Introduction
Nearly all cells generate and eject vesicles, and these vesicles constitute major vehicles for intercellular communication. Exosomes, as nanosized vesicles (~30–100 nm in diameter1), target cell function by delivering proteins, lipids, and nucleic acids. Exosomes are emerging as a valuable source for disease stage-specific information and as fingerprints of disease progression, and as potential biomarkers in different pathophysiological states2–5. However, since exosomes provide a major medium of intercellular communication6, they likely also impact the treatment of diseases7, 8. Recent reports have highlighted the critical application of exosomes as personalized targeted drug delivery vehicles6, 9. Exosomes harvested from multipotent mesenchymal stromal cells (MSCs) mediate the restorative therapeutic effects of MSCs for stroke10. Here, we review the biogenesis of exosomes, their molecular composition and role as messengers of intercellular communication, and describe using exosomes for treatment of stroke. We also focus on therapeutic effects and underlying mechanisms of action of exosomes as therapeutic vectors for stroke11, but do not discuss the role of exosomes as disease or injury biomarkers. Capitalizing on the function of exosomes as vehicles for intercellular communication in physiological and patho-physiological conditions such as stroke, provides a paradigm shift and enormous potential for safe and effective therapeutic approaches for stroke and for other diseases/injury.
Exosome biogenesis and content
Exosomes are highly conserved among most eukaryotic organisms, from microorganisms up to mammals12. Exosomes originate from the endocytic route, and are formed by the inward budding of the plasma membrane. The membrane of late endosomes invaginates and forms small vesicles that are pinched off into the endosomal space. The internal intralumenal vesicles with their cargo secreted into the extracellular space are exosomes 13.
Exosomes contain conserved proteins such as CD81, CD63 (membrane associated proteins like LAMP-3), and CD9, Alix and tumor susceptibility gene 101 protein9, as well as tissue/cell type specific proteins that reflect their cellular source14. The exosome membranes are enriched with cholesterol, sphingomyelin, and ceramide15. Exosomes contain many biologically active molecules, such as proteins, ribonucleic acids (RNAs), deoxyribonucleic acids, lipids and microRNAs (miRs)16. These bioactive molecules mediate exosomal intercellular communication and may target specific cell types, and thereby modify their target cell function by delivering proteins, lipids, and nucleic acids17. Most proteins within exosomes derive from parent cell membranes, the cytosol, and Golgi, but rarely from endoplasmic reticulum or mitochondria12. Cytosolic proteins remain within the exosomes, and those derived from the plasma membrane are retained in the vesicle membrane, maintaining the same topology of the cell, with potential roles in sequestering soluble ligands18. Exosome proteins participate in antigen presentation, cell adhesion, cell structure and motility, and are stress regulators, involved in transcription and protein synthesis, and in trafficking and membrane fusion19. Many functional effects of exosomes may be attributed to the transfer of their RNA and miR content17. RNAs and miRs are the most relevant cargo in exosomes in terms of the ability of a small number of molecules to influence several proteins/enzymes within one or more cellular pathways in target cells 20.
Exosome isolation and storage
Many methods for exosome isolation have been described21, 22. They include: 1) differential centrifugation coupled with ultracentrifugation (UC)15, 23; 2) using anti-EpCAM (epithelial cell adhesion molecule) coated magnetic beads immunoaffinity pull-down24, 25; 3) density gradient separation25; 4) sequential centrifugal ultrafiltration by tangential flow filtration26; 5) using ExoQuick-TC27; 6) rapid isolation of exosome by alternating current electrokinetic microarray chip device 28; 7) using a commercially available size exclusion chromatography column (SEC) for rapid vesicle purification29. Ultracentrifugation isolated exosomes have the highest protein purity, but the lowest recovery of particles.30 Specific g-force/k factor usage during differential ultracentrifugation also influences the purity and yield of exosomes31. Baranyai et al compared the purity of exosomes using differential ultracentrifugation and SEC. They found that using ultracentrifugation isolation, the diameter of the majority of isolated particles fell into the size range of exosomes; however, albumin was also present in the preparations, when 1h of ultracentrifugation at 4°C was applied32. SEC isolation showed good reproducibility, and rapid vesicle purification (less than 10 minutes); however, post-column exosome concentration steps resulted in some protein loss and also leads to low exosome recovery and reduced purity (assessed by the particle-to-protein ratio)29. Van Deun et al, 33 compared four methods of exosome extraction: differential ultracentrifugation, OptiPrep density gradient centrifugation (ODG), ExoQuick™ precipitation and Total Exosome Isolation precipitation. They found that ultracentrifugation and ODG showed better clean exosome preparations measured by CD63-immuno-TEM than ExoQuick™ and Total Exosome Isolation precipitation. ODG showed the purest exosome preparations 33. However, Taylor et al, indicated that circulating exosomes isolated by ExoQuick precipitation produces exosomal RNA and protein with greater purity and quantity than chromatography, ultracentrifugation, and DynaBeads.34 Therefore, to-date there is no gold-standard method for exosome isolation, which complicates inter-laboratory comparisons of data 21.
Exosome storage conditions (such as temperature and duration) influence the therapeutic utility of exosomes and their stability. Protein and RNA content of exosomes decrease at 10 days of room temperature storage compared with storage at −70°C and 4°C35. Exosomes are stable at 4°C for short term (within 7 days) and when stored at −80°C for at least 90 days25. Storage at below −70°C is the most favorable condition for long term preservation of fresh exosomes for clinical application and basic research35.
Interaction of exosomes with target cells
Exosomes are taken up by target cells by several mechanisms, most of which are mediated by the endocytosis route, such as clathrin mediated endocytosis36, phagocytosis37, lipid raft mediated internalization38, and macropinocytosis39 as well as by direct fusion with the plasma membrane40. Exosomes bind to target cells via ligand-receptor interactions, such as integrins, tetraspanins and intercellular adhesion molecules. Tetraspanins as a functionally important component of exosomes, also have specific effects on distinct cell fission and fusion machineries41. After binding, exosomal contents are internalized by recipient cells via fusion with the plasma membrane of recipient cells and direct release of contents into the cytoplasm, or by exosome internalization by endocytosis into recipient cells. The exosomal tetraspanin web regulates target cell selection, as well as facilitates tailoring exosomes for drug delivery42. Human brain endothelial cell derived exosomes (hBEC-Exos) contain several receptors to carry macromolecules across the BBB, including transferrin receptor, insulin receptor, low density lipoprotein (LDL), LDL receptor-related protein, and TMEM30A (a putative antigen for the single-domain antibody), and hBEC-Exos act as cell communication vesicles with both brain astrocytes and cortical neurons43. Therefore, exosomes are promising tools to target drugs or biological material to specific cells across different biological barriers, and exosomes mediate cell-to-cell interaction6, 8.
Exosome effects on immunoresponse
Exosomes communicate with cells, participate in the cascade of antigen presentation, and are implicated in various essential immunological processes such as immune surveillance44. Exosomes derived from dendritic cells are antigen-presenting, and have been used for treatment of brain tumor in phase I and II clinical trials45, 46. Exosomes can cross the BBB and transfer brain antigens to the periphery, and regulate the peripheral immune system47, 48. Exosomes secreted by resident brain cells in response to pathogenic stimuli also influence bystander cells by the transfer of dysregulated miRs that suppress the expression of essential genes in the recipient cells49. Stroke and central nervous system (CNS) neuroinflammatory diseases, such as multiple sclerosis, regulate peripheral immune response via exosomes47, 50. Microglial-Exos and astrocyte-Exos store and release the inflammatory cytokine IL-1β51, 52.
Exosomes also transfer pro-inflammatory messages from the periphery to recipient brain cells. Balusu et al. found that the choroid plexus epithelium (CPE) cells sense and transmit peripheral inflammatory signals to the brain via the release of exosomes.53 These CPE-Exos enter brain parenchyma and are taken up by astrocytes and microglia, inducing miR target repression and inflammatory gene up-regulation53. Microglia-derived extracellular vesicles can stimulate neuronal activity and participate in the propagation of inflammatory signals54. Exosomes isolated from circulating immune cells from conditions of environmental enrichment increase oligodendrocyte progenitor cell differentiation into myelinating cells in cultured hippocampal slices and promote myelination in vivo when intranasally administered to naïve rats55. Thus, exosomes as mediators of neuroinflammation may impact stroke outcome49.
Exosome effects on thrombosis
Circulating exosomes participate in the coagulation cascade by providing a surface for the assembly of clotting factors 56. In intracerebral hemorrhage (ICH), CSF and plasma pro-coagulant microvesicle (MV)/Exo levels are significantly increased, and may contribute to stroke pathogenesis57. Platelet-MVs/Exos have 50–100 fold higher specific pro-coagulant activity than activated platelets58. Circulating MVs/Exos derived from endothelial cells and blood cells may promote procoagulant activity and thrombin generation59. However, compared with MVs and apoptotic vesicles, exosomes have reduced coagulation and immunogenic effects60. Manipulating thrombosis and the coagulation cascade via exosomes as an intervention for ischemic stroke, warrants investigation.
Therapeutic effects of exosomes on stroke
Exosomes transport cell type-specific molecular cargo extracellularly and over large distances. In addition, the same exosomes may evoke differential response in different cells. Neural released exosomes not only regulate the onset and progression of neurodegenerative and neuroinflammatory diseases, but also may play a role in the regeneration and remodeling of the nervous system after stroke61. Neural secreted exosomes contribute to local synaptic plasticity, and also influence neuronal networks by long-range communication within the CNS. Therefore, by inhibiting their release from diseased cells and/or by manipulating their cargo to enable shuttling of secretory RNA, miR or molecules such as cytokines, chemokines, and growth factors62, exosomes may function as therapeutic agents56.
Advantages of using stem cell-derived exosomes for stroke therapy
Cell-based therapies for stroke improve neurological outcome63–66. The mechanisms of cell-based therapy induced therapeutic effects after stroke are not mediated via cell-replacement or transplanted cell differentiation into brain cells14, 66. Secreted paracrine factors from stem cells are the principal mechanism underlying their therapeutic action in stroke14. Using stem cell-secreted paracrine factors and cell–free therapy are likely safer alternatives in promoting brain plasticity after stroke, and in neurodegenerative disease. Recently, a variety of cell types have been shown to secrete paracrine factors that are contained within membrane vesicles, such as exosomes, microvesicles, ectosomes, membrane particles, and apoptotic bodies18. Extracellular vesicles have emerged as important mediators of intercellular communication, being involved in the transmission of biological signals between cells56. Treatment of stroke and neural injury with extracellular vesicles, i.e. exosomes, harvested from MSCs, rather than the exosome parent MSCs supplants the therapeutic benefits of administration of the parent MSCs8, 10. Exosomes are specifically internalized by recipient cells, which avoids a multiplicity of potential concerns associated with administration of living cells, and exosomes provide therapeutic benefit, at least the equivalent of their cellular source. Compared to cell-therapy, the advantages of exosome-based therapy include9, 14: 1) low immunogenicity56; 2) no vascular obstructive effect, and reduced risk of secondary microvascular thrombosis14; 3) systemically injected exosomes are able to cross the BBB and enter the brain parenchyma67, 68; 4) the potential to develop large scale cellular factories of engineered therapeutic vesicles17; 5) exosomes have higher surface/volume ratio and amplify ligand gated signaling pathways and the transfer of biomolecules from stem cells to target tissues; 6) ability to readily modify exosome microRNA (miR) content. Here, we review the possible application of exosomes for the treatment of stroke, to provide a safe and effective alternative to cell-based therapy7, 69. In the following section, we discuss exosome therapy for stroke, which is summarized in Figure 1.
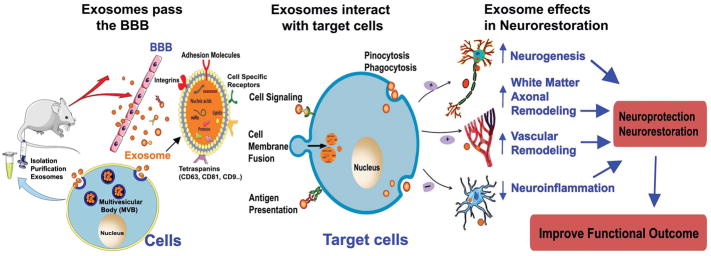
Figure 1. Summary the therapeutic effects of exosome in stroke.
1) Intravenous administration of exosomes can pass the BBB and are taken up by endogenous brain cells. 2) Exosomes contain miRs, mRNA and proteins (such as CD81, CD63, CD9, Alix et al, as well as cell type specific antigens); 3) Exosomes interact with target cells and transfer their RNA, miR and protein content by: A: the endocytosis route (pinocytosis and phagocytosis); B: direct fusion with the plasma membrane; C: binding to target cell via ligand-receptor interactions (such as integrins, tetraspanins and intercellular adhesion molecules). 4) Exosomes communicate with endogenous brain cells and induce neurogenesis, white matter/axonal and vascular remodeling, as well as inhibit neuroinflammation, and thereby promote neuroprotective or neurorestorative effects, as well as improve functional outcome after stroke.
MSC-Exosome (MSC-Exo) for treatment of stroke
Compared to other cell types, the MSC is a prolific exosome producer70. Using proteomic analysis, Otero-Ortega identified more than 2000 proteins in MSC-Exo, many of which may be implicated in brain repair67. MSCs induce neurological recovery post stroke and neural injury, primarily by paracrine effects via MSC secreted exosomes, which mediate restorative actions of MSCs10, 71–73. MSC-Exos taken up by endothelial cells, dose-dependently increase endothelial cell proliferation, migration, and capillary tube formation, as well as impair T-cell function by inhibiting T cell proliferation in vitro74. Systemically injecting bone marrow derived MSC-Exo at one day after ischemic stroke or traumatic brain injury (TBI) significantly improves functional outcome, as well as enhances angiogenesis, neurogenesis and neurite remodeling in rats10, 75–78. Similarly, hMSC-Exo treatment of stroke increases long-term neuroprotection, promotes neuroregeneration, enhances neurological recovery, and modulates peripheral post-stroke immune responses, but does not affect cerebral immune cell infiltration in mice79. MSC-Exos significantly improve functional outcome and reduce structural injury, and show promise in treating global hypoxic-ischemic injury of the fetal brain80. hMSC-Exo treatment dose-dependently reduces brain neuroinflammation after TBI in mice81, and significantly ameliorates inflammation-induced neuronal cellular degeneration, reduces microgliosis and prevents reactive astrogliosis, as well as improves functional recovery in TBI animals82, 83. Adipose-derived MSC-Exo treatment initiated at 3h after ischemic stroke was safe, decreasing lesion volume, increasing angiogenesis, demonstrating anti-inflammatory and immunomodulatory capacity, as well as improving neurological function in rats84. Intravenous administration of MSC-Exos to rats subjected to intracerebral hemorrhage (ICH) significantly promotes white matter/axonal remodeling identified by fiber tract integrity, white matter repair and axonal sprouting, as well as decreases neurological functional deficits compared with the control group at 28 days after ICH67.
Multicellular sources of microparticles or exosomes as a treatment of stroke
In addition to MSC-Exo, exosomes derived from other cell types also induce neuroprotective and neurorestorative effects after stroke. Exosomes derived from human adipose-derived stem cells increase expression of protein-kinaseCδII in immortalized mouse hippocampal cell line and increase neuronal survival and proliferation85. Platelet derived microparticles dose-dependently increase endogenous neural stem cell proliferation, neurogenesis and angiogenesis in the ischemic brain and significantly improve neurological functional outcome after ischemic stroke in rats86. Microparticles derived from activated platelets contain a variety of growth factors augmenting endogenous neural progenitor cell proliferation and neurovascular remodeling, which may be utilized for stroke therapy86, 87. Altmann et al. showed that secretomes derived from rat and human apoptotic mononuclear cells also induce neuroprotective effects identified by decreased lesion volume and improved functional neurological outcome88. Therefore, exosomes and microvesicles derived from a variety of cells induce angiogenesis, suppress apoptosis and stimulate cell proliferation, promote neurogenesis and synaptic plasticity, deliver immunomodulatory signals, as well as recruit and/or reprogram cells, restorative events, that in-concert improve functional recovery after stroke.
Exosome treatment effects by transfer of microRNA (miR)
Exosomes contain miRs which play important roles in cell function, disease, and immunomodulation7, 8, 89. Systemic administration of MSC-Exos improve neurological functional outcome in animal models of stroke, impacting post transcriptional gene expression and ensuing protein expression in their target cells via transfer of miRs76. MiRs are short sequences of non-coding RNA that function in RNA silencing and post-transcriptional regulation of gene expression90. Among their myriad of functional properties, miRs also regulate neurovascular remodeling, inflammation and stem cell biology91. We also note, that MSC harvested exosomes also stimulate endogenous brain cells to subsequently release miRs, in a “chain-reaction” like manner, ultimately promoting brain plasticity after stroke92. In addition, MSCs inhibit macrophage activation by shedding miR-containing exosomes89. In vitro data show that exosomes derived from environmental enrichment serum promote oligodendrocyte precursor cell differentiation into myelinating cells and reduce oxidative stress55. MSC-Exos promote axonal growth, and inhibition of argonaut 2 protein (a primary miR machinery protein) abolishes MSC-Exos induced axonal growth75. MSCs communicate with brain parenchymal cells and regulate neurite outgrowth by transfer of miRs, such as, miR-133b to neural cells via exosomes76. Collectively, these data demonstrate that exosomes mediate their functional benefit in stroke at least partially by the transfer of miRs to parenchymal cells.
Modification of exosomes
Exosomes have low toxicity, high stability in the circulation, and high efficiency of transport to donor cells. Modified exosomes have been used as vehicles to transport exogenous chemical compounds to recipient cells. Exosomes are valuable for the delivery of RNA interference and miR regulatory molecules, in addition to other single-stranded oligonucleotides93. Intravenous administration of neuronal-targeted exosomes loaded with specific siRNA knocked down their target genes in neurons94. Curcumin, an anti-inflammatory agent, can be encapsulated in exosomes95. Intranasal administration of curcumin-encapsulated exosomes in ischemic stroke mice significantly reduced astrogliosis and increased the expression of NeuN as well as vascular endothelial tight junction proteins expression when compared to non-treated stroke mice95.
Exosomes also contain distinct subsets of miRs depending upon the cell type source96. Modulation of miRs within stem cells and thereby within exosomes derived from the parent cell, may enhance exosome induced therapeutic efficacy. MSC-Exo can be enriched with specific miRs to enhance recovery of injured tissues8, 14. MSCs release functional small RNAs via their exosomes10, 71–73. In vitro, the miR-17-92 cluster promotes oligodendrogenesis, neurogenesis, and axonal outgrowth97. Treatment of stroke in rat with MSC-Exo and miR-17-92 enriched MSC-Exo both significantly improved neurological functional recovery, but miR-17-92 cluster enriched exosome treatment induced significantly more improvement of functional outcome and enhancement of neurogenesis, oligodendrogenesis and neuronal dendrite plasticity in the ischemic brain than the control MSC-Exo treatment97. Tailored exosomes derived from MSCs further enhance neurite growth via the phosphatase and tensin homologue/mammalian target of rapamycin signals by increasing the miR-17-92 cluster98. Exosomes derived from miR-133b-overexpressed MSCs as well as miR-17-92 cluster enriched exosomes significantly increase brain plasticity and neurological functional recovery after stroke compared to MSC-Exo treated stroke rats97, 99. In vitro data also show that exosomes harvested from astrocytes subjected to oxygen glucose deptrivation (OGD) treated with miR-133b-overexpressing MSC-Exo significantly increased neurite outgrowth in cultured primary cortical neurons compared with the exosomes derived from OGD astrocytes subjected to MSC-Exo-Control99. Thus, in vivo and in vitro data suggest that modulating miR content of exosome may be an effective means to amplify the therapeutic effects of exosomes for the treatment of stroke and neurological injury, as well as degenerative diseases8.
Caveats and future studies
Although exosomes exhibit very promising therapeutic effects, exosome-based therapy for stroke has just recently emerged, and many additional studies are warranted in order to move this therapy to the clinic. Among the considerations and studies to be performed are the following: 1) Scaling production of exosomes for human clinical trials will be required. Recent publications suggest that these production and scaling methods are actively being pursued21, 22. In addition, cell culture conditions and storage methods may have a major impact on the exosome content and their functionality. Appropriate exosome isolation methods, storage, and functional read-out systems need to be standardized. 2) Exosome content, function and activity depend on the generating cells of origin. Therefore, exosome cell source, including age, gender, comorbidities and other factors associated with the exosome generating cells should be optimized. 3) Although initial preclinical studies have shown that a single dose of exosomes administered post stroke is highly efficacious in promoting neurological recovery, we cannot exclude the possibility that multiple dosing, particularly for different types of stroke may further improve neurological outcomes. 4) Dose-response and therapeutic window studies are required. Given the reported very extended therapeutic window for treatment of stroke with cell based therapies, exosomes may likewise provide an extended therapeutic window. Previous studies have shown that exosome treatment initiated at 24h after brain hemorrhage or ischemic stroke, significantly improves functional outcome, decreases lesion volume and promotes axonal/WM remodeling for both hemorrhage and stroke10, 67, 76, 79, 97, 99. Adipose-derived mesenchymal stem cell (ADMSC) derived exosome treatment of stroke initiated at 50 mins or 3h after stroke reduced lesion volume and enhanced neurological recovery in rat84. Thus, exosome treatment of stroke not only induces neurorestoration, but also promotes neuroprotection. However, some therapeutic interventions provide therapeutic benefit in the acute phase of stroke, but impair regeneration in the chronic phase, and vice versa100. Therefore, investigation of the optimal timing of exosome therapy, which may be affected by the parental cell source, is required. 5) Of primary importance, is the performance of safety studies for stroke using exosomes. These studies should include safety in patients with co-morbidities. Particularly, studies should be performed to ensure that the restorative exosomes are not oncogenic, and further promote tumor growth. Enhancing tissue regeneration after stroke in the CNS may increase the risk to activate cancer100. Induction of neurovascular remodeling is itself associated with angiogenic and cell proliferative events 101. In addition, exosomes contain many microRNAs, such as miR-9, miR-223 and miR-126 which not only induce neural repair or axonal growth responses, but are also closely linked with oncogenesis102–105. Potential oncogenic features of extracellular vesicles or exosomes are being addressed in the literature 106. Further studies, however, are warranted to evaluate the oncogenic potential of restorative therapy for stroke. Therefore, safety, time window, dose-response, multiple dosing, and oncogenic potential studies, among others, are required for effective and safe translation of this very promising restorative and neuroprotective therapeutic approach for the treatment of stroke.
Conclusion
Exosomes have multifaceted roles in the regulation of physiological and pathological processes, and importantly may also function as therapeutic agents. Exosomes derived from different cells can induce neuroprotection and neurorestorative effects by modulating gene, protein and miR expression in their target cells and tissues. Exosomes from specific cells, such as MSCs, and likely other cells, reduce inflammation, and increase angiogenesis, neurogenesis and white matter remodeling after stroke. Modified exosomes can be used as vehicles to transport exogenous genes, proteins and chemical compounds to recipient cells. Modulation of genes and miRs in exosome parent cells enhances exosome induced therapeutic efficacy72, 76, 97. Therefore, exosomes may potentially be used as personalized targeted drug delivery vehicles6, 9. However, we should cautiously and carefully develop this promising therapy. Safety, time window and dose-response studies are just a part of the range of investigations that will be performed prior to the translation of exosomes into the clinic for the treatment of stroke, and likely other forms of neurological injury and degenerative diseases.
Acknowledgments
This work was supported by National Institute of Neurological Disorders and Stroke R01NS083078 (JC), R01NS099030 (JC), R01NS097747 (JC) and R01NS088656 (MC).
Footnotes
Disclosures: None
References
- 1.Ailawadi S, Wang X, Gu H, Fan GC. Pathologic function and therapeutic potential of exosomes in cardiovascular disease. Biochimica et biophysica acta. 2015;1852:1–11. doi: 10.1016/j.bbadis.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belting M, Christianson HC. Role of exosomes and microvesicles in hypoxia-associated tumour development and cardiovascular disease. Journal of internal medicine. 2015;278:251–263. doi: 10.1111/joim.12393. [DOI] [PubMed] [Google Scholar]
- 3.Colombo E, Borgiani B, Verderio C, Furlan R. Microvesicles: Novel biomarkers for neurological disorders. Frontiers in physiology. 2012;3:63. doi: 10.3389/fphys.2012.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Musante L, Tataruch DE, Holthofer H. Use and isolation of urinary exosomes as biomarkers for diabetic nephropathy. Frontiers in endocrinology. 2014;5:149. doi: 10.3389/fendo.2014.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Javeed N, Mukhopadhyay D. Exosomes and their role in the micro-/macro-environment: A comprehensive review. Journal of biomedical research. 2016:30. doi: 10.7555/JBR.30.20150162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chopp M, Zhang ZG. Emerging potential of exosomes and noncoding micrornas for the treatment of neurological injury/diseases. Expert opinion on emerging drugs. 2015;20:523–526. doi: 10.1517/14728214.2015.1061993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki E, Fujita D, Takahashi M, Oba S, Nishimatsu H. Stem cell-derived exosomes as a therapeutic tool for cardiovascular disease. World journal of stem cells. 2016;8:297–305. doi: 10.4252/wjsc.v8.i9.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang ZG, Chopp M. Exosomes in stroke pathogenesis and therapy. The Journal of clinical investigation. 2016;126:1190–1197. doi: 10.1172/JCI81133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luarte A, Batiz LF, Wyneken U, Lafourcade C. Potential therapies by stem cell-derived exosomes in cns diseases: Focusing on the neurogenic niche. Stem cells international. 2016;2016:5736059. doi: 10.1155/2016/5736059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2013;33:1711–1715. doi: 10.1038/jcbfm.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kourembanas S. Exosomes: Vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annual review of physiology. 2015;77:13–27. doi: 10.1146/annurev-physiol-021014-071641. [DOI] [PubMed] [Google Scholar]
- 12.Lopez-Verrilli MA, Court FA. Exosomes: Mediators of communication in eukaryotes. Biological research. 2013;46:5–11. doi: 10.4067/S0716-97602013000100001. [DOI] [PubMed] [Google Scholar]
- 13.Simons M, Raposo G. Exosomes--vesicular carriers for intercellular communication. Current opinion in cell biology. 2009;21:575–581. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Xin H, Li Y, Chopp M. Exosomes/mirnas as mediating cell-based therapy of stroke. Front Cell Neurosci. 2014;8:377. doi: 10.3389/fncel.2014.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Current protocols in cell biology. 2006;Chapter 3(Unit 3.22) doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- 16.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mrnas and micrornas is a novel mechanism of genetic exchange between cells. Nature cell biology. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 17.Choi DS, Kim DK, Kim YK, Gho YS. Proteomics of extracellular vesicles: Exosomes and ectosomes. Mass spectrometry reviews. 2015;34:474–490. doi: 10.1002/mas.21420. [DOI] [PubMed] [Google Scholar]
- 18.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nature reviews Immunology. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 19.Lakkaraju A, Rodriguez-Boulan E. Itinerant exosomes: Emerging roles in cell and tissue polarity. Trends in cell biology. 2008;18:199–209. doi: 10.1016/j.tcb.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ling H, Fabbri M, Calin GA. Micrornas and other non-coding rnas as targets for anticancer drug development. Nature reviews Drug discovery. 2013;12:847–865. doi: 10.1038/nrd4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu R, Greening DW, Zhu HJ, Takahashi N, Simpson RJ. Extracellular vesicle isolation and characterization: Toward clinical application. The Journal of clinical investigation. 2016;126:1152–1162. doi: 10.1172/JCI81129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greening DW, Xu R, Ji H, Tauro BJ, Simpson RJ. A protocol for exosome isolation and characterization: Evaluation of ultracentrifugation, density-gradient separation, and immunoaffinity capture methods. Methods in molecular biology. 2015;1295:179–209. doi: 10.1007/978-1-4939-2550-6_15. [DOI] [PubMed] [Google Scholar]
- 23.Lasser C, Eldh M, Lotvall J. Isolation and characterization of rna-containing exosomes. Journal of visualized experiments : JoVE. 2012:e3037. doi: 10.3791/3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tauro BJ, Greening DW, Mathias RA, Ji H, Mathivanan S, Scott AM, et al. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line lim1863-derived exosomes. Methods (San Diego, Calif) 2012;56:293–304. doi: 10.1016/j.ymeth.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Kalra H, Adda CG, Liem M, Ang CS, Mechler A, Simpson RJ, et al. Comparative proteomics evaluation of plasma exosome isolation techniques and assessment of the stability of exosomes in normal human blood plasma. Proteomics. 2013;13:3354–3364. doi: 10.1002/pmic.201300282. [DOI] [PubMed] [Google Scholar]
- 26.Xu R, Simpson RJ, Greening DW. A protocol for isolation and proteomic characterization of distinct extracellular vesicle subtypes by sequential centrifugal ultrafiltration. Methods in molecular biology. 2017;1545:91–116. doi: 10.1007/978-1-4939-6728-5_7. [DOI] [PubMed] [Google Scholar]
- 27.Katakowski M, Buller B, Zheng X, Lu Y, Rogers T, Osobamiro O, et al. Exosomes from marrow stromal cells expressing mir-146b inhibit glioma growth. Cancer letters. 2013;335:201–204. doi: 10.1016/j.canlet.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ibsen SD, Wright J, Lewis JM, Kim S, Ko SY, Ong J, et al. Rapid isolation and detection of exosomes and associated biomarkers from plasma. 2017 doi: 10.1021/acsnano.7b00549. [DOI] [PubMed] [Google Scholar]
- 29.Welton JL, Webber JP, Botos LA, Jones M, Clayton A. Ready-made chromatography columns for extracellular vesicle isolation from plasma. Journal of extracellular vesicles. 2015;4:27269. doi: 10.3402/jev.v4.27269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang YT, Huang YY, Zheng L, Qin SH, Xu XP, An TX, et al. Comparison of isolation methods of exosomes and exosomal rna from cell culture medium and serum. International journal of molecular medicine. 2017;40:834–844. doi: 10.3892/ijmm.2017.3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeppesen DK, Hvam ML, Primdahl-Bengtson B, Boysen AT, Whitehead B, Dyrskjot L, et al. Comparative analysis of discrete exosome fractions obtained by differential centrifugation. Journal of extracellular vesicles. 2014;3:25011. doi: 10.3402/jev.v3.25011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baranyai T, Herczeg K, Onodi Z, Voszka I, Modos K, Marton N, et al. Isolation of exosomes from blood plasma: Qualitative and quantitative comparison of ultracentrifugation and size exclusion chromatography methods. PloS one. 2015;10:e0145686. doi: 10.1371/journal.pone.0145686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Deun J, Mestdagh P, Sormunen R, Cocquyt V, Vermaelen K, Vandesompele J, et al. The impact of disparate isolation methods for extracellular vesicles on downstream rna profiling. Journal of extracellular vesicles. 2014:3. doi: 10.3402/jev.v3.24858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor DD, Zacharias W, Gercel-Taylor C. Exosome isolation for proteomic analyses and rna profiling. Methods in molecular biology. 2011;728:235–246. doi: 10.1007/978-1-61779-068-3_15. [DOI] [PubMed] [Google Scholar]
- 35.Lee M, Ban J-J, Im W, Kim M. Influence of storage condition on exosome recovery. Biotechnology and Bioprocess Engineering. 2016;21:299–304. [Google Scholar]
- 36.Tian T, Zhu YL, Zhou YY, Liang GF, Wang YY, Hu FH, et al. Exosome uptake through clathrin-mediated endocytosis and macropinocytosis and mediating mir-21 delivery. The Journal of biological chemistry. 2014;289:22258–22267. doi: 10.1074/jbc.M114.588046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feng D, Zhao WL, Ye YY, Bai XC, Liu RQ, Chang LF, et al. Cellular internalization of exosomes occurs through phagocytosis. Traffic (Copenhagen, Denmark) 2010;11:675–687. doi: 10.1111/j.1600-0854.2010.01041.x. [DOI] [PubMed] [Google Scholar]
- 38.Svensson KJ, Christianson HC, Wittrup A, Bourseau-Guilmain E, Lindqvist E, Svensson LM, et al. Exosome uptake depends on erk1/2-heat shock protein 27 signaling and lipid raft-mediated endocytosis negatively regulated by caveolin-1. The Journal of biological chemistry. 2013;288:17713–17724. doi: 10.1074/jbc.M112.445403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fitzner D, Schnaars M, van Rossum D, Krishnamoorthy G, Dibaj P, Bakhti M, et al. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. Journal of cell science. 2011;124:447–458. doi: 10.1242/jcs.074088. [DOI] [PubMed] [Google Scholar]
- 40.Tian T, Zhu YL, Hu FH, Wang YY, Huang NP, Xiao ZD. Dynamics of exosome internalization and trafficking. Journal of cellular physiology. 2013;228:1487–1495. doi: 10.1002/jcp.24304. [DOI] [PubMed] [Google Scholar]
- 41.Rana S, Zoller M. Exosome target cell selection and the importance of exosomal tetraspanins: A hypothesis. Biochemical Society transactions. 2011;39:559–562. doi: 10.1042/BST0390559. [DOI] [PubMed] [Google Scholar]
- 42.Rana S, Yue S, Stadel D, Zoller M. Toward tailored exosomes: The exosomal tetraspanin web contributes to target cell selection. The international journal of biochemistry & cell biology. 2012;44:1574–1584. doi: 10.1016/j.biocel.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 43.Haqqani AS, Delaney CE, Tremblay TL, Sodja C, Sandhu JK, Stanimirovic DB. Method for isolation and molecular characterization of extracellular microvesicles released from brain endothelial cells. Fluids and barriers of the CNS. 2013;10:4. doi: 10.1186/2045-8118-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raposo G, Stoorvogel W. Extracellular vesicles: Exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katakowski M, Chopp M. Exosomes as tools to suppress primary brain tumor. Cellular and molecular neurobiology. 2016;36:343–352. doi: 10.1007/s10571-015-0280-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pitt JM, Andre F, Amigorena S, Soria JC, Eggermont A, Kroemer G, et al. Dendritic cell-derived exosomes for cancer therapy. The Journal of clinical investigation. 2016;126:1224–1232. doi: 10.1172/JCI81137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Selmaj I, Mycko MP, Raine CS, Selmaj KW. The role of exosomes in cns inflammation and their involvement in multiple sclerosis. Journal of neuroimmunology. 2017;306:1–10. doi: 10.1016/j.jneuroim.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 48.de Rivero Vaccari JP, Brand F, 3rd, Adamczak S, Lee SW, Perez-Barcena J, Wang MY, et al. Exosome-mediated inflammasome signaling after central nervous system injury. Journal of neurochemistry. 2016;136(Suppl 1):39–48. doi: 10.1111/jnc.13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gupta A, Pulliam L. Exosomes as mediators of neuroinflammation. Journal of neuroinflammation. 2014;11:68. doi: 10.1186/1742-2094-11-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen J, Cui C, Yang X, Xu J, Venkat P, Zacharek A, et al. Mir-126 affects brain-heart interaction after cerebral ischemic stroke. Translational stroke research. 2017;8:374–385. doi: 10.1007/s12975-017-0520-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bianco F, Pravettoni E, Colombo A, Schenk U, Moller T, Matteoli M, et al. Astrocyte-derived atp induces vesicle shedding and il-1 beta release from microglia. Journal of immunology. 2005;174:7268–7277. doi: 10.4049/jimmunol.174.11.7268. [DOI] [PubMed] [Google Scholar]
- 52.Bianco F, Perrotta C, Novellino L, Francolini M, Riganti L, Menna E, et al. Acid sphingomyelinase activity triggers microparticle release from glial cells. The EMBO journal. 2009;28:1043–1054. doi: 10.1038/emboj.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Balusu S, Van Wonterghem E, De Rycke R, Raemdonck K, Stremersch S, Gevaert K, et al. Identification of a novel mechanism of blood-brain communication during peripheral inflammation via choroid plexus-derived extracellular vesicles. 2016;8:1162–1183. doi: 10.15252/emmm.201606271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turola E, Furlan R, Bianco F, Matteoli M, Verderio C. Microglial microvesicle secretion and intercellular signaling. Frontiers in physiology. 2012;3:149. doi: 10.3389/fphys.2012.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pusic KM, Pusic AD, Kraig RP. Environmental enrichment stimulates immune cell secretion of exosomes that promote cns myelination and may regulate inflammation. Cellular and molecular neurobiology. 2016;36:313–325. doi: 10.1007/s10571-015-0269-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.SELA, Mager I, Breakefield XO, Wood MJ. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nature reviews Drug discovery. 2013;12:347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 57.Huang M, Hu YY, Dong XQ. High concentrations of procoagulant microparticles in the cerebrospinal fluid and peripheral blood of patients with acute basal ganglia hemorrhage are associated with poor outcome. Surgical neurology. 2009;72:481–489. doi: 10.1016/j.surneu.2008.12.016. discussion 489. [DOI] [PubMed] [Google Scholar]
- 58.Sinauridze EI, Kireev DA, Popenko NY, Pichugin AV, Panteleev MA, Krymskaya OV, et al. Platelet microparticle membranes have 50- to 100-fold higher specific procoagulant activity than activated platelets. Thrombosis and haemostasis. 2007;97:425–434. [PubMed] [Google Scholar]
- 59.Horn P, Erkilet G, Veulemans V, Kropil P, Schurgers L, Zeus T, et al. Microparticle-induced coagulation relates to coronary artery atherosclerosis in severe aortic valve stenosis. PloS one. 2016;11:e0151499. doi: 10.1371/journal.pone.0151499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muhsin-Sharafaldine MR, Saunderson SC, Dunn AC, Faed JM, Kleffmann T, McLellan AD. Procoagulant and immunogenic properties of melanoma exosomes, microvesicles and apoptotic vesicles. Oncotarget. 2016;7:56279–56294. doi: 10.18632/oncotarget.10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Porro C, Trotta T, Panaro MA. Microvesicles in the brain: Biomarker, messenger or mediator? Journal of neuroimmunology. 2015;288:70–78. doi: 10.1016/j.jneuroim.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 62.Martinez MC, Tual-Chalot S, Leonetti D, Andriantsitohaina R. Microparticles: Targets and tools in cardiovascular disease. Trends in pharmacological sciences. 2011;32:659–665. doi: 10.1016/j.tips.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 63.Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke; a journal of cerebral circulation. 2001;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 64.Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, et al. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke; a journal of cerebral circulation. 2001;32:2682–2688. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- 65.Steinberg GK, Kondziolka D, Wechsler LR, Lunsford LD, Coburn ML, Billigen JB, et al. Clinical outcomes of transplanted modified bone marrow-derived mesenchymal stem cells in stroke: A phase 1/2a study. Stroke; a journal of cerebral circulation. 2016;47:1817–1824. doi: 10.1161/STROKEAHA.116.012995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen J, Venkat P, Zacharek A, Chopp M. Neurorestorative therapy for stroke. Frontiers in human neuroscience. 2014;8:382. doi: 10.3389/fnhum.2014.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Otero-Ortega L, Gomez de Frutos MC, Laso-Garcia F, Rodriguez-Frutos B, Medina-Gutierrez E, Lopez JA, et al. Exosomes promote restoration after an experimental animal model of intracerebral hemorrhage. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2017 doi: 10.1177/0271678X17708917. 271678x17708917. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 68.Chen CC, Liu L, Ma F, Wong CW, Guo XE, Chacko JV, et al. Elucidation of exosome migration across the blood-brain barrier model in vitro. Cellular and molecular bioengineering. 2016;9:509–529. doi: 10.1007/s12195-016-0458-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Beer L, Mildner M, Gyongyosi M, Ankersmit HJ. Peripheral blood mononuclear cell secretome for tissue repair. Apoptosis : an international journal on programmed cell death. 2016;21:1336–1353. doi: 10.1007/s10495-016-1292-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yeo RW, Lai RC, Zhang B, Tan SS, Yin Y, Teh BJ, et al. Mesenchymal stem cell: An efficient mass producer of exosomes for drug delivery. Advanced drug delivery reviews. 2013;65:336–341. doi: 10.1016/j.addr.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 71.Xu JF, Yang GH, Pan XH, Zhang SJ, Zhao C, Qiu BS, et al. Altered microrna expression profile in exosomes during osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. PloS one. 2014;9:e114627. doi: 10.1371/journal.pone.0114627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xin H, Li Y, Liu Z, Wang X, Shang X, Cui Y, et al. Mir-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem cells. 2013;31:2737–2746. doi: 10.1002/stem.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baglio SR, Rooijers K, Koppers-Lalic D, Verweij FJ, Perez Lanzon M, Zini N, et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive mirna and trna species. Stem cell research & therapy. 2015;6:127. doi: 10.1186/s13287-015-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bian S, Zhang L, Duan L, Wang X, Min Y, Yu H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. Journal of molecular medicine. 2014;92:387–397. doi: 10.1007/s00109-013-1110-5. [DOI] [PubMed] [Google Scholar]
- 75.Zhang Y, Chopp M, Liu XS, Katakowski M, Wang X, Tian X, et al. Exosomes derived from mesenchymal stromal cells promote axonal growth of cortical neurons. Molecular neurobiology. 2017;54:2659–2673. doi: 10.1007/s12035-016-9851-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang X, et al. Exosome-mediated transfer of mir-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem cells. 2012;30:1556–1564. doi: 10.1002/stem.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xiong Y, Mahmood A, Chopp M. Emerging potential of exosomes for treatment of traumatic brain injury. Neural regeneration research. 2017;12:19–22. doi: 10.4103/1673-5374.198966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xiong Y, Zhang Y, Mahmood A, Chopp M. Investigational agents for treatment of traumatic brain injury. Expert opinion on investigational drugs. 2015;24:743–760. doi: 10.1517/13543784.2015.1021919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Doeppner TR, Herz J, Gorgens A, Schlechter J, Ludwig AK, Radtke S, et al. Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem cells translational medicine. 2015;4:1131–1143. doi: 10.5966/sctm.2015-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ophelders DR, Wolfs TG, Jellema RK, Zwanenburg A, Andriessen P, Delhaas T, et al. Mesenchymal stromal cell-derived extracellular vesicles protect the fetal brain after hypoxia-ischemia. Stem cells translational medicine. 2016;5:754–763. doi: 10.5966/sctm.2015-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim DK, Nishida H, An SY, Shetty AK, Bartosh TJ, Prockop DJ. Chromatographically isolated cd63+cd81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after tbi. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:170–175. doi: 10.1073/pnas.1522297113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang Y, Chopp M, Meng Y, Katakowski M, Xin H, Mahmood A, et al. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. Journal of neurosurgery. 2015;122:856–867. doi: 10.3171/2014.11.JNS14770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Drommelschmidt K, Serdar M, Bendix I, Herz J, Bertling F, Prager S, et al. Mesenchymal stem cell-derived extracellular vesicles ameliorate inflammation-induced preterm brain injury. Brain, behavior, and immunity. 2017;60:220–232. doi: 10.1016/j.bbi.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 84.Chen KH, Chen CH, Wallace CG, Yuen CM, Kao GS, Chen YL, et al. Intravenous administration of xenogenic adipose-derived mesenchymal stem cells (admsc) and admsc-derived exosomes markedly reduced brain infarct volume and preserved neurological function in rat after acute ischemic stroke. Oncotarget. 2016;7:74537–74556. doi: 10.18632/oncotarget.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.El Bassit G, Patel RS, Carter G, Shibu V, Patel AA, Song S, et al. Malat1 in human adipose stem cells modulates survival and alternative splicing of pkcdeltaii in ht22 cells. Endocrinology. 2017;158:183–195. doi: 10.1210/en.2016-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hayon Y, Dashevsky O, Shai E, Brill A, Varon D, Leker RR. Platelet microparticles induce angiogenesis and neurogenesis after cerebral ischemia. Current neurovascular research. 2012;9:185–192. doi: 10.2174/156720212801619018. [DOI] [PubMed] [Google Scholar]
- 87.Hayon Y, Shai E, Varon D, Leker RR. The role of platelets and their microparticles in rehabilitation of ischemic brain tissue. CNS & neurological disorders drug targets. 2012;11:921–925. doi: 10.2174/1871527311201070921. [DOI] [PubMed] [Google Scholar]
- 88.Altmann P, Mildner M, Haider T, Traxler D, Beer L, Ristl R, et al. Secretomes of apoptotic mononuclear cells ameliorate neurological damage in rats with focal ischemia. F1000Research. 2014;3:131. doi: 10.12688/f1000research.4219.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Phinney DG, Di Giuseppe M, Njah J, Sala E, Shiva S, St Croix CM, et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle micrornas. 2015;6:8472. doi: 10.1038/ncomms9472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ambros V. The functions of animal micrornas. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 91.Sen CK. Micrornas as new maestro conducting the expanding symphony orchestra of regenerative and reparative medicine. Physiological genomics. 2011;43:517–520. doi: 10.1152/physiolgenomics.00037.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Juranek JK, Geddis MS, Song F, Zhang J, Garcia J, Rosario R, et al. Rage deficiency improves postinjury sciatic nerve regeneration in type 1 diabetic mice. Diabetes. 2013;62:931–943. doi: 10.2337/db12-0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.O’Loughlin AJ, Woffindale CA, Wood MJ. Exosomes and the emerging field of exosome-based gene therapy. Current gene therapy. 2012;12:262–274. doi: 10.2174/156652312802083594. [DOI] [PubMed] [Google Scholar]
- 94.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of sirna to the mouse brain by systemic injection of targeted exosomes. Nature biotechnology. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 95.Kalani A, Chaturvedi P, Kamat PK, Maldonado C, Bauer P, Joshua IG, et al. Curcumin-loaded embryonic stem cell exosomes restored neurovascular unit following ischemia-reperfusion injury. The international journal of biochemistry & cell biology. 2016;79:360–369. doi: 10.1016/j.biocel.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cui C, Ye X, Chopp M, Venkat P, Zacharek A, Yan T, et al. Mir-145 regulates diabetes-bone marrow stromal cell-induced neurorestorative effects in diabetes stroke rats. Stem cells translational medicine. 2016;5:1656–1667. doi: 10.5966/sctm.2015-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xin H, Katakowski M, Wang F, Qian JY, Liu XS, Ali MM, et al. Microrna cluster mir-17-92 cluster in exosomes enhance neuroplasticity and functional recovery after stroke in rats. Stroke; a journal of cerebral circulation. 2017;48:747–753. doi: 10.1161/STROKEAHA.116.015204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang Y, Ueno Y, Liu XS, Buller B, Wang X, Chopp M, et al. The microrna-17-92 cluster enhances axonal outgrowth in embryonic cortical neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:6885–6894. doi: 10.1523/JNEUROSCI.5180-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xin H, Wang F, Li Y, Lu QE, Cheung WL, Zhang Y, et al. Secondary release of exosomes from astrocytes contributes to the increase in neural plasticity and improvement of functional recovery after stroke in rats treated with exosomes harvested from microrna 133b-overexpressing multipotent mesenchymal stromal cells. Cell transplantation. 2017;26:243–257. doi: 10.3727/096368916X693031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Carmichael ST. Emergent properties of neural repair: Elemental biology to therapeutic concepts. Annals of neurology. 2016;79:895–906. doi: 10.1002/ana.24653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, et al. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circulation research. 2003;92:692–699. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- 102.Jayaraman M, Radhakrishnan R, Mathews CA, Yan M, Husain S, Moxley KM, et al. Identification of novel diagnostic and prognostic mirna signatures in endometrial cancer. Genes & cancer. 2017;8:566–576. doi: 10.18632/genesandcancer.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Latchana N, Abrams ZB, Howard JH, Regan K, Jacob N, Fadda P, et al. Plasma microrna levels following resection of metastatic melanoma. Bioinformatics and biology insights. 2017;11:1177932217694837. doi: 10.1177/1177932217694837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang H, Mao F, Shen T, Luo Q, Ding Z, Qian L, et al. Plasma mir-145, mir-20a, mir-21 and mir-223 as novel biomarkers for screening early-stage non-small cell lung cancer. Oncology letters. 2017;13:669–676. doi: 10.3892/ol.2016.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen P, Price C, Li Z, Li Y, Cao D, Wiley A, et al. Mir-9 is an essential oncogenic microrna specifically overexpressed in mixed lineage leukemia-rearranged leukemia. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:11511–11516. doi: 10.1073/pnas.1310144110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fatima F, Nawaz M. Stem cell-derived exosomes: Roles in stromal remodeling, tumor progression, and cancer immunotherapy. Chinese journal of cancer. 2015;34:541–553. doi: 10.1186/s40880-015-0051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]