Abstract
Background
Conventional coronary artery disease risk factors might potentially explain at least 90% of the attributable risk of coronary artery disease. To better understand the association between the pre-industrial lifestyle and low prevalence of coronary artery disease risk factors, we examined the Tsimane, a Bolivian population living a subsistence lifestyle of hunting, gathering, fishing, and farming with few cardiovascular risk factors, but high infectious inflammatory burden.
Methods
We did a cross-sectional cohort study including all individuals who self-identified as Tsimane and who were aged 40 years or older. Coronary atherosclerosis was assessed by coronary artery calcium (CAC) scoring done with non-contrast CT in Tsimane adults. We assessed the difference between the Tsimane and 6814 participants from the Multi-Ethnic Study of Atherosclerosis (MESA). CAC scores higher than 100 were considered representative of significant atherosclerotic disease. Tsimane blood lipid and inflammatory biomarkers were obtained at the time of scanning, and in some patients, longitudinally.
Findings
Between July 2, 2014, and Sept 10, 2015, 705 individuals, who had data available for analysis, were included in this study. 596 (85%) of 705 Tsimane had no CAC, 89 (13%) had CAC scores of 1–100, and 20 (3%) had CAC scores higher than 100. For individuals older than age 75 years, 31 (65%) Tsimane presented with a CAC score of 0, and only four (8%) had CAC scores of 100 or more, a five-fold lower prevalence than industrialised populations (p≤0-0001 for all age categories of MESA). Mean LDL and HDL cholesterol concentrations were 2.35 mmol/L (91 mg/dL) and 1.0 mmol/L (39.5 mg/dL), respectively; obesity, hypertension, high blood sugar, and regular cigarette smoking were rare. High- sensitivity C-reactive protein was elevated beyond the clinical cutoff of 3.0 mg/dL in 360 (51%) Tsimane participants.
Interpretation
Despite a high infectious inflammatory burden, the Tsimane, a forager-horticulturalist population of the Bolivian Amazon with few coronary artery disease risk factors, have the lowest reported levels of coronary artery disease of any population recorded to date. These findings suggest that coronary atherosclerosis can be avoided in most people by achieving a lifetime with very low LDL, low blood pressure, low glucose, normal body-mass index, no smoking, and plenty of physical activity. The relative contributions of each are still to be determined.
Funding
National Institute on Aging, National Institutes of Health; St Luke's Hospital of Kansas City; and Paleocardiology Foundation.
Introduction
Large-scale population studies have identified conventional coronary artery disease risk factors as important to the clinical manifestation of coronary artery disease events, and might explain more than 90% of the attributable risk of coronary artery disease.1 These data are supported by findings that nearly 50% of coronary artery disease risk can be reduced on adoption of a heart-healthy lifestyle, even in individuals with a higher genetic susceptibility to coronary artery disease.2 Additionally, multiple primary prevention studies have conclusively shown that the control of risk factors including cholesterol, smoking, hypertension, and diabetes lead to significant, consistent, and dose-related reduction in major adverse cardiac events.3-6 Populations with a lower prevalence of risk factors such as those that preceded modern lifestyles in industrialised settings should naturally show less prevalence of coronary disease.
Previous research by the HORUS Study Team on atherosclerosis in a pre-modern era has focused on mummies, observing peripheral atherosclerosis to be common in multiple ancient populations.7,8 Atherosclerosis was confirmed by demonstration of calcific deposits in arterial beds. High inflammatory exposure from infection and presumptive abundance of risk factors was hypothesised to play a part in the pathogenesis of coronary artery disease in these populations.9 Conversely, contemporary pre-industrial populations have shown either lower prevalence of peripheral atherosclerosis,10,11 or coronary risk factors.12,13 On the basis of carotid and femoral ultrasound studies (Heart Attack Prevention Programme for You, HAPPY), atherosclerosis in a physically active, strictly vegetarian, non-smoking, and regularly meditating suburban population in India was encountered a decade and a half later than the comparable population in Texas, USA.10,11 Similarly, the Tsimane Health and Life History Project team (THLHP) has been working with the indigenous Tsimane of Bolivia, a population of 16 000 individuals that live a pre-industrial lifestyle of hunting, gathering, fishing, and farming along the Maniqui River, an Amazon River tributary. The indirect measures of vascular ageing indicate that the Tsimane have low rates of hypertension and arterial stiffness.12,13 Although the Tsimane have a low prevalence of coronary risk factors, they are similar to ancestral subsistence populations in their high infectious burden associated with high levels of biomarkers of inflammation.
To better understand the association between the preindustrial lifestyle and low prevalence of coronary artery disease risk factors, the HORUS Study Team and THLHP joined to collaboratively examine the hypothesis that an active subsistence lifestyle, resembling an era before sedentary urbanisation and large-scale economic specialisation, would be associated with low levels of manifest coronary artery disease, despite high inflammation from parasites and pathogens. To do so, we used direct coronary artery visualisation by CT for quantification of extent, severity, and location of coronary artery calcium (CAC) in 705 Tsimane adults. CAC scoring is a safe, non-invasive test commonly done by CT that enables quantification of CAC extent, severity and location; CAC scoring can now be accomplished with minimal radiation burden.14 An array of population-based and observational cohort registries have proved the prognostic usefulness of CAC scores beyond traditional coronary artery disease risk factors and non-coronary measures of peripheral atherosclerosis.15
Methods
Study design and participants
The THLHP has been working with the Tsimane population since 2002, and in 2011, expanded its coverage to 85 Tsimane villages, sampling individuals aged 40 years or older in a cohort-based panel design. This cross-sectional sample includes all individuals who met the inclusion criteria of self-identifying as Tsimane and who were aged 40 years or older (see appendix for age estimation methods). The THLHP makes regularly scheduled visits to Tsimane villages every 18 months, during which clinical examinations are performed and blood samples collected to examine within and cross population variations of the ageing process.12,13,16,17
Individuals ranged between 40 years and 94 years of age. We focused our efforts on individuals older than 60 years. Due to the pyramidal population structure, we used a random number generator to select communities in which to sample individuals aged 40–59 years. We elected to randomly sample at the community level rather than the individual level for reasons of cultural appropriateness, so specific individuals would not feel targeted or left out.
This study was approved by the University of New Mexico and University of California, Santa Barbara Human Subjects Review Committees. Written informed consent was obtained from participants, after having had the procedure and risks explained to them in their native language, from each village, and the Tsimane Government (Gran Consejo). The radiation dose was relatively low, and all the participants were mature adults older than 40 years.
Procedures
Individuals underwent CAC scanning by electrocardiogram (ECG)-gated CT imaging using a 16-detector row scanner (GE Brightspeed, Milwaukee, WI, USA) between July, 2014, and September, 2015. A licensed radiological technician acquired a single, ECG-gated scan (appendix). Scans were supervised and reviewed by at least one of the team cardiologists. A central core laboratory, whose members were masked to participant data, performed calcium scoring of the coronaries using semi-automatic software (GE SmartScore 4.0, Milwaukee, WI, USA) according to the method of Agatston and colleagues.18
Tsimane CAC scores were compared with published scores of population-based studies in high-income countries, including the US Multiethnic Study of Atherosclerosis (MESA),15 the German Heinz-Nixdorf Recall (HNR) study,19 and eleven other CT studies20-25 (appendix). Because of the variable reporting of age categories across published studies, we could not directly compare studies in Tsimane with other studies at the individual level, and instead assessed differences in CAC by age across broader age categories. Most of these studies excluded adults with coronary artery disease or symptoms at baseline, therefore providing lower-bound population estimates of CAC. Tsimane CAC protocols were similar to these studies.24 As published reports8,21-23 typically stratify CAC scores as 0 (almost no risk), 1–99 (low risk), 100–399 (moderate risk), and 400 or greater (high risk), we compared our findings with those published results. We used the same categories as those previously published, except that we combined the two highest categories since there was only one Tsimane individual with a CAC score of 400 or above.
Following fasting morning blood draws, blood testing included lipids, oxidised LDL cholesterol, apolipoprotein A and B, glucose, high sensitivity C-reactive protein (hs- CRP), erythrocyte sedimentation rate, five-part differential of white blood cells, and nine cytokines (appendix).
Statistical analysis
To assess the association between coronary artery disease risk factors and Tsimane CAC scores, we did a multivariate zero-inflated negative binomial regression;26 this approach is specifically designed for distributions with a large fraction of “zero” CAC scores and overdispersion, as are the Tsimane CT data. The model simultaneously performs a logistic regression to test for factors that inflate CAC absence, and a negative binomial regression to examine factors associated with CAC scores (including zeros and positive scores), while controlling for age and sex. Standard linear models perform poorly with such distributions, although a comparison using the standard approach shows that the zero-inflated negative binomial model explains more of the variance and fits the data better (appendix). Hs-CRP was log transformed for normality, and all statistics were run in Stata, version 14.2. All risk factors were tested for interaction with sex, and none of those interactions were significant. The model presented in this study is a post- hoc subset of variables that significantly predict attributable CAC risk; Akaike’s Information Criteria were used for model selection.
Role of the funding source
The funders of the study had no role in the study design, data collection, or data analysis, data interpretation, or writing of the report. HK, RCT, BCT, CEF, MG, and GST had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Between July 2, 2014, and Sept 10, 2015, 705 individuals were included in this study. Individuals older than 60 years who were not sampled had either recently migrated between communities after major flooding (n=49), did not wish to participate because they were hunting, working in their fields, or engaged in childcare (n=15), or refused to participate (n=2). Only one individual refused to participate because of poor health or inability to travel (recovering from a hernia surgery; figure 1). To address potential sources of bias, analyses comparing individuals who had a CT versus those who did not have a CT showed no significant differences in sex (p=0.634), systolic (p=0.301) or diastolic (p=0.301) blood pressure, or body fat (p=0.942); our sample is therefore representative of all individuals over the age of 40 years.
Figure 1.
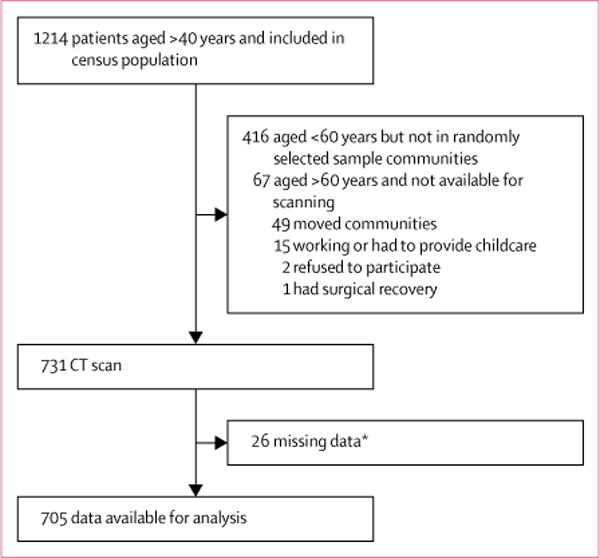
Study profile
*Data were missing mainly because of broken laboratory equipment, electricity issues, missing supplies, or absent or sick laboratory personnel; data were missing for blood biomarkers, cholesterol, body fat, and confidence in the validity of the age estimate.
There were several reasons for the extremely high participation rate. First, the long-term presence of the THLHP, combined with medical assistance, created an atmosphere of trust and collaboration. Second, individuals who had travelled to the city of Trinidad for CT scanning were given free access to medical specialists and treatment for any ailments they presented with. Third, all food, travel, and lodging were provided free of cost and administered by the THLHP team. Finally, we provided compensation for participants’ time, including subsistence tools, needles, and yarn. CT scanning showed incidental findings (appendix) in 72 people who were referred to specialists (92% to pulmonologists for Mycobacterium tuberculosis).
Baseline characteristics for the study population are listed in table 1. The mean age of the study population was 57.6 years (95% CI 41–92); 356 (50%) of 705 participants were women (table 1). Younger individuals had a higher weight, height, and body-mass index (BMI). Older individuals had higher systolic and diastolic blood pressures and higher heart rates.
Table 1.
Baseline characteristics by age
| 40–44 years (n=31) | 45–54 years (n=298) | 55–64 years (n=204) | 65–74 years (n=124) | 75+ years (n=48) | Total (n=705) | p value | |
|---|---|---|---|---|---|---|---|
| Anthropometry | |||||||
|
| |||||||
| Proportion of men (%) | 50 (0.5) | 50 (0.5) | 50 (0.5) | 50 (0.5) | 40 (0.5) | 50 (0.5) | 0.6301 |
| Mean weight (kg) | 58.8 (9.0) | 60.3 (9.0) | 58.5 (10.9) | 56.2 (9.5 | 52.1 (9.5) | 58.4 (9.9) | <0.0001 |
| Height (cm) | 157.4 (7.5) | 157.2 (7.0) | 154.9 (8.0) | 154.5 (8.3) | 151.2 (8.3) | 155.7 (7.8) | <0.0001 |
| Body.mass index (kg/m2) | 23.7 (3.0) | 24.4 (3.1) | 24.4 (4.1) | 23.5 (3.3) | 22.6 (2.8) | 24.1 (3.5) | 0.0220 |
| Body fat (%) | 21.8% (8.3) | 21.9% (8.2) | 22.8% (8.3) | 21.7% (8.4) | 21.7% (6.5) | 22.1% (8.2) | 0.6726 |
|
| |||||||
| Physiology | |||||||
|
| |||||||
| Systolic blood pressure (mm Hg) | 113.1 (12.0) | 114.8 (11.2) | 115.4 (11.5) | 120.2 (15.0) | 117.1 (15.6) | 116.0 (12.5) | 0.0007 |
| Diastolic blood pressure (mm Hg) | 76.4 (12.5) | 73.0 (9.1) | 73.4 (10.0) | 74.0 (10.8) | 73.1 (11.5) | 73.4 (10.0) | 0.0055 |
| Heart rate (beats per min) | 63.4 (9.2) | 64.3 (7.8) | 66.2 (9.5) | 68.5 (11.2) | 69.0 (9.8) | 65.9 (9.3) | <0.0001 |
|
| |||||||
| Lipids | |||||||
|
| |||||||
| Total cholesterol (mmol/L) | 4.0 (0.7) | 3.9 (0.7) | 4.0 (0.9) | 3.9 (0.8) | 3.6 (0.7) | 3.9 (0.8) | 0.0203 |
| LDL cholesterol (mmol/L) | 2.5 (0.6) | 2.4 (0.7) | 2.4 (0.8) | 2.3 (0.7) | 2.1 (0.7) | 2.4 (0.7) | 0.3200 |
| HDL cholesterol (mmol/L) | 1.0 (0.2) | 1.0 (0.2) | 1.0 (0.2) | 1.0 (0.2) | 1.0 (0.2) | 1.0 (0.2) | 0.4630 |
| Triglycerides (mmol/L) | 1.0 (0.5) | 1.1 (0.5) | 1.2 (0.6) | 1.2 (0.5) | 1.1 (0.4) | 1.2 (0.5) | 0.2780 |
| ApoA (mg/dL) | 90.0 (60.5) | 109.6 (77.8) | 141.1 (86.2) | 142.7 (87.8) | 140.7 (99.5) | 126.0 (84.9) | <0.0001 |
| ApoB (mg/dL) | 86.4 (24.0) | 86.9 (33.8) | 109.4 (47.0) | 104.1 (44.0) | 97.1 (44.7) | 97.2 (41.5) | <0.0001 |
| Oxidised LDL (U/L) | 82.9 (22.6) | 78.0 (21.8) | 74.8 (22.9) | 77.4 (23.4) | 70.4 (21.2) | 76.6 (22.4) | 0.0374 |
|
| |||||||
| Inflammatory markers | |||||||
|
| |||||||
| Leucocyte count (cells per µL) | 9642 (2222) | 9013(2307) | 9316 (2284) | 9280 (2681) | 9325 (2375) | 9199 (2368) | 0.4800 |
| Lymphocyte count (cells per µL) | 2673 (922) | 2357 (718) | 2497 (707) | 2343 (770) | 2415 (657) | 2414 (733) | 0.0690 |
| Eosinophil count (cells per µL) | 1648 (1101) | 1497 (1057) | 1228(884) | 1371 (946) | 1143 (870) | 1378 (987) | 0.0110 |
| Neutrophil count (cells per µL) | 5137 (1504) | 5066 (1740) | 5479 (1780) | 5495 (2125) | 5785 (1774) | 5315 (1826) | 0.0270 |
| Monocyte count (cells per µL) | 132 (135) | 93 (117) | 84 (102) | 68 (97) | 62 (61) | 86 (108) | 0.0170 |
| ESR (mm/h) | 22.2 (16.0) | 21.4 (13.3) | 21.1 (12.4) | 21.8 (12.5) | 29.8 (21.1) | 22.0 (13.9) | 0.0031 |
| hs.CRP (mg/L) | 3.2 (2.6) | 3.5 (3.0) | 3.8 (3.6) | 4.0 (3.0) | 4.0 (3.9) | 3.7 (3.2) | 0.6320 |
| Interleukin 5 (pg/mL) | 4.3 (12.2) | 2.5 (2.7) | 3.0 (6.1) | 2.6 (3.3) | 2.8 (2.2 | 2.8 (4.7) | 0.3500 |
| Interleukin 10 (pg/mL) | 5.4 (10.6) | 4.1 (3.8) | 5.2 (9.1) | 4.1 (3.5) | 5.1 (5.6) | 4.5 (6.3) | 0.3170 |
| Framingham risk score (10 years) | 0.01 (0.01) | 0.02 (0.02) | 0.04 (0.04) | 0.08 (0.05) | 0.12 (0.07) | 0.04 (0.05) | <0.0001 |
|
| |||||||
| Proportions above high-risk cutoffs | |||||||
|
| |||||||
| Body-mass index >30 kg/m2 | 0.03 (0.03) | 0.05 (0.01) | 0.10 (0.02) | 0.03 (0.02) | 0.02 (0.02) | 0.06 (0.01) | 0.0670 |
| Hypertensive* | 0.10 (0.05) | 0.04 (0.01) | 0.05 (0.02) | 0.06 (0.02) | 0.08 (0.04) | 0.05 (0.01) | 0.5306 |
| Total cholesterol >6.2 mmol/L | 0.00 (0.00) | 0.00 (0.00) | 0.01 (0.01) | 0.01 (0.01) | 0.00 (0.00) | 0.00 (0.00) | 0.4710 |
| LDL cholesterol >3.4 mmol/L | 0.11 (0.06) | 0.09 (0.02) | 0.10 (0.02) | 0.11 (0.03) | 0.02 (0.02) | 0.09 (0.01) | 0.4875 |
| Triglycerides >2.3 mmol/L | 0.03 (0.03) | 0.05 (0.01) | 0.05 (0.02) | 0.03 (0.02) | 0.00 (0.00) | 0.04 (0.01) | 0.6010 |
| HDL cholesterol <1.0 mmol/L | 0.61 (0.09) | 0.57 (0.03) | 0.55 (0.03) | 0.53 (0.04) | 0.57 (0.07) | 0.56 (0.02) | 0.9460 |
| Glucose >6.9 mmol/L | 0.00 (0.00) | 0.00 (0.00) | 0.01 (0.01) | 0.01 (0.01) | 0.00 (0.00) | 0.00 (0.00) | 0.6330 |
| Leucocytes >10 700 cells per µL | 0.32 (0.08) | 0.21 (0.02) | 0.24 (0.03) | 0.25 (0.04) | 0.22 (0.06) | 0.23 (0.02) | 0.5780 |
| Elevated ESR† | 0.30 (0.08) | 0.27 (0.03) | 0.25 (0.03) | 0.25 (0.04) | 0.41 (0.07) | 0.27 (0.02) | 0.3390 |
| hs.CRP >3.0 mg/L | 0.43 (0.09) | 0.48 (0.03) | 0.46 (0.03) | 0.55 (0.04) | 0.45 (0.07) | 0.48 (0.02) | 0.5100 |
Data are mean (SD) or proportion (SE), unless otherwise specified. ApoA=apolipoprotein A. ApoB=apolipoprotein B. ESR=erythrocyte sedimentation rate. hs.CRP=high sensitivity C.reactive protein. Conversion factors from mmol/L to mg/dL: for total cholesterol, LDL, and HDL, multiply mmol/L by 3867; for triglycerides, multiply mmol/L by 88.57; and for glucose, multiply mmol/L by l8.02.
Hypertension was defined as a systolic blood pressure of more than 140 mm Hg or a diastolic blood pressure of more than 90 mm Hg.
Elevated ESR was considered as higher than 22 mm/h for men and higher than 29 mm/h for women.
Prevalence of coronary artery disease risk factors, including blood pressure, and cholesterol and glucose concentrations, were low in all age groups (table 1). Prevalence of smoking was also low. Although 201 (28%) adults reported occasional smoking, the mean number of cigarettes smoked was roughly ten per month in those who smoked (ie, 0.5 pack years over a lifetime). No differences were observed across age groups for hypertension, hypercholesterolaemia, obesity, or diabetes. Hs-CRP concentrations of higher than 3.0 mg/L were deemed clinically elevated,27 and were reported in 360 (51%) of Tsimane participants; mean CRP was 3.7 mg/L (table 1). No differences were observed across age groups for the extent of inflammation as determined by erythrocyte sedimentation rate or hs- CRP.
We observed a very low prevalence of coronary atherosclerosis in Tsimane as measured by CAC scoring. 596 (85%) of 705 participants had no CAC, 89 (13%) participants had CAC scores lower than 100, and only 20 (3%) had CAC scores of 100 or higher (figures 2–4, appendix). Figure 2 compares the age-specific prevalence of Tsimane and the US MESA sample with either no CAC or CAC scores of more than 100. Compared with MESA, a 24-year lag is observed before Tsimane reach a CAC score of more than 0, and a 28-year lag before Tsimane reach a CAC score of 100 or higher. Figure 3 shows that the rate of progression of atherosclerosis to coronary artery disease is much slower among the Tsimane than in the MESA cohort, and even Tsimane men show a much flatter progression with age than MESA women (of note, the scale for MESA men [0-4800] is three times the scale for MESA women and for Tsimane men and women [0-1600]). These findings extended to later life where, after age 75 years, 31 (65%) Tsimane were free from CAC and only four (8%) had moderately increased CAC scores. These findings of apparent athero-protection experienced by the Tsimane extended to both sexes. CAC scores are normally two to four times higher in men than in women.15,20-23 Although Tsimane men had higher CAC scores than Tsimane women, a finding more prominent in the older population, Tsimane men had lower CAC scores than Japanese women, a population previously regarded as having the lowest CAC scores reported for any ethnicity (figure 4).
Figure 2.
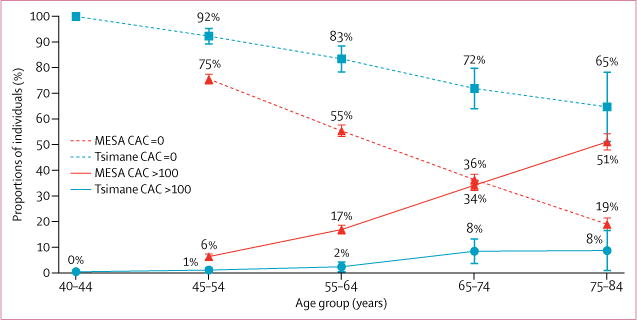
CAC scores by age for US MESA, and Tsimane samples
Tsimane have significantly lower CAC for each age category (all p ≤0.0001). Raw data and t tests for differences between Tsimane and MESA23 are given in the appendix. Error bars show 95% CI. CAC=coronary artery calcium.
Figure 4.
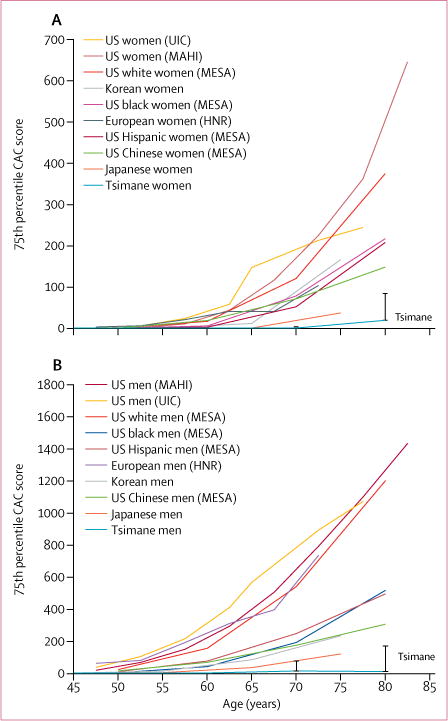
75th percentile of CAC score in women (A) and men (B) across populations
Data sources are in the appendix. Data were used from the Heinz Nixdorf RECALL Study (HNR),19 the Multi-Ethnic Study of Atherosclerosis (MESA),20-21,23 Japan,22 the Mid America Heart Institute (MAHI),24 the University of Illinois at Chicago,25 and Korea.28 A bootstrapped 95% CI is displayed for the Tsimane data. CAC=coronary artery calcium.
Figure 3.
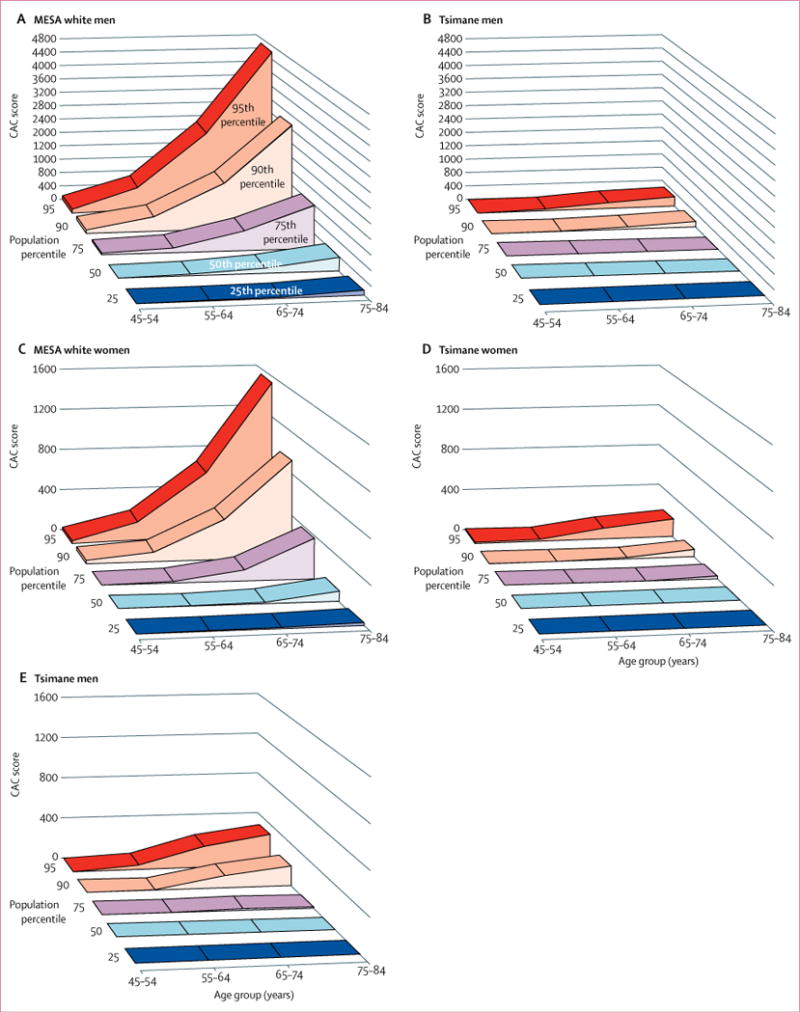
CAC score by age and CAC percentile in MESA white men (A) and Tsimane men (scaled to MESA men at 0-4800; B) and in MESA white women (C) and Tsimane women (D) and Tsimane men (scaled to MESA women at 0-1600; E)
Raw Tsimane data in five percentile groups for each age group15 are in the appendix. MESA=Multi-Ethnic Study of Atherosclerosis. CAC=coronary artery calcium.
Significant predictors of a CAC score greater than 0 were age, body-fat percentage, hs-CRP, and erythrocyte sedimentation rate. Other inflammatory markers, including neutrophil and monocyte count, and interleukin 10 and interleukin 5, were increased for individuals with CAC scores higher than 0. Table 2 shows the results of the best-fit model of predictors of CAC, derived from the zero- inflated negative binomial regression model and delineating significant predictors of both CAC absence (lower portion) and CAC score (upper portion). The coefficients report the attributable risk in terms of incidence rate ratios for unit changes in the independent variable. The lower portion shows that younger age, female sex, and lower triglycerides are associated with greater likelihood of CAC absence. The upper portion of the table shows that age, and to a lesser extent body fat and hs-CRP, are positively associated with CAC scores. Monocyte counts, however, are negatively associated with CAC scores. With the very low prevalence and level of CAC within the Tsimane population, the model explains only 19% of the attributable risk, with age and sex accounting for 14% of the attributable risk, and all other risk factors accounting for the remaining 5%. Risk factors from table 1 across categories of CAC score risk group and a comparison of this model to a more standard log linear model in which log (CAC+1) is regressed on risk factors are shown in the appendix.
Table 2.
Zero-inflated negative binomial model predicting Tsimane CAC scores
| IRR | 95% CI | p value | |
|---|---|---|---|
| Predictor of CAC score | |||
|
| |||
| Age (years) | 1 07 | 1 04 to 1.11 | <0.0001 |
| Male sex | 2 86 | 1 10.to 745 | 0 0311 |
| Body fat (%) | 1 11 | 1 06 to 1.18 | 0 0001 |
| Interleukin-10 (pg/mL) | 0 85 | 075 to 0.96 | 0 0106 |
| Interleukin-5 (pg/mL) | 0 77 | 0 62 to 0.95 | 0 0145 |
| Log hs-CRP (mg/L) | 1 71 | 1 16 to 2.51 | 0 0070 |
| Monocyte count (per 1000) | 0 005 | 0 00 to 0.23 | 0 0070 |
| Neutrophil count (per 1000) | 0 83 | 0 70 to 0.98 | 0 0282 |
| ESR (mm/h) | 0 97 | 0 95 to 1.00 | 0 0377 |
| Intercept | 0 32 | 0 03 to 398 | 0 3765 |
|
| |||
| Predictor of CAC absence | |||
|
| |||
| Age | –0.07 | –0.10 to –0.04 | <0.0001 |
| Male sex | –1.23 | –1.84 to –0.63 | 0 0001 |
| HDL | –0.04 | –0.07 to 0.00 | 0 0474 |
| Triglycerides | –0.01 | –0.02 to 0.00 | 0 0125 |
| Intercept | 8 63 | 6 14 to 11.13 | 0 0006 |
IRRs indicate the proportional change in risk for each dependent variable. IRRs greater than 1 indicate increased risk, and less than 1 increase reduced risk. The upper section displays significant predictors of non-zero scores, while the lower section displays the significant predictors of “inflated” scores of zero. Overall, the model accounts for 19% of the variance in CAC. IRR=incident rate ratio. CAC=coronary artery calcium. hs-CRP=high sensitivity C-reactive protein. ESR=erythrocyte sedimentation rate.
Discussion
In this population-based study of the indigenous Tsimane population of Bolivia, we observed a very low prevalence of coronary atherosclerosis, as measured by CAC scoring. The low prevalence of atherosclerosis extends to the older ages wherein up to 31 (65%) Tsimane octogenarians were free from atherosclerosis; only four (8%) of octogenarians showed moderately elevated CAC scores. This makes the Tsimane the population with the least reported coronary atherosclerosis. This contrasts starkly with the US MESA population in which only 14% of the MESA population had no CAC, and more than 50% exhibited CAC scores of at least 100 Agatston units. These findings translate to a 24-year lag before Tsimane reach a CAC score of above 0 and a 28-year lag before they reach a CAC score of at least 100 compared with an unselected US population (figures 2–4). By these findings, an 80-year old Tsimane possesses the “vascular age” of an American individual in their mid fifties. These findings of apparent protection from coronary atherosclerosis in the Tsimane extend to old age.
The pathophysiological mechanisms by which the Tsimane seem to be protected against CAC development are unknown. Numerous potential explanations exist, and might relate to coronary artery disease risk factors, subsistence lifestyle, genetics, and inflammation or immune regulation. Tsimane have low total and LDL cholesterol. From 2004 to 2011, the mean LDL cholesterol was 1.84 mmol/L (SD 0.57; appendix).13 Since 2011, average LDL cholesterol has increased by 0.16 mmol/L per year and now averages 2.35 mmol/L. This change is coincident with the availability of small gasoline motors that preclude the need for manual canoeing or rafting for river travel to the market town, San Borja, where nonsubsistence food can be obtained. Whether the increasing LDL-cholesterol concentrations coincide with increasing CAC will require assessment in future studies. This low prevalence and extent of CAC exists in the presence of low HDL, a known coronary artery disease risk factor. In the Tsimane, the average HDL was 1.0 mmol/L. This finding might highlight a differential weight to LDL versus HDL cholesterol for CAC formation or, alternatively, might be explained by ratios of total cholesterol or LDL cholesterol to HDL cholesterol that place Tsimane individuals in a low-risk category when using US-based classifications. Lower HDL cholesterol might also contribute to a higher likelihood of infections and inflammation.29
The potentially atheroprotective effect of low LDL or LDL-to-HDL ratio might be partly augmented by the Tsimane lifestyle. The Tsimane live a traditional forager- horticultural lifestyle, in huts of thatched roofs, typically in villages of roughly 60-200 people (figure 5). An estimated 14% of their average caloric diet is protein, 14% is fat, and 72% is carbohydrate.30 Meat protein and fat are acquired by hunting with guns and bow and arrow, or fresh water fishing with arrows, hook and line, or nets. Non-processed carbohydrates are grown in the form of rice, plantain, manioc, and corn via slash-and-burn horticulture, and the Tsimane also gather wild nuts and fruits. Importantly, carbohydrates are high in fibre and very low in saturated fat and simple sugars, which might further explain our study findings. The Tsimane diet lacks trans fats, and is a low fat diet, with an average estimated daily consumption of 38 g fat, with 11 g saturated fat, 14 g monounsaturated fat, and 8 g polyunsaturated fat.30 Most of a typical Tsimane day involves the physical activity of farming, hunting, food preparation, household chores, and parenting. The average hunt lasts more than 8 h and covers nearly 18 km, while horticultural labour includes using metal axes to chop large areas of primary forest. Men and women spend a mean of 6–7 h and 4–6 h per day engaging in physical activity, respectively.16 Less than 10% of Tsimane daylight hours are spent engaged in sedentary activity, while more than 54% of waking hours are sedentary in industrial populations.16,31 The Tsimane lack access to clean water, sewerage, or electricity; over two- thirds of adults suffer from intestinal helminths at any given time.32 The low prevalence of coronary artery disease in the Tsimane extends indirect evidence found in other populations33,34 that an active subsistence lifestyle is atheroprotective.
Figure 5.

An extended Tsimane family at the entrance to their home (A), two sisters and their children harvesting manioc root (B), a man and his son fishing with bow and arrow (C), and two men butchering a deer after hunting in the forest (D)
This present study findings are in agreement with, and extend the findings of, a MESA substudy regarding western heart-healthy behaviours and arterial ageing, which assessed later life adults exhibiting CAC scores of 0 at baseline by serial CT at 5 years after index CT.35 Importantly, this study noted that absence of traditional coronary artery disease risk factors in aggregate were associated with slower progression of CAC, but that the absence of the risk factors individually might not be associated with slower CAC progression. Recently, a heart-healthy western lifestyle was associated with a 46% decrease in coronary artery disease events.2 This lifestyle was defined as meeting three of four healthy lifestyle factors from the American Heart Association goals, including no smoking, a BMI of less than 30, physical activity of moderate intensity at least once a week, and a healthy diet pattern.2 These findings are directly applicable to the Tsimane, almost all of whom meet and exceed the American Heart Association goals.
Although genetics plays a minor part in causation of coronary artery disease,1 individuals with loss of function mutation of PCSK9 have been described with very low levels of LDL cholesterol and substantial freedom from major adverse coronary events.36,37 Because of the gradually increasing cholesterol levels in Tsimane over time with marker integration, they do not seem to be genetically protected. Presumptively, if the genetic makeup of the South American Tsimane is similar to North Americans, the Tsimane diet and exercise lifestyle might offer evidence to advocate for targets for effective primary prevention of atherosclerosis. However, the subsistence lifestyle in which each individual hunts, grows or gathers his own food, and exercises half the day, is impractical in an industrialised society. Furthermore, whether there is a threshold effect for diet and exercise above which no gain is achieved for slowing progression of CAC is unknown. Future studies addressing this issue might have strong implications on defining healthy behaviours for heart disease prevention.
Previous clinical trials and observational studies have supported the hypothesis of atherosclerosis as an inflammatory disease.9 However, whether inflammation is associative or causal in the coronary artery disease pathway remains unknown. Previous studies in industrialised civilisations have observed a dose-response association between inflammatory markers such as hs- CRP and future adverse coronary artery disease events.9 In this study, a high inflammatory burden was observed in the Tsimane with a low prevalence of CAC for all inflammatory markers (table 1). The current study used single measures of hs-CRP and other inflammatory biomarkers, previous work by the THLHP showed strong consistency in immune activation across multiple time points, suggesting much of the increased inflammation among Tsimane is chronic.30 Hs-CRP was marginally associated with CAC presence, but other inflammatory markers such as erythrocyte sedimentation rate, neutrophil count, interleukin 5, and interleukin 10 were noted to be inversely proportional to CAC. These findings run counter to previous industrialised population studies, for which the opposite has been found.9 Inflammation in the presence of low LDL concentrations might not potentiate the atherosclerotic process, although this might depend on the type and severity of infection.
Our study is not without limitations. We employed CAC scoring as a direct measure of coronary atherosclerosis, but its non-contrast nature precludes assessment of non- calcified plaque constituents. Although other methods of coronary assessment using contrast-enhanced coronary CT angiography or intravascular imaging might have afforded more information gain, we elected not to do so for reasons of safety. Yet, to date, CAC has emerged as a robust indicator of future coronary artery disease risk in population-based and observational cohort studies in industrialised environments.
While the prognostic implications of CAC in the Tsimane were beyond the scope of this study, increasing CAC extent and severity has been associated with higher rates of death and adverse coronary artery disease outcomes in previous investigations. Tsimane adults die at a modal age of 70 years (the most common age at the time of death), with cause-specific death ascertained by verbal autopsies using the WHO instrument. In a sample of 50 adult deaths occurring over the past 5 years, we identified only one potential case of death due to myocardial infarction (appendix).
Although we aimed to study all Tsimane individuals aged at least 40 years, this was not achievable because some individuals were not available for consenting and a few others refused consent. Yet, in this asymptomatic population of individuals, those who did versus those did not consent to study participation did not differ for coronary artery disease risk factors. We further compared CAC findings of the Tsimane to that of the population- based MESA study, which possessed a different make-up and frequency of sex and ethnicity to the current study. We considered a matched population of Tsimane to a US population, but elected to maximise the number of participants who could be effectively compared by including all individuals undergoing CAC scanning. With a low prevalence of coronary artery disease risk factors, heart-healthy lifestyle, high infectious inflammation, and low CAC, the Tsimane, a forager-horticulturalist population of the Bolivian Amazon, have the lowest reported levels of coronary artery disease of any population ever recorded to date. These findings suggest that coronary atherosclerosis can be avoided in most people by achieving a lifetime with very low LDL, low blood pressure, low glucose, normal BMI, no smoking, and plenty of physical activity. The relative contributions of each are still to be determined.
Supplementary Material
Research in context.
Evidence before this study
We searched both PubMed and Google Scholar for articles with terms “coronary calcium” and “CAC” (coronary calcium scoring) published after 1990 with no language restrictions. We searched for all population-based studies of coronary artery disease using CAC to determine whether data have been published on prevalence and severity of disease in small-scale subsistence populations and to integrate all available data. We included studies that were either population-representative or of very large sample size with explicit sampling methodologies described.
Added value of this study
The findings of the present study show that, in comparison to existing evidence, Tsimane indigenous South Americans have the lowest prevalence of coronary atherosclerosis of any population yet studied. This was achieved despite a high infectious inflammatory burden.
Implications of all the available evidence
The available evidence suggests that a lifetime with very low LDL cholesterol, a subsistence diet of wild game, fish, and high-fibre carbohydrates that are very low in saturated fat, combined with physical activity throughout much of the day sets a new target in the prevention of coronary atherosclerosis. Although consuming a subsistence diet is generally not feasible in urban industrialised populations, adoption of certain aspects of subsistence lifestyles could benefit individuals in sedentary industrialised populations. Urbanisation and the elimination of a subsistence diet and lifestyle might represent a novel risk factor for coronary artery disease. The general lack of prediction of CAC by inflammatory biomarkers in this study suggests the potential of a threshold effect for either degree of coronary atherosclerosis or LDL cholesterol level required for inflammation to either cause or predict coronary artery disease. Alternatively, inflammation secondary to infection might not be causal.
Acknowledgments
We thank the HORUS and THLHP teams listed in the appendix and our collaborators at the Hospital Presidente German Busch of Trinidad, Bolivia, including Edila Arteaga and Editha Alpire and CT core lab leader Kimberly Elmore of the Dalio Institute of Cardiovascular Imaging, Weill Cornell Medical College; Andrei Irimia, University of Southern California; Megan Costa, Arizona State University; and Khurram Nasir, Baptist Health South Florida. JS acknowledges support from the Agence Nationale de la Recherche—Labex Institute of Advanced Study in Toulouse.
Footnotes
Declaration of interests
JKM reported grant support from GE Medical. All other authors declared no competing interests.
Contributors
CEF introduced the HORUS and THLHP teams. Thereafter, the conception, design, and implementation of the study was developed by HK, RCT, BCT, LSW, AHA, MLS, BB, JDS, JS, BF, DER, DEM, CJR, GPL, RB, ARG, JKM, JN, CEF, MG, and GST. All authors also contributed to the interpretation of the data and drafting of the report. BCT, HK, MG, and ARG supervised laboratory measurements. RCT, LSW, AHA, MLS, JDS, BF, DEM, CJR, GPL, JN, CEF, and GST supervised CT scanning and calcium artery scoring in Bolivia. JKM and RCT supervised core lab coronary artery scoring. DEM served as the lead examining physician in Bolivia. HK, BCT, and BB led the statistical analysis.
Contributor Information
Hillard Kaplan, Department of Anthropology, University of New Mexico, Albuquerque, NM, USA
Prof Randall C Thompson, Saint Luke's Mid America Heart Institute, University of Missouri-Kansas City, Kansas City, MO, USA.
Benjamin C Trumble, School of Human Evolution and Social Change, and Center for Evolution and Medicine, Arizona State University, Tempe, AZ, USA.
L Samuel Wann, Ascension Healthcare, Milwaukee, WI, USA.
Adel H Allam, Al Azhar University, Cairo, Egypt
Bret Beheim, Department of Human Behavior, Ecology and Culture, Max Planck Institute for Evolutionary Anthropology, Leipzig, Germany.
Prof Bruno Frohlich, National Museum of Natural History, Smithsonian Institution, Washington, DC, USA; Dartmouth College, Hanover, NH, USA.
M Linda Sutherland, Newport Diagnostic Center, Newport Beach, CA, USA.
James D Sutherland, South Coast Radiological Medical Group, Laguna Hills, CA, USA.
Jonathan Stieglitz, Institute for Advanced Study in Toulouse, Toulouse, France; Department of Anthropology, University of New Mexico, Albuquerque, NM, USA.
Daniel Eid Rodriguez, Department of Medicine, Universidad de San Simón, Cochabamba, Bolivia.
David E Michalik, University of California, Irvine School of Medicine, Irvine, CA, USA; Miller Women's and Children's Hospital Long Beach, CA, USA.
Chris J Rowan, Renown Institute for Heart and Vascular Health; University of Nevada, Reno, NV, USA.
Guido P Lombardi, Laboratorio de Paleopatologia, Catedra Pedro Weiss, Universidad Peruana Cayetano Heredia, Lima, Peru.
Ram Bedi, Department of Bioengineering, University of Washington, Seattle WA, USA.
Angela R Garcia, Department of Anthropology University of California Santa Barbara, Santa Barbara, CA, USA.
Prof James K Min, Weill Cornell Medical College and the NewYork-Presbyterian Hospital, NY, USA.
Jagat Narula, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Prof Caleb E Finch, University of Southern California Leonard Davis School of Gerontology, Los Angeles, CA, USA; Dornsife College, University of Southern California, Los Angeles, CA, USA.
Prof Michael Gurven, Department of Anthropology University of California Santa Barbara, Santa Barbara, CA, USA.
Prof Gregory S Thomas, Long Beach Memorial, Long Beach, CA, USA; and University of California Irvine, Orange, CA, USA.
References
- 1.Yusuf S, Hawken S, Ôunpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 2.Khera AV, Emdin CA, Drake I, et al. Genetic risk, adherence to a healthy lifestyle, and coronary disease. New Engl J Med. 2016;375:2349–58. doi: 10.1056/NEJMoa1605086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Group MR. The Multiple Risk Factor Intervention Trial (MRFIT). A national study of primary prevention of coronary heart disease. JAMA. 1976;35:825–27. [PubMed] [Google Scholar]
- 4.Nissen S, Tuzcu E, Schoenhagen P, et al. Reversal of atherosclerosis with aggressive lipid lowering (REVERSAL) investigators. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med. 2005;352:29–38. doi: 10.1056/NEJMoa042000. [DOI] [PubMed] [Google Scholar]
- 5.Sipahi I, Tuzcu EM, Schoenhagen P, et al. Effects of normal, prehypertensive, and hypertensive blood pressure levels on progression of coronary atherosclerosis. J Am Coll Cardiol. 2006;48:833–38. doi: 10.1016/j.jacc.2006.05.045. [DOI] [PubMed] [Google Scholar]
- 6.Holman RR, Paul SK, Bethel MA, Neil HAW, Matthews DR. Long-term follow-up after tight control of blood pressure in type 2 diabetes. New Engl J Med. 2008;359:1565–76. doi: 10.1056/NEJMoa0806359. [DOI] [PubMed] [Google Scholar]
- 7.Allam AH, Thompson RC, Wann LS, et al. Atherosclerosis in ancient Egyptian mummies: the Horus study. JACC Cardiovasc Imaging. 2011;4:315–27. doi: 10.1016/j.jcmg.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Thompson RC, Allam AH, Lombardi GP, et al. Atherosclerosis across 4000 years of human history: the Horus study of four ancient populations. Lancet. 2013;381:1211–22. doi: 10.1016/S0140-6736(13)60598-X. [DOI] [PubMed] [Google Scholar]
- 9.Abd TT, Eapen DJ, Bajpai A, Goyal A, Dollar A, Sperling L. The role of C-reactive protein as a risk predictor of coronary atherosclerosis: implications from the JUPITER trial. Curr Atheroscler Rep. 2011;13:154–61. doi: 10.1007/s11883-011-0164-5. [DOI] [PubMed] [Google Scholar]
- 10.Bedi R, Nagra A, Fukumoto T, et al. Detection of subclinical atherosclerosis in peripheral arterial beds with B-mode ultrasound: a proposal for guiding the decision for medical intervention and an artifact-corrected volumetric scoring index. Glob Heart. 2014;9:367–78. doi: 10.1016/j.gheart.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Singh S, Nagra A, Maheshwari P, et al. Rapid screening for subclinical atherosclerosis by carotid ultrasound examination: the HAPPY (Heart Attack Prevention Program for You) substudy. Glob Heart. 2013;8:83–89. doi: 10.1016/j.gheart.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Gurven M, Blackwell AD, Rodríguez DE, Stieglitz J, Kaplan H. Does blood pressure inevitably rise with age?: longitudinal evidence among forager-horticulturalists. Hypertension. 2012;60:25–33. doi: 10.1161/HYPERTENSIONAHA.111.189100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vasunilashorn S, Crimmins EM, Kim JK, et al. Blood lipids, infection, and inflammatory markers in the Tsimane of Bolivia. Am J Hum Biol. 2010;22:731–40. doi: 10.1002/ajhb.21074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hecht HS, de Siqueira MEM, Cham M, et al. Low-vs. standard-dose coronary artery calcium scanning. Eur Heart J Cardiovasc Imaging. 2015;16:358–63. doi: 10.1093/ehjci/jeu218. [DOI] [PubMed] [Google Scholar]
- 15.Budoff MJ, Young R, Lopez VA, et al. Progression of coronary calcium and incident coronary heart disease events: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2013;61:1231–39. doi: 10.1016/j.jacc.2012.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gurven M, Jaeggi AV, Kaplan H, Cummings D. Physical activity and modernization among Bolivian Amerindians. PLoS One. 2013;8:e55679. doi: 10.1371/journal.pone.0055679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurven M, Kaplan H. Longevity among hunter-gatherers: a cross-cultural examination. Popul Dev Rev. 2007;33:321–65. [Google Scholar]
- 18.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. JAm Coll Cardiol. 1990;15:827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 19.Schmermund A, Möhlenkamp S, Stang A, et al. Assessment of clinically silent atherosclerotic disease and established and novel risk factors for predicting myocardial infarction and cardiac death in healthy middle-aged subjects: rationale and design of the Heinz Nixdorf RECALL Study. Am Heart J. 2002;144:212–18. doi: 10.1067/mhj.2002.123579. [DOI] [PubMed] [Google Scholar]
- 20.Bild DE, Detrano R, Peterson D, et al. Ethnic differences in coronary calcification the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2005;111:1313–20. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 21.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. New Engl J Med. 2008;358:1336–45. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 22.Fujiyoshi A, Miura K, Ohkubo T, et al. Cross-sectional comparison of coronary artery calcium scores between caucasian men in the United States and Japanese men in Japan The Multi-Ethnic Study of Atherosclerosis and the Shiga Epidemiological Study of Subclinical Atherosclerosis. Am J Epidemiol. 2014;180:590–98. doi: 10.1093/aje/kwu169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tota-Maharaj R, Blaha MJ, Blankstein R, et al. Association of coronary artery calcium and coronary heart disease events in young and elderly participants in the Multi-Ethnic Study of Atherosclerosis: a secondary analysis of a prospective, population-based cohort. Mayo Clin Proc. 2014;89:1350–59. doi: 10.1016/j.mayocp.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson RC, McGhie Al, Moser KW, et al. Clinical utility of coronary calcium scoring after nonischemic myocardial perfusion imaging. J Nucl Cardiol. 2005;12:392–400. doi: 10.1016/j.nuclcard.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Hoff JA, Quinn L, Sevrukov A, et al. The prevalence of coronary artery calcium among diabetic individuals without known coronary artery disease. J Am Coll Cardiol. 2003;41:1008–12. doi: 10.1016/s0735-1097(02)02975-3. [DOI] [PubMed] [Google Scholar]
- 26.Gudmundsson EF, Gudnason V, Sigurdsson S, Launer LJ, Harris TB, Aspelund T. Coronary artery calcium distributions in older persons in the AGES-Reykjavik study. Eur J Epidemiol. 2012;27:673–87. doi: 10.1007/s10654-012-9730-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 28.Park HE, Kim M-K, Choi S-Y, et al. The prevalence and distribution of coronary artery calcium in asymptomatic Korean population. Int J Cardiovasc Imaging. 2012;28:1227–35. doi: 10.1007/s10554-011-9922-2. [DOI] [PubMed] [Google Scholar]
- 29.Grion C, Cardoso LT, Perazolo TF, et al. Lipoproteins and CETP levels as risk factors for severe sepsis in hospitalized patients. Eur J Clin Invest. 2010;40:330–38. doi: 10.1111/j.1365-2362.2010.02269.x. [DOI] [PubMed] [Google Scholar]
- 30.Martin MA, Lassek WD, Gaulin SJ, et al. Fatty acid composition in the mature milk of Bolivian forager-horticulturalists: controlled comparisons with a US sample. Matern Child Nutr. 2012;8:404–18. doi: 10.1111/j.1740-8709.2012.00412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matthews CE, Chen KY, Freedson PS, et al. Amount of time spent in sedentary behaviors in the United States, 2003-2004. Am J Epidemiol. 2008;167:875–81. doi: 10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blackwell A, Trumble B, Maldonado Suarez I, et al. Immune function in Amazonian horticulturalists. Ann Hum Biol. 2016;43:382–96. doi: 10.1080/03014460.2016.1189963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koopman JJ, van Bodegom D, Jukema JW, Westendorp RG. Risk of cardiovascular disease in a traditional African population with a high infectious load: a population-based study. PLoS One. 2012;7:e46855. doi: 10.1371/journal.pone.0046855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kennelly B, Truswell A, Schrire V. A clinical electrocardiographic study of Kung Bushmen. S Afr: Med J. 1972;46:1093–97. [PubMed] [Google Scholar]
- 35.Whelton SP, Silverman MG, McEvoy JW, et al. Predictors of long-term healthy arterial aging: coronary artery calcium nondevelopment in the MESA study. JACC Cardiovasc Imaging. 2015;8:1393–400. doi: 10.1016/j.jcmg.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 36.Benn M, Nordestgaard BG, Grande P, Schnohr P, Tybjærg-Hansen A. PCSK9R46L, low-density lipoprotein cholesterol levels, and risk of ischemic heart disease: 3 independent studies and meta-analyses. J Am Coll Cardiol. 2010;55:2833–42. doi: 10.1016/j.jacc.2010.02.044. [DOI] [PubMed] [Google Scholar]
- 37.Cohen JC, Boerwinkle E, Mosley TH, Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. New Engl J Med. 2006;354:1264–72. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


