Abstract
Diffuse pulmonary ossification (DPO) is a rare disease characterized by metaplastic bone formation in the lung. There are few reports with a long-term follow-up of this disease. We herein report a 47-year-old man diagnosed with idiopathic DPO at 30 years of age. The patient's vital capacity was normal until 36 years of age (3.39 L, 82.4% predicted), but it was severely decreased when he visited the hospital again at 47 years of age due to cough and dyspnea (1.98 L, 44.6% predicted). Chest computed tomography showed a significant increase in the number of high-density nodules, suggesting that the progression of DPO had caused restrictive ventilatory impairment.
Keywords: pulmonary ossification, restrictive ventilatory impairment, prognosis
Introduction
Diffuse pulmonary ossification (DPO) is a rare disease characterized by the ectopic formation of mature bone in the bilateral lung parenchyma. This disease was first reported by Luschka in 1856 (1). There are few cases in which living patients are diagnosed with DPO, as the symptoms are generally subclinical, and radiographical abnormalities are usually masked by other pulmonary diseases. According to autopsy data, the incidence of DPO ranges from 0.16% to 1.6% (2-4).
DPO can be classified into two subtypes: nodular and dendriform DPO, both of which have secondary and idiopathic forms (2, 5). Most cases of dendriform DPO arise secondary to acute or chronic damage of the lung, such as idiopathic pulmonary fibrosis, acute respiratory distress syndrome, and chronic obstructive pulmonary disease (6). In contrast, idiopathic DPO is extremely rare: to our knowledge, only around 20 cases have been reported, even including possible cases (5, 7-21). Few of these reports included detailed pulmonary function data, and even the reports with available data showed inconsistent results. Among them, only one report presented the long-term follow-up data (10). In that case report, the lung volumes and diffusing capacity of the lung for carbon monoxide (DLCO) declined slightly over 14 years (detailed data were lacking), suggesting that idiopathic dendriform DPO may progress very slowly.
We herein report a case of idiopathic dendriform DPO that progressed over 11 years and resulted in severe restrictive ventilatory impairment.
Case Report
The patient was a 47-year old man (height, 172.9 cm; weight, 75.6 kg). He was admitted with spontaneous pneumothorax at 27 years of age. His pneumothorax was cured by chest drainage, but chest X-ray and computed tomography (CT) revealed bilateral diffuse nodular opacities. A transbronchial lung biopsy was performed, but a definitive diagnosis could not be reached.
He presented to the hospital again with diffuse nodular opacities at 30 years of age. A video-assisted thoracoscopic lung biopsy of the right lobe was performed. Multiple dendric foci of bone were observed in the alveolar spaces (Fig. 1). The histopathological image showed subpleural fibrosis, predominantly around the ossified areas, but it was not specific. There were no findings of interstitial lung disease associated with these lesions. Other underlying heart and pulmonary diseases were excluded, so he was diagnosed with idiopathic dendriform DPO. He had a history of hypertension. He had been a smoker from age 16 and quit smoking at age 45 (29 pack-years). He had been working as a plumber from 26 years of age and then started working in industrial waste disposal at 43 years of age, wearing a dust mask. Regarding his family medical history, his mother has been diagnosed with pulmonary calcification.
Figure 1.
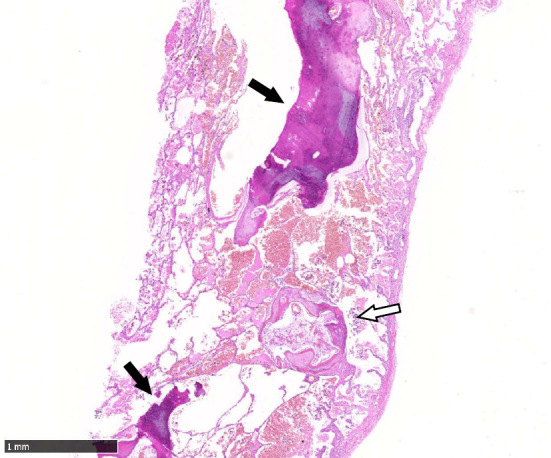
A surgical lung biopsy specimen shows multiple bone formations in the alveolar spaces (arrows).
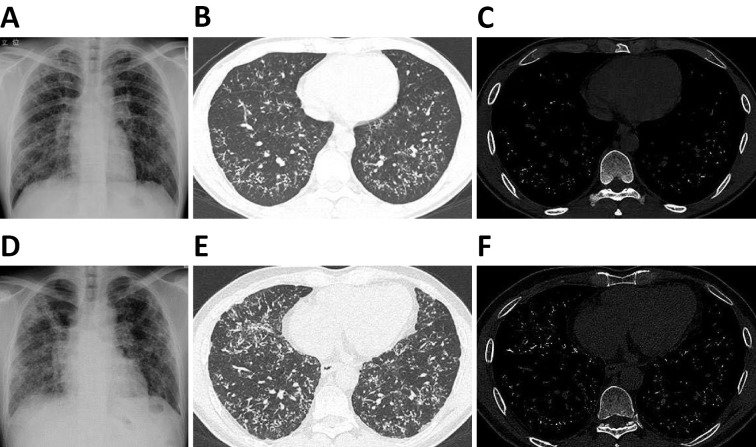
The images of chest X-ray and CT at 33 years of age are shown in Fig. 2A-C. Chest X-ray showed bilateral ground-glass opacity, and CT revealed high-density nodules bilaterally in the lower lobes. Despite the abnormal chest images, the pulmonary function test conducted at 36 years of age showed normal results (Table 1): the vital capacity (VC) was 3.39 L (82.4% predicted), and the forced expiratory volume in 1 second/forced vital capacity ratio (FEV1/FVC) was 81.9%.
Figure 2.
Chest X-ray (A), CT image with lung window (B), and CT image with bone window (C) at 33 years of age. Chest X-ray (D), CT image with lung window (E), and CT image with bone window (F) at 46 years of age. Chest X-ray showed bilateral ground-glass opacity, and CT showed high-density nodules bilaterally in the lower lobes. Compared with the images at 33 years of age, the bilateral ground-glass opacity on chest X-ray had progressed, and the number of high-density nodules on CT had increased significantly at 46 years of age.
Table 1.
Pulmonary Function Data at Ages 36 and 47 Years.
| Age: 36 | Age: 47 | |||||
|---|---|---|---|---|---|---|
| Value | % Predicted | Value | % Predicted | |||
| VC | (L) | 3.39 | 82.4 | 1.98 | 44.6 | |
| FVC | (L) | 3.20 | 77.8 | 1.92 | 44.1 | |
| FEV1 | (L) | 2.62 | 68.9 | 1.58 | 42.4 | |
| FEV1/FVC | (%) | 81.87 | 104.4 | 82.29 | 96.6 | |
| DLCO | (mL/min/mmHg) | N/A | N/A | 15.39 | 53.8 | |
| RV/TLC | (%) | N/A | - | 45.58 | - | |
VC: vital capacity, FVC: forced vital capacity, FEV1: forced expiratory volume in 1 second, DLCO: diffusing capacity of the lung for carbon monoxide, RV: residual volume, TLC: total lung capacity, N/A: not available
He visited the hospital again at 46 years of age after a 10-year interval due to cough and dyspnea when climbing stairs. The laboratory data showed no abnormal results, except for elevation of Krebs von den Lungen (KL)-6 (870 U/mL) (Table 2). Echocardiogram also showed no abnormalities. Chest X-ray revealed the progression of bilateral ground-glass opacity (Fig. 2D). The number of high-density nodules had increased significantly in lower lobes (Fig. 2E, F). Pulmonary function tests revealed that the VC had decreased severely (1.98 L, 44.6% predicted) (Table 1), while the FEV1/FVC was 82.3% (96.6% predicted). The DLCO was decreased (15.4 mL/min/mmHg, 53.8% predicted), and the residual volume/total lung capacity ratio (RV/TLC) was increased (45.58%). Given these data, we diagnosed the patient with severe restrictive ventilatory impairment due to the progression of DPO. Because there is no established medical treatment, he is now being evaluated for listing for lung transplantation.
Table 2.
Laboratory Data at Age 46 Years.
| Hematology | Biochemistry | Immunology | ||||||||
| WBC | 9.77×109 | /L | AST | 22 | U/L | PR3-ANCA | <1.0 | U/mL | ||
| Neutrophil | 54.1 | % | ALT | 15 | U/L | MPO-ANCA | <1.0 | U/mL | ||
| Lymphocyte | 31.9 | % | LDH | 200 | U/L | KL-6 | 870 | U/mL | ||
| Monocyte | 7.4 | % | ALP | 289 | U/L | Anti-ds DNA antibody | <7 | IU/mL | ||
| Eosinophil | 5.9 | % | γ-GTP | 28 | U/L | Anti-centromere antibody | <5.0 | |||
| Basophil | 0.7 | % | TP | 7.2 | g/dL | Anti-nuclear antibody | <1:40 | |||
| RBC | 4.99×1012 | /L | ALB | 4.2 | g/dL | SP-D | 96.1 | ng/mL | ||
| HGB | 15.3 | g/dL | Ch-E | 407 | U/L | Anti-CCP antibody | <0.6 | U/mL | ||
| HCT | 45.8 | % | T-Bil | 0.5 | mg/dL | Anti-ARS antibody | <5.0 | U/mL | ||
| MCV | 91.8 | fL | CRE | 0.83 | mg/dL | Anti-Sm antibody | <5.0 | U/mL | ||
| MCH | 30.7 | pg | eGFR | 79.3 | mL/min/1.73m2 | Anti-RNP antibody | <5.0 | U/mL | ||
| MCHC | 33.4 | % | UA | 6.3 | mg/dL | Anti-SS-A antibody | <5.0 | U/mL | ||
| PLT | 306×109 | /L | BUN | 10 | mg/dL | Anti-SS-B antibody | <5.0 | U/mL | ||
| T-CHO | 199 | mg/dL | Anti-Scl-70 antibody | <5.0 | U/mL | |||||
| Coagulation | TG | 54 | mg/dL | |||||||
| PT | 11.4 | s | CK | 147 | U/L | Blood gas analysis | ||||
| PT-INR | 0.99 | Na | 140 | mEq/L | pH | 7.37 | ||||
| APTT | 43.5 | s | K | 4.1 | mEq/L | PaO2 | 91.1 | Torr | ||
| Fibrinogen | 328 | mg/dL | Cl | 106 | mEq/L | PaCO2 | 40.5 | Torr | ||
| Ca | 9.5 | mg/dL | HCO3- | 23 | mmol/L | |||||
| IP | 2.7 | mg/dL | BE | -2.0 | mmol/L | |||||
| CRP | <0.1 | mg/dL | ||||||||
| ACE | 12.4 | U/L | 6-min walking distance | 555 | m | |||||
| Aldolase | 6 | U/L | ||||||||
WBC: white blood cell, RBC: red blood cell, HGB: hemoglobin, HCT: hematocrit, MCV: mean corpuscular volume, MCH: mean corpuscular hemoglobin, MCHC: mean corpuscular hemoglobin concentration, PT: prothrombin time, PT-INR: prothrombin time international normalized ratio, APTT: activated partial thromboplastin time, AST: aspartate aminotransferase, ALT: alanine aminotransferase, LDH: lactate dehydrogenase, ALP: alkaline phosphatase, γ-GTP: gamma glutamyl transpeptidase, TP: total protein, ALB: albumin, Ch-E: cholinesterase, T-Bil: total bilirubin, CRE: creatinine, eGFR: epidermal growth factor receptor, UA: uric acid, BUN: blood urea nitrogen, T-CHO: total cholesterol, TG: triglycerides, CK: creatine kinase, Na: sodium, K: potassium, Cl: chloride, Ca: calcium, IP: inorganic phosphorus, CRP: C-reactive protein, ACE: angiotensin-1-converting enzyme, PR3: proteinase3, ANCA: anti neutrophil cytoplasmic antibody, MPO: myeloperoxidase, KL-6: Krebs von den Lungen-6, SP-D: surfactant protein-D, CCP: cyclic citrullinated peptide, ARS: aminoacyl tRNA synthetase, Sm: Smith, RNP: ribonucleoprotein, SS-A: Sjögren syndrome-A, SS-B: Sjögren syndrome-B, BE: base excess
Discussion
We encountered a case of idiopathic DPO. Secondary DPO can be derived from not only preexisting disorders but also rare earth metal and heavy metal exposure (22, 23). This patient has an occupational history as a plumber before the diagnosis. The association between his occupation as a plumber and the pathogenesis of DPO is not apparent, as the exposure period until the episode of spontaneous pneumothorax was only one year. We summarized the published cases of idiopathic DPO in Table 3. Some reports have shown that spontaneous pneumothorax can be caused by pulmonary ossification (14, 18-20); therefore, DPO might have been present when our patient suffered from spontaneous pneumothorax. Based on the published cases, idiopathic DPO appears to occur predominantly in younger man, which is compatible with our case.
Table 3.
Summary of the Published Cases of Idiopathic Diffuse Pulmonary Ossification.
| Case No. | Age, years | Sex | Past medical history | Family history | Smoking history | Environmental / occupational exposures | Pulmonary function test, % predicted | Reference No. |
|---|---|---|---|---|---|---|---|---|
| 1 | 34 | M | Intermittent hemoptysis | Not mentioned | 10 pack-years | Never | FVC: 109%, FEV1: 87%, DLCO: 72% | 5 |
| 2 | 29 | M | Pneumothorax | Father: Dendriform pulmonary ossification | Not mentioned | Not mentioned | Not mentioned | 9 |
| 3 | 43 | M | Allergic rhinitis/ Asthma/ Diabetes mellitus/ Coronary atherosclerotic heart disease | Not mentioned | Never | Never | "Lung volumes and DLCO have declined slightly over 14 years" | 10 |
| 4 | 51 | M | None | Not mentioned | Not mentioned | Never | "Normal except for an isolated reduction in the transfer factor (73% predicted)" | 11 |
| 5 | 26 | M | Cleft lip and palate/ Chronic sinusitis | None | Never | Never | "Normal" | 12 |
| 6 | 32 | M | Pneumonia | Not mentioned | Never | Never | VC: 93%, FVC: 82%, FEV1: 71%, DLCO: 102% | 13 |
| 7 | 47 | M | Pneumothorax | Not mentioned | Not mentioned | Exposure to methylmethacrylate, quartz cristobalite, chrome cobalt, alginates, asbestos and silica as a dental technician | "Normal" | 14 |
| 8 | 49 | M | None | Not mentioned | Never | Not mentioned | "A slow and progressive decline in pulmonary function" | 15 |
| 9 | 58 | M | Left renal artery stenosis/ Ischemic stroke | Not mentioned | Not mentioned | Never | Not mentioned | 16 |
| 10 | 43 | M | None | Not mentioned | Never | Never | VC: 110%, FVC: 99%, FEV1: 92%, DLCO: 71% | 17 |
| 11 | 33 | M | Pneumothorax/ Asthma | Not mentioned | Never | Not mentioned | Not mentioned | 18 |
| 12 | 53 | M | Pneumothorax | Not mentioned | Not mentioned | Not mentioned | Not mentioned | 19 |
| 13 | 26 | M | Pneumothorax | Father: Similar lung condition in his 30s with computed tomography (CT) images | Never | Never | "Normal" (FEV1: 91%) | 20 |
| 14 | 47 | F | Hypoplasia and deformity of the middle fingers/ Diabetes mellitus/ Schizophrenia | Father: Multiple small high density nodules by CT | Never | Never | VC: 101%, FEV1: 71% | 21 |
In addition to the case with "idiopathic" in the publication, the possible cases were included. Some old publications which have only abstracts with little information of the cases on the PubMed were excluded. Not mentioned: No available information in the publication, VC: vital capacity, FVC: forced vital capacity, FEV1: forced expiratory volume in one second, DLCO: diffusing capacity of the lung for carbon monoxide
Idiopathic dendriform DPO has been thought to be an indolent condition (5, 10). Our patient's VC decreased approximately 1.4 L (41.6% of the predicted VC at 36 years of age) over 11 years, which is the most severe case on record of idiopathic dendriform DPO. Although the decreased lung volume based on the images and the decreased DLCO along with the VC suggest the progression of fibrosis, there were no apparent findings of interstitial pneumonia by CT. Based on the histological and radiological findings, we speculate that the non-specific lung fibrosis did not precede ossification but developed secondary to ossification. However, its mechanism remains unclear. During the video-assisted thoracoscopic lung biopsy, the lung showed incomplete collapse, suggesting that lung compliance was decreased. Our patient's chest CT image showed abundant high-density nodules bilaterally compared with the literature cases (5, 11, 14, 17). This result may explain the severity of the restrictive ventilatory impairment in our case. The pulmonary function test also showed an impaired diffusing capacity (DLCO), which is compatible with the previous case (10).
Some biological hypotheses of DPO pathogenesis have been proposed. DPO is strongly associated with fibrosing interstitial lung diseases (24); however, the pathogenesis of idiopathic DPO is still uncertain. As a candidate, transforming growth factor-beta (TGF-β)/bone morphogenetic protein (BMP) is known to be associated with ectopic bone formation, as well as normal bone organogenesis (25). Furthermore, three reports of familial DPO have been published (9, 20, 21). Interestingly, two of these reports are from Japan. In all three cases, the patients' fathers suffered from DPO. Some genetic abnormalities may be involved in DPO pathogenesis; therefore, when a patient is diagnosed with idiopathic DPO, the patient's family should also be checked, especially male relatives. We examined the present patient's mother, who had been diagnosed with pulmonary calcification, but her chest CT image showed only mild calcification, suggesting old inflammatory change. Unfortunately, we have no data for the patient's father or son at present.
In this case, the follow-up period from the first diagnosis was approximately 17 years, which is the longest period yet among such reports. However, it should be noted that whether the restrictive ventilatory impairment progressed gradually through the entire period or progressed rapidly in recent years is unknown, as the patient had missed appointments for approximately 10 years. In a previous report, lung transplantation was performed for end-stage pulmonary fibrosis, and the patient was diagnosed with DPO based on an examination of the extirpated lung (26); however, the prognosis after the transplantation is unknown. Further reports of idiopathic dendriform DPO with long-term follow-up data are required to determine the optimum timing for lung transplantation.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Luschka H. Ramified ossification of the pulmonary parenchyma. Wirchows Arch 10: 500-505, 1856. [Google Scholar]
- 2. Tseung J, Duflou J. Diffuse pulmonary ossification: an uncommon incidental autopsy finding. Pathology 38: 45-48, 2006. [DOI] [PubMed] [Google Scholar]
- 3. Friedrich T, Steinecke R, Horn LC, Eichfeld U. Idiopathic pulmonary ossification. Rofo 169: 267-273, 1998(in German, Abstract in English). [DOI] [PubMed] [Google Scholar]
- 4. Lara JF, Catroppo JF, Kim DU, da Costa D. Dendriform pulmonary ossification, a form of diffuse pulmonary ossification: report of a 26-year autopsy experience. Arch Pathol Lab Med 129: 348-353, 2005. [DOI] [PubMed] [Google Scholar]
- 5. Reddy TL, von der Thüsen J, Walsh SL. Idiopathic dendriform pulmonary ossification. J Thorac Imaging 27: W108-W110, 2012. [DOI] [PubMed] [Google Scholar]
- 6. Peros-Golubicić T, Tekavec-Trkanjec J. Diffuse pulmonary ossification: an unusual interstitial lung disease. Curr Opin Pulm Med 14: 488-492, 2008. [DOI] [PubMed] [Google Scholar]
- 7. Gortenuti G, Portuese A. Disseminated pulmonary ossification. Eur J Radiol 5: 14-16, 1985. [PubMed] [Google Scholar]
- 8. Ndimbie OK, Williams CR, Lee MW. Dendriform pulmonary ossification. Arch Pathol Lab Med 111: 1062-1064, 1987. [PubMed] [Google Scholar]
- 9. Azuma A, Miyamoto H, Enomoto T, Usuki J, Kudoh S. Familial clustering of dendriform pulmonary ossification. Sarcoidosis Vasc Diffuse Lung Dis 20: 152-154, 2003. [PubMed] [Google Scholar]
- 10. Ahari JE, Delaney M. Dendriform pulmonary ossification: a clinical diagnosis with 14 year follow-up. Chest 132: 701S, 2007. [Google Scholar]
- 11. Ryan CF, Flint JD, Müller NL. Idiopathic diffuse pulmonary ossification. Thorax 59: 1004, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mizushina Y, Bando M, Hosono T, et al. A rare case of asymptomatic diffuse pulmonary ossification detected during a routine health examination. Intern Med 51: 2923-2927, 2012. [DOI] [PubMed] [Google Scholar]
- 13. Bai P, Sun YC, Chen DN, Jin JM, Zhuo J, Liu HG. Idiopathic diffuse pulmonary ossification: a case report and review of the literature. Zhonghua Jie He He Hu Xi Za Zhi 32: 588-592, 2009(in Chinese, Abstract in English). [PubMed] [Google Scholar]
- 14. Harvey NT, Heraganhally S, Au V, Ellis D, Klebe S, Henderson DW. Idiopathic diffuse dendriform pulmonary ossification in a dental technician. Pathology 44: 363-365, 2012. [DOI] [PubMed] [Google Scholar]
- 15. Martinez JB, Ramos SG. Dendriform pulmonary ossification. Lancet 382: e22, 2013. [DOI] [PubMed] [Google Scholar]
- 16. Bisceglia M, Chiaramonte A, Panniello G, Tucci A, Orcioni GF, Colby TV. Selected case from the Arkadi M. Rywlin international pathology slide series: diffuse dendriform pulmonary ossification: report of 2 cases with review of the literature. Adv Anat Pathol 22: 59-68, 2015. [DOI] [PubMed] [Google Scholar]
- 17. Fernández-Bussy S, Labarca G, Pires Y, Díaz JC, Caviedes I. Dendriform pulmonary ossification. Respir Care 60: e64-e67, 2015. [DOI] [PubMed] [Google Scholar]
- 18. Kato T, Ishikawa K, Kadoya M, Okamoto K, Kaji M. Spontaneous pneumothorax in a patient with dendriform pulmonary ossification: report of a case. Surg Today 42: 903-908, 2012. [DOI] [PubMed] [Google Scholar]
- 19. Abe J, Oura H, Niikawa H, Yaegashi H, Kondo T. Dendriform pulmonary ossification: unusual cause of spontaneous pneumothorax. Thorax 69: 97-98, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tsai AP, English JC, Murphy D, Sin DD. Recurrent pneumothorax related to diffuse dendriform pulmonary ossification in genetically predisposed individual. Respirol Case Rep 5: e00211, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kinoshita Y, Mizuguchi I, Hidaka K, Ishii H, Watanabe K. Familial diffuse pulmonary ossification: a possible genetic disorder. Respir Investig 55: 79-82, 2017. [DOI] [PubMed] [Google Scholar]
- 22. Yoon HK, Moon HS, Park SH, Song JS, Lim Y, Kohyama N. Dendriform pulmonary ossification in patient with rare earth pneumoconiosis. Thorax 60: 701-703, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ohtsuki Y, Yamaka A, Ohyama H, et al. Histochemical demonstration of aluminium and iron deposition in pulmonary bony tissues in three cases of diffuse pulmonary ossification. Histol Histopathol 23: 137-141, 2008. [DOI] [PubMed] [Google Scholar]
- 24. Egashira R, Jacob J, Kokosi MA, et al. Diffuse pulmonary ossification in fibrosing interstitial lung diseases: prevalence and associations. Radiology 2017(Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 25. Wu M, Chen G, Li YP. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res 4: 16009, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gevenois PA, Abehsera M, Knoop C, Jacobovitz D, Estenne M. Disseminated pulmonary ossification in end-stage pulmonary fibrosis: CT demonstration. AJR Am J Roentgenol 162: 1303-1304, 1994. [DOI] [PubMed] [Google Scholar]