Summary
Identification of tissue transglutaminase (tTG) as a major target antigen of IgA anti-endomysial antibodies and detection of auto-antibodies against tTG in the serum pointed out a new direction in the serologic diagnosis of coeliac disease.
Clinical utility of determination of anti-tTGIgA antibodies, with recombinant human tTG used as antigen, was evaluated for the diagnosis of coeliac disease and monitoring the adherence to the diet in children and adolescents.
Patients
The study was performed in 169 patients aged 2-24 years, including 42 children (26 girls, 16 boys, mean age 8.01 ± 5.69, range 2-18) with newly diagnosed coeliac disease (CD) (group I), 60 patients (39 females, 21 males, mean age 15.68 ± 4.74, range 5-24) with CD recognized at least 3 years before entering the study, non-compliants with gluten-free diet (group II) and 67 children (34 girls, 33 boys, mean age 6,28± 4.48, range 2-16) suspected of malabsorption, in whom diagnosis of CD had been excluded.
Methods
Serum samples were taken from all patients and tested for total IgA, anti-endomysial IgA (IgAEmA) or IgG autoantibodies (IgGEmA), only in cases with IgA deficiency, by indirect immunofluorescence method and anti-tTGIgA antibodies by ELISA.
Results
| Anti-tTGIgA | IgEmA | Total | |||||
|---|---|---|---|---|---|---|---|
| group I | group II | group III | |||||
| positive | negat. | positive | negat. | positive | negat. | % | |
| positive | 85.7% | - | 85% | 1.7% | - | - | 52.1% |
| borderline | - | 2.4% | 5% | - | 3% | - | 3.5% |
| negative | 4.8% | 7.1% | 6.6% | 1.7% | - | 97% | 44.4% |
Strong significant associations between anti-tTGIgA present in the serum and IgAEmA (Kendall τ 0.7748, p<0.0001) and good correlation between the levels of anti-tTGIgA and IgAEmA (r=0,488, p=0.001) were found in group I. We have not shown the relationship between the presence of both types of antibodies in patients of group II (Kendall τ 0.2102, p=0.0937). However, a good significant correlation between the levels of these parameters was observed (r=0,813, p<0,0001). Anti-tTGIgA concentration was nificantly higher in patients of group I compared to group II (38.35 U/ml v. 23.13 U/ml, p=0,0356). The sensitivity of anti- tTGIgA test in group I was 88.1%, in group II - 91.7% while specificity reached 97%.
Conclusions
Determination of anti-tTGIgA shows high sensitivity (88.1%) and specificity (97%) for the detection of coeliac disease. This test can be used alternatively with the immunofluorescent IgAEmA in diagnosis of coeliac disease, and also as a marker of compliance with gluten-free diet. However, both IgAEmA and anti-tTGIgA tests do not reach 100% sensitivity and specificity for diagnosis and nitoring of celiac disease. Therefore small intestinal biopsy is still recommended as a ? gold standard?.
Key words: coeliac disease, anti-endomysial antibodies, anti-tissue transglutaminase antibodies
Introduction
Coeliac disease (CD) is a genetically determined chronic inflammatory intestinal disease induced by gluten, the storage protein of wheat (gliadin), barley (hordein) and rey (secalin). It can be diagnosed in the presence of characteristic abnormalities in a small intestinal biopsy sample and by improvement on a gluten-free diet. The major histopathological changes are suggestive of coeliac disease in different grades of villous atrophy with crypt hyperplasia and intraepithelial lymphocytosis [1].
The clinical classification of coeliac disease is based on the presence of gastrointestinal symptoms. Difficulties in the diagnosis of atypical forms of the disease where gastrointestinal symptoms are absent or not prominent, along with the need for identification of CD cases in high-risk populations and monitoring the effects of adherence to the diet, led to the development of sensitive, specific and simple in vitro serologic assays [1].
Until now, testing for anti-endomysial antibodies (EmA) and anti-reticulin antibodies (ARA) seemed to be the most useful in the diagnosis and the treatment of CD patients with gluten-free diet. However, the indirect immunofluorescence methods for the detection of these antibodies have some disadvantages like observer-dependence, interferences with anti-nuclear or smooth muscle antibodies and difficulties in inter-laboratory standardization [2,3]. Moreover, there are ethical concerns about the use of monkey oesophagus as a substrate [4]. It is well known that EmA testing alone has no sufficient diagnostic accuracy for CD and for monitoring the effects of gluten-free diet, because the presence of these antibodies depends on villous and crypt architecture of small intestinal mucosa (5). Lower sensitivity of EmA screening in cases with moderate abnormalities of mucosal pattern may result in worse detection of CD cases [5,6]. EmA seems to be not a reliable enough marker for slight dietary transgressions [4].
A most frequent pitfall of serological testing of EmA is selective IgA deficiency which occurs 10- to 16-fold more often than in the general population. Selective IgA deficient individuals usually have a raised concentration of IgG antibodies; so IgG-EmA test appears to be useful for identification of coeliac disease in these patients [7]. Another pitfall of serological testing is that children younger than 2 years of age are often negative for anti-endomysial antibodies. In this group of patients antibodies against gliadin (AGA) seem to be more specific and sensitive. The results of serological analysis will alter the use of immunosuppressive therapy and the amount of gluten consumed by patients; so after one month of gluten-free diet they can be negative [1].
Identification of tissue transglutaminase (tTG) as the major autoantigen in coeliac disease and the antigenic target recognised by anti-endomysial antibodies and detection of anti-tTG antibodies in the serum of CD patients has allowed a new diagnostic approach to serologic testing [8]. Most studies evaluating sensitivity and specificity of anti-tTGIgA antibodies for diagnosis and follow-up were promising, especially, after introducing human recombinant tTG (rh-tTG) instead of tissue transglutaminase from guinea pig (gp-tTG) as antigen in commercially available tests [9,10]. It has been suggested that simple and less expensive ELISA tests could replace the imunofluorescence method for the detection of anti-endomysial antibodies [11].
The aim of this study was to estimate the value of IgA antibodies against tTG for detection and monitoring coeliac disease.
Patients and methods
The study included 169 patients, divided into three groups. Group I consisted of 42 children (26 girls, 16 boys, mean age 8.01 ± 5.69, range 2-18) with newly diagnosed CD, which fulfil ESPGHAN criteria. Group II consisted of 60 patients (39 females, 21 males, mean age 15.68 ± 4.74, range 5-24) with CD recognized at least 3 years before entering the study, which reported non-compliance with the gluten-free diet. 67 children (34 girls, 33 boys, mean age 6,28 ± 4.48, range 2-16) who were suspected of malabsorption, and in whom diagnosis of CD had been excluded, formed group III. The study protocol was approved by the Bioethics Committee at L.Rydygier Medical University and informed consent was obtained from each patient.
Methods
Serum samples were taken from all patients and stored at -20?C before being assayed. All samples were tested for total IgA, anti-endomysial IgA (IgAEmA) or anti-endomysial IgG (IgGEmA) autoantibodies, only in cases with IgA deficiency, and anti-tTGIgA antibodies. In patients with newly recognized CD endoscopic intestinal biopsy was performed with histopathological evaluation according to four-grade Shmerling scale.
EmA were detected with indirect immunofluorescence method using monkey oesophagus as antigen. Each positive antibody titre was considered as a positive result for EmA test.
Anti-tTGIgA antibodies were measured by a commercially available ELISA technique (Pharmacia Upjohn, Sweden) based on recombinant human tTG as antigen. The measuring range of this test is 0.1-100 U/ml. According to the manufacturer recommendation, we established our own cutoffs: anti-tTGIgA < 4 U/ml were considered negative, 4-9 U/ml - borderline, > 9 U/ml were considered positive.
Statistical analysis was performed using statistical package Statistica for Windows. The Chi2 test was used for analysis of data with non-Gaussian distribution. Kendall tau and contingency coefficients were calculated for estimation of the association between two variables. Non-parametric Mann-Whitney test and Spearman's correlation coefficients were calculated. P<0.05 was used as being statistically significant.
Results
Anti-tTGIgA were detected in 36 of 42 children (85.7%) with newly diagnosed CD (group I, Table 1). Total IgA values in this group were within a reference range, except in one case which showed a decreased value for its age. In 6 patients (14.3%) negative or borderline results were found. Four of them were IgA deficient but only 3 were negative with anti-tTGIgA; in one patient a borderline result was shown (4.66 U/ml).
Table 1.
Number of cases positive, negative or borderline for serum IgAEmA and anti-tTGIgA in children with newly diagnosed coeliac disease (group I)
| IgAEmA | Anti-tTGIgA | all | |||||
|---|---|---|---|---|---|---|---|
| positive | borderline | negative | |||||
| N | % | N | % | N | % | N | |
| Positive | 36 | 10000 | – | – | 2 | 40.00 | 38 |
| negative | – | – | 1 | 100.00 | 3 | 60.00 | 4 |
| All | 36 | 100.00 | 1 | 100.00 | 5 | 100.00 | 42 |
EmA were detected in all patients positive with anti-tTGIgA but also in 2 cases with negative results. These two patients had high IgAEmA titers (+2560 IF and +640 IF; in histopathological findings, III/IV and IV grade of villous atrophy, respectively).
The mean value of anti-tTGIgA in children of group I was 38.35 U/ml (quartile1 and quartile 3-2.18 and 107.14, respectively). Statistically significant association was found between the presence of both types antibodies (Kendall τ coefficient 0.7748, p<0.0001) and their levels (r=0.4880, p=0.001).
Among 42 children of group I, total villous atrophy in histopathological findings of small intestine was observed in 30 cases (71.4%), grade III/IV in 6 (14.3%), grade III in 5 (11.9%) and grade II in 1 case (2.4%). Positive results with anti-tTGIgA test were found in 90% of children with grade IV, 66.7% with grade III/IV and in 100% of children with grade III of villous atrophy. Statistical analysis has shown that the relationship between the degree of morphological damage of small intestinal mucosa and positive result of anti-tTGIgA test was rather weak (p=0.2741) (Table 2).
Table 2.
Relationship between anti-tTGIgA and degree of villous atrophy in children with newly diagnosed CD
| Anti-fTGIgA | Degree of Villains atrophy in histological picture of intestinal mucosa biopsy | dl | |||||||
|---|---|---|---|---|---|---|---|---|---|
| IV° | III/IV° | III° | II° | ||||||
| N | % | N | % | N | % | N | % | N | |
| positive | 27 | 90.00 | 4 | 66.67 | 5 | 100.00 | – | – | 36 |
| borderline | 1 | 3.33 | – | – | – | – | – | – | 1 |
| negative | 2 | 6.67 | 2 | 33.33 | – | – | 1 | 100.00 | 5 |
| all | 30 | 100.00 | 6 | 100.00 | 5 | 100.00 | 1 | 42 | |
Chi2 = 11.8144 (p = 0.0663)
Contingency coefficient = 0.4686
γcorrelation = 0.1970 (p = 0.5136)
Spearman's rank correlation = 0.1727 (p = 0 2741)
In 60 CD patients of group II, monitored for at least 3 years, non-compliance with gluten-free diet and normal IgA levels were found. Of 60 patients, 52 were positive with anti-tTGIgA (86.7%). In all these cases but one EmAs were detected (+2.5 IF to + 640 IF). Borderline results with anti-tTGIgA were found in 3 patients with EmA titers +2.5 IF, +5 IF and + 40 IF. Among 5 cases with negative anti-tTGIgA 4 were positive with IgAEmA (+2.5, +5, +5 and +40 IF).
IgAEmA were detected in 58 of 60 patients (96.7%). Two patients of this group were reported to be on a gluten-rich diet. Nevertheless, they had normal total IgA levels and were negative with IgAEmA and IgG EmA. In these 2 patients anti-tTGIgA were negative or borderline (0.542 and 4.257 U/ml).
The mean level of anti-tTGIgA in group II was 23.13 U/ml [(Q1;Q3) (12.07; 55.22)]. There was no association between the presence of both types of antibodies (p=0.0937, Kendall τ 0.2102) but a good positive correlation between the levels of these variables was observed (r=0.8134, p<0.0001). Results are presented in Table 3.
Table 3.
Number of cases positive, negative and borderline for serum IgAEmA and anti- tTGIgA in patients non-compliants with gluten-free diet (group II)
| IgAEmA | Anti-tTGIgA | all | |||||
|---|---|---|---|---|---|---|---|
| positive | borderline | negative | |||||
| N | % | N | % | N | % | N | |
| Positive | 51 | 58.08 | 3 | 100.00 | 4 | 80.00 | 58 |
| Negative | 1 | 1.92 | – | – | 1 | 20.00 | 2 |
| All | 52 | 100.00 | 3 | 100.00 | 5 | 100.00 | 60 |
Chi-2 = 4.7347 (p = 0.0937)
Contingency coefficient = 0.2704
Kendall τ = 0.2102
Interestingly, statistical analysis has revealed significantly higher level of IgAEmA and anti-tTGIgA in patients with newly diagnosed CD than in patients which reported non-compliance with the gluten-free diet (p=0.0008 and p=0.0356, respectively) (Figure 1).
Figure 1.
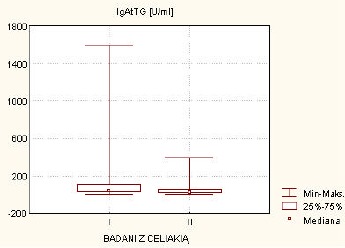
Comparison between anti-tTGIgA in both groups of patients with coeliac disease
In 67 children (group III) with other gastrointestinal diseases, in which CD was excluded, EmAs were not detected and borderline anti-tTGIgA was found only in 2 cases (5.59 i 7.44 U/ml).
The sensitivity of 88.1% in group I (85.1% positive, 2.4% borderline as positive) and 91.7% in group II (86.7% positive, 5% borderline as positive) was obtained, while specificity reached 97% for anti-tTGIgA ELISA (Table 5). Positive and negative predictive value were: in group I - 94.9 and 92.9%, in group II - 96.5 and 92.9%, respectively (Table 4).
Table 4.
Assesment of the utility of anti-tTGIgA-ELISA for diagnosis and monitoring of coeliac disease
| Patients with coeliac disease | |||
|---|---|---|---|
| Patients with newly diagnosed coeliac disease | Patients non-compliant with gluten-Free diet | All patients | |
| Sensitivity | 88.10% | 91.67% | 90.20% |
| Specificity | 97.01% | 97.01% | 97.01% |
| Positive predictive value | 94.87% | 96.49% | 97.87% |
| Negative predictive value | 92.85% | 92.86% | 86.67% |
| Precision | 93.58% | 94.49% | 92.90% |
Discussion
Tissue transglutaminase belongs to a diverse family of enzymes that are widely distributed in tissues and body fluids of mammals and some plants. In humans, there are several other enzymes which belong to this family: three of epithelial origin and two extracellular (coagulation factor XIII and prostate TG). Tissue transglutaminase is found in the small bowel mucosa, endothelial cells, smooth muscle cells and thymus. Tissue TG is a cytosolic enzyme, physiologically inactive. In the tissues damaged after mechanical injury, inflammation or during apoptosis tTG is released from the cells. tTG plays a role in the aetiopathogenesis of several diseases like these of the central nervous system (Huntington's chorea), malignancies, HIV infections, atherosclerosis, non-specific enteritis, cirrhosis, cataract or several autoimmune diseases [12, 13, 14]. The finding of anti-tTG antibodies is of special interest in the pathogenesis of CD. tTG is present in all layers of the intestine wall with the highest activity in the submucosa but almost undetectable in the epithelium. Transglutaminase is absent from crypt epithelium but increased expression of the enzyme was shown in mature epithelial cells migrating to small intestine villi [15].
tTG induces the deamidation of gluten peptides present in the diet and the formation of neoepitopes that, in association with HLA-DQ2 molecules on the surface of T-lymphocytes, presents antigen drive of the antibody response to both gliadin and tTG [12,16].
Since identification of tTG in 1997 as a major autoantigen recognized by anti-endomysium antibodies [17] commercially available tests for IgA class anti-tTG [10], using guinea pig tTG as an antigen were developed. However, recently recombinant human antigen was recommended because of higher sensitivity and specificity [18-23].
In our study the ELISA test, using rhtTG, provided encouraging results with 88.1% sensitivity in children with newly diagnosed CD, which was comparable to 75-100% in the previous data [10, 11, 19, 21-32]. Test sensitivity could probably be increased by measuring IgG class anti-tTG antibodies in cases with decreased or deficient IgA, which is often found in CD patients. Therefore, it is suggested that in suspected cases first total IgA should be measured and then patients with IgA deficiency checked for anti-tTGIgG antibodies [7,29,33-34].
A weak association between anti-tTGIgA positivity and mucosal pattern in children with CD may result from a small number of cases with moderate villous atrophy (11 patients). Presumably, test sensitivity increases in cases with total villous atrophy while decreases in patients with subtle changes of mucosal architecture as reported by others [31, 35]. This may lead to negative results in patients with gluten-sensitive enteropathy with normal or slightly changed mucosa.
In CD patients monitored for 3 years, which reported high gluten consumption, anti-tTGIgA sensitivity of 91.7% was achieved; this was lower than the IgAEmA sensitivity (96.7%) found by us earlier [36]. EmAs seem to be a better marker of gluten-free diet compliance. According to data reported elsewhere, anti-tTGIgA show positive correlation with the amount of gluten in the diet before CD recognition and during the gluten challenge [37]; also with duration of gluten-free diet or gluten challenge [28]. Several CD patients committing dietetic errors are negative with anti-tTGIgA; on the other hand negative serology in CD patients is not related to histologic regeneration of small intestine mucosa [38].
CD patients on gluten-free diet present significantly lower values of anti-tTGIgA compared with non-compliants [31]; that is also observed in our study.
The anti-tTGIgA test in our hands had 97% specificity which was very high and comparable to other data -90.1 to 99.2% [10, 18, 19, 21-31, 39]. In two cases with diverse gastrointestinal diseases bordeline positive anti-tTGIgA values were found while IgAEmA results were negative. One of these two patients, a 12 years old girl with a family history of CD and gastrointestinal symptoms of unknown etiology, had a normal biopsy, trace amounts of IgAEmA (+2.5 IF) and recently found borderline anti-tTGIgA of 7.44 U/ml which may not be a false positive but suggests an early silent atypical form of CD. Indeed, very recent data suggest that anti-tTGIgA test can be used to detect CD in patients unrecognized by IgAEmA [21,40].
The 94.1% accordance of anti-tTGIgA detection with the presence of IgAEmA, measured by immunofluorescence method, observed in all patients in the study was quite high, however does not allow the complete replacement of EmA testing with rh anti-tTG ELISA, that is in agreement with previous reports [18,20,22,37,41]. We suggest, in agreement with some others, that anti-tTG antibodies can be used as the first-step tool in the routine diagnostic panel for CD and in doubtful cases EmA should be tested [29,32]. According to Dickey et al. serology screening should be based on both EmA and anti-tTGIgA detection because in every third patient only one type of antibody is present [30]. It is worth noting that, at present, serologic markers are not reliable enough to become a “gold standard” in diagnosis and monitoring of coeliac disease. In fact, a proportion of CD cases, especially these with subtotal villous atrophy, is negative for any antibodies characteristic for CD that makes avoiding biopsy by clinicians, impossible [24,30].
We have shown that anti-tTGIgA ELISA with recombinant human antigen may be used interchangeably with IgAEmA in serology screening for diagnosis of CD and adherence to the gluten-free diet but, providing sensitivity and specificity below 100%, should not replace small intestinal biopsy.
Acknowledgements
We thank Dr G.Dymek and Ms A.Stefanska B.Sc. for technical help. This work was supported by grant KBN 4PO5E 06819 from Committee for Scientific Research in Poland.
References
- 1.Green PHR, Jabri B. Coeliac disease. Lancet, 2003, 9381, 383-386 [DOI] [PubMed] [Google Scholar]
- 2.Vitoria J.C., Arrieta A., Arranz C. Antibodies to gliadin, endomysium, and tissue transglutaminase for the diagnosis of celiac disease. JPGN, 1999, 29, 571-574 [DOI] [PubMed] [Google Scholar]
- 3.Russo P.A., Chartrand L.J., Seidman E. Comparative analysis of serologic screening tests for the initial diagnostics of celiac disease. Pediatrics, 1999, 104, 75-78 [DOI] [PubMed] [Google Scholar]
- 4.Baudon JJ, Johanet C, Absalon YB, Morgant G, et al. Diagnosing celiac disease: a comparison of human tissue transglutaminase antibodies with antigliadin and antiendomysium antibodies. Arch. Ped. Adol.Med., 2004, 158, 6, 584-588 [DOI] [PubMed] [Google Scholar]
- 5.Rostami K., Mulder C.J.J., van Overbeek F.M., Kerckhaert J., Meijer J.W.R., von Blomberg M.B.E., et al. Should relatives of coeliacs with mild clinical complaints undergo a small-bowel biopsy despite negative serology? Eur.J.Gastroenterol.Hepatol., 2000, 12[1], 51-55 [DOI] [PubMed] [Google Scholar]
- 6.Rostami K., Kerckhaert J., Tiemessen R., von Blomberg B.M.E., Meijer J.W.R., Mulder C.J.J. Sensitivity of antyendomysium and antigliadin antibodies in untreated celiac disease: disappointing in clinical practice. Am.J.Gastroenterol., 1999, 94, 888-894 [DOI] [PubMed] [Google Scholar]
- 7.Cataldo F., Lio D., Marino V., Picarelli A., Ventura A., Corazza G.R. IgG(1) antiendomysium and IgG antitissue transglutaminase (anti-tTG) antibodies in coeliac patients with selective IgA deficiency. Gut, 2000, 47(3), 366-369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dieterich W., Ehnis T., Bauer M., Donner P., Volta U., Riecken E.O., et al. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat.Med., 1997, 3, 797-801 [DOI] [PubMed] [Google Scholar]
- 9.Bazzigaluppi E., Lampasona V., Barera G., Venerando A., Bianchi C., Chiumello G., et al. Comparison of tissue transglutaminase-specific antibody assays with established antibody measurements for celiac disease. J.Autoimmunol., 1999, 12(1), 51-56 [DOI] [PubMed] [Google Scholar]
- 10.Troncone R., Maurano F., Rossi M., Micillo M., Greco L., Auricchio R., et al. IgA antibodies to tissue transglutaminase: an effective diagnostic test for coeliac disease. J.Pediatr., 1999, 134, 166-171 [DOI] [PubMed] [Google Scholar]
- 11.Sblattero D., Berti I., Trevisiol C., Marzari R., Tommasini A., Bradbury A., et al. Human recombinant tissue transglutaminase ELISA: an innovate diagnostic assay for celiac disease. Am.J.Gastroenterol., 2000, 95, 5, 1253-1257 [DOI] [PubMed] [Google Scholar]
- 12.Schuppan D. Current concepts of celiac disease pathogenesis. Gastroenterology, 2000, 119, 234-242 [DOI] [PubMed] [Google Scholar]
- 13.Aeschlimann D., Thomazy V. Protein crosslinking in assembly and remodeling of extracellular matrices. Connect. Tissue Res., 2000, 41(1), 1-27 [DOI] [PubMed] [Google Scholar]
- 14.Skovbjerg H., Norem O., Anthonsen D., Moller J., Sjostrom H. Gliadin is a good substrate of several transglutaminases: possible implication in the pathogenesis of celiac disease. Scand. J.Gastroenterol., 2002, 37(7), 812-817 [PubMed] [Google Scholar]
- 15.Molberg O., McAdam S.N., Sollid L.M. Role of tissue transglutaminase in celiac disease. J.Ped.Gastroenterol.Nutr., 2000, 30, 232-240 [DOI] [PubMed] [Google Scholar]
- 16.Molberg O., McAdam S.N., Korner R., Quarsten H., Kristiansen C., Madsen L., et al. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat.Med., 1998, 4, 6, 713-717 [DOI] [PubMed] [Google Scholar]
- 17.Dieterich W., Laag E., Schopper H., Volta U., Ferguson A., Gillett H., et al. Autoantibodies to tissue transglutaminase as predictors of celiac disease. Gastroenterology, 1998, 115, 1317-1321 [DOI] [PubMed] [Google Scholar]
- 18.Wolters V., Vooijs-Moulaert A.F., Burger H., Brooimans R., DeSchryver J., Rijkers G., et al. Human tissue transglutaminase enzyme linked immunosorbent assay outperforms both the guinea pig based tissue transglutaminase assay and anti-endomysium antibodies when screening for coeliac disease. Eur.J.Ped., 2002, 161(5), 284-287 [DOI] [PubMed] [Google Scholar]
- 19.Sardy M., Odenthal U., Karpati S., Paulsson M., Smyth N. Recombinant human tissue transglutaminase ELISA for the diagnosis of gluten-sensitive enteropathy. Clin.Chem., 1999, 4512, 2142-2149 [PubMed] [Google Scholar]
- 20.Wong R.C., Wilson R.J., Steele R.H., Radford-Smith G., Adelstein S. A comparison of 13 guinea pig and human anti-tissue transglutaminase antibody ELISA kits. J.Clin.Pathol., 2002, 55(7), 488-494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tonutti E., Visentini D., Bizzaro N., Caradonna M., Cerni L., Villalta D., et al. The role of antitissue transglutaminase assay for the diagnosis and monitoring of coeliac disease: a French-Italian multicentre study. J.Clin.Pathol., 2003, 56(5), 389-393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martini S., Mengozzi G., Aimo G., Giorda L., Pangi R., Guidetti C.S. Comparative evaluation of serologic tests for celiac disease diagnosis and follow-up. Clin.Chem., 2002, 48, 960-963 [PubMed] [Google Scholar]
- 23.Kocna P., Vanickova Z., Perusicova J., Dvorak M. Tissue transglutaminase-serology markers for celiac disease. Clin.Chem.Lab.Med., 2002, 40, 485-492 [DOI] [PubMed] [Google Scholar]
- 24.Biagi F., Ellis H.J., Yiannakou J.Y. Tissue transglutaminase antibodies in coeliac disease. Am.J.Gastroenterol., 1999, 94, 2187-2192 [DOI] [PubMed] [Google Scholar]
- 25.Sugai E., Selvaggio G., Vazquez H., Viola M., Mazure R., Pizarro B., Smecuol E., Flores D., Pedreira S., Maurino E., Gomez J.C., Bai J.C. Tissue transglutaminase antibodies in celiac disease: assessment of a commercial kit. Am.J.Gastroenterol., 2000, 95(9), 2318-2322 [DOI] [PubMed] [Google Scholar]
- 26.Sardy M., Karpati S., Peterfy F., Rasky K., Tomsits E., Zagoni T., et al. Comparison of a tissue transglutaminase ELISA with the endomysium antibody test in the diagnosis of gluten-sensitive enteropathy. Z.Gastroenterol., 2000, 38(5), 357-364 [DOI] [PubMed] [Google Scholar]
- 27.Hansson T., Dahlbom T., Hall J., Holtz A., Elfman L., Dannaeus A., et al. Antibody reactivity against human and guinea pig tissue transglutaminase in children with celiac disease. J.Pediatr.Gastroenterol.Nutr., 2000, 30(4), 379-384 [DOI] [PubMed] [Google Scholar]
- 28.Burgin-Wolff A., Dahlbom I., Hadziselimovic F., Petersson C.J. Antibodies against human tissue transglutaminase and antiendomysium in diagnosing and monitoring coeliac disese. Scand.J.Gastroenterol., 2002, 37(6), 685-691 [DOI] [PubMed] [Google Scholar]
- 29.West J., Lloyd C.A., Hill P.G., Holmes G.K. IgA-antitissue transglutaminase: validation of a commercial assay for diagnosing coeliac disease. Clin.Lab., 2002, 48(5-6), 241-246 [PubMed] [Google Scholar]
- 30.Dickey W., McMillan S.A., Hughes D.F. Sensitivity of serum tissue transglutaminase antibodies for endomysial antibody positive and negative coeliac disease. Scand.J.Gastroenterol., 2001, 36(5), 511-514 [DOI] [PubMed] [Google Scholar]
- 31.Fabiani E., Catassi C. The serum IgA class anti-tissue transglutaminase antibodies in the diagnosis and follow up of coeliac disease. Results of an international multi-centre study. International Working Group on Eu-tTG. Eur.J.Gastroenterol.Hepatol., 2001, 13(6), 659-665 [DOI] [PubMed] [Google Scholar]
- 32.Biagi F., Pezzimenti D., Campanella J., Vadacca G.B., Corazza G.R. Endomysial and tissue transglutaminase antibodies in coeliac sera: a comparison not influenced by previous serological testing. Scand.J.Gastroenterol., 2001, 36(9), 955-958 [DOI] [PubMed] [Google Scholar]
- 33.Gillett H.R., Gillett P.M., Kingstone K., Marshall T., Ferguson A. IgA deficiency and coeliac disease. J.Ped.Gastroenterol.Nutr., 1997, 25(3), 366-367 [DOI] [PubMed] [Google Scholar]
- 34.Beutner E.H., Kumar V., Chorzelski T.P., Czerwionka-Szaflarska M. IgG endomysial antibodies in IgA deficient patient with coeliac disease. Lancet, 1989, 1, 1261-1262 [DOI] [PubMed] [Google Scholar]
- 35.Trevisiol C., Ventura A., Baldas V., Tommasini A., Santon D., Martelossi S., Torre G., Berti I., Spano A., Crovella S., Amorosa A., Sblattero D., Marzari R., Bradbury A., Not T. A reliable screening procedure for coeliac disease in clinical practise. Scand.J.Gastroenterol., 2002, 37(6), 679-684 [DOI] [PubMed] [Google Scholar]
- 36.Szaflarska-Szczepanik A., Odrowaz-Sypniewska G., Dymek G. Tissue transglutaminase antibodies as a marker of gluten-free diet compliance in patients with coeliac disease (in Polish). Pol.Merk.Lek., 2001, 11, 65, 411-413 [PubMed] [Google Scholar]
- 37.Weile B., Heegaard N.H., Hoier-Madsen M., Wiik A., Krasilnikoff P.A. Tissue transglutaminase and antiendomysial autoantibodies measured in an historical cohort of children and yound adults in whom coeliac disease was suspected. Eur.J.Gastroenterol.Hepatol., 2002, 14(1), 71-76 [DOI] [PubMed] [Google Scholar]
- 38.Kaukinen K., Sulkanen S., Maki M., Collin P. IgA-class transglutaminase antibodies in evaluating the efficacy of gluten-free diet in celiac disease. Eur.J.Gastroenterol. Hepatol., 2002, 14(3), 311-315 [DOI] [PubMed] [Google Scholar]
- 39.Basso D., Guariso G., Plebani M. Serologic testing for celiac disease. Clin.Chem., 2002, 11, 2082-2083 [PubMed] [Google Scholar]
- 40.Tesei N., Sugai E., Vazquez H., Smecuol E., Niveloni S., Mazure R., et al. Antibodies to human recombinant tissue transglutaminase may detect celiac disease patients undiagnosed by endomysial antibodies. Aliment.Pharmacol.Ther., 2003, 17, 1415-1423 [DOI] [PubMed] [Google Scholar]
- 41.Wolters V., Vooijs-Moulaert A.F., Burger H., Brooimans R., DeSchryver J., Rijkers G., Houwen R. Human tissue transglutaminase enzyme linked immunosorbent assay outperforms both the guinea pig based tissue transglutaminase assay and anti-endomysium antibodies when screening for coeliac disease. Eur.J.Pediatr., 2002, 161(5), 284-287 [DOI] [PubMed] [Google Scholar]


