SUMMARY
After a long-standing tradition of analytical quality and analytical quality control programs, most medical laboratories that are aware of the need for total quality management, are experiencing new systems designed to assure quality throughout the entire total testing process, from the pre-analytical to the post-analytical steps. The availability of a new International Standard, ISO 15189:2003, specifically developed and designed to satisfy the requirements for quality management and competence in medical laboratories, should promote the harmonization of accreditation programs at an international level, and implementation of an effective quality system at a local level. The importance of the pre- and post-analytical phases are well recognized in the new International Standard and, therefore, efforts to comply with this standard might assure an approach that safeguards and continuously improves total quality in medical laboratories.
Key words: total testing process, laboratory errors, quality specifications
INTRODUCTION
Laboratory testing is a highly complex process. The testing cycle, commonly called the total testing process (TTP), was well described several years ago by George D. Lundberg, who pictured it as a “brain-to-brain loop” (1). The starting point for a laboratory test, a question made by the physician to the laboratory, can concern diagnostic, prognostic and monitoring processes, and/or health maintenance and promotion. The end result of the testing cycle is patient outcome and the effectiveness of laboratory information in improving medical and economical outcomes. In this cyclical process, the laboratory test is ordered, the patient identified, and the specimen collected, transported and prepared for analysis. After the specimen has been analyzed, the results are interpreted and reported to the physician or whoever ordered the tests. The action finally taken is based on the interpretation of the test results (Figure 1). Traditionally, clinical laboratories have focused their attention on quality control methods and quality assessment programs dealing with analytical aspects. However, a growing body of evidence accumulated in recent decades demonstrates that quality in clinical laboratories cannot be assured by simply focusing on purely analytical aspects (2). A recent review of errors in laboratory medicine concluded that in the delivery of laboratory testing, mistakes occur more frequently before (pre-analytic), and after, the test has been performed (3). Many of the mistakes in TTP are referred to as “laboratory errors”, but are actually due to poor communication, actions taken by others involved in the testing process (e.g. physicians, nurses and phlebotomists), or poorly designed processes which are outside the laboratory’s control (4). Likewise, there is evidence that laboratory information is only partially utilized: a recent report demonstrates that 45% of the results for urgent laboratory tests requested by the Emergency Department of one Hospital were never accessed, or were accessed far too late (5). In a modern approach to total quality, that is centred on patients’ needs and satisfaction, the risk of errors and mistakes in pre- and post-examination steps must be minimized in order to guarantee total quality to laboratory services.
Figure 1:
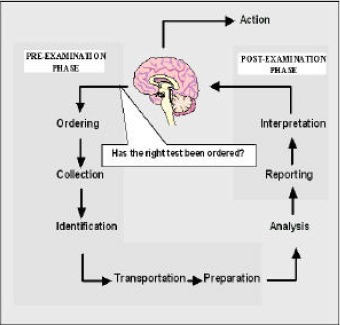
The total testing process and the pre-/post-analytical phases.
The recent International Standard developed for clinical laboratories, entitled the ISO 15189: 2003 “Medical laboratories: particular requirements for quality and competence”, enables laboratory professionals to use a quality system encompassing all the steps and processes within the simplified three-phase (pre-, intra- and post-analytical phase) framework. According to this new International Standard, laboratory services “include arrangements for requisition, patient preparation, patient identification, collection of samples, transportation, storage, processing and examination of clinical samples, together with subsequent validation, interpretation, reporting and advice, in addition to the consideration of safety and ethics in medical laboratory work”.
THE PRE-EXAMINATION PHASE
According to the ISO 15189: 2003 International Standard, pre-examination processes include “steps starting in chronological order from the clinician’s request, including the examination requisition, preparation of the patient, collection of the primary sample, transportation to and within the laboratory and ending when the analytical examination starts”. From a theoretical viewpoint, the pre-analytical phase can be further subdivided into two parts: in one, the so-called “pre-pre-analytical phase”, the clinician decides which laboratory test should be performed on the basis of his/her knowledge and experience; the other, the “conventional” pre-analytical phase, involves a series of related processes starting from patient identification, through the choice of right collection tubes, and ending with transportation and preparation for analysis of the samples (6).
The “pre-pre-analytical” phase includes the formulation of a clinical question and the selection of appropriate examinations. The inappropriate utilization of laboratory services is under scrutiny worldwide because of its possible effects on total costs, and the increased risk of medical errors and injury that it incurs. It is therefore unanimously agreed that it is important to provide consultancy as part of a laboratory service in order to improve appropriateness. There are great variations in the estimates of inappropriate laboratory use, ranging from 11 to 70 percent for general biochemistry and haematology tests, 5 to 95 percent for urine screens and microbiology, and 17.4 to 55 percent for cardiac enzymes and thyroid tests (7). Numerous studies have been conducted on interventions to reduce the excessive and inappropriate use of laboratory tests. Combined interventions are more effective than single interventions. Moreover, the use and diffusion of evidence based laboratory guidelines should be associated with continuous monitoring and clinical advice from laboratory specialists (8).
The conventional pre-analytical phase includes ordering, collecting and handling, transporting and receiving samples prior to the examination itself. In these practical steps, it is important to safeguard integrity between the primary sample(s) and the patient, between the primary sample(s) and the request documentation, and then finally, in the preparation process, between the primary sample and secondary preparations from the primary sample. In forensic medicine, these relationships are referred to as a 'chain of custody' (9) A basic pre-requisite, however, is the quality of the primary sample(s), to be collected in a standardized way, using appropriate materials, at specific times or after the (specific) preparation of the patient (10).
Complying with ISO 15189:2003
ISO 15189:2003 clause 5.4 ‘Pre-examination procedures’, includes requirements for a request form, a primary sample collection manual, the traceability of primary samples to an identified individual (the patient), monitoring of samples in transport, recording of receipt of samples, processing of urgent samples and policies for rejection of samples. The clinical laboratory must assure “the right test and the right order to the right patient, for the right question, at the right time”.
Request form
Request (or requisition) forms (whether a hard copy or an electronic version) from clinicians, and the reports issued by the laboratory, are the most important means of communication. In ISO 15189:2003, clause 5.4.1, the ‘Pre-examination procedures’ section specifies that the request form should have space for the inclusion of certain items of information. In addition to the minimum information (patient’s name, date of birth, identification number, and date of collection) necessary for the complete and clear identification of the patient, physicians should provide an added value to their requests by indicating the clinical question and other information on the patient, thus enabling laboratory professionals to select the most appropriate tests, or test cascade. The advantages and disadvantages of electronic requesting of laboratory tests have been well addressed. Here, it is particularly important to stress the potential role of ward order systems in encouraging clinicians to select the most appropriate tests, in facilitating dissemination of protocols and guidelines and in effecting real-time consultation by phlebotomists regarding specimen type, sample timings, and providing any other information useful for a state-of-the-art specimen collection (11).
Sample collection manual
As the various tests ordered may call for more than one type of specimen (e.g serum, plasma, whole blood, and/or urine), a collection manual is of crucial importance. Information in this manual should be checked whenever the phlebotomist is unsure about the right specimen(s) to obtain. The manual should contain information on the appropriate tube(s), any anticoagulant of choice, the amount of blood to be collected, any need for immediate refrigeration, and any other aspect that might affect testing quality.
Identification and collection
The mechanism by which the specimen is associated with the patient and the request card is of utmost importance. Thanks to the introduction of “positive specimens identification” with unique identification labels (barcodes) and the reduction achieved in transcription errors, the risk of errors in the pre-analytical phase has significantly decreased. Sample collection is a key-step for quality in laboratory services: in, for example, blood gas analysis, arterial blood is needed. Arteries are more difficult to access because they are buried deep in the tissue and arterial pressure is greater than venous pressure. Damage to the artery, which is more serious than damage to vein, may call for repair surgery. Extensive training and practice are therefore required for those who perform arterial punctures, which also call for special syringes and immediate transport. The metabolic processes should be slowed down or prevented by rapid cooling of specimens, and a speedy analysis should be guaranteed. All this information, including specifications as to the level of competence required by phlebotomists, should be provided in the collection manual.
Specimen transportation
Problems pertaining to specimen transportation fall into two categories: those associated with the timely and safe delivery of the specimen to the laboratory in a fit condition for examination, and those concerned with the health and safety of all personnel who might come into contact with the specimen, or its container, in transit (12). Both portering services and pneumatic tube systems have advantages and disadvantages, risks and associated problems that require specific standard operating procedures, and staff training.
Specimen reception and preparation
ISO 15189:2003 requires that ‘all primary samples received shall be recorded in an accession book, worksheet, computer or comparable system’ and that ‘the date and time of receipt of samples, as well as the identity of the receiving officer, shall be recorded’. Specimen preparation includes all the activities required to make a sample suitable for analysis on plasma or serum, including centrifugation, aliquoting, pipetting, dilution and sorting specimens into batches for automated analysis. The specimen preparation step has attracted considerable attention both because of the recognition of hazards for laboratory staff, and the its significant contribution to total cost and testing time (turnaround time) (13). Automated pre-analytical processing units are effective in reducing the work involved in specimen processing, and the laboratory errors that occur during specimen sorting, labelling, and aliquoting. Furthermore, these instruments improve the integrity of specimen handling throughout the steps of specimen processing, and the safety for laboratory staff (14). Another tool in shortening turnaround time may be the use of point-of-care instruments measuring on whole blood where applicable.
Acceptance/rejection criteria
ISO 15189:2003 requires that ‘criteria shall be developed and documented for acceptance or rejection of primary samples’ and ‘if compromised primary samples are accepted, the final report shall indicate the nature of the problem and if applicable, that caution is required when interpreting the result’. Specimens or samples can be compromised by uncertain identity (e.g. a request card received with an inadequately labelled specimen container in the same plastic envelope) or by inadequacy of the specimen (e.g. analysis vitiated by haemolysis) (15,16). Mechanisms for categorising and recording these incidents enable corrective and/or preventative action to be taken.
THE POST EXAMINATION PHASE
The overall purpose of all post-examination activities is to ensure that the results of examinations are presented accurately and clearly, and that they reach the user in a timely and secure manner. However, in Annex C, “Ethics in laboratory medicine”, the new International Standard underlines that “in addition to the accurate reporting of laboratory results, the laboratory has an additional responsibility to ensure that, as far as possible, the examinations are correctly interpreted and applied in the patient’s best interest. Specialist advice with regard to the selection and interpretation of examinations is part of the laboratory service”.
Complying with ISO 15189:2003
According to ISO 15189: 2003, the main aspects of post examination procedures are: a) review, evaluation in conformity with available clinical information and release of laboratory results by authorized personnel (subclause 5.7.1); b) storage of the primary and other laboratory samples according to an approved policy (5.7.2); and c) safe disposal of samples (5.7.3). While sample storage and safe disposal are aspects of “internal” quality, reporting of results strongly affects the communication to clinicians and the effective translation of results into clinical information. The reporting of results, in turn, involves three main issues:
content and presentation of the report;
responsibility for its validation and authorisation; and
method and security of communication and ownership.
CONTENT AND PRESENTATION OF THE REPORT
Table 1 shows the essential data that should be included in the report.
Table 1. Contents of laboratory reports (from Burnett D, (10), modified)
The report should ideally include but not be limited to the………
Identification of the laboratory issuing the report (and if different the identity of the laboratory undertaking the investigation)
Report destination
Identification of the requester (and his/her address)
Identification and location of the patient
Date and time of primary sample collection
Date and time of receipt by the laboratory
Date and time of issuing the report
Type of primary sample and its source
Results of the examination including information on factors (e.g. haemolysis, inadequate labelling of specimen container) that could compromise the results
Biological reference intervals where applicable
Clinical limits and reference change values (critical difference)
Intepretive comments, where appropriate
Identity of the person authorising the report
Fundamental issues in improving the utilization and effectiveness of laboratory data are the development of appropriate reference ranges, the addition to the report of information related to analytical and biological variation, and the inclusion of interpretative comments.
The concept of reference values, first introduced in 1969(17) is not adhered to rigorously as it should be by laboratory scientists and the clinicians as rigorously. All laboratorians should read the recent issue of Clinical Chemistry and Laboratory Medicine dedicated to the debate on reference values and reference intervals (18), in order to improve upon their knowledge and use of these concepts in clinical practice.
The inclusion of information based on quality specifications in addition to numerical results and reference ranges has recently been debated (19). By definition, quality specifications (i.e. precision and bias) are the level of performance required to facilitate clinical decision-making. Thus, this information should be used not only within the clinical laboratory to guarantee state-of-the-art service, but it should also be communicated to clinicians in order to improve upon their reasoning and decision-making.
The importance of interpretative comments on reports has now been recognized. The Royal College of Pathologists has produced guidelines for interpretative comments on biochemical reports, and experience is being gained in the assessment of the inter-laboratory quality of comments (20). Lim et al. have described the quality assessment of interpretative comments in clinical chemistry (21) and Laposata has made useful suggestions concerning the qualification required for providing interpretative comments, reimbursement for this activity (22). Finally, Kilpatrick has demonstrated the influence that interpretative comments have on patient outcomes (23)
VALIDATION AND AUTHORIZATION
Establishing that data are correct and appropriate, and authorizing their release, are important steps in the reporting process, and should be defined in writing. Two types of validation, referred to as technical and clinical validation, are widely discussed. Validation is defined as ‘confirmation by examination and provision of objective evidence that the particular requirements for a specific intended use are fulfilled’ (10). It is not easy to clearly distinguish between technical and clinical validation, but the former should guarantee that ‘requirements set for the examination in terms of its performance’, have been met, while the latter should deal with the plausibility check, based on screening each laboratory result in the context of all other test results and the patient information available. It has now been well established that validation systems, such as VALAB and LabRespond, are useful tools for performing plausibility checks and detecting any erroneous results in routine practice (24).
COMMUNICATION OF REPORTS
As stated by Burnett, reports can be communicated either in a hard copy or in an electronic form, but each method has disadvantages (in terms of the content remaining uncorrupted (fidelity) and it being securely transferred (security)) (10). The need to communicate laboratory information in real time must not compromise the fidelity and security of reports. Crucial aspects of data communication are procedures for immediate notification of physicians when the results “fall within alert or critical intervals” (subclause 5.8.7) fixed the establishment of turnaround times/ for each examination (subclause 5.8.11), and well defined policies and practice for the “telephoned report” and for any results communicated verbally (subclause 5.8.14).
CONCLUSIONS
Quality in the pre- and post-analytical phases of laboratory activity can be assured by implementing a quality system that complies with the ISO 15189: 2003 requirements. This International Standard, developed for medical laboratories, takes into account both quality issues and the competence needed to deliver a state-of-the art laboratory service. ISO 15189:2003 identifies several requirements for quality and competence in the pre-analytical phase, as well as in the intra- and post-analytical phases of laboratory testing, but it does not specify quality indicators and related quality specifications. While there is a consensus on analytical quality specifications, only recently have some indicators and specifications for the pre- and post-analytical phases been proposed. For example, quality indicators have been identified for requests, sampling, transport and receiving samples (25,26). The related quality specifications, or limits of acceptability, derive from a literature review or benchmark and reflect the present situation (state-of-the-art), but have to be verified in routine practice. Moreover, it has yet to be demonstrated that monitoring these indicators and related specifications, leads to reduced error rates and improves clinical outcomes.
REFERENCES
- 1).Lundberg GD. How clinicians should use the diagnostic laboratory in a changing medical world. Clin Chim Acta 1999; 280: 3-11. [DOI] [PubMed] [Google Scholar]
- 2).Plebani M, Carraro P. Mistakes in a stat laboratory: types and frequency. Clin Chem 1997: 43: 1348-1351 [PubMed] [Google Scholar]
- 3).Bonini P, Plebani M, Ceriotti F, Rubboli F. Errors in laboratory medicine. Clin Chem 2001; 48: 691-698. [PubMed] [Google Scholar]
- 4).Plebani M, Bonini P. Interdepartmental cooperation may help avoid errors in medical laboratories. BMJ 2002; 324: 423-424. [PubMed] [Google Scholar]
- 5).Kilpatrick ES. Use of computer terminals on wards to access emergency test results: a retrospective audit. BMJ 2001;322 1101-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Stroobants AK, Goldschmidt HMJ, Plebani M. Error budget calculations in laboratory medicine: linking the concepts of biological variation and allowable medical errors. Clin Chim Acta 2003; 333: 169-176. [DOI] [PubMed] [Google Scholar]
- 7).Silverstein MD. An approach to medical errors and patient safety in laboratory services. A white paper. The Quality Institute Meeting, Atlanta, April 2003. [Google Scholar]
- 8).Solomon DH, Hashimoto H, Daltroy L, Liang MH. Techniques to improve physicians’ use of diagnostic tests: a new conceptual framework. JAMA 1998; 280: 2020-2027. [DOI] [PubMed] [Google Scholar]
- 9).Burnett D. Understanding accreditation in laboratory medicine. London, ACB Venture Publications, 1996. [Google Scholar]
- 10).Burnett D. A practical guide to accreditation in laboratory medicine. London, ACB Venture Publications, 2002. [Google Scholar]
- 11).Jones R, O’Connor J. Information management and informatics: need for a modern pathology service. Ann Clin Biochem 2004; 41: 183-191. [DOI] [PubMed] [Google Scholar]
- 12).Miller JJ. Specimen collection, handling, preparation, and storage. In Clinical Diagnostic Technology: The total testing process (Volume 1: The preanalytical phase). Ward-Cook KM, Lehman CA, Schoeff LE, Williams RH. Eds. [Google Scholar]
- 13).Godolphin W, Bodker K, Uyeno D, Goh LO. Automated blood-sample handling in the clinical laboratory. Clin Chem 1990; 36: 1551-1555. [PubMed] [Google Scholar]
- 14).Holman JW, Mifflin TE, Felder RA, Demers LM. Evaluation of an automated preanalytical robotic workstation at two academic centers. Clin Chem 2002; 48: 540-548. [PubMed] [Google Scholar]
- 15).Jones Ba, Calam RR, Howanitz PJ. Chemistry specimen acceptability. A College of American Pathologists Q-Probes study of 453 laboratories. Arch Pathol Lab Med 1997; 121: 19-26. [PubMed] [Google Scholar]
- 16).Carraro P, Servidio G, Plebani M. Hemolyzed specimens: a reason for rejection or a clinical challenge? Clin Chem 2000; 46 306-307. [PubMed] [Google Scholar]
- 17).Grasbeck R, Saris NE. Establishment and use of normal values. Scand J Clin Lab Invest. 1969; 26 Suppl 110: 62-63. [Google Scholar]
- 18).Hyltoft Petersen P, Henny J. Special issue on reference values and reference intervals. Clin Chem Lab Med 2004; 42(7): 685-868. [DOI] [PubMed] [Google Scholar]
- 19).Plebani M. What information on quality specifications should be communicated to clinicians, and how? Clin Chim Acta 2004; 346: 25-35. [DOI] [PubMed] [Google Scholar]
- 20).The Royal College of Pathologists. Guidelines for the provision of interpretative comments on biochemical reports. Bull R Coll Pathol. 1998, 104: 25. [Google Scholar]
- 21).Lim EM, sikaris KA, Gill J, Calleja J, Hickman PE, Beilby J. Quality assessment of interpretative commenting in clinical chemistry. Clin Chem 2004; 50: 632-637. [DOI] [PubMed] [Google Scholar]
- 22).Laposata M. Patient-specific narrative interpretations of complex clinical laboratory evaluations: who is competent to provide them? Clin Chem 2004; 50: 471-472. [DOI] [PubMed] [Google Scholar]
- 23).Kilpatrick ES. Can the addition of interpretative comments to laboratory reports influence outcome? An example involving patients taking thyroxine. Ann Clin biochem 2004; 41(3): 227-229. [DOI] [PubMed] [Google Scholar]
- 24).Oosterhuis WP, Ulenkate HJLM, Goldschmidt HMJ. Evaluation of LabRespond, a new automated validation system for clinical laboratory test results. Clin Chem 2000; 46: 1811-1817. [PubMed] [Google Scholar]
- 25).Plebani M. Towards quality specifications in extra-analytical phases of laboratory activity. Clin Chem Lab med 2004; 42: 576-577 [DOI] [PubMed] [Google Scholar]
- 26).Ricos C, Garcia-Victoria M, de la Fuente B. Quality indicators and specifications for the extra analytical phases in clinical laboratory management. Clin Chem Lab Med 2004; 42: 578-582. [DOI] [PubMed] [Google Scholar]


