 50.2 | 43.7±6.0 | 46.7±5.8 |
| Hippocampus | 40.6 | 35.0±4.6 | 36.8±3.6 | 37.4 | 35.6±4.6 | 35.7±3.5 |
| Cerebellum | 48.5 | 53.6±7.0 | 52.8±4.2 | 45.5 | 52.6±6.5 | 52.4±4.3 |
Discussion
This is the first reported case of SCA31 complicated by blepharospasm. In our patient, blepharospasm occurred in the early stage of the disease. Although the association between SCA31 and blepharospasm in our patient is unclear, blepharospasm has also been reported to occur in the early stages of SCA types 1 (8, 9) and 3 (10).
In our patient, the rCBF measurements, which were performed using the 3DSRT software program, demonstrated hyperperfusion in various regions, including the right lenticular nucleus and left thalamus. Although we cannot explain the pathologic mechanisms of the hyperperfusion in these regions, the hyperperfusion of the right lenticular nucleus and left thalamus was considered to be associated with the blepharospasm. The 3DSRT software program was useful for demonstrating abnormalities of the rCBF.
Recent studies demonstrated the involvement of the cerebellum in patients with blepharospasm (4, 6). Since the cerebello-thalamo-cortical tract facilitates inhibition via projections to the interneurons in the sensorimotor cortices, the reduction of its fiber tract integrity is correlated with cortical activation and hyperexcitability (6). Magnetic cerebellar stimulation inducing Purkinje cell activation, if it had been performed, might have demonstrated inhibition of the supression of mortor cortex excitability (14). In conclusion, although the association between SCA31 and blepharospasm in our patient remains unclear, this combination might be more than coincidental. Further studies involving a large number of SCA31 patients are needed to clarify the prevalence of blepharospasm in SCA31 patients.
The authors state that they have no Conflict of Interest (COI).
Supplementary Material
The patient could transiently open his bilateral eyelids by touching the lateral portion of the unilateral eyebrow.
Acknowledgement
The authors thank Mr. Norikazu Yamashita, Masato Koshiji, and Hironobu Nemoto for their technical assistance with the rCBF measurements.
References
- 1. Sato N, Amino T, Kobayashi K, et al. Spinocerebellar ataxia type 31 is associated with “inserted” penta-nucleotide repeats containing (TGGAA)n. Am J Hum Genet 85: 544-557, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ishikawa K, Mizusawa H. The chromosome 16q-linked autosomal dominant cerebellar ataxia (16q-ADCA): a newly identified degenerative ataxia in Japan showing peculiar morphological changes of the Purkinje cell: The 50th Anniversary of Japanese Society of Neuropathology. Neuropathology 30: 490-494, 2010. [DOI] [PubMed] [Google Scholar]
- 3. Nakamura K, Yoshida K, Matsushima A, et al. Natural history of spinocerebellar ataxia type 31: a 4-year prospective study. Cerebellum 16: 518-524, 2017. [DOI] [PubMed] [Google Scholar]
- 4. Valls-Sole J, Defazio G. Blepharospasm: update on epidemiology, clinical aspects, and pathophysiology. Front Neurol 7: 45, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Defazio G, Hallett M, Jinnah HA, Berardelli A. Development and validation of a clinical guideline for diagnosing blepharospasm. Neurology 81: 236-240, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ramdhani RA, Kumar V, Velickovic M, Frucht SJ, Tagliati M, Simonyan K. What's special about task in dystonia? A voxel-based morphometry and diffusion weighted imaging study. Mov Disord 29: 1141-1150, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yoon WT, Chung EJ, Lee SH, Kim BJ, Lee WY. Clinical analysis of blepharospasm and apraxia of eyelid opening in patients with parkinsonism. J Clin Neurol 1: 159-165, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu YR, Lee-Chen GJ, Lang AE, Chen CM, Lin HY, Chen ST. Dystonia as a presenting sign of spinocerebellar ataxia type 1. Mov Disord 19: 586-587, 2004. [DOI] [PubMed] [Google Scholar]
- 9. Thurtell MJ, Biousse V, Newman NJ. Rod-cone dystrophy in spinocerebellar ataxia type 1. Arch Ophthalmol 129: 956-958, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cardoso F, de Oliveira JT, Puccioni-Sohler M, Fernandes AR, de Mattos JP, Lopes-Cendes I. Eyelid dystonia in Machado-Joseph disease. Mov Disord 15: 1028-1030, 2000. [DOI] [PubMed] [Google Scholar]
- 11. Matsuda H, Mizumura S, Nagao T, et al. Automated discrimination between very early Alzheimer disease and controls using an easy Z-score imaging system for multicenter brain perfusion single-photon emission tomography. AJNR Am J Neuroradiol 28: 731-736, 2007. [PMC free article] [PubMed] [Google Scholar]
- 12. Takeuchi R, Yonekura Y, Matsuda H, Konishi J. Usefulness of a three-dimensional stereotaxic ROI template on anatomically standardised 99mTc-ECD SPET. Eur J Nucl Med Mol Imaging 29: 331-341, 2002. [DOI] [PubMed] [Google Scholar]
- 13. Takeuchi R, Matsuda H, Yoshioka K, Yonekura Y. Cerebral blood flow SPET in transient global amnesia with automated ROI analysis by 3DSRT. Eur J Nucl Med Mol Imaging 31: 578-589, 2004. [DOI] [PubMed] [Google Scholar]
- 14. Ugawa Y, Day BL, Rothwell JC, Thompson PD, Merton PA, Marsden CD. Modulation of motor cortical excitability by electrical stimulation over the cerebellum in man. J Physiol 441: 57-72, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The patient could transiently open his bilateral eyelids by touching the lateral portion of the unilateral eyebrow.