Abstract
The relationship between mechanical force and alveolar bone remodeling is an important issue in orthodontics because tooth movement is dependent on the response of bone tissue to the mechanical force induced by the appliances used. Mechanical cyclical stretch (MCS), fluid shear stress (FSS), compression, and microgravity play different roles in the cell differentiation and proliferation involved in bone remodeling. However, the underlying mechanisms are unclear, particularly the molecular pathways regulated by non-coding RNAs (ncRNAs) that play essential roles in bone remodeling. Amongst the various ncRNAs, miRNAs act as post-transcriptional regulators that inhibit the expression of their target genes. miRNAs are considered key regulators of many biologic processes including bone remodeling. Here, we review the role of miRNAs in mechanical force-induced bone metabolism.
Keywords: Bone remodeling, compression, fluid shear stress, mechanical cyclical stretch, microgravity, microRNA
Introduction
Bone remodeling, which involves a cross-talk between osteoclasts and osteoblasts, is regulated by a number of proteins that interact through complex mechanisms [1]. Mechanical force can induce resident cell populations to adapt, maintain, and repair the bone structure. The in vivo milieu to which osteoblasts and osteoclasts are exposed is dynamic and changeable, and is where strain, stress, shear, pressure, fluid flow, streaming potential, and acceleration forces regulate bone remodeling [2]. Thus, studying the effects of mechanical forces on bone cells in vitro will improve our understanding of bone remodeling.
Mechanical forces play essential roles in bone remodeling. Mechanical cyclical stretching (MCS), fluid shear stress (FSS), compression, and microgravity play different roles in cell differentiation and proliferation by affecting intracellular interactions. Although the mechanisms are unclear, regulation by non-coding RNAs (ncRNAs) play an indispensable role in bone metabolism. ncRNAs, which are not translated into proteins [3], include miRNAs, siRNAs, piwi-interacting RNAs, small non-translated nucleolar RNAs (snoRNAs), small nRNAs, and long ncRNAs (lncRNAs). miRNAs, which are short ssRNAs of 18–25 nts, modulate orthodontic tooth movement (OTM) and alveolar bone remodeling in normal and inflammatory microenvironments in vivo [4,5]. LncRNAs are associated with the osteodifferentiation of human adipose-derived stem cells (ASCs) [6]. As part of the complex miRNA–mRNA–lncRNA regulatory network, lncRNAs influence bone formation and resorption in patients with osteoporosis [7]. Moreover, constitutive expression or silencing of lncRNA H19 is related to BMP9-induced osteogenic differentiation [8]. snoRNAs are also involved in bone formation. The snoRNA Snord116 is closely related to the bone-mass phenotype in people with Prader–Willi syndrome [9]. Circular RNAs (circRNAs) may play important roles in bone formation [10], and the circRNA–miRNA–mRNA network may function in osteogenesis [11].
Few studies have investigated mechanical force-induced changes in the expression of ncRNAs related to bone metabolism. Indeed, the effects of mechanical forces on lncRNA and snoRNA expression have been investigated less extensively than their effects on miRNAs. Hence, we reviewed the effects of mechanical force on miRNAs during bone remodeling.
Effects of mechanical forces on bone metabolism
MCS
MCS is involved in bone formation. Long-term mechanical stretching of human adipose tissue multipotential stromal cells (hAT-MSCs) leads to early osteogenesis [12], which has been confirmed in vivo [2,13–16]. Production of PDL-specific markers, including periostin and tenascin, can be stimulated by mechanical stress and can enhance cell proliferation [17]. The NGF-TrkA signaling pathway promotes communication between osteoblasts and sensory nerves in mice undergoing MCS [2]. The NOTCH and PERK-eIF2 α-ATF4 signaling pathways are also involved in osteodifferentiation [18,19]. These processes alter the expression levels of diverse osteogenesis-associated genes including osteopontin (OPN), osteocalcin, runt-related transcription factor 2 (RUNX2), and type I collagen [20,21].
FSS
FSS promotes osteoblast proliferation [22] and induces Ca2+ influx via transient receptor potential cation channel subfamily V member 4 (TRPV4) channels and osteogenic differentiation of MSCs. These effects are inhibited by a selective TRPV4 blocker and TRPV4 siRNA [23]. FSS-induced up-regulation of cyclin B1 and CDK1 through the Gq-dependent ERK5 signaling pathway promotes the proliferation of MC3T3-E1 cells [22]. AMP-activated protein kinase signaling in BMSCs is involved in adiponectin-mediated prevention of FSS-induced cell death [24]. Moreover, the effects of FFS differ according to cell surface chemistry; osteoblasts have higher sensitivity to, and lower tolerance for, –OH and –CH3 surfaces compared with –NH2 surfaces [25]. FSS enhances and weakens calcium oscillations in osteoblasts in the early (4 days) and late (8 days) stages of induction, respectively [26]. The effects of FSS on osteoclasts are mediated by signaling pathways involving mechanosensitive and cation-selective channels, phospholipase C, and the endoplasmic reticulum [27].
Compressive force
Compressive force plays an important role in osteoclastogenesis. Both compressive force and hypoxia may initiate osteoclastogenesis during OTM [28]. Heavy compression causes bone fracture of finger-like patterns [29]. TNF-α levels are higher on the compression side of periodontal ligament fibroblasts than on the tension side, which may influence RANKL expression during OTM [30]. Moreover, compression influences the osteodifferentiation of osteoblasts through the ClC-3 chloride channel in MC3T3-E1 cells, and regulates EphB4 and ephrinB2 expression [13,31]. Furthermore, nfatc-1, trap, rank, cath-K, clc7, mmp-9, atp6i, dc-stamp, and oc-stamp, and integrin-αv and -β3 are up-regulated by compression [32]. The effects of compression in OTM are dependent on the intensity of the force applied and are influenced by caffeine [33].
Other forces
The application of high-frequency, low-intensity mechanical vibrations in the bone marrow mesenchymal stem cells of D1-ORL-UVA mice induces adipogenesis and alters their morphology [34]. Both photobiomodulation and low-amplitude high-frequency ultrasound enhance bone-fracture healing [35]. Low-intensity pulsed ultrasound (LIPUS) of appropriate strength and frequency significantly increases the bone tissue mineral density in mice. Thus, LIPUS may be clinically useful for maintaining bone integrity [36–38]. The effects of LIPUS involve the up-regulation of cyclooxygenase-2 and prostaglandin-E2, which are important in bone remodeling [39].
A brief introduction to miRNAs
Classified as ssRNA molecules comprising an average of 22 nts, miRNAs inhibit gene expression at the DNA, RNA, and protein levels [40,41]. Almost 50% of miRNA genes are present in intergenic regions. Their expression is controlled by their own promoters, or in the case of polycistronic miRNA clusters, shared promoters [42]. In cancer [43], cardiovascular disease [41], COPD muscle dysfunction [44], osteoarthritis [45], and skin disorders [46], miRNAs play important roles as diagnostic biomarkers and molecular targetted therapies. miRNAs are regulated by, amongst others, TCF, β-catenin, and dickkopf-related protein 1 (Wnt signaling pathway) [47–50], and by Smad proteins (Smad7-Smad1/5/8-RUNX2 and Smad4-mediated pathways) [51–53]. miRNAs modulate the expression of histone deacetylase 4, forkhead box protein O1, Osterix (Osx), and growth/differentiation factor 5 during cell growth and differentiation [54–56].
Some reviews have addressed the role of miRNAs in bone remodeling and summarized the molecular pathways and specific proteins involved [57,58]. miRNAs function in the process of osteoporosis and bone resorption [58,59], indicating their therapeutic potential in tissue engineering in terms of interfering with bone resorption [60]. However, these reviews focussed on the effect of miRNAs on bone remodeling in the absence of mechanical forces. Therefore, we next discuss the role of miRNAs in bone formation induced by mechanical stimuli.
miRNAs are involved in the mechanical force modulated bone metabolism
MCS
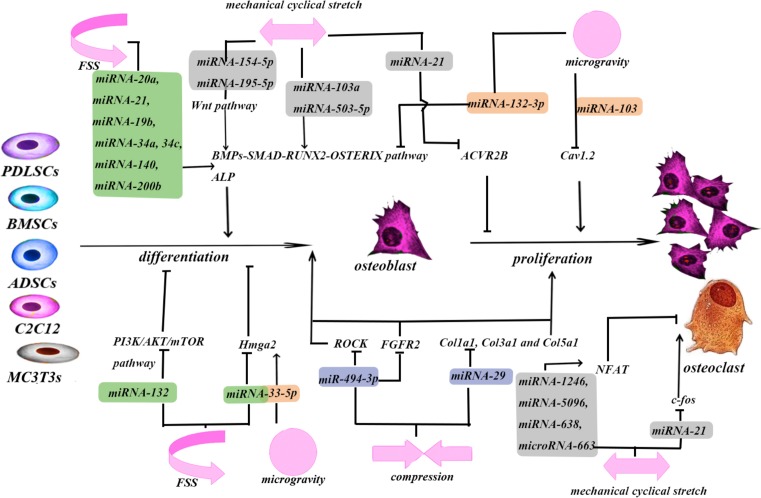
miRNAs and their target genes form a complex network, the balance of which can be altered by mechanical forces; however, the underlying mechanism is unclear, despite the ever-increasing number of studies on the effects of MCS on bone metabolism. Most of these studies have used miRNA microarrays. The effects of changes in miRNA expression are mediated by proteins related to bone formation (e.g. RUNX2 and ALP). Overexpression of miR-503-5p in BMSCs attenuated stretch-induced osteogenic differentiation and decreased RUNX2 and ALP expression both in vitro and in vivo [61]. miR-103-a, which functions as an endogenous attenuator of RUNX2 in osteoblasts together with its target gene, pank3, was down-regulated during cyclical mechanical stretching-induced osteoblast differentiation, which led to an increase in RUNX2 protein levels [62]. Application of stretching force to periodontal ligament stem cells (PDLSCs) led to osteoblastic differentiation as well as changes in miR-1246, miR-5096, miR-638, miR-663, miR-21, miR-4492, and miR-4734 expression. miR-1246 is a novel target of p53 that can activate nuclear factor of activated T cells (NFAT) [63]. Moreover, activation of the NFAT pathway by RANKL attenuates osteoclast differentiation [64]. Under tensile stress, miR-154-5p, by targetting the Wnt/PCP pathway, prevents osteogenic differentiation of adipose-derived mesenchymal stem cells. miR-195-5p, which targets WNT3A, fibroblast growth factor 2 (FGF2), and bone morphogenetic protein receptor type IA (BMPR1A) [65] also inhibited osteogenic differentiation in PDLSCs. Indeed, inhibition of endogenous miR-154-5p using an antisense oligonucleotide significantly promoted osteogenic differentiation [66]. This may partly explain how mechanical stimulation activates the Wnt/β-catenin signaling pathway and promotes bone formation [67]. Exposure of PDLSCs to stretching force decreased the expression level of activin receptor type IIB (ACVR2B) via a direct interaction of miR-21 with the 3′-untranslated repeat sequence of ACVR2B mRNA [68]. ACVR2B can regulate osteoblasts directly and negatively; therefore, miR-21 plays a role in osteogenic differentiation [69]. The expression of miR-138 in human bone marrow mesenchymal stem cells was decreased by mechanical tension. miR-138 targets PTK2, which encodes focal adhesion kinase, a key mechanotransduction factor in osteogenesis. H19 may also be involved in this process [70].
Mechanical stretching down-regulates miR-500, miR-1934, miR-31, miR-378, and miR-331 expression and up-regulates miR-1941 expression by activating NF-κB in C2C12 myoblasts. Thus, miRNA expression during stretch-induced myoblast proliferation is dependent on NF-κB, which implies a mechanism for the intracellular transmission of external mechanical stimuli [71]. miRNAs are closely related to the components of regulatory networks involved in osteogenesis under mechanical stretching forces. miR-33, by acting through the miR-33-BMP3-Smad signaling pathway, prevents proliferation of venous SMCs in response to arterial stretching. AgomiR-33 negatively regulates BMP3 expression and Smad2 and Smad5 phosphorylation [72]. BMP3, which inhibits Smad signaling and osteoblast differentiation, interacts with ACVR2B, as knockdown of endogenous ACVR2B in bone marrow stromal cells ameliorated the BMP3-mediated suppression of osteoblast differentiation [73,74]. In human trabecular meshwork cells, CMS induces the expression of miR-24, resulting in down-regulation of the subtilisin-like proprotein convertase Furin, which plays a major role in the processing of transforming growth factor β1 (TGF-β1). Down-regulation of Furin leads to weight loss; reduced bone mineral density (BMD), serum osteocalcin, total calcium, and intact parathyroid hormone levels; and an increased serum C telopeptide level. A similar finding was reported in osteoporotic postmenopausal females [75–77].
FSS
Mechanical signals produced by FSS are sensed by mechanosensitive cells in bone and translated into biochemical signals [78]. This process is highly dependent on the sensitivity of cells to mechanical forces, which is closely associated with the chemistry of cell scaffolds on the surface [25]. It is thought that interstitial fluid flow and short-term FSS promote the terminal differentiation of pre-osteoblasts. Several miRNAs (miR-20a, miR-21, miR-19b, miR-34a and -34c, miR-140, and miR-200b) influence RUNX2 and ALP expression by targetting PPARy, Bambi, Crim l, TGFBR, SMAD3, PLAP-1, and TGFP1, which are key negative regulators of the BMP-SMAD-RUNX2-Osx signaling pathway [79]. Exposure to FSS significantly up-regulates miR-132 expression in PDLCs. miR-132 has regulatory effects through the PI3K/AKT/mTOR signaling pathway, as determined using the PI3K/AKT and mTOR signaling pathway inhibitor, BEZ235, which blocks FSS-induced differentiation in human PDL cells [80]. Wang et al. [81] reported that after FSS miR-33-5p and its target gene Hmga2 negatively regulated osteoblast differentiation by modulating the proliferation of stem cells and MSCs [82]. Although few studies have focussed on the roles of miRNAs in the effects of FSS on bone formation, the signaling pathways and target genes involved have been identified. Further work should clarify the relationships between mechanical force and miRNA expression levels.
Compressive force
Numerous studies have investigated the effects of compressive force on bone formation; however, few identified the underlying mechanism(s) in osteoblasts and osteoclasts. Iwawaki et al. [83] assessed miRNA expression during compressive treatment in MC3T3-E1 cells by microarray analysis. miR-494-3p was up-regulated after compression and inhibited the proliferation of MC3T3-E1 cells by modulating the mRNA levels of fibroblast growth factor receptor 2 (FGFR2) and Rho-associated coiled-coil kinase 1 (ROCK1). Both FGFR2 and ROCK1 are predicted to be targets of miR-494-3p and harbor miR-494-3p binding sites within the 3′-UTRs [83]. FGFR2 is a tyrosine kinase receptor involved in cell proliferation and differentiation [84]. Activation of the RhoA/ROCK pathway stimulates osteogenic and chondrogenic differentiation of mesenchymal stem cells [85]. The inhibition may be due to a reduced sealing zone area, which is an osteoclast-specific cytoskeletal structure that contributes to osteoclast-mediated bone resorption [86]. Consequently, compressive force inhibits osteoblast proliferation by up-regulating miR-494-3p, which suppresses FGFR2 and ROCK1 expression. Expression of miR-29 in PDLCs is altered by compressive force, which affects the expression of several genes encoding major extracellular matrix (ECM) components negatively. In addition, Col1a1, Col3a1, and Col5a1 are direct targets of the miR-29 family in PDLCs [87]. Indeed, Col3 reportedly plays a role in trabecular bone formation and maintenance by modulating osteogenesis [88]. In conclusion, compression regulates alveolar bone formation and ECM homeostasis in periodontal ligament. Further studies should focus on identifying the mechanism involved.
Microgravity
The metabolism of osteoblasts is influenced by microgravity. Human mesenchymal stem cell (hMSC) differentiation into osteoblasts was suppressed in a ground-based, simulated microgravity environment, as indicated by the lack of expression of ALP, collagen 1, and osteonectin [89]. However, the function of miRNAs in microgravity-induced bone formation is unclear. miR-132-3p inhibits osteoblast differentiation and participates in the regulation of bone loss induced by simulated microgravity by targetting the gene encoding E1A-binding protein p300, a histone acetyltransferase important for the activity and stability of RUNX2 [90]. Moreover, miR-33-5p partially attenuates the microgravity-induced inhibition of MC3T3-E1 differentiation by targetting Hmga2; si targetting by miR-495 prevents osteoblast proliferation and promotes apoptosis [81,91]. miR-103 inhibits osteoblast proliferation by suppressing Cav1.2 expression in simulated microgravity [92,93].
Discussion
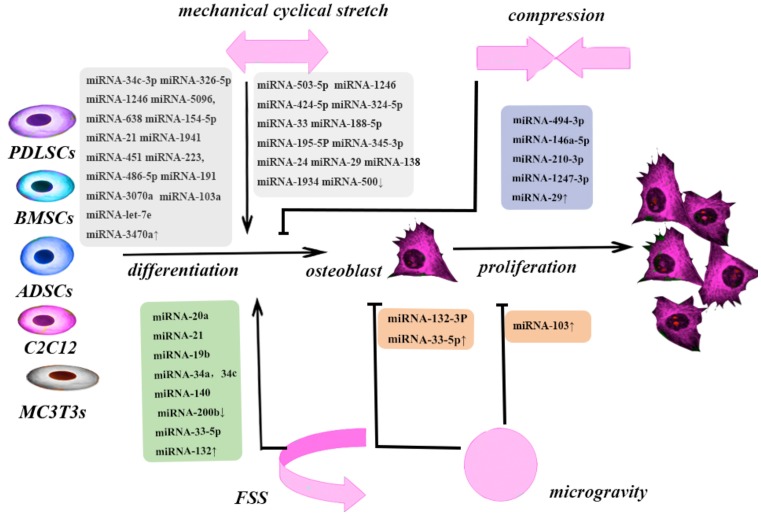
The relationship between mechanical force and miRNAs plays an important role in bone remodeling (Figure 1 and Table 1). The regulatory effects of miRNAs depend on a complex molecular network (Figure 2). miRNA microarrays are the most frequently used methods of detecting changes in miRNA expression. A mimic or inhibitor miRNA can be used to identify the target genes of candidate miRNAs. Dual-luciferase reporters may enable identification of combined sites of miRNAs on their target mRNAs. Wang et al. [81] assessed the relationship between miR33-5p and its target gene, Hmga2, by co-transfecting inhibitor-33 with siRNA-Hmga2. This resulted in partial blocking of the inhibitor-33-induced reduction in RUNX2, Osx, and ALP expression. Moreover, co-transfection of mimic-33 with pc-DNA3.1-Hmga2 or blank pcDNA3.1 also reduced the magnitude of the mimic-33-induced increases in RUNX2, Osx, and ALP expression [81]. Most studies on the effects of mechanical forces did not assess the effects of the intensity of the force applied. In animal studies, miR-21 responded to orthodontic force in periodontal tissue in a dose- and time-dependent manner [5]. The levels of miR195-5p differed at 24, 48, and 72 h after application of tension force [65]. Therefore, the intensity of the mechanical force applied is also important. The optimum force can be different amongst types of stem cells. This could lead to clinical use of inhibitors of miRNAs.
Figure 1. Different mechanical forces play different roles in bone remodeling.
Table 1. Studies about the role of miRNA in different forces induced bone metabolism.
| Mechanical force | miRNAs (express change with mechanical force) | Sample cells | Functions | Author |
|---|---|---|---|---|
| Compression | miR-29↑ | PDLSCs and ABCs | Remodeling of alveolar bone (Col1a1, Col3a1, and Col5a1) | Chen [87] |
| Compression | miR-494-3p, miR-146a-5p, miR-210-3p, miR-1247-3p ↑ | MC3T3-E2 cells | Osteoblast proliferation | Iwawaki [83] |
| FSS | miR-20a miR-21 miR-19b miR-34a, 34c, miR-140 miR-200b↓ | MC3T3-E1 cells | Osteogenic differentiation | Mai [79] |
| FSS | miR-132↑ | PDLSCs | Osteoblast differentiation and proliferation | Qi [80] |
| FSS | miR-33-5p↑ | MC3T3-E1 cells | Osteoblast differentiation | Wang [81] |
| MCS | miR-34c-3p miR-326-5p ↑ miR-503-5p, miR-324-5p, miR-188-5p, miR-345-3p, miR-30a-5p, miR-29b-3p, miR-351-3p↓ | RBMSCs | Osteogenic differentiation and bone formation. | Liu [61] |
| MCS | miR-1246, miR-5096, miR-638, miR-663, miR-21, miR-4492, miR-4734↑miR-107, miR-423-5p and miR-3195↓ | PDLSCs | Osteoblast differentiation | Wei [119] |
| MCS | miR-103a↓ | hFOB 1.19 cells | Osteoblast differentiation and bone formation | Zuo [62] |
| MCS | miR-154-5p↑ | ADSC | Osteogenic differentiation of ADSCs | Li [66] |
| MCS | miR-21↑ | PDLSCs | Osteogenic differentiation | Wei [68] |
| MCS | miR-24↓ | HTM cell | Regulate the response to CMS | Luna [75] |
| MCS | miR-29↓ | PDLSCs and ABCs | Remodeling of alveolar bone (Col1a1, Col3a1, and Col5a1) | Chen [87] |
| MCS | miR-33↓ | SMCs | Cell proliferation | Huang [6] |
| MCS | miR-500, miR-1934, miR-31, miR-378, miR-331, and miR-5097↓ miR-1941 ↑ | C2C12 cells | Cell proliferation | Hua [71] |
| MCS | miR-195-5p, miR-424-5p, miR-1297, miR-3607-5p miR-145-5p, miR-4328, and miR-224-5p↓ | PDLSCs | Bone formation | Chang [120] |
| MCS | miR-195-5p | PDLSCs | Osteogenic differentiation | Chang [65] |
| MCS | miR-451, miR-223, miR-486-5p↑ miR-1246, miR-1260, miR-141↓ | PDLSCs | Periodontal tissue homeostasis | Stoecklin-Wasmer [121] |
| MCS | miR-191*, miR-3070a, miR-M1-2-3p miR-let-7e*, miR-3470a↑ miR-32, miR-33, miR-5110, and miR-5121↓ | MC3T3-E1 cells | Osteoblast differentiation | Guo [122] |
| MCS | miR-138↓ | hBMMSCs | Osteogenic differentiation | Wu [70] |
| Microgravity | miR-103↑ | MC3T3-E1 cells | Osteoblast proliferation | Sun [92] |
| Microgravity | miR-103↑ | MC3T3-E1 cells | Osteoblast proliferation | Sun [93] |
| Microgravity | miR-132-3P↑ | MC3T3-E1 cells | Osteoblast differentiation | Hu [90] |
| Microgravity | miR-33-5p↓ | MC3T3-E1 cells | Osteoblast differentiation | Wang [81] |
| Orthodontic force | miR-21↑ | PDLSCs of mice | Osteogenesis of human PDLSCs following OTM | Chen [5] |
Abbreviations: ABC, alveolar bone cell; hBMMSC, human marrow mesenchymal stem cell; HTM, human trabecular meshwork.
Figure 2. miRNAs mediate the process of bone remodeling as a post-transcriptional inhibitor.
Irrespective of the amount of force used, MC3T3-E1 and PDLSC cells are the most frequently used in vitro studies of the effects of mechanical forces due to their function in osteogenesis. However, whether this occurs in vivo is unclear. miR-21 expression in hPDLSE scraped from tooth’s roots is altered by application of orthodontic force for 1 month [5]. Rat models of OTM were used to assess the effects of stretching force on miR-503-5p and miR-195-5p expression [61,65]. Further in vivo studies are needed before miRNAs can be considered safe for clinical use.
Identifying the functions of miRNAs in the presence of mechanical force is problematic [94]. miRNAs play multiple roles in osteoblasts and osteoclasts in the presence of MCS, FSS, compressive force, and microgravity. miRNAs may regulate osteogenesis, which could be translated into novel therapeutic approaches for orthodontic conditions and bone fractures, as well as for systemic diseases, such as osteoporosis. miRNAs may be useful as diagnostic biomarkers and therapeutic agents [95]. The expression levels of miRNAs are significantly altered in fractured bone tissue; this is likely related to fracture healing. miR-196a-3, with its target gene fgf2, is associated with BMD [96]. miR-145 attenuates TNF-α-driven cartilage matrix degradation in osteoarthritis by suppressing mitogen-activated protein kinase kinase 4 (MKK4) expression [97]. miR-21, miR-22, and miR-30 regulate H2S production, which plays a role in bone formation as an osteoprotective factor [98]. In particular, miR-21 regulates osteoblastogenesis and osteoclastogenesis to prevent bone resorption [99]. miRNAs can modulate the activity of bone formation-related factors and signaling pathways (e.g. BMPs and the NF-κB, RUNX2, Osx, and WNT signaling pathways). This facilitates the effects of other factors (e.g. Rorβ) on bone metabolism and enables these factors to function as regulators for treatment of bone-defect diseases [100–102]. Although few studies have evaluated the efficacy of miRNAs in orthodontics, miRNAs and miRNA inhibitors can be used clinically to regulate the tooth movement rate. The amount of secretory miR-29 in gingival crevicular fluid was altered by orthodontic force, possibly due to the recruitment of osteoclasts [103]. This suggested the utility of adding specific miRNAs to gingival crevicular fluid during orthodontic treatment to change the tooth movement rate. Furthermore, use of exosomes as a tool for RNA transfer in therapeutic engineering has been reported [60], and exosomes from osteoclast precursors are involved in osteoclastogenesis [104]. Other biomaterials that mediated miRNA delivery, such as electrospun nanofiber scaffolds, chitosan, and CaP coated with PEGylated compounds, may be useful [105]. These technologies will lead to therapeutic applications of miRNAs as regulators of bone remodeling induced by orthodontic forces.
Mechanotransduction is critical for maintaining bone strength and quality under physiological conditions [106], and mechanical forces have been used to treat diseases involving bone loss. For instance, distraction osteogenesis is used to treat orthognathic and bone defects caused by trauma or a congenital cleft palate. Dentoalveolar distraction osteogenesis for canine retraction reportedly results in earlier tooth movement with minimal anchorage loss and a reduced treatment time compared with traditional distalization [107]; this has been confirmed in other studies [108–110]. Extracorporeal shockwave therapy is widely used in the treatment of bone-healing disturbances, vascular bone diseases, femoral hip osteonecrosis, and bone resorption in periodontal disease [111–114]. A previous study shed light on the relationship between mechanical force and bone metastasis in breast cancer [115]. Low intensity vibration (LIV) can increase bone density and can be used in the treatment of osteoporosis and fracture [116–117]. Moreover, mechanical loading is widely used to treat craniofacial deformities such as jaw discrepancies and cranial suture [118]. The above findings imply that modulating the expression levels of miRNAs could enhance the efficacy of mechanical force in the treatment of bone diseases
Conclusion
The various effects of mechanical forces in bone remodeling are mediated by miRNAs as post-transcriptional regulators. Although the underlying mechanisms are unclear, previous studies suggest the therapeutic potential of miRNAs [95–105]. The antisense oligonucleotides of some miRNAs, such as anti-miR-503, anti-miR-103, anti-miR-195, may be used to promote the expression of RUNX2 and enhance osteoblast differentiation on the stretch side during orthodontic treatment. Other miRNAs, such as miR-29, may be used to promote osteoclast recruitment following compressive force. However, the dose and the time dependence of the effects of miRNAs should be investigated prior to their clinical application. Optimum vehicles for the transfer of miRNAs are also needed. Although miRNA-based orthodontic treatment is not yet available, this review may facilitate the development of novel miRNA-based therapies for orthodontics and for treatment of bone diseases such as osteoporosis and fracture.
Abbreviations
- ACVR2B
activin receptor type IIB
- BMD
bone mineral density
- circRNA
circular RNA
- ECM
extracellular matrix
- FGF2
fibroblast growth factor 2
- FGFR2
fibroblast growth factor receptor 2
- FSS
fluid shear stress
- LIPUS
low-intensity pulsed ultrasound
- lncRNA
long non-coding RNA
- MCS
mechanical cyclical stretch
- MSC
marrow mesenchymal stem cells
- ncRNA
non-coding RNA
- NFAT
nuclear factor of activated T-cell
- OSX
Osterix
- OTM
orthodontic tooth movement
- PDL
periodontal ligament
- PDLSC
periodontal ligament stem cell
- RANKL
receptor activator of nuclear factor kappa-Β ligand
- ROCK1
Rho-associated coiled-coil kinase 1
- RUNX2
runt-related transcription factor 2
- snoRNA
small non-translated nucleolar RNA
- TRPV4
transient receptor potential cation channel subfamily V member 4
- TNF-α
tumor necrosis factor alpha
Funding
This work was supported by the National Natural Science Foundation of China [grant numbers 81670957, 81700938]; the Beijing Natural Science Foundation [grant number 7172239]; the National Natural Science Foundation of China (NSFC) [grant number 81679557 (to W.L.)]; the National Natural Science Foundation of China (NSFC) [grant number 81700938 (to Y.Z.)]; and the Beijing Municipal Natural Science Foundation [grant number 7172239 (to W.L.)]. The funders had no role in study design, data collection and analysis, decision to publish, or the preparation of the article.
Author contribution
Y.W. and Y.Z. conceived and designed the study strategy. L.J. was responsible for reference collection and data management. Y.W. wrote the manuscript and prepared the tables and figures. Y.Z. reviewed and edited the manuscript. W.L. and Y.Z. were responsible for study supervision. All the authors reviewed the manuscript.
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Kular J., Tickner J., Chim S. et al. (2012) An overview of the regulation of bone remodelling at the cellular level. Clin. Biochem. 45, 863–873 10.1016/j.clinbiochem.2012.03.021 [DOI] [PubMed] [Google Scholar]
- 2.Thompson W.R., Rubin C.T. and Rubin J. (2012) Mechanical regulation of signaling pathways in bone. Gene 503, 179–193 10.1016/j.gene.2012.04.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maiorano N. and Hindges R. (2012) Non-coding RNAs in retinal development. Int. J. Mol. Sci. 13, 558–578 10.3390/ijms13010558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartel D.P. (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297 10.1016/S0092-8674(04)00045-5 [DOI] [PubMed] [Google Scholar]
- 5.Chen N., Sui B., Hu C. et al. (2016) microRNA-21 contributes to orthodontic tooth movement. J. Dent. Res. 95, 1425–1433 10.1177/0022034516657043 [DOI] [PubMed] [Google Scholar]
- 6.Huang G., Kang Y., Huang Z. et al. (2017) Identification and characterization of long non-coding RNAs in osteogenic differentiation of human adipose-derived stem cells. Cell. Physiol. Biochem. 42, 1037–1050 10.1159/000478751 [DOI] [PubMed] [Google Scholar]
- 7.Hao L., Fu J., Tian Y. et al. (2017) Systematic analysis of lncRNAs, miRNAs and mRNAs for the identification of biomarkers for osteoporosis in the mandible of ovariectomized mice. Int. J. Mol. Med. 40, 689–702 10.3892/ijmm.2017.3062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liao J., Yu X., Hu X. et al. (2017) lncRNA H19 mediates BMP9-induced osteogenic differentiation of mesenchymal stem cells (MSCs) through Notch signaling. Oncotarget 8, 53581–53601 10.18632/oncotarget.18655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khor E., Fanshawe B., Qi Y. et al. (2016) Prader-Willi critical region, a non-translated, imprinted central regulator of bone mass: possible role in skeletal abnormalities in Prader-Willi syndrome. PLoS ONE 11, e0148155 10.1371/journal.pone.0148155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li X., Peng B., Zhu X. et al. (2017) Changes in related circular RNAs following ERβ knockdown and the relationship to rBMSC osteogenesis. Biochem. Biophys. Res. Commun. 493, 100–107 10.1016/j.bbrc.2017.09.068 [DOI] [PubMed] [Google Scholar]
- 11.Zheng Y., Li X., Huang Y. et al. (2017) The circular RNA landscape of periodontal ligament stem cells during osteogenesis. J. Periodontol. 88, 906–914 10.1902/jop.2017.170078 [DOI] [PubMed] [Google Scholar]
- 12.Vlaikou A., Kouroupis D., Sgourou A. et al. (2017) Mechanical stress affects methylation pattern of GNAS isoforms and osteogenic differentiation of hAT-MSCs. Biochim. Biophys. Acta 1864, 1371–1381 10.1016/j.bbamcr.2017.05.005 [DOI] [PubMed] [Google Scholar]
- 13.Wang D., Wang H., Gao F. et al. (2017) ClC-3 promotes osteogenic differentiation in MC3T3-E1 cell after dynamic compression. J. Cell. Biochem. 118, 1606–1613 10.1002/jcb.25823 [DOI] [PubMed] [Google Scholar]
- 14.Shi G., Zheng X., Zhu C. et al. (2017) Evidence of the role of R-Spondin 1 and its receptor Lgr4 in the transmission of mechanical stimuli to biological signals for bone formation. Int. J. Mol. Sci. 18 10.3390/ijms18030564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liedert A., Kaspar D., Blakytny R. et al. (2006) Signal transduction pathways involved in mechanotransduction in bone cells. Biochem. Biophys. Res. Commun. 349, 1–5 10.1016/j.bbrc.2006.07.214 [DOI] [PubMed] [Google Scholar]
- 16.Killion C., Mitchell E., Duke C. et al. (2017) Mechanical loading regulates organization of the actin cytoskeleton and column formation in postnatal growth plate. Mol. Biol. Cell 28, 1862–1870 10.1091/mbc.e17-02-0084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim J., Kang M., Eltohamy M. et al. (2016) Dynamic mechanical and nanofibrous topological combinatory cues designed for periodontal ligament engineering. PLoS ONE 11, e0149967 10.1371/journal.pone.0149967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang S., Wei F., Hu L. et al. (2016) PERK-eIF2α-ATF4 pathway mediated by endoplasmic reticulum stress response is involved in osteodifferentiation of human periodontal ligament cells under cyclic mechanical force. Cell. Signal. 28, 880–886 10.1016/j.cellsig.2016.04.003 [DOI] [PubMed] [Google Scholar]
- 19.Wang J., Wang C., Zhang N. et al. (2016) Mechanical stimulation orchestrates the osteogenic differentiation of human bone marrow stromal cells by regulating HDAC1. Cell Death Dis. 7, e2221 10.1038/cddis.2016.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao J., Fu S., Zeng Z. et al. (2016) Cyclic stretch promotes osteogenesis-related gene expression in osteoblast-like cells through a cofilin-associated mechanism. Mol. Med. Rep. 14, 218–224 10.3892/mmr.2016.5239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng Z., Yin X., Zhang X. et al. (2015) Cyclic stretch enhances bone morphogenetic protein-2-induced osteoblastic differentiation through the inhibition of Hey1. Int. J. Mol. Med. 36, 1273–1281 10.3892/ijmm.2015.2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bo Z., Bin G., Jing W. et al. (2016) Fluid shear stress promotes osteoblast proliferation via the Gαq-ERK5 signaling pathway. Connect. Tissue Res. 57, 299–306 10.1080/03008207.2016.1181063 [DOI] [PubMed] [Google Scholar]
- 23.Hu K., Sun H., Gui B. et al. (2017) TRPV4 functions in flow shear stress induced early osteogenic differentiation of human bone marrow mesenchymal stem cells. Biomed. Pharmacother. 91, 841–848 10.1016/j.biopha.2017.04.094 [DOI] [PubMed] [Google Scholar]
- 24.Zhao L., Fan C., Zhang Y. et al. (2016) Adiponectin enhances bone marrow mesenchymal stem cell resistance to flow shear stress through AMP-activated protein kinase signaling. Sci. Rep. 6, 28752 10.1038/srep28752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y., Wang J., Xing J. et al. (2016) Surface chemistry regulates the sensitivity and tolerability of osteoblasts to various magnitudes of fluid shear stress. J. Biomed. Mater. Res. A 104, 2978–2991 10.1002/jbm.a.35848 [DOI] [PubMed] [Google Scholar]
- 26.Li P., Hu M., Sun S. et al. (2012) Fluid flow-induced calcium response in early or late differentiated osteoclasts. Ann. Biomed. Eng. 40, 1874–1883 10.1007/s10439-012-0554-z [DOI] [PubMed] [Google Scholar]
- 27.Li P., Liu C., Hu M. et al. (2014) Fluid flow-induced calcium response in osteoclasts: signaling pathways. Ann. Biomed. Eng. 42, 1250–1260 10.1007/s10439-014-0984-x [DOI] [PubMed] [Google Scholar]
- 28.Li M., Yi J., Yang Y. et al. (2016) Compression and hypoxia play independent roles while having combinative effects in the osteoclastogenesis induced by periodontal ligament cells. Angle Orthod. 86, 66–73 10.2319/121414.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu B., Kou X., Zhang C. et al. (2017) Stretch force guides finger-like pattern of bone formation in suture. PLoS ONE 12, e0177159 10.1371/journal.pone.0177159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kook S., Jang Y. and Lee J. (2011) Human periodontal ligament fibroblasts stimulate osteoclastogenesis in response to compression force through TNF-α-mediated activation of CD4+ T cells. J. Cell. Biochem. 112, 2891–2901 10.1002/jcb.23205 [DOI] [PubMed] [Google Scholar]
- 31.Hou J., Chen Y., Meng X. et al. (2014) Compressive force regulates ephrinB2 and EphB4 in osteoblasts and osteoclasts contributing to alveolar bone resorption during experimental tooth movement. Korean J. Orthod. 44, 320–329 10.4041/kjod.2014.44.6.320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayakawa T., Yoshimura Y., Kikuiri T. et al. (2015) Optimal compressive force accelerates osteoclastogenesis in RAW264.7 cells. Mol. Med. Rep. 12, 5879–5885 10.3892/mmr.2015.4141 [DOI] [PubMed] [Google Scholar]
- 33.Yi J., Yan B., Li M. et al. (2016) Caffeine may enhance orthodontic tooth movement through increasing osteoclastogenesis induced by periodontal ligament cells under compression. Arch. Oral Biol. 64, 51–60 10.1016/j.archoralbio.2015.12.009 [DOI] [PubMed] [Google Scholar]
- 34.Baskan O., Mese G. and Ozcivici E. (2017) Low-intensity vibrations normalize adipogenesis-induced morphological and molecular changes of adult mesenchymal stem cells. Proc. Inst. Mech. Eng. H 231, 160–168 10.1177/0954411916687338 [DOI] [PubMed] [Google Scholar]
- 35.Rajaei Jafarabadi M., Rouhi G., Kaka G. et al. (2016) The effects of photobiomodulation and low-amplitude high-frequency vibration on bone healing process: a comparative study. Lasers Med. Sci. 31, 1827–1836 10.1007/s10103-016-2058-9 [DOI] [PubMed] [Google Scholar]
- 36.Uddin S. and Qin Y. (2015) Dynamic acoustic radiation force retains bone structural and mechanical integrity in a functional disuse osteopenia model. Bone 75, 8–17 10.1016/j.bone.2015.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y., Chai Z., Zhang Y. et al. (2014) Influence of low-intensity pulsed ultrasound on osteogenic tissue regeneration in a periodontal injury model: X-ray image alterations assessed by micro-computed tomography. Ultrasonics 54, 1581–1584 10.1016/j.ultras.2014.03.015 [DOI] [PubMed] [Google Scholar]
- 38.Chan Y., Hsu K., Kuo C. et al. (2010) Using low-intensity pulsed ultrasound to improve muscle healing after laceration injury: an in vitro and in vivo study. Ultrasound Med. Biol. 36, 743–751 10.1016/j.ultrasmedbio.2010.02.010 [DOI] [PubMed] [Google Scholar]
- 39.Veronick J., Assanah F., Piscopo N. et al. (2017) Mechanically loading cell/hydrogel constructs with low-intensity pulsed ultrasound for bone repair. Tissue Eng. Part A 24, 254–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Majidinia M. and Yousefi B. (2016) DNA damage response regulation by microRNAs as a therapeutic target in cancer. DNA Repair (Amst.) 47, 1–11 10.1016/j.dnarep.2016.09.003 [DOI] [PubMed] [Google Scholar]
- 41.Barwari T., Joshi A. and Mayr M. (2016) MicroRNAs in cardiovascular disease. J. Am. Coll. Cardiol. 68, 2577–2584 10.1016/j.jacc.2016.09.945 [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez A., Griffiths-Jones S., Ashurst J.L. et al. (2004) Identification of mammalian microRNA host genes and transcription units. Genome Res. 14, 1902–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Majeed W., Iftikhar A., Khaliq T. et al. (2016) Gastric carcinoma: recent trends in diagnostic biomarkers and molecular targeted therapies. Asian Pac. J. Cancer Prev. 17, 3053–3060 [PubMed] [Google Scholar]
- 44.Barreiro E. (2016) The role of MicroRNAs in COPD muscle dysfunction and mass loss: implications on the clinic. Expert Rev. Respir. Med. 10, 1011–1022 10.1080/17476348.2016.1206819 [DOI] [PubMed] [Google Scholar]
- 45.Nugent M. (2016) MicroRNAs: exploring new horizons in osteoarthritis. Osteoarthr. Cartil. 24, 573–580 10.1016/j.joca.2015.10.018 [DOI] [PubMed] [Google Scholar]
- 46.Wang J., Zhang Y., Zhang N. et al. (2015) An updated review of mechanotransduction in skin disorders: transcriptional regulators, ion channels, and microRNAs. Cell. Mol. Life Sci. 72, 2091–2106 10.1007/s00018-015-1853-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu W., Liu Y., Guo T. et al. (2013) TCF3, a novel positive regulator of osteogenesis, plays a crucial role in miR-17 modulating the diverse effect of canonical Wnt signaling in different microenvironments. Cell Death Dis. 4, e539 10.1038/cddis.2013.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Du K., Li Z., Fang X. et al. (2017) Ferulic acid promotes osteogenesis of bone marrow-derived mesenchymal stem cells by inhibiting microRNA-340 to induce β-catenin expression through hypoxia. Eur. J. Cell Biol. 96, 496–503 10.1016/j.ejcb.2017.07.002 [DOI] [PubMed] [Google Scholar]
- 49.Long H., Sun B., Cheng L. et al. (2017) miR-139-5p represses BMSC osteogenesis via targeting Wnt/β-catenin signaling pathway. DNA Cell Biol. 36, 715–724 10.1089/dna.2017.3657 [DOI] [PubMed] [Google Scholar]
- 50.Tang X., Lin J., Wang G. et al. (2017) MicroRNA-433-3p promotes osteoblast differentiation through targeting DKK1 expression. PLoS ONE 12, e0179860 10.1371/journal.pone.0179860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li X., Guo L., Liu Y. (2017) MicroRNA-21 promotes osteogenesis of bone marrow mesenchymal stem cells via the Smad7-Smad1/5/8-Runx2 pathway. Biochem. Biophys. Res. Commun. 493, 928–933 10.1016/j.bbrc.2017.09.119 [DOI] [PubMed] [Google Scholar]
- 52.Xu R., Zhao M., Yang Y. et al. (2017) MicroRNA-449c-5p inhibits osteogenic differentiation of human VICs through Smad4-mediated pathway. Sci. Rep. 7, 8740 10.1038/s41598-017-09390-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gu Y., Ma L., Song L. et al. (2017) miR-155 inhibits mouse osteoblast differentiation by suppressing SMAD5 expression. Biomed Res. Int. 2017, 1893520 10.1155/2017/1893520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu D., Gao Y., Hu N. et al. (2017) miR-365 ameliorates dexamethasone-induced suppression of osteogenesis in MC3T3-E1 cells by targeting HDAC4. Int. J. Mol. Sci. 18, 977, 10.3390/ijms18050977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yin J., Zhuang G., Zhu Y. et al. (2017) MiR-615-3p inhibits the osteogenic differentiation of human lumbar ligamentum flavum cells via suppression of osteogenic regulators GDF5 and FOXO1. Cell Biol. Int. 41, 779–786 10.1002/cbin.10780 [DOI] [PubMed] [Google Scholar]
- 56.Wang C., Liao H. and Cao Z. (2016) Role of osterix and microRNAs in bone formation and tooth development. Med. Sci. Monit. 22, 2934–2942 10.12659/MSM.896742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jing D., Hao J., Shen Y. et al. (2015) The role of microRNAs in bone remodeling. Int. J. Oral Sci. 7, 131–143 10.1038/ijos.2015.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun M., Zhou X., Chen L. et al. (2016) The regulatory roles of microRNAs in bone remodeling and perspectives as biomarkers in osteoporosis. Biomed. Res. Int. 2016, 1652417 10.1155/2016/1652417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taipaleenmäki H. (2018) Regulation of bone metabolism by microRNAs. Curr. Osteoporos. Rep. 16, 1–12 10.1007/s11914-018-0417-0 [DOI] [PubMed] [Google Scholar]
- 60.Holliday L., McHugh K., Zuo J. et al. (2017) Exosomes: novel regulators of bone remodelling and potential therapeutic agents for orthodontics. Orthod. Craniofac. Res. 20 (Suppl. 1), 95–99 10.1111/ocr.12165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu L., Liu M., Li R. et al. (2017) MicroRNA-503-5p inhibits stretch-induced osteogenic differentiation and bone formation. Cell Biol. Int. 41, 112–123 10.1002/cbin.10704 [DOI] [PubMed] [Google Scholar]
- 62.Zuo B., Zhu J., Li J. et al. (2015) microRNA-103a functions as a mechanosensitive microRNA to inhibit bone formation through targeting Runx2. J. Bone Miner. Res. 30, 330–345 10.1002/jbmr.2352 [DOI] [PubMed] [Google Scholar]
- 63.Liao J., Zhou X., Zhang Y. et al. (2012) MiR-1246: a new link of the p53 family with cancer and Down syndrome. Cell Cycle 11, 2624–2630 10.4161/cc.20809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cui Y., Zhao X., Mei L. et al. (2018) Osteon myospalacem baileyi attenuates osteoclast differentiation through RANKL induced NFAT pathways. J. Ethnopharmacol., 213, 65–71, 10.1016/j.jep.2017.10.007 [DOI] [PubMed] [Google Scholar]
- 65.Chang M., Lin H., Fu H. et al. (2017) MicroRNA-195-5p regulates osteogenic differentiation of periodontal ligament cells under mechanical loading. J. Cell. Physiol. 232, 3762–3774 10.1002/jcp.25856 [DOI] [PubMed] [Google Scholar]
- 66.Li J., Hu C., Han L. et al. (2015) MiR-154-5p regulates osteogenic differentiation of adipose-derived mesenchymal stem cells under tensile stress through the Wnt/PCP pathway by targeting Wnt11. Bone 78, 130–141 10.1016/j.bone.2015.05.003 [DOI] [PubMed] [Google Scholar]
- 67.Zhao C., Li Y., Wang X. et al. (2017) The effect of uniaxial mechanical stretch on Wnt/β-catenin pathway in bone mesenchymal stem cells. J. Craniofac. Surg. 28, 113–117 10.1097/SCS.0000000000003252 [DOI] [PubMed] [Google Scholar]
- 68.Wei F., Liu D., Feng C. et al. (2015) microRNA-21 mediates stretch-induced osteogenic differentiation in human periodontal ligament stem cells. Stem Cells Dev. 24, 312–319 10.1089/scd.2014.0191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goh B., Singhal V., Herrera A. et al. (2017) Activin receptor type 2A (ACVR2A) functions directly in osteoblasts as a negative regulator of bone mass. J. Biol. Chem. 292, 13809–13822 10.1074/jbc.M117.782128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu J., Zhao J., Sun L. et al. (2017) Long non-coding RNA H19 mediates mechanical tension-induced osteogenesis of bone marrow mesenchymal stem cells via FAK by sponging miR-138. Bone 108, 62–70 10.1016/j.bone.2017.12.013 [DOI] [PubMed] [Google Scholar]
- 71.Hua W., Zhang M., Wang Y. et al. (2016) Mechanical stretch regulates microRNA expression profile via NF-κB activation in C2C12 myoblasts. Mol. Med. Rep. 14, 5084–5092 10.3892/mmr.2016.5907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang K., Bao H., Yan Z. et al. (2017) MicroRNA-33 protects against neointimal hyperplasia induced by arterial mechanical stretch in the grafted vein. Cardiovasc. Res. 113, 488–497 [DOI] [PubMed] [Google Scholar]
- 73.Kokabu S., Gamer L., Cox K. et al. (2012) BMP3 suppresses osteoblast differentiation of bone marrow stromal cells via interaction with Acvr2b. Mol. Endocrinol. 26, 87–94 10.1210/me.2011-1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Matzelle M., Shaw A., Baum R. et al. (2016) Inflammation in arthritis induces expression of BMP3, an inhibitor of bone formation. Scand. J. Rheumatol. 45, 379–383 10.3109/03009742.2015.1126347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Luna C., Li G., Qiu J. et al. (2011) MicroRNA-24 regulates the processing of latent TGFβ1 during cyclic mechanical stress in human trabecular meshwork cells through direct targeting of FURIN. J. Cell. Physiol. 226, 1407–1414 10.1002/jcp.22476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li Y., Jie L., Tian A. et al. (2017) Transforming growth factor beta is regulated by a glucocorticoid-dependent mechanism in denervation mouse bone. Sci. Rep. 7, 9925 10.1038/s41598-017-09793-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Faraji A., Abtahi S., Ghaderi A. et al. (2016) Transforming growth factor β1 (TGF-β1) in the sera of postmenopausal osteoporotic females. Int. J. Endocrinol. Metab. 14, e36511 10.5812/ijem.36511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Papachroni K., Karatzas D., Papavassiliou K. et al. (2009) Mechanotransduction in osteoblast regulation and bone disease. Trends Mol. Med. 15, 208–216 10.1016/j.molmed.2009.03.001 [DOI] [PubMed] [Google Scholar]
- 79.Mai Z., Peng Z., Zhang J. et al. (2013) miRNA expression profile during fluid shear stress-induced osteogenic differentiation in MC3T3-E1 cells. Chin. Med. J. 126, 1544–1550 [PubMed] [Google Scholar]
- 80.Qi L. and Zhang Y. (2014) The microRNA 132 regulates fluid shear stress-induced differentiation in periodontal ligament cells through mTOR signaling pathway. Cell. Physiol. Biochem. 33, 433–445 10.1159/000358624 [DOI] [PubMed] [Google Scholar]
- 81.Wang H., Sun Z., Wang Y. et al. (2016) miR-33-5p, a novel mechano-sensitive microRNA promotes osteoblast differentiation by targeting Hmga2. Sci. Rep. 6, 23170 10.1038/srep23170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kalomoiris S., Cicchetto A., Lakatos K. et al. (2016) Fibroblast growth factor 2 regulates high mobility group A2 expression in human bone marrow-derived mesenchymal stem cells. J. Cell. Biochem. 117, 2128–2137 10.1002/jcb.25519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Iwawaki Y., Mizusawa N., Iwata T. et al. (2015) MiR-494-3p induced by compressive force inhibits cell proliferation in MC3T3-E1 cells. J. Biosci. Bioeng. 120, 456–462 10.1016/j.jbiosc.2015.02.006 [DOI] [PubMed] [Google Scholar]
- 84.Hong L., Han Y., Liu J. et al. (2013) Fibroblast growth factor receptor 2: a therapeutic target in gastric cancer. Expert Rev. Gastroenterol. Hepatol. 7, 759–765 10.1586/17474124.2013.837804 [DOI] [PubMed] [Google Scholar]
- 85.Strzelecka-Kiliszek A., Mebarek S., Roszkowska M. et al. (2017) Functions of Rho family of small GTPases and Rho-associated coiled-coil kinases in bone cells during differentiation and mineralization. Biochim. Biophys. Acta 1861, 1009–1023 10.1016/j.bbagen.2017.02.005 [DOI] [PubMed] [Google Scholar]
- 86.Song R., Fullerton D., Ao L. et al. (2017) An epigenetic regulatory loop controls pro-osteogenic activation by TGF-β1 or bone morphogenetic protein 2 in human aortic valve interstitial cells. J. Biol. Chem. 292, 8657–8666 10.1074/jbc.M117.783308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen Y., Mohammed A., Oubaidin M. et al. (2015) Cyclic stretch and compression forces alter microRNA-29 expression of human periodontal ligament cells. Gene 566, 13–17 10.1016/j.gene.2015.03.055 [DOI] [PubMed] [Google Scholar]
- 88.Volk S., Shah S., Cohen A. et al. (2014) Type III collagen regulates osteoblastogenesis and the quantity of trabecular bone. Calcif. Tissue Int. 94, 621–631 10.1007/s00223-014-9843-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zayzafoon M., Gathings W. and McDonald J. (2004) Modeled microgravity inhibits osteogenic differentiation of human mesenchymal stem cells and increases adipogenesis. Endocrinology 145, 2421–2432 10.1210/en.2003-1156 [DOI] [PubMed] [Google Scholar]
- 90.Hu Z., Wang Y., Sun Z. et al. (2015) miRNA-132-3p inhibits osteoblast differentiation by targeting Ep300 in simulated microgravity. Sci. Rep. 5, 18655 10.1038/srep18655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tian Z., Zhou H., Xu Y. et al. (2017) MicroRNA-495 inhibits new bone regeneration via targeting high mobility group AT-Hook 2 (HMGA2). Med. Sci. Monit. 23, 4689–4698 10.12659/MSM.904404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sun Z., Cao X., Hu Z. et al. (2015) MiR-103 inhibits osteoblast proliferation mainly through suppressing Cav1.2 expression in simulated microgravity. Bone 76, 121–128 10.1016/j.bone.2015.04.006 [DOI] [PubMed] [Google Scholar]
- 93.Sun Z., Cao X., Zhang Z. et al. (2015) Simulated microgravity inhibits L-type calcium channel currents partially by the up-regulation of miR-103 in MC3T3-E1 osteoblasts. Sci. Rep. 5, 8077 10.1038/srep08077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wei F., Yang S. and Wang S., (2018) MicroRNAs: a critical regulator under mechanical force. Histol. Histopathol. 33, 335–342, 10.14670/HH-11-924 [DOI] [PubMed] [Google Scholar]
- 95.Nugent M. (2017) MicroRNAs and fracture healing. Calcif. Tissue Int. 101, 355–361, 10.1007/s00223-017-0296-x [DOI] [PubMed] [Google Scholar]
- 96.Zhu D., Guo Y., Zhang Y. et al. (2017) A functional SNP regulated by miR-196a-3p in the 3′UTR of FGF2 is associated with bone mineral density in the Chinese population. Hum. Mutat. 38, 725–735 10.1002/humu.23216 [DOI] [PubMed] [Google Scholar]
- 97.Hu G., Zhao X., Wang C. et al. (2017) MicroRNA-145 attenuates TNF-α-driven cartilage matrix degradation in osteoarthritis via direct suppression of MKK4. Cell Death Dis. 8, e3140 10.1038/cddis.2017.522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhai Y., Tyagi S. and Tyagi N. (2017) Cross-talk of MicroRNA and hydrogen sulfide: a novel therapeutic approach for bone diseases. Biomed. Pharmacother. 92, 1073–1084 10.1016/j.biopha.2017.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hu C., Sui B., Du F. et al. (2017) miR-21 deficiency inhibits osteoclast function and prevents bone loss in mice. Sci. Rep. 7, 43191 10.1038/srep43191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Goldring S.R. and Goldring M.B. (2007) Eating bone or adding it: the Wnt pathway decides. Nat. Med. 13, 133–134 10.1038/nm0207-133 [DOI] [PubMed] [Google Scholar]
- 101.Hess K., Ushmorov A., Fiedler J. et al. (2009) TNFalpha promotes osteogenic differentiation of human mesenchymal stem cells by triggering the NF-kappaB signaling pathway. Bone 45, 367–376 10.1016/j.bone.2009.04.252 [DOI] [PubMed] [Google Scholar]
- 102.Farr J., Weivoda M., Nicks K. et al. (2018) Osteoprotection through the deletion of the transcription factor Rorβ in mice. J. Bone Miner. Res., 33, 720–731, 10.1002/jbmr.3351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Atsawasuwan P., Lazari P., Chen Y. et al. (2018) Secretory microRNA-29 expression in gingival crevicular fluid during orthodontic tooth movement. PLoS ONE 13, e0194238 10.1371/journal.pone.0194238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huynh N., VonMoss L., Smith D. et al. (2016) Characterization of regulatory extracellular vesicles from osteoclasts. J. Dent. Res. 95, 673–679 10.1177/0022034516633189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Scimeca J. and Verron E. (2017) The multiple therapeutic applications of miRNAs for bone regenerative medicine. Drug Discov. Today 22, 1084–1091 10.1016/j.drudis.2017.04.007 [DOI] [PubMed] [Google Scholar]
- 106.Magrey M. and Khan M. (2017) The paradox of bone formation and bone loss in ankylosing spondylitis: evolving new concepts of bone formation and future trends in management. Curr. Rheumatol. Rep. 19, 17 10.1007/s11926-017-0644-x [DOI] [PubMed] [Google Scholar]
- 107.Kurt G., İşeri H., Kişnişçi R. et al. (2017) Rate of tooth movement and dentoskeletal effects of rapid canine retraction by dentoalveolar distraction osteogenesis: a prospective study. Am. J. Orthod. Dentofacial Orthop. 152, 204–213 10.1016/j.ajodo.2016.12.019 [DOI] [PubMed] [Google Scholar]
- 108.Sayin S., Bengi A., Gürton A. et al. (2004) Rapid canine distalization using distraction of the periodontal ligament: a preliminary clinical validation of the original technique. Angle Orthod. 74, 304–315 [DOI] [PubMed] [Google Scholar]
- 109.Işeri H., Kişnişci R., Bzizi N. et al. (2005) Rapid canine retraction and orthodontic treatment with dentoalveolar distraction osteogenesis. Am. J. Orthod. Dentofacial Orthop. 127, 533–541, 10.1016/j.ajodo.2004.01.022 [DOI] [PubMed] [Google Scholar]
- 110.Kharkar V. and Kotrashetti S. (2010) Transport dentoalveolar distraction osteogenesis-assisted rapid orthodontic canine retraction. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 109, 687–693 10.1016/j.tripleo.2009.10.014 [DOI] [PubMed] [Google Scholar]
- 111.d’Agostino M., Craig K., Tibalt E. et al. (2015) Shock wave as biological therapeutic tool: from mechanical stimulation to recovery and healing, through mechanotransduction. Int. J. Surg. 24, 147–153 10.1016/j.ijsu.2015.11.030 [DOI] [PubMed] [Google Scholar]
- 112.d’Agostino C., Romeo P., Lavanga V. et al. (2014) Effectiveness of extracorporeal shock wave therapy in bone marrow edema syndrome of the hip. Rheumatol. Int. 34, 1513–1518 10.1007/s00296-014-2991-5 [DOI] [PubMed] [Google Scholar]
- 113.Romeo P., Lavanga V., Pagani D. et al. (2014) Extracorporeal shock wave therapy in musculoskeletal disorders: a review. Med. Princ. Pract. 23, 7–13 10.1159/000355472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang Q., Liu L., Sun W. et al. (2017) Extracorporeal shockwave therapy in osteonecrosis of femoral head: a systematic review of now available clinical evidences. Medicine (Baltimore) 96, e5897 10.1097/MD.0000000000005897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lynch M. and Fischbach C. (2014) Biomechanical forces in the skeleton and their relevance to bone metastasis: biology and engineering considerations. Adv. Drug Deliv. Rev. 79-80, 119–134 10.1016/j.addr.2014.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Uribe F., Dutra E. and Chandhoke T. (2017) Effect of cyclical forces on orthodontic tooth movement, from animals to humans. Orthod. Craniofac. Res. 20 (Suppl. 1), 68–71 10.1111/ocr.12166 [DOI] [PubMed] [Google Scholar]
- 117.Ozcivici E., Luu Y., Adler B. et al. (2010) Mechanical signals as anabolic agents in bone. Nat. Rev. Rheumatol. 6, 50–59 10.1038/nrrheum.2009.239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Karamesinis K. and Basdra E. (2018) The biological basis of treating jaw discrepancies: an interplay of mechanical forces and skeletal configuration. Biochim. Biophys. Acta 1864, 1675–1683 10.1016/j.bbadis.2018.02.007 [DOI] [PubMed] [Google Scholar]
- 119.Wei F., Wang J., Ding G. et al. (2014) Mechanical force-induced specific microRNA expression in human periodontal ligament stem cells. Cells Tissues Organs (Print) 199, 353–363 10.1159/000369613 [DOI] [PubMed] [Google Scholar]
- 120.Chang M., Lin H., Luo M. et al. (2015) Integrated miRNA and mRNA expression profiling of tension force-induced bone formation in periodontal ligament cells. In Vitro Cell. Dev. Biol. Anim. 51, 797–807 10.1007/s11626-015-9892-0 [DOI] [PubMed] [Google Scholar]
- 121.Stoecklin-Wasmer C., Guarnieri P., Celenti R. et al. (2012) MicroRNAs and their target genes in gingival tissues. J. Dent. Res. 91, 934–940 10.1177/0022034512456551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Guo Y., Wang Y., Liu Y. et al. (2015) MicroRNA-218, microRNA-191*, microRNA-3070a and microRNA-33 are responsive to mechanical strain exerted on osteoblastic cells. Mol. Med. Rep. 12, 3033–3038 10.3892/mmr.2015.3705 [DOI] [PubMed] [Google Scholar]