Abstract
Fecal incontinence and evacuation disorders are common, impair quality of life and incur substantial economic costs worldwide. As symptoms alone are poor predictors of underlying pathophysiology and aetiology, diagnostic tests of anorectal function could facilitate patient management in those cases that are refractory to conservative therapies. In the last decade several major technological advances have improved our understanding of anorectal structure, co-ordination and sensorimotor function.
This Consensus Statement provides the reader with an appraisal of the current indications, study performance characteristics, clinical utility, and strengths and limitations of the most widely available tests of anorectal structure (ultrasonography and MRI) and function (anorectal manometry, neurophysiological investigations, rectal distension techniques and tests of evacuation including defecography). Additionally this article provides our consensus on the clinical relevance of these tests.
INTRODUCTION
Evacuation of bowel contents is a highly regulated and coordinated function of the combined activities of the colon, rectum and anus1. Dysfunction of this unit can lead to fecal incontinence and/or symptoms of an evacuation disorder, and can have a devastating effect on quality of life2. In North America, between 7% and 18% of community dwelling adults fecal incontinence3, 4 and 12–19% of the population report evacuation disorders5 with an age and sex-adjusted incidence approximately 3-fold greater than Crohn’s disease6. Since the underlying aetiology and pathophysiology of fecal incontinence and evacuation disorders is multifactorial, reliance on symptoms alone to guide therapy is inadequate7.
The primary approach to a patient presenting for the first time with fecal incontinence or constipation with difficult defecation should be to exclude serious underlying pathology (such as colorectal malignancy and IBD)8. In patients with symptoms refractory to first-line therapies such as lifestyle modification and optimization of stool consistency, it is justifiable to proceed with evaluation of anorectal structure, motor and sensory function 9, 10. The selection of appropriate investigations is often guided by the clinical history and examination. Such an evaluation should focus on determining the duration, type and severity of the patient’s symptoms as well as identification of risk factors for symptom onset11. Epidemiological studies have identified a number of such risk factors including increasing age, elevated BMI and presence of diarrhea12, 13. In women, obstetric injury is particularly relevant14–16 owing to the risk of damage to the pelvic floor, anal sphincters and pudendal nerves during the second stage of labour17, 18. In men, iatrogenic injury to the sphincter complex secondary to anal surgery is a factor in up to 59% presenting for assessment19, and co-existent benign perianal disease (such as hemorrhoids, fistula-in-ano and radiation proctitis) is also common20. In all patients, particular attention should be paid towards symptoms of other anorectal complaints (for example, fecal incontinence in a patient presenting with constipation) as data increasingly suggest that both fecal incontinence and evacuation disorders commonly co-exist21. Also, anorectal evaluation begins with a carefully performed digital rectal examination that can reveal several abnormalities including dyssynergia, weak anal sphincters, sphincter defects, fecal impaction and others15,23 .
No single test can fully characterize the causes of faecal incontinence and/or an evacuation disorder. Instead, several tests are used to assess anorectal structure, motor and sensory function (Table 1). Data on the usefulness of these tests is conflicting, and some studies suggest that clinical examination alone is an adequate method for stratification of treatment22, 23; however, there are data to suggest that quantification of function can directly influenced clinical decision-making24, 25 and could provide biomarkers that predict response to treatment26–28. However, despite several attempts at consensus29–31, there is widespread discordance in practices between institutions32. Such a disparity probably reflects factors such as access to technology, resource availability and local expertise; nevertheless, it is generally accepted that the clinical utility of tests improves when anorectal function is assessed in a structured and systematic manner33. Thus, the aim of this Consensus Statement is to provide the practicing clinician with a background and framework regarding the indication(s), application and clinical interpretation(s) of tests of anorectal function and notable key advances (Box 1). Recommendations are based on a review of the literature and discussion by members of the International Anorectal Physiology Working Group (IAPWG) under the auspices of the International Working Group for Disorders of Gastrointestinal Motility and Function, a collective of clinicians and academics with particular interest and experience in the field of anorectal function testing.
TABLE 1.
Clinical utility for commonly performed investigations of anorectal physiological function.
| Function | Investigation | Clinical utility |
|---|---|---|
| Anus | ||
| Motor | Anorectal manometry (conventional) | **** |
| Anorectal manometry (high resolution) | **** | |
| Anorectal manometry (3D) | *** | |
| Electromyography | *** | |
| Pudendal nerve terminal motor latencies | * | |
| Structure | Endoanal ultrasonography | **** |
| Transperineal ultrasonography | *** | |
| Endoanal or pelvic MRI | *** | |
| MRI muscle fiber tracking | * | |
| Electrostimulation | * | |
| Sensory | Light touch stimulation | * |
| Anal evoked potentials | ** | |
| Rectum | ||
| Sensory | Balloon distension | **** |
| Rectal barostat | *** | |
| Rectal evoked potentials | ** | |
| Motor | Distal colonic manometry | ** |
| Rectal barostat | *** | |
| Rectal motor evoked potentials | * | |
| Anorectal unit | ||
| Motor | Anorectal manometry(conventional, high-resolution or 3D high resolution) | **** |
| Balloon expulsion | **** | |
| Motor, sensory and structure | Barium defecography | **** |
| Magnetic resonance defecography | *** | |
| Functional lumen imaging probe | * |
Limited clinical utility or of research interest only.
Emerging technology with limited data of clinical utility.
Recognized clinical utility but less commonly performed.
Good clinical utility and commonly performed.
Box 1. Key advances in evaluating anorectal function.
Investigations of anorectal structure, function and sensation are indicated for the assessment of patients with symptoms suggestive of an evacuation disorder and/or faecal incontinence that are unresponsive to conservative therapy
No single investigation can fully assess anorectal function; for this reason, a range of techniques are generally used to characterize pathophysiology and aetiology of symptoms
Anal endosonography and anorectal manometry (ARM) provide an assessment of sphincter structure and function in patients with symptoms of faecal incontinence; ARM with balloon expulsion and defecography identify functional and/or structural pathology in patients with evacuation disorders
Investigations of anorectal sensation are a vital component of assessment in both faecal incontinence and evacuation disorders
Owing to the overlap of normal and abnormal values, the results of such functional investigations should be interpreted carefully taking into context the clinical picture and the multifactorial etiology of anorectal disorders
METHODS
The IAPWG steering committee (E.V.C., S.M.S., H.H., M.F. and S.S.R.) was appointed by the International Working Group for Disorders of Gastrointestinal Motility and Function. Under the guidance of the steering committee, the authors performed focused literature reviews in the following areas: anorectal manometry, anorectal neurophysiology, endoanal ultrasonography, pelvic floor ultrasonography, rectal sensory testing, balloon expulsion and defecography. Consensus was achieved through careful evaluation and discussion of available literature, and expert agreement when recommendations lacked supporting evidence
This Consensus Statement will focus on the following tests that are widely available to the practicing clinician, and can be divided into: tests of anal motor function, anorectal manometry (ARM), high resolution (HR-ARM) and high-definition 3D anorectal manometry [3D-HR-ARM) and neurophysiology; tests of anal and pelvic floor structure, endoanal or pelvic floor ultrasonography; tests of rectal sensory and motor function, simple balloon distension and rectal barostat; tests of evacuation, balloon expulsion and both barium and magnetic resonance (MR) defecography; emerging technologies are also briefly addressed.
TESTS OF ANAL MOTOR FUNCTION
Anorectal manometry
ARM is the most widely used technique for the detection of abnormalities of sphincter function and/or rectoanal co-ordination15, 29, 31. This investigation consists of a series of pressure measurements that assess: involuntary function of the anal canal during rest; voluntary function during squeeze; reflex rectoanal co-ordination during rectal distension; and rectoanal co-ordination during simulated defecation (‘push’)7, 29.
Manometric equipment can record pressure data from single points in the anal canal (termed ‘conventional anal manometry’) or can record and display detailed information simultaneously from the whole anal canal and distal rectum (high-resolution manometry)34, 35. Although conventional anal manometry using water perfused system remains in clinical practice, studies suggest a move towards the more detailed high-resolution solid state methodology, probably in part due to the ability of this technique to more accurately characterise sphincteric function36.
Within the paradigm of high-resolution manometry, two technologies exist. HR-ARM records luminal pressures circumferentially from sensors mounted on a flexible catheter with data presented either topographically in color plots or as an average circumferential pressure at different longitudinal levels of the anorectum34. On the other hand, 3D-ARM records point pressures longitudinally and radially from sensors mounted on a rigid probe with morphology represented in both 2D and 3D34.
Study indications
ARM is typically indicated for the assessment of fecal incontinence and constipation, especially if characterized by symptoms of disordered evacuation. Relative indications for ARM include assessment of functional anorectal pain, pre-operative assessment of anorectal function and assessment of anorectal function in patients after obstetric injury and/or traumatic birth to inform treatment decisions concerning future mode of delivery7, 29.
Study performance
Although not expected to be fully diagnostic, a digital rectal examination should be performed before intubation to provide an overview of anorectal and pelvic floor structure and function, to exclude fecal loading, stricture, bleeding and pain. It is also helpful to check patient understanding of instructions such as ‘squeeze’ and ‘push’. Studies are typically performed in the left lateral position and any lubricant to aid probe placement should be non-anesthetizing. The probe is then positioned ensuring that the sensors span the distal rectum to beyond the anal verge. Both conventional and high-resolution techniques can use either water-perfused or solid state technology for data collection and detailed description of hardware and software setup and catheter design is described elsewhere37.
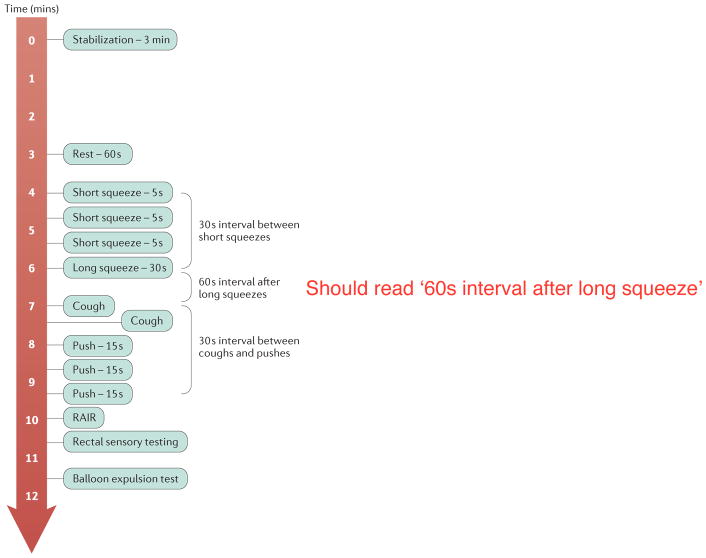
A standardized protocol for ARM would improve utility of the procedure, peer-acceptance, translation and dissemination of results. The protocol recommended by the IAPWG is shown in Figure 1. This protocol consists of the following standardized measurements: rest - basal anal pressures at rest over 60 s; squeeze - anal pressure during voluntary effort; long squeeze - anal pressure during sustained voluntary effort; cough - anorectal pressure changes during cough (that is, reflex increase in rectal and anal sphincter pressure during abrupt change in intra-abdominal pressure); push - anorectal pressure changes during simulated defecation; RAIR - reflex anal response to rectal distension; rectal sensation - assessment of rectal sensitivity to distension, typically performed as part of an ARM protocol (discussed in detail later).
Figure 1.
Schematic of standardized protocol for high-resolution anorectal manometry.
Normal reference values for many of these variables have been described in three studies using HR-ARM38–40 and five using 3D-ARM (three adult populations41–43, one pediatric population44, one series of primigravid women (first pregnancy)45. A number of similar reference values exist for conventional manometry, however, many studies use historical set-ups and protocols no longer in current use.
Clinical utility
A number of contemporary and historical studies have demonstrated differences in manometric findings between healthy volunteer (Figure 2a) and patient groups. Several clinically relevant features have been observed. Sphincter hypotonia (low anal resting pressure), though of low sensitivity, is associated with passive fecal incontinent46–51 (Figure 2b), whereas sphincter hypertonia (high anal resting pressure) can be a feature of anal fissure52–54 or constipation55. Sphincter hypocontractility (impaired ability to voluntarily contract the anal sphincter) is associated with fecal incontinence, particularly fecal urgency47, 56 (Figure 2c) and poor propulsion (impaired rectal force during push), dyssynergia (paradoxical anal sphincter contraction during push) (Figure 2d) and pelvic floor akinesia (failure of movement of the pelvic floor)57–59 have been noted. An absent rectoanal inhibitory reflex is classically seen in Hirschsprung disease60; however, abnormal responses can also be observed in patients with fecal incontinence61 and constipation62. and after anorectal surgery63 .
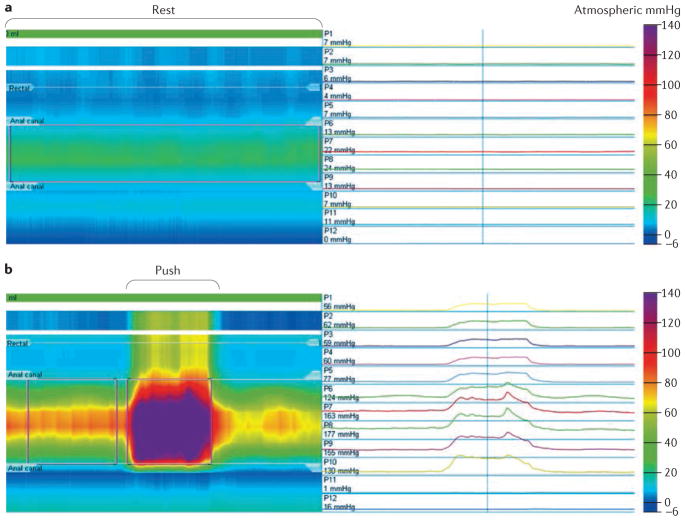
Figure 2.
Representative high-resolution anorectal manometry traces and resultant line traces to assess anorectal function. a | Sphincter hypotonia in a patient with fecal incontinence, visualized in the color contour plot as a band of pale green (~ 20–25mmHg) set between normal blue (~5mmHg) rectal (superiorly) and atmospheric (inferiorly) pressures. b | Dyssynergia (paradoxical anal sphincter contraction during push visualized in the color contour plot as a band of purple (~ 150–175mmHg) within the anal canal and band of yellow (~50mmHg) in a patient with evacuation disorder.
In addition to these findings, 3D-ARM has the ability to illustrate the normal asymmetry of pressures within the anal canal, with higher pressures in the posterior proximal and anterior distal regions of the sphincter. Deviation from this normal manometric anatomy can be detected on either 3D or 2D pressure plots at rest and/or squeeze, and can be suggestive of pathology, though studies only demonstrate slight concordance with anal sphincter defects detected by endoanal ultrasonography64, 65. Thus, pressure defects detected by 3D-ARM should not be used as surrogate markers of anatomical anal sphincter defects without exercising caution.
Some evidence supports the hypothesis that pelvic floor abnormalities not previously identified by conventional anorectal manometry can be found with 3D-ARM. Pilot studies suggest that this technique can measure pelvic floor descent66 and that results have a high positive predictive value (up to 100%) for the presence of an intra-anal intussusception diagnosed by defecography67, 68. These findings might be a useful indicator of the existence of pelvic floor disorders and help direct further investigation (such as defecography), especially in patients with symptoms of evacuation disorders.
Strengths and limitations
ARM is the best-established technology that provides a direct assessment of anal sphincter pressure and rectoanal co-ordination during simulated defecation. It is widely available, easy to perform and well accepted by patients. However, interpretation of findings can be difficult due to the wide variability (and overlap) of manometric measurements in health and disease50, 69–72. Furthermore, some studies suggest that ARM offers little additional utility over digital rectal examination for planning patient management22. Additionally, due to the striking variability in practices between institutions32, appropriate caution should be paid to using reference ranges unless equipment design and test protocol mirrors one’s own. Study set-up and patient position, presence or absence of the perception of the desire to defecate among others can each have a major effect on absolute values reported73–78.
Anorectal neurophysiology
Although less commonly used in clinical practice36 (due to the adoption of less invasive surrogate measures of sphincter function), anal electromyography and pudendal nerve terminal motor latencies (PNTML) remain an important component of anorectal neurophysiological function. The branches of the pudendal nerve, which course over the pelvic floor, are vulnerable to stretch injury (during the third trimester, second stage of labor and forceps-assisted vaginal delivery), which can lead to denervation of the external anal sphincter (EAS), muscle weakness and fecal incontinence79, 80. Owing to the complex nature of symptom generation, such assessment is always performed in conjunction with other investigations (for example, ARM) to enable accurate understanding of the pathophysiological mechanisms.
Recording of pelvic floor electromyography (from both the EAS and levator ani muscles) enables mapping of the EAS to identify sphincter defects, determination of striated muscle function and assessment of denervation-reinnervation potentials (indicative of neural injury)81. PNTML measurement evaluates the neuromuscular integrity between the pudendal nerve and the anal sphincter7, 31, 81.
Study indications
Neurophysiological assessment is typically indicated for the investigation of symptoms of fecal incontinence thought to be secondary to neurological injury. Relative indications for neurophysiology include symptoms of anorectal pain and characterization of complex pelvic floor disorders, especially prior to anorectal surgery7.
Study performance
Studies of electromyography activity are typically performed using a needle, skin or anal plug electrode. Disposable needle electrodes are inserted into the muscle under study and parameters are recorded to describe insertional activity, spontaneous activity, motor unit action potential morphology and recruitment during voluntary or reflex activity. For PNTML, stimulation of the pudendal nerve using a disposable bipolar electrode generates a compound muscle action potential response of the EAS82. The PNTML is the time between stimulus artifact and the onset of the compound muscle action potential response.
Clinical utility
In the striated anal musculature of patients with fecal incontinence, motor unit action potential activity, fiber density and jitter (the stability of consecutive muscle fiber discharges, reflecting the stability of terminal motor axons and neuromuscular transmission) through recording of electromyography activity, have all been shown to be altered in comparison with controls81, 83–85. For PTNML, abnormal results (prolonged latencies) are used as a surrogate marker of pudendal neuropathy and indicate either demyelination or damage to a number of fast firing fibers86.
Strengths and limitations
Electromyography recordings correlate well with sphincter pressures87 (e.g. electromyographic recruitment is seen simultaneously with increased anal pressures during squeeze). Intraluminal electrodes are believed to be more accurate because they are closer to the external anal sphincter muscle and are less likely to pick up gluteal or other muscles88. Currently, the most frequent application of electromyography is as a biofeedback signal for pelvic floor retraining of the external anal sphincter in patients with fecal incontinence or constipation89–91, and it can be especially useful to detect paradoxical contraction or impaired relaxation of the sphincter in those with evacuation disorders91.
The clinical utility of PNTML remains controversial, as there are several test limitations: it is operator-dependent31; sensitivity and specificity are poor83, 84; normal latencies can be recorded in a damaged nerve as long as some fast-conducting fibers remain92; and prediction of clinical outcomes after intervention are conflicting93. In addition, the upper limit of normal for latency is ill-defined due to substantial variability in healthy individuals 7.
TESTS OF ANAL STRUCTURE
Endoanal and pelvic floor ultrasonography
Endoanal ultrasonography94 is a simple and well-tolerated technique that is widely used for detecting both internal anal sphincter (IAS) and EAS defects and has substantially increased our understanding of the pathogenesis of fecal incontinence. Total pelvic floor ultrasonography (integrating endoanal, transvaginal and transperineal ultrasonography) has been used to assess pelvic organ prolapse (that is, rectocele, enterocele, intussusception and cystocele)95. Endoanal and/or pelvic MRI, although not widely available, are alternative imaging modalities only available in specialist centers95
Study indications
Endoanal or pelvic floor ultrasonography can be used for a number of indications: to evaluate morphological integrity of the anal sphincters in patients with fecal incontinence, particularly when surgery is being considered and to provide information on the pelvic viscera and pelvic floor movement in patients with symptoms of an evacuation disorder as a complementary investigation to ARM and defecography96. Moreover, these approaches can be used to assess obstetric anal sphincter injuries (OASIS) after childbirth either to guide early repair and/or to inform planning of subsequent deliveries95. Other indications exist, particularly for the staging of rectoanal neoplasms (beyond the scope of this article)97. Transperineal ultrasonography has additional indications such as assessment of urinary incontinence, voiding difficulties, pelvic organ prolapse symptoms (again considered outside the scope of this article)98, 99.
Study performance
Endoanal ultrasonography is performed in the lateral or prone position using a rigid endoprobe (3–20 MHz), providing 360° axial views of the sphincter complex94. Modern systems enable continuous capturing of images as the probe is withdrawn through the anal canal with post-hoc multiplanar 3D image reconstruction and calculation of anal sphincter volumes.
During the performance of total pelvic floor ultrasonography, the patient is placed in the dorsal lithotomy position with the hips flexed and abducted. Transperineal ultrasonography uses a curved array probe (5–9 MHz), whereas transvaginal scanning is performed using a linear array endoscopic probe (12 MHz) to obtain dynamic 2D posterior and anterior mid-sagittal views. In addition to the detection of anal sphincter defects95, this technique enables evaluation of the dynamic interaction between the pelvic floor and viscera.
Anal anatomy is complex. On endoanal ultrasonography, two discrete rings of tissue are principally visualized, the inner hypoechoic IAS and the outer hyperechoic EAS, which is caudad to puborectalis94. Several other structures including the subepithelial tissues and conjoined longitudinal muscle are also imaged100. By contrast to 2D ultrasonography, 3D ultrasonography can also measure the length and volume of the EAS and might be better for distinguishing EAS defects from defects in surrounding structures (that is, transverse perineii and puboanalis muscle)94. Transperineal ultrasonography, in addition to characterization of the sphincter complex, provides a dynamic evaluation of the interaction of the pelvic floor viscera and musculature, comprising assessment of the posterior, central and anterior compartments101.
Clinical utility
Endoanal ultrasonography is widely available and should be considered the cornerstone for anal imaging102. The IAS can be classified as normal or pathological, with the latter defined by several factors: sphincter discontinuity (defect)103 (Figure 3b), atrophy (identified by diffuse thinning of the sphincter [thickness ≤1 mm]50) (Figure 3c) and/or degeneration104 or hypertrophy48, 105. IAS defects and/or atrophy are appreciated to be associated with symptoms of (particularly passive) fecal incontinence106, 107. IAS hypertrophy is primarily seen in relation to rectal intussusception and/or prolapse47, 48, 50.
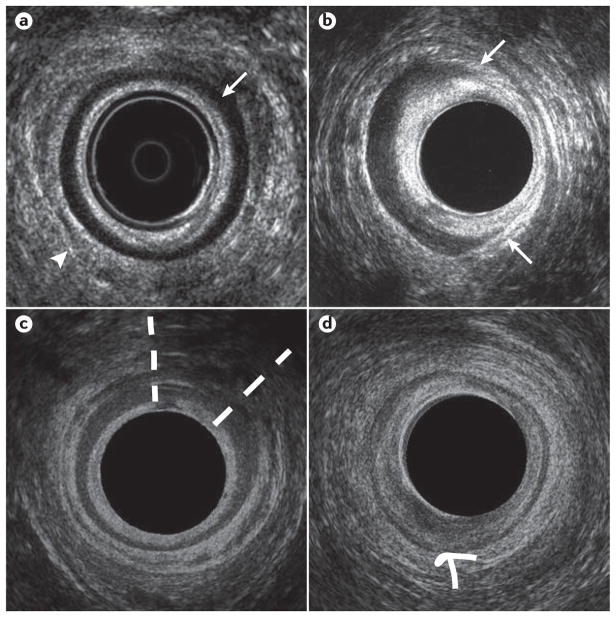
Figure 3.
Representative endoanal ultrasonography images. a | The mid anal canal in healthy volunteer, demonstrating an intact internal anal sphincter (IAS) (arrow) appearing hypoechoic, and an intact external anal sphincter (EAS) (arrowhead) appearing hyperechoic. b Mid anal canal in a patient with fecal incontinence, demonstrating an IAS defect between the 1 and 5 o’clock positions (between the arrows). c | Mid anal canal in a patient with fecal incontinence demonstrating an EAS defect, evident as an area of hypoechoic discontinuity between the 12 and 2 o’clock positions (extent of defect between dashed lines). d | Mid anal canal in a patient with fecal incontinence demonstrating IAS atrophy (global thinning of the smooth muscle ring, which is of mixed echogenicity difficult to distinguish from surrounding structures, arrow). The EAS is intact.
The EAS can be characterized as normal, or pathological by sphincter discontinuity (defect) (Figure 3d), or interruption of its fibrillar echotexture manifest as focal thinning, scarring, or atrophy108, 109. All abnormalities can be described in cross-section according to a clock face (for example, defect between 1 and 3 o’clock), and also in the longitudinal plane. EAS disruption is a characteristic feature of OASIS94, is associated with anal hypocontractility110, 111 and can be found in up to 68% of individuals presenting with symptoms of fecal incontinence (n = 200) 112, 113.
Total pelvic ultrasonography has the additional ability to enable classification of organ prolapse, structural and functional causes contributing to evacuation disorders114 and the identification of pubovisceral avulsion (abnormal insertion of the levator ani on the inferior pubic ramus), which is an important pathophysiological mechanism for pelvic organ prolapse and fecal incontinence95, 102. Though not widely available, studies have also used a 4D approach to evaluate dynamic pelvic floor movement in real time115.
Strengths and limitations
Imaging of the anal sphincters can identify defects that are often clinically unrecognized and might be amenable to surgical repair. However, whereas abnormalities of anal structure are associated with anal hypotonia and hypocontractility,116 it can be challenging to interpret the clinical relevance of this finding, because ~10% of women have postpartum (occult) sphincter defects without any symptoms117.
TESTS OF ANORECTAL SENSORY AND MOTOR FUNCTION
Simple balloon distension and rectal barostat
Awareness of rectal filling is critical to normal bowel function101, 118. Abnormal visceral sensitivity and/or biomechanical function (most commonly described by evaluation of rectal compliance) are often found in fecal incontinence and evacuation disorders7, providing the rationale for measurement of anorectal sensory and motor function via balloon distention and rectal barostat in clinical practice.
Study indications
Rectal sensory testing should be considered integral to physiological assessment of anorectal function. This testing is most commonly performed with simple balloon distension. Assessment with a rectal barostat, which is less widely available, should be considered in select patients with alterations of rectal sensation on ‘standard’ balloon distension, and/or in whom there is a high index of suspicion of abnormal rectal compliance or capacity 7. These tests enable the detection of heightened (hypersensitivity) or impaired and/or blunted (hyposensitivity)) rectal sensation and/or abnormal rectal compliance or capacity (that is, ‘stiff’ or small (hypocompliant) or ‘lax’ or large (hypercompliant)).
Study performance
Rectal sensation is evaluated by assessing the perception of rectal distension7, 119, 120. For simple balloon distension, this test is performed by distending an elastic balloon, secured to a catheter placed within the rectum, with air (manually using a handheld syringe or via a pump). Either ramp (continuous at 1–2 ml/s) or intermittent (phasic or stepwise) distension paradigms can be used7. During balloon inflation, individuals are instructed to report perceived sensations (first sensation; desire to defecate; urgency; and maximum toleration or pain). The distending volume (or, less frequently pressure) at each of these sensory thresholds is then recorded7.
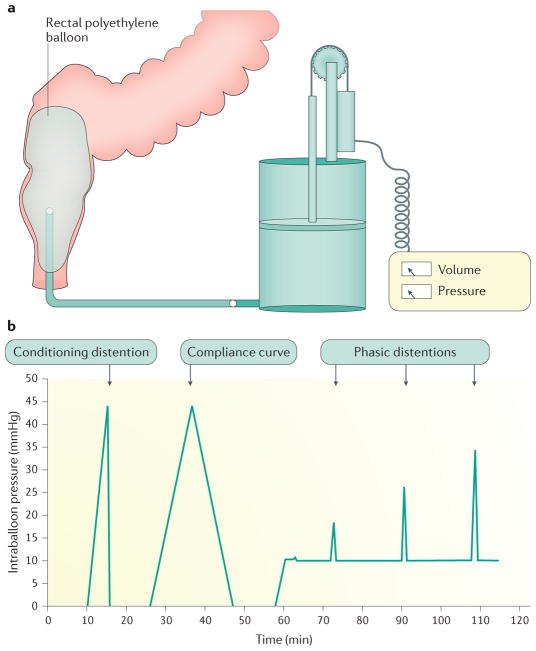
Evaluation of both motor and sensory function is performed with a computerized barostat that enables distension at a specified and precise rate, thereby minimizing observer bias and measurement error7, 119. An ‘oversized’, non-elastic bag is used that can be regarded as infinitely compliant (in that its own properties have no influence on internal pressure) (Figure 4a). This is preferable to measurements using balloons, which require correction to account for their intrinsic elasticity7, 119. Patients are examined in a semi-prone or lateral position to reduce pelvic hydrostatic pressure. An initial ‘conditioning’ distention documents the minimal distending (that is, intra-abdominal) pressure and ensures that subsequent measurements are reproducible (Figure 4b)121. Similar to the ‘simple balloon’ test described earlier, sensory thresholds are recorded during ramp or phasic distensions. Intra-bag (intra-rectal) volumes and pressures are recorded concurrently enabling rectal compliance to be calculated from the derived pressure-volume curve. Rectal capacity can also be measured (defined as barostat bag volume at a prospectively defined, supra-physiological pressure; for example, 40mmHg122). Perceived sensations can alternatively be described using visual analog scores (for example, urgency, pain) recorded on a 0–100 mm scale during distensions at set pressures (typically at 8, 16, 24, and 32 mmHg above operating pressure)123.
Figure 4.
Schematic of rectal barostat setup. a | Typical rectal barostat setup. b | Barostat conditioning distension protocol.
Clinical utility
The thresholds for rectal sensation can be normal, reduced (hypersensitivity) or increased (hyposensitivity) in both fecal incontinence and chronic constipation124. Demonstration of altered sensation can guide therapeutic measures aimed at normalizing sensory thresholds and relieving bowel symptoms.
Rectal hypersensitivity is a common finding in patients with symptoms of urgency and frequent defecation in diarrhea-predominant IBS, ulcerative colitis and radiation proctitis50. In IBS, this finding can be associated with increased symptom severity69, 117, 125. Rectal hypersensitivity is also a feature of fecal urgency126, urge fecal incontinence127 and low anterior resection syndrome128. Such hypersensitivity can be related to to reduced compliance, capacity or an exaggerated response to rectal distension129. Normalization of sensory thresholds in patients with hypersensitivity has been associated with positive clinical outcomes following the use of behavioral therapy, pharmacological agents and surgical interventions117, 130; however, good outcomes are not necessarily associated with post-intervention changes in sensory parameters.
Rectal hyposensitivity (often found alongside an attenuated or absent call to stool131–133) is observed in 18–66% of patients with chronic constipation134, constipation-predominant IBS, fecal incontinence, and evacuatory dysfunction secondary to spinal cord injury135. This finding can be ‘primary’ (due to direct impairment of afferent pathway function), ‘secondary’ (due to altered biomechanical properties, e.g. megarectum), or both135, 136. When hyposensitivity is present, the assumed mechanism is that stool is involuntarily expelled before the individual is alerted to the need to respond137. In such patients, sensory retraining has been shown to facilitate timely contraction of the external sphincter and improve continence138.
Demonstration of rectal hyposensitivity can indicate a severe clinical phenotype and predict a poor response to treatments such as biofeedback or bowel retraining139, 140 and surgery with colectomy141. However, in those patients who do respond, normalization of impaired sensation is generally associated with an improvement in symptoms142, most notably during treatment with neuromodulation143, 144.
Strengths and limitations
Several consensus statements and technical reviews acknowledge that evaluation of rectal sensory function has an accepted place in the clinical management of patients with anorectal disorders27. Nevertheless, methods for simple, elastic balloon distension are poorly standardized29, 30, 145, 146. Though the use of a barostat overcomes these limitations, this technology is not widely available. However, development of a ‘rapid’ barostat40 could enable this approach to be performed in routine clinical practice (as a typical barostat protocol may take approximately 1 hour to complete)7. For either technique, measurements can be affected by age, the rate and pattern of distention, patient position, and biomechanical and structural properties of the rectum147, hence it is essential for results to be interpreted in the context of appropriate normative values.
TESTS OF EVACUATION
Balloon expulsion test
Inability to expel solid stool from the rectum is a key feature of patients with constipation characterized by symptoms of an evacuation disorder. The balloon expulsion test (BET) is a direct method by which to assess this function.
Study indications and performance
Balloon expulsion is a simple, office-based test, which is indicated as a first-line screening investigation for assessment of the ability to evacuate. With a patient lying in the left lateral position with hips and knees flexed, a lubricated, preferably non-latex balloon attached to a plastic catheter is inserted into the rectum and inflated with 50 ml of warm water. The patient is then seated on a commode in privacy and asked to expel the balloon. The ability (or inability) to expel the balloon and the time taken for expulsion is recorded.7, 148, 149 Although reported cut-offs for normality vary, the generally accepted limit for expulsion is between 1–3 min. Expulsion times longer than this can indicate disordered evacuation 150.
Clinical utility
Though often considered to be synonymous with dyssynergic defecation, it is pertinent to note that the sensitivity and specificity of this test is variable (ranging between 68–94% and 71–81%, respectively29, 150, 151). BET in isolation is, therefore, not sufficient to clearly diagnose an evacuation disorder150, 151. Furthermore, agreement with other tests of evacuation is suboptimal (a prospective study of 100 patients with functional constipation published in 2016 showed only fair agreement between BET and defecography in diagnosing evacuation disorders, and no agreement between BET and anorectal manometry152) and the BET provides no information about anatomical phenomena that might impair evacuation (for example, rectocele or occluding intussusception).
Nevertheless, studies do indicate that a positive result can predict response to biofeedback therapy with demonstration of a clinical response in up to 85% of patients,153 though this finding is not consistent across all studies154–156.
Strengths and limitations
In spite of good reproducibility157, test setup and study performance are poorly standardized150. Demographic factors have an influence, with male participants having a shorter balloon expulsion time than women, and expulsion time increasing with age150, 152.
As a simple to perform, office-based screening test, balloon expulsion can be considered useful for the initial assessment of patients with symptoms of evacuation disorder, however a firm diagnosis requires confirmation with other allied tests of evacuation.
Defecography
Barium (X-ray) defecography, or evacuation proctography, is an established clinical tool for the diagnosis of evacuation disorders158. Barium defecography evaluates rectal wall morphology, pelvic floor motion and evacuation in real time159. MRI defecography enables imaging of all pelvic compartments160. In comparison with other tests of evacuatory function (for example, manometry, balloon expulsion and transperineal ultrasonography), defecography provides better overall evaluation of the defecatory process and structure and/or function of the anorectum161–163.
Study indications
Primary indications for both investigations are: to identify structural or ‘functional’ obstructive features associated with impaired evacuation in patients with refractory symptoms of constipation consistent with an evacuation disorder; to identify impaired evacuation and/or pelvic organ prolapse in patients with fecal incontinence7, 164; and to evaluate the effects of treatment, for example after surgical repair of anorectal or pelvic floor pathology21, 165.
Study performance
Barium defecography involves fluoroscopic imaging of the anorectum during contraction of pelvic floor muscles and rectal evacuation (that is, simulated defecation) after barium paste, composed of barium sulfate, porridge oats and water, has been instilled into the rectum166, 167. As rectal sensory function is critical to normal defecation159, 168, introduction of a thick paste (approximating normal stool), to a volume individualized to a patients’ desire to defecate,1 might be preferable to a fixed volume of liquid barium. The patient is then seated upright on a radiolucent commode (in privacy behind a screen) during fluoroscopic screening, and instructed to squeeze the anal sphincter then expel rectal contents until evacuation is felt to be complete, or the patient reports that they are unable to empty further. Rectal dimensions (length, diameter, capacity), anorectal angles, rectal wall morphological features, perineal descent, and evacuatory efficacy (rate, percentage of contrast expelled)169 can be measured. However, the technique lacks standardization and numerous modifications to the original method168 have been described, most notably opacification of bladder and/or vagina and/or small bowel (for example colpocystodefecography) which enables concurrent visualization of cul-de-sac hernias (for example, enterocele), and other pelvic organ prolapses.
MRI defecography can be performed either with the patient supine, within a closed-configuration magnet, or upright (sitting) within an open-configuration system163. Limited availability of the latter, however, means that the majority of studies are performed with the patient in a non-physiological supine position. The rectum is filled with a stool substitute (for example, mashed potatoes or ultrasonography gel) mixed with gadolinium if necessary.
Clinical utility
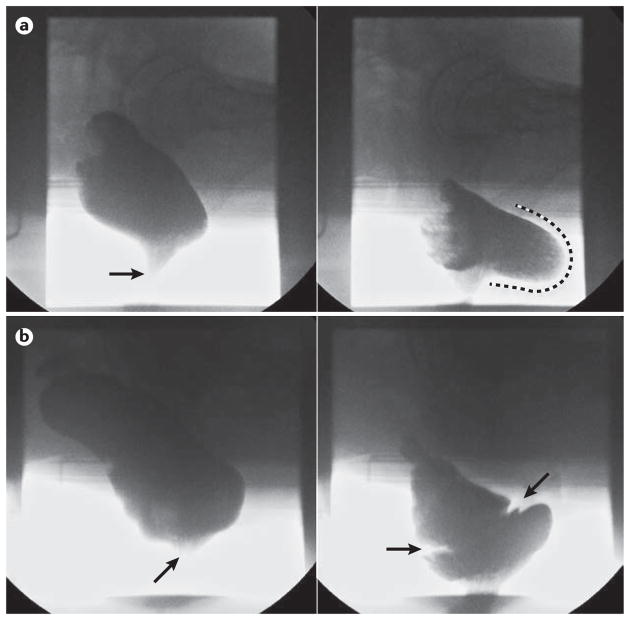
Barium defecography can identify impaired rectal evacuation and diagnose anatomical and ‘functional’ features that can contribute to symptoms of an evacuation disorder, such as rectocele (Figure 5a), obstructing intussusception (Figure 5b), rectal prolapse and megarectum, as well as dyssynergic defecation, levator ani and descending perineum syndrome159. With small bowel opacification, the effect of enterocele can be assessed. MR defecography additionally enables concurrent evaluation of bladder and vaginal vault descent, and enables imaging in different orthogonal planes160.
Figure 5.
Representative barium defecography images. a | A significant rectocele; the left panel shows a lateral view of the rectum at rest, opacified by barium neostool with the anal canal closed (arrow). The right panel clearly demonstrates a large retaining rectocele at end evacuation (extent of anterior bulging highlighted by dashed line) b) Obstructing full thickness intussusception; the left panel shows a lateral view of the rectum at rest, with the anal canal closed (arrow). The right panel shows an image at mid evacuation with clear invagination of the mid rectum (between arrows) secondary to a full thickness rectal intussusception; this is causing occlusion of the distal rectal lumen with retention of neostool proximal to this.
Various grading systems exist for defining structural abnormalities and pelvic organ descent, both for barium163 and MRI modalities,170–172 and these can be used as a basis for guiding therapy, particularly in those abnormalities deemed amenable to surgical repair. However, anatomical findings such as a small rectocele or minor intussusception are frequently found in healthy individuals161, 173, and failure to recognize such variants of normal can lead to overdiagnosis. A study using barium defecography has shown that an anterior rectocele is almost always present in asymptomatic female study participants168, 170, and that only large (>4 cm) and/or retentive rectoceles (that might be smaller) should be regarded as clinically relevant. Intra-anal (as opposed to intra-rectal) intussusception168 and enterocele168 are also appreciated to be pathological.
Limited comparative studies exist comparing modalities. Barium defecography better detects intussusceptions than MR, either using supine174 or upright imaging175, and is more sensitive for identifying retentive rectoceles176. Upright MR defecography is superior to dynamic supine MRI in imaging intussusceptions; diagnostic utility is otherwise equivalent177, 178.
Strengths and limitations
Of the available tests used to investigate patients with symptoms of an evacuation disorder, diagnostic agreement is imperfect179; however, only defecography evaluates evacuation and pelvic organ structure. For this reason, consensus documents recommend defecography either as a first-line180, 181 or second-line182, 183 test. MRI has several advantages over barium defecography, in that it lacks exposure to ionizing radiation, and provides excellent soft tissue resolution of all pelvic floor compartments and supporting structures, enabling assessment of coexisting cystocele and uterovaginal prolapse10, 184.
There remain several limitations to defecography in general. Radiation exposure for barium studies make this test unsuitable for certain patient groups, particularly pregnant women. As with all tests of evacuatory function, defecography is not performed in response to the spontaneous desire to defecate and embarrassment in the patient can inhibit normal behavior, leading to overdiagnosis of impaired evacuation163; nevertheless, diagnostic yield of functional disorders on defecography is approximately half that using other tests of evacuatory function185. A paucity of normative data are available,186 particularly for MRI defecography168 and a large degree of overlap in results between patients and controls limits interpretability of results174.
OTHER TECHNOLOGIES
A number of further technologies designed to describe anorectal function exist, some of which are established but not in routine clinical use (e.g. endoanal MRI), and others which are emerging (e.g. the functional lumen imaging probe). Primarily acknowledged as tools in the research setting, they provide novel insights into the mechanisms of anorectal (dys)function. Though the following list is not exhaustive, the following is a brief introduction to those modalities which the Consensus group believe to have some diagnostic capability.
Functional lumen imaging probe
The anal canal’s passive ability to withstand opening pressure (that is, distensibility) is thought to have a role in the continence mechanism187. A novel device, the functional lumen imaging probe (Flip; Crospon, Ireland) measures the cross-sectional diameter of a saline-filled balloon within the anal canal as distension pressure increases168, 170. Early studies show that patients with fecal incontinence have increased distensibility of the anal canal and this finding can be more sensitive to clinically relevant pathology than anorectal manometry.
Rectal and anal motor evoked potentials
The integrity of spinoanorectal pathways that govern anorectal neuronal function can be assessed using magnetic stimulation of the lumbar and sacral regions overlying the nerve plexi and recording the motor evoked potentials188. More specifically, it can reveal either unilateral or bilateral prolonged motor evoked potentials at the lumbar region and/or sacral region and at the rectal and/or anal sites189.
A study showed that translumbar and trans-sacral MEPs of the rectum and anus provide better delineation of peripheral neuromuscular injury in individuals with fecal incontinence and spinal cord injury than PNTML190. This approach is relatively easy to perform but it is not widely available, which is the major limitation.
Endoanal MRI
Endoanal MRI114, 192, 193 is a technique that enables high-resolution imaging of the EAS (with the ability to differentiate between defects, scaring and atrophy) together with visualization of surrounding structures pertinent to pelvic organ prolapse and fecal incontinence (detecting, for example, pubovisceral avulsion). Additionally, MRI muscle fibre-tracking is a research technique that has enabled detailed functional anatomy of the continence mechanism, including morphology of the EAS and puborectalis complex191.
ANORECTAL DYSFUNCTION
Physiological classification
No single test can fully characterize the cause(s) of fecal incontinence and evacuation disorders, and instead, a range of investigations should be applied to assess anorectal structure, function and sensitivity (Table 1). We also recognize that there is no widely accepted consensus on the physiological nomenclature for the classification of anorectal disorders or the use of findings from anorectal investigations to broadly describe phenotypes.
A broad summary of the expert consensus view regarding findings of each investigation discussed earlier is presented in Table 2, with the clinical relevance classified as ‘major’, ‘minor’ and ‘of questionable significance’.
TABLE 2.
Clinical relevance of findings of commonly performed investigations of anorectal physiological function.
| Function | Investigation | Finding | Clinical relevance |
|---|---|---|---|
| Anus | |||
| Motor | Anorectal manometry | Anal hypotonia | *** |
| Anal hypertonia | ** | ||
| Anal hypocontractility | *** | ||
| Electromyography | Reduced or abnormal myogenic activity | *** | |
| Pudendal nerve terminal motor latencies | Prolonged latency | * | |
| Structure | Endoanal ultrasonography | IAS defect | *** |
| IAS degeneration or atrophy | ** | ||
| IAS hypertrophy | ** | ||
| EAS atrophy | ** | ||
| EAS defect | *** | ||
| Sensory | Anal mucosal electrosensitivity | Anal hyposensitivity | ** |
| Rectum | |||
| Sensory | Balloon distension or rectal barostat | Rectal hypersensitivity | *** |
| Rectal hyposensitivity | *** | ||
| Motor, sensory and structure | Rectal barostat | Rectal hypercompliance | ** |
| Rectal hypocompliance | ** | ||
| Increased rectal capacity | ** | ||
| Decreased rectal capacity | ** | ||
| Anorectal unit | |||
| Motor | Balloon expulsion Anorectal manometry | Prolonged expulsion time | *** |
| Pelvic akinesia (can be described as type IV dyssynergia) | *** | ||
| Poor propulsion with dyssynergia(can be described as type II dyssynergia) | ** | ||
| Normal propulsion with dyssynergia(can be described as type I or III dyssynergia) | ** | ||
| Anorectal areflexia | *** | ||
| Motor, sensory and structure | Defecography (barium or MRI) | Obstructing intussusceptione | *** |
| Retaining rectocelef | *** | ||
| Megarectum | * | ||
| Rectal prolapse | *** | ||
| Enterocele or sigmoidocele | ** | ||
| Cystocele | ** | ||
| Vaginal vault prolapse | ** | ||
| Excessive perineal descent | ** | ||
| Impaired rectal emptyinga | *** | ||
| Impaired anorectal angle openinga | ** | ||
EAS, external anal sphincter; IAS, internal anal sphincter
Finding of questionable clinical significance
Finding of minor clinical significance
Finding of major clinical significance
Functional (as opposed to structural) abnormality of evacuation
Fecal incontinence
In the context of fecal incontinence, major physiological findings with clear implications for clinical management and/or prognosis include anal hypotonia, anal hypocontractility and large sphincter defects on endoanal ultrasonography (Table 2). However, due to the multifactorial nature of symptom generation, such abnormal physiological findings are rarely found alone, and are often seen in overlapping clusters. Examples of well recognized physiological phenotypes include: anal hypotonia, IAS hypertrophy and rectoanal intussusception or full thickness rectal prolapse in patients with symptoms of passive fecal leakage;188–190, 192–194 and anal hypocontractility, rectal hypersensitivity and rectal hypocompliance in patients with fecal urgency or incontinence or diarrhoea-predominant IBS108, 195, 196. However, although anecdotally recognized, prospective studies are required to better characterize these phenotypes, symptom clusters and disorders.
Evacuation disorders
For evacuation disorders, major findings include impaired emptying secondary to abnormal rectal structure (intussusception, prolapse or rectocele) or function (impaired propulsion or dyssynergia) and abnormalities of rectal sensitivity (in particular rectal hyposensitivity). Again, recognized phenotypes are often characterized by more than one physiological abnormality (for example, rectal hyposensitivity with impaired evacuation secondary to enlarged rectal capacity, hypercompliance or megarectum127, 130, 197–199), and these also merit further study.
Effect of physiological evaluation on management
Epidemiological research has demonstrated that diarrhea is the most important cause of fecal incontinence (odds ratio 53, 95% CI 6.1–147, compared with continent controls)137 and hard stool is the most common finding in constipation165. However, symptoms of disordered defecation can be secondary to abnormalities outside the anorectum, such as colonic dysfunction200, or due to obsessive compulsive disorders and/or somatization201. Hence, physiological assessments should be considered for patients whose symptoms have not responded to stool regulation.
The clinical utility of physiological measurement has previously been confirmed in large case series21; however, high-quality data from prospective trials using contemporary technology with outcome data is limited. Study design is challenging due to difficulties defining patient groups based on (non-specific) symptoms, the wide range of normal values, and the variety of potential physiological findings.
The problem of overlap between health and disease is particularly pertinent in this field: as demonstrated in a blinded study of ARM in healthy individuals and patients with constipation in which 90% of healthy individuals were classified as ‘abnormal’ using conventional descriptors of rectoanal co-ordination24, 202. Test results should, therefore, be interpreted with an appropriate degree of caution (particularly when planning irreversible surgical correction of a physiological or structural ‘abnormality’) until further robustly performed stratified medicine studies become available.
Furthermore, it should not be assumed that a single measureable physiological change directly correlates with a single symptom, as these diseases are often multifactorial. As has been demonstrated elsewhere, treatment directed at one physiological abnormality might yield poor results when the true pathological driver is not fully appreciated (e.g. gastric acid suppression therapy for duodenal ulceration secondary to Helicobacter pylori infection)203.
However, particular findings on physiological testing do currently act as a basis for clinical management (for example, anal hypocontractility with an EAS defect after obstetric injury) but there remains a need for further development of an evidence-based classification system of physiological phenotypes. This development will require serial diagnostic and outcome studies to assess the clinical utility of the system for the direction of specific behavioral, medical, and surgical interventions.
Conclusions
If anorectal symptoms persist despite empiric stool regulation therapy without identification of a treatable cause, anorectal function testing can provide information that might explain the causes of fecal incontinence or evacuation disorders. This Consensus Statement has identified challenges concerning data acquisition, analysis and interpretation of results (Box 2); however, there is a high level of agreement that the evaluation of anorectal structure and function and a mechanistic understanding of anorectal pathophysiology can identify disease phenotypes and direct effective management.
Box 2. Open research questions.
Optimal manometric measurements for diagnosis of sphincter dysfunction and rectoanal co-ordination need to be refined and better defined
The clinical importance of measurements and tools used to assess rectal capacity, compliance and sensory function in patients with anorectal disorders require further validation in clinical practice
Classification systems for physiological characterization of fecal incontinence and evacuation disorders that integrate the results of ARM, anorectal sensation, anal endosonography and defecography are required
Serial diagnostic and outcome studies are needed to assess the clinical utility of anorectal investigations for stratifying patients to behavioral, medical or surgical therapies
Acknowledgments
The International Working Group for Disorders of Gastrointestinal Motility and Function initiated the consensus meetings and provided material support for the consensus process. Five groups, each focused on a specific region or disease, reviewed the current state-of-the-art in clinical measurement of gastrointestinal motility and function (oropharynx, esophagus, reflux disease, stomach / intestine and anorectum). The consensus process was endorsed by the European Society of Neurogastroenterology and Motility, American Neurogastroenterology and Motility Society, South American and Latin Society, Asian Neurogastroenterology and Motility Association, Australasian Neurogastroenterology and Motility Association and the European Society of Colo-Proctology. Financial support was provided by a long-term grant from the United European Gastroenterology Education Committee, registration fees for meetings and sponsorship from major manufacturers of physiological measurement equipment.
Footnotes
Author contributions
All authors made equal contributions to all aspects of this manuscript.
Related links
International Working Group for Disorders of Gastrointestinal Motility and Function https://www.idigest.ch/
COMPETING INTERESTS
E.V.C. has received honoraria for teaching from MMS/Laborie.
S.M.S. has received honoraria for teaching from MMS/Laborie.
J.R.-T. has received research funding from Newton Foundation-CONACYT, Sanfer and Asofarma Laboratories, speaker fees from Covidien/Medtronics, Takeda, Allergan, Astra Zeneca, Sanofi and Sanfer.
F.M. has served as consultant for Medtronic, Laborie.
A.B. has licensed intellectual property in a portable anorectal manometry device to Medspira Inc.
M.F. has received research funding from Covidien/Medtronic, speaker fees from Covidien/Medtronic, Sandhill, MMS/Laborie, Reckitt Benckiser, and Mui Scientific.
S.S.R. has served on advisory boards for Forest labs, Synergy Pharmaceuticals, Vibrant Ltd, Intone, and has received research grants from Forest labs, Synergy, InTone, and Medtronic. H.H. and A.M. declare no competing interests.
References
- 1.Palit S, Lunniss PJ, Scott SM. The physiology of human defecation. Dig Dis Sci. 2012;57:1445–64. doi: 10.1007/s10620-012-2071-1. [DOI] [PubMed] [Google Scholar]
- 2.Perry S, Shaw C, McGrother C, et al. Prevalence of faecal incontinence in adults aged 40 years or more living in the community. Gut. 2002;50:480–4. doi: 10.1136/gut.50.4.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitehead WE, Borrud L, Goode PS, et al. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology. 2009;137:512–7. 517e1–2. doi: 10.1053/j.gastro.2009.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johanson JF, Lafferty J. Epidemiology of fecal incontinence: the silent affliction. Am J Gastroenterol. 1996;91:33–6. [PubMed] [Google Scholar]
- 5.Higgins PD, Johanson JF. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol. 2004;99:750–9. doi: 10.1111/j.1572-0241.2004.04114.x. [DOI] [PubMed] [Google Scholar]
- 6.Noelting J, Eaton J, Choung R, et al. The incidence rate and characteristics of clinically diagnosed defecatory disorders in the community. Neurogastroenterology & Motility. 2016;28:1690–1697. doi: 10.1111/nmo.12868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scott SM, Gladman MA. Manometric, sensorimotor, and neurophysiologic evaluation of anorectal function. Gastroenterol Clin North Am. 2008;37:511–38. vii. doi: 10.1016/j.gtc.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Norton C, Thomas L, Hill J, et al. Management of faecal incontinence in adults: summary of NICE guidance. BMJ. 2007;334:1370–1. doi: 10.1136/bmj.39231.633275.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keighley MRB. Faecal incontinence. In: Keighley MRB, editor. Surgery of the anus, rectum and colon. Vol. 1. London: W. B. Saunders; 1993. pp. 516–608. [Google Scholar]
- 10.Bharucha AE, Dorn SD, Lembo A, et al. American Gastroenterological Association medical position statement on constipation. Gastroenterology. 2013;144:211–217. doi: 10.1053/j.gastro.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 11.Bharucha AE. Fecal incontinence. Gastroenterology. 2003;124:1672–1685. doi: 10.1016/s0016-5085(03)00329-9. [DOI] [PubMed] [Google Scholar]
- 12.Quander CR, Morris MC, Melson J, et al. Prevalence of and factors associated with fecal incontinence in a large community study of older individuals. Am J Gastroenterol. 2005;100:905–9. doi: 10.1111/j.1572-0241.2005.30511.x. [DOI] [PubMed] [Google Scholar]
- 13.Alimohammadian M, Ahmadi B, Janani L, et al. Suffering in silence: a community-based study of fecal incontinence in women. Int J Colorectal Dis. 2014;29:401–6. doi: 10.1007/s00384-013-1809-3. [DOI] [PubMed] [Google Scholar]
- 14.Fynes M, Donnelly V, Behan M, et al. Effect of second vaginal delivery on anorectal physiology and faecal continence: a prospective study. Lancet. 1999;354:983–6. doi: 10.1016/S0140-6736(98)11205-9. [DOI] [PubMed] [Google Scholar]
- 15.Rao SS. Pathophysiology of adult fecal incontinence. Gastroenterology. 2004;126:S14–22. doi: 10.1053/j.gastro.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 16.Damon H, Guye O, Seigneurin A, et al. Prevalence of anal incontinence in adults and impact on quality-of-life. Gastroenterol Clin Biol. 2006;30:37–43. doi: 10.1016/s0399-8320(06)73076-7. [DOI] [PubMed] [Google Scholar]
- 17.Kamm MA. Obstetric damage and faecal incontinence. Lancet. 1994;344:730–3. doi: 10.1016/s0140-6736(94)92213-6. [DOI] [PubMed] [Google Scholar]
- 18.Goldberg RP, Abramov Y, Botros S, et al. Delivery mode is a major environmental determinant of stress urinary incontinence: results of the Evanston-Northwestern Twin Sisters Study. Am J Obstet Gynecol. 2005;193:2149–53. doi: 10.1016/j.ajog.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 19.Lunniss PJ, Gladman MA, Hetzer FH, et al. Risk factors in acquired faecal incontinence. J R Soc Med. 2004;97:111–6. doi: 10.1258/jrsm.97.3.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim T, Chae G, Chung SS, et al. Faecal incontinence in male patients. Colorectal Dis. 2008;10:124–30. doi: 10.1111/j.1463-1318.2007.01266.x. [DOI] [PubMed] [Google Scholar]
- 21.Nurko S, Scott SM. Coexistence of constipation and incontinence in children and adults. Best Pract Res Clin Gastroenterol. 2011;25:29–41. doi: 10.1016/j.bpg.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lam T, Felt-Bersma R. Clinical examination remains more important than anorectal function tests to identify treatable conditions in women with constipation. International urogynecology journal. 2013;24:67–72. doi: 10.1007/s00192-012-1796-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tantiphlachiva K, Rao P, Attaluri A, et al. Digital rectal examination is a useful tool for identifying patients with dyssynergia. Clinical Gastroenterology and Hepatology. 2010;8:955–960. doi: 10.1016/j.cgh.2010.06.031. [DOI] [PubMed] [Google Scholar]
- 24.Vaizey CJ, Kamm MA. Prospective assessment of the clinical value of anorectal investigations. Digestion. 2000;61:207–14. doi: 10.1159/000007759. [DOI] [PubMed] [Google Scholar]
- 25.Liberman H, Faria J, Ternent CA, et al. A prospective evaluation of the value of anorectal physiology in the management of fecal incontinence. Dis Colon Rectum. 2001;44:1567–74. doi: 10.1007/BF02234373. [DOI] [PubMed] [Google Scholar]
- 26.Altomare DF, Rinaldi M, Petrolino M, et al. Reliability of electrophysiologic anal tests in predicting the outcome of sacral nerve modulation for fecal incontinence. Dis Colon Rectum. 2004;47:853–7. doi: 10.1007/s10350-004-0524-0. [DOI] [PubMed] [Google Scholar]
- 27.Knowles CH, Thin N, Gill K, et al. Prospective randomized double-blind study of temporary sacral nerve stimulation in patients with rectal evacuatory dysfunction and rectal hyposensitivity. Ann Surg. 2012;255:643–9. doi: 10.1097/SLA.0b013e318247d49f. [DOI] [PubMed] [Google Scholar]
- 28.Chiarioni G, Bassotti G, Stanganini S, et al. Sensory retraining is key to biofeedback therapy for formed stool fecal incontinence. Am J Gastroenterol. 2002;97:109–17. doi: 10.1111/j.1572-0241.2002.05429.x. [DOI] [PubMed] [Google Scholar]
- 29.Rao SS, Azpiroz F, Diamant N, et al. Minimum standards of anorectal manometry. Neurogastroenterol Motil. 2002;14:553–9. doi: 10.1046/j.1365-2982.2002.00352.x. [DOI] [PubMed] [Google Scholar]
- 30.Azpiroz F, Enck P, Whitehead WE. Anorectal functional testing: review of collective experience. Am J Gastroenterol. 2002;97:232–40. doi: 10.1111/j.1572-0241.2002.05450.x. [DOI] [PubMed] [Google Scholar]
- 31.Diamant NE, Kamm MA, Wald A, et al. AGA technical review on anorectal testing techniques. Gastroenterology. 1999;116:735–60. doi: 10.1016/s0016-5085(99)70195-2. [DOI] [PubMed] [Google Scholar]
- 32.Carrington EV, Heinrich H, Knowles CH, et al. Methods of anorectal manometry vary widely in clinical practice: Results from an international survey. Neurogastroenterology & Motility. 2017 doi: 10.1111/nmo.13016. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 33.Rao SS, Patel RS. How useful are manometric tests of anorectal function in the management of defecation disorders? Am J Gastroenterol. 1997;92:469–75. [PubMed] [Google Scholar]
- 34.Dinning P, Carrington E, Scott SM. Colonic and anorectal motility testing in the high-resolution era. Curr Opin Gastroenterol. 2016;32:44–48. doi: 10.1097/MOG.0000000000000229. [DOI] [PubMed] [Google Scholar]
- 35.Carrington EV, Heinrich H, Knowles CH, et al. Methods of anorectal manometry vary widely in clinical practice: results from an international survey. Neurogastroenterol Motil. 2017 doi: 10.1111/nmo.13016. in press. [DOI] [PubMed] [Google Scholar]
- 36.Carrington EV, Heinrich H, Knowles CH, et al. Methods of anorectal manometry vary widely in clinical practice: Results from an international survey. Neurogastroenterology & Motility. 2017;29:e13016. doi: 10.1111/nmo.13016. n/a. [DOI] [PubMed] [Google Scholar]
- 37.Dinning PG, Carrington EV, Scott SM. The use of colonic and anorectal high-resolution manometry and its place in clinical work and in research. Neurogastroenterol Motil. 2015;27:1693–708. doi: 10.1111/nmo.12632. [DOI] [PubMed] [Google Scholar]
- 38.Noelting J, Ratuapli SK, Bharucha AE, et al. Normal values for high-resolution anorectal manometry in healthy women: effects of age and significance of rectoanal gradient. Am J Gastroenterol. 2012;107:1530–6. doi: 10.1038/ajg.2012.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carrington EV, Brokjaer A, Craven H, et al. Traditional measures of normal anal sphincter function using high-resolution anorectal manometry (HRAM) in 115 healthy volunteers. Neurogastroenterol Motil. 2014;26:625–35. doi: 10.1111/nmo.12307. [DOI] [PubMed] [Google Scholar]
- 40.Sauter M, Heinrich H, Fox M, et al. Toward more accurate measurements of anorectal motor and sensory function in routine clinical practice: validation of high-resolution anorectal manometry and Rapid Barostat Bag measurements of rectal function. Neurogastroenterol Motil. 2014;26:685–95. doi: 10.1111/nmo.12317. [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Yang X, Xu C, et al. Normal values and pressure morphology for three-dimensional high-resolution anorectal manometry of asymptomatic adults: a study in 110 subjects. Int J Colorectal Dis. 2013;28:1161–8. doi: 10.1007/s00384-013-1706-9. [DOI] [PubMed] [Google Scholar]
- 42.Coss-Adame E, Rao SS, Valestin J, et al. Accuracy and Reproducibility of High-definition Anorectal Manometry and Pressure Topography Analyses in Healthy Subjects. Clin Gastroenterol Hepatol. 2015 doi: 10.1016/j.cgh.2014.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mion F, Garros A, Brochard C, et al. 3D High-definition anorectal manometry: values obtained in asymptomatic volunteers, fecal incontinence and chronic constipation. Results of a prospective multicenter study (NOMAD) Neurogastroenterol Motil. 2017 doi: 10.1111/nmo.13049. [DOI] [PubMed] [Google Scholar]
- 44.Banasiuk M, Banaszkiewicz A, Dziekiewicz M, et al. Values from three-dimensional high-resolution anorectal manometry analysis of children without lower gastrointestinal symptoms. Clin Gastroenterol Hepatol. 2016;14:993–1000. e3. doi: 10.1016/j.cgh.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 45.Wickramasinghe DP, Perera CS, Senanayake H, et al. Three-Dimensional Anorectal Manometry Findings in Primigravida. Dig Dis Sci. 2015;60:3764–70. doi: 10.1007/s10620-015-3784-8. [DOI] [PubMed] [Google Scholar]
- 46.Lestar B, Penninckx F, Kerremans R. The composition of anal basal pressure. An in vivo and in vitro study in man. Int J Colorectal Dis. 1989;4:118–22. doi: 10.1007/BF01646870. [DOI] [PubMed] [Google Scholar]
- 47.Engel AF, Kamm MA, Bartram CI, et al. Relationship of symptoms in faecal incontinence to specific sphincter abnormalities. Int J Colorectal Dis. 1995;10:152–5. doi: 10.1007/BF00298538. [DOI] [PubMed] [Google Scholar]
- 48.Vaizey CJ, Kamm MA, Bartram CI. Primary degeneration of the internal anal sphincter as a cause of passive faecal incontinence. Lancet. 1997;349:612–5. doi: 10.1016/S0140-6736(96)09188-X. [DOI] [PubMed] [Google Scholar]
- 49.Felt-Bersma RJ, Klinkenberg-Knol EC, Meuwissen SG. Anorectal function investigations in incontinent and continent patients. Differences and discriminatory value. Dis Colon Rectum. 1990;33:479–85. doi: 10.1007/BF02052142. [DOI] [PubMed] [Google Scholar]
- 50.Bharucha AE, Fletcher JG, Harper CM, et al. Relationship between symptoms and disordered continence mechanisms in women with idiopathic faecal incontinence. Gut. 2005;54:546–55. doi: 10.1136/gut.2004.047696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prichard D, Harvey DM, Fletcher JG, et al. Relationship among anal sphincter injury, patulous anal canal, and anal pressures in patients with anorectal disorders. Clinical Gastroenterology and Hepatology. 2015;13:1793–1800. e1. doi: 10.1016/j.cgh.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Farouk R, Duthie GS, MacGregor AB, et al. Sustained internal sphincter hypertonia in patients with chronic anal fissure. Dis Colon Rectum. 1994;37:424–9. doi: 10.1007/BF02076185. [DOI] [PubMed] [Google Scholar]
- 53.Xynos E, Tzortzinis A, Chrysos E, et al. Anal manometry in patients with fissure-in-ano before and after internal sphincterotomy. International journal of colorectal disease. 1993;8:125–128. doi: 10.1007/BF00341183. [DOI] [PubMed] [Google Scholar]
- 54.Jones OM, Ramalingam T, Lindsey I, et al. Digital rectal examination of sphincter pressures in chronic anal fissure is unreliable. Diseases of the colon & rectum. 2005;48:349–352. doi: 10.1007/s10350-004-0753-2. [DOI] [PubMed] [Google Scholar]
- 55.Staller K, Barshop K, Kuo B, et al. Resting anal pressure, not outlet obstruction or transit, predicts healthcare utilization in chronic constipation: a retrospective cohort analysis. Neurogastroenterology & Motility. 2015;27:1378–1388. doi: 10.1111/nmo.12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Telford K, Ali A, Lymer K, et al. Fatigability of the external anal sphincter in anal incontinence. Diseases of the colon & rectum. 2004;47:746–752. doi: 10.1007/s10350-003-0122-6. [DOI] [PubMed] [Google Scholar]
- 57.Rao SS, Welcher KD, Leistikow JS. Obstructive defecation: a failure of rectoanal coordination. Am J Gastroenterol. 1998;93:1042–50. doi: 10.1111/j.1572-0241.1998.00326.x. [DOI] [PubMed] [Google Scholar]
- 58.Bharucha AE, Fletcher JG, Seide B, et al. Phenotypic variation in functional disorders of defecation. Gastroenterology. 2005;128:1199–210. doi: 10.1053/j.gastro.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 59.Carrington EV, Grossi U, Knowles C, et al. 43-'Pelvic Floor Akinesia'-A Highly Specific Manometric Finding in Patients with Defecatory Dysfunction. Gastroenterology. 2017;152:S16. [Google Scholar]
- 60.Aaronson I, Nixon HH. A clinical evaluation of anorectal pressure studies in the diagnosis of Hirschsprung's disease. Gut. 1972;13:138–46. doi: 10.1136/gut.13.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sangwan YP, Coller JA, Schoetz DJ, et al. Spectrum of abnormal rectoanal reflex patterns in patients with fecal incontinence. Dis Colon Rectum. 1996;39:59–65. doi: 10.1007/BF02048271. [DOI] [PubMed] [Google Scholar]
- 62.Xu X, Pasricha PJ, Sallam HS, et al. Clinical significance of quantitative assessment of rectoanal inhibitory reflex (RAIR) in patients with constipation. Journal of clinical gastroenterology. 2008;42:692–698. doi: 10.1097/MCG.0b013e31814927ba. [DOI] [PubMed] [Google Scholar]
- 63.Zbar AP, Beer-Gabel M, Chiappa AC, et al. Fecal incontinence after minor anorectal surgery. Dis Colon Rectum. 2001;44:1610–9. doi: 10.1007/BF02234380. discussion 1619–23. [DOI] [PubMed] [Google Scholar]
- 64.Rezaie A, Iriana S, Pimentel M, et al. Can 3D high resolution anorectal manometry detect anal sphincter defects in patients with faecal incontinence? Colorectal Dis. 2016 doi: 10.1111/codi.13530. [DOI] [PubMed] [Google Scholar]
- 65.Vitton V, Ben Hadj Amor W, Baumstarck K, et al. Comparison of three-dimensional high-resolution manometry and endoanal ultrasound in the diagnosis of anal sphincter defects. Colorectal Dis. 2013;15:e607–11. doi: 10.1111/codi.12319. [DOI] [PubMed] [Google Scholar]
- 66.Benezech A, Bouvier M, Grimaud JC, et al. Three-dimensional high-resolution anorectal manometry and diagnosis of excessive perineal descent: a comparative pilot study with defaecography. Colorectal Dis. 2014;16:O170–5. doi: 10.1111/codi.12522. [DOI] [PubMed] [Google Scholar]
- 67.Benezech A, Cappiello M, Baumstarck K, et al. Rectal intussusception: can high resolution three - dimensional ano - rectal manometry compete with conventional defecography? Neurogastroenterology & Motility. 2016 doi: 10.1111/nmo.12978. [DOI] [PubMed] [Google Scholar]
- 68.Heinrich H, Sauter M, Fox M, et al. Assessment of Obstructive Defecation by High-Resolution Anorectal Manometry Compared With Magnetic Resonance Defecography. Clin Gastroenterol Hepatol. 2015;13:1310–1317. e1. doi: 10.1016/j.cgh.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 69.Sun WM, Donnelly TC, Read NW. Utility of a combined test of anorectal manometry, electromyography, and sensation in determining the mechanism of 'idiopathic' faecal incontinence. Gut. 1992;33:807–13. doi: 10.1136/gut.33.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maeda Y, Vaizey CJ, Hollington P, et al. Physiological, psychological and behavioural characteristics of men and women with faecal incontinence. Colorectal Dis. 2009;11:927–32. doi: 10.1111/j.1463-1318.2008.01717.x. [DOI] [PubMed] [Google Scholar]
- 71.Zbar AP, Kmiot WA, Aslam M, et al. Use of vector volume manometry and endoanal magnetic resonance imaging in the adult female for assessment of anal sphincter dysfunction. Dis Colon Rectum. 1999;42:1411–8. doi: 10.1007/BF02235038. [DOI] [PubMed] [Google Scholar]
- 72.Grossi U, Carrington EV, Bharucha AE, et al. Diagnostic accuracy study of anorectal manometry for diagnosis of dyssynergic defecation. Gut. 2015 doi: 10.1136/gutjnl-2014-308835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jones MP, Post J, Crowell MD. High-resolution manometry in the evaluation of anorectal disorders: a simultaneous comparison with water-perfused manometry. Am J Gastroenterol. 2007;102:850–5. doi: 10.1111/j.1572-0241.2007.01069.x. [DOI] [PubMed] [Google Scholar]
- 74.Fang JC, Hilden K, Tuteja AK, et al. Comparison of air-coupled balloon esophageal and anorectal manometry catheters with solid-state esophageal manometry and water-perfused anorectal manometry catheters. Dig Dis Sci. 2004;49:1657–63. doi: 10.1023/b:ddas.0000043382.59539.d3. [DOI] [PubMed] [Google Scholar]
- 75.Simpson RR, Kennedy ML, Nguyen MH, et al. Anal manometry: a comparison of techniques. Dis Colon Rectum. 2006;49:1033–8. doi: 10.1007/s10350-006-0549-7. [DOI] [PubMed] [Google Scholar]
- 76.Vitton V, Ben Hadj Amor W, Baumstarck K, et al. Water-Perfused Manometry Versus 3-D High Resolution Manometry: A Comparative Study On A Large Patient Population With Ano-Rectal Disorders. Colorectal Dis. 2013 doi: 10.1111/codi.12397. [DOI] [PubMed] [Google Scholar]
- 77.Kang HR, Lee JE, Lee JS, et al. Comparison of high-resolution anorectal manometry with water-perfused anorectal manometry. J Neurogastroenterol Motil. 2015;21:126–32. doi: 10.5056/jnm14025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thekkinkattil DK, Lim MK, Nicholls MJ, et al. Contribution of posture to anorectal manometric measurements: are the measurements in left-lateral position physiologic? Dis Colon Rectum. 2007;50:2112–9. doi: 10.1007/s10350-007-9043-0. [DOI] [PubMed] [Google Scholar]
- 79.Snooks S, Swash M, Mathers S, et al. Effect of vaginal delivery on the pelvic floor: a 5 - year follow - up. British Journal of Surgery. 1990;77:1358–1360. doi: 10.1002/bjs.1800771213. [DOI] [PubMed] [Google Scholar]
- 80.Parks A, Swash M, Urich H. Sphincter denervation in anorectal incontinence and rectal prolapse. Gut. 1977;18:656–665. doi: 10.1136/gut.18.8.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lefaucheur JP. Neurophysiological testing in anorectal disorders. Muscle Nerve. 2006;33:324–33. doi: 10.1002/mus.20387. [DOI] [PubMed] [Google Scholar]
- 82.Rogers J, Henry M, Misiewicz J. Disposable pudendal nerve stimulator: evaluation of the standard instrument and new device. Gut. 1988;29:1131–1133. doi: 10.1136/gut.29.8.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thomas C, Lefaucheur J-P, Galula G, et al. Respective value of pudendal nerve terminal motor latency and anal sphincter electromyography in neurogenic fecal incontinence. Neurophysiologie Clinique/Clinical Neurophysiology. 2002;32:85–90. doi: 10.1016/s0987-7053(01)00287-8. [DOI] [PubMed] [Google Scholar]
- 84.Gregory WT, Lou J-S, Stuyvesant A, et al. Quantitative electromyography of the anal sphincter after uncomplicated vaginal delivery. Obstetrics & Gynecology. 2004;104:327–335. doi: 10.1097/01.AOG.0000134527.07034.81. [DOI] [PubMed] [Google Scholar]
- 85.Wexner SD, Marchetti F, Salanga VD, et al. Neurophysiologic assessment of the anal sphincters. Diseases of the Colon & Rectum. 1991;34:606–612. doi: 10.1007/BF02049902. [DOI] [PubMed] [Google Scholar]
- 86.Olsen AL, Rao SS. Clinical neurophysiology and electrodiagnostic testing of the pelvic floor. Gastroenterology Clinics of North America. 2001;30:33–54. doi: 10.1016/s0889-8553(05)70166-7. [DOI] [PubMed] [Google Scholar]
- 87.Sorensen M, Tetzschner T, Rasmussen O, et al. Relation between electromyography and anal manometry of the external anal sphincter. Gut. 1991;32:1031–1034. doi: 10.1136/gut.32.9.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Merletti R, Bottin A, Cescon C, et al. Multichannel surface EMG for the non-invasive assessment of the anal sphincter muscle. Digestion. 2004;69:112–22. doi: 10.1159/000077877. [DOI] [PubMed] [Google Scholar]
- 89.Wexner SD, Cheape JD, Jorge JM, et al. Prospective assessment of biofeedback for the treatment of paradoxical puborectalis contraction. Diseases of the colon & rectum. 1992;35:145–150. doi: 10.1007/BF02050669. [DOI] [PubMed] [Google Scholar]
- 90.Heymen S, Jones KR, Ringel Y, et al. Biofeedback treatment of fecal incontinence. Diseases of the colon & rectum. 2001;44:728–736. doi: 10.1007/BF02234575. [DOI] [PubMed] [Google Scholar]
- 91.Enck P, Van der Voort I, Klosterhalfen S. Biofeedback therapy in fecal incontinence and constipation. Neurogastroenterology & Motility. 2009;21:1133–1141. doi: 10.1111/j.1365-2982.2009.01345.x. [DOI] [PubMed] [Google Scholar]
- 92.Cheong DM, Vaccaro CA, Waxner SD, et al. Electrodiagnostic evaluation of fecal incontinence. Muscle & nerve. 1995;18:612–619. doi: 10.1002/mus.880180608. [DOI] [PubMed] [Google Scholar]
- 93.Lacima G, Pera M, Gonzalez-Argente X, et al. Is electromyography a predictive test of patient response to biofeedback in the treatment of fecal incontinence? Neurourology and urodynamics. 2016;35:390–394. doi: 10.1002/nau.22715. [DOI] [PubMed] [Google Scholar]
- 94.Abdool Z, Sultan AH, Thakar R. Ultrasound imaging of the anal sphincter complex: a review. British Journal of Radiology. 2012;85:865–75. doi: 10.1259/bjr/27314678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hainsworth AJ, Solanki D, Hamad A, et al. Integrated total pelvic floor ultrasound in pelvic floor defaecatory dysfunction. Colorectal Dis. 2017;19:O54–O65. doi: 10.1111/codi.13568. [DOI] [PubMed] [Google Scholar]
- 96.Stoker J, Rociu E, Wiersma TG, et al. Imaging of anorectal disease. Br J Surg. 2000;87:10–27. doi: 10.1046/j.1365-2168.2000.01338.x. [DOI] [PubMed] [Google Scholar]
- 97.Bipat S, Glas AS, Slors FJ, et al. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging--a meta-analysis. Radiology. 2004;232:773–83. doi: 10.1148/radiol.2323031368. [DOI] [PubMed] [Google Scholar]
- 98.Karmarkar R, Bhide A, Digesu A, et al. Mode of delivery after obstetric anal sphincter injury. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2015;194:7–10. doi: 10.1016/j.ejogrb.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 99.Harvey M-A, Pierce M, Walter J-E, et al. Obstetrical anal sphincter injuries (oasis): Prevention, recognition, and repair. Journal of Obstetrics and Gynaecology Canada. 2015;37:1131–1148. doi: 10.1016/s1701-2163(16)30081-0. [DOI] [PubMed] [Google Scholar]
- 100.Oom DM, West RL, Schouten WR, et al. Detection of anal sphincter defects in female patients with fecal incontinence: a comparison of 3-dimensional transperineal ultrasound and 2-dimensional endoanal ultrasound. Diseases of the Colon & Rectum. 2012;55:646–52. doi: 10.1097/DCR.0b013e318251dca1. [DOI] [PubMed] [Google Scholar]
- 101.Williams AB, Bartram CI, Halligan S, et al. Anal sphincter damage after vaginal delivery using three-dimensional endosonography. Obstetrics and Gynecology. 2001;97:770–5. doi: 10.1016/s0029-7844(01)01318-7. [DOI] [PubMed] [Google Scholar]
- 102.Albuquerque A, Pereira E. Current applications of transperineal ultrasound in gastroenterology. World journal of radiology. 2016;8:370. doi: 10.4329/wjr.v8.i4.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Steensma AB. The central and posterior compartments. In: Dietz HP, Hoyte LP, Steensma AB, editors. Atlas of pelvic floor ultrasound. London: Springer; 2008. pp. 63–75. [Google Scholar]
- 104.Beets-Tan RG, Morren GL, Beets GL, et al. Measurement of anal sphincter muscles: endoanal US, endoanal MR imaging, or phased-array MR imaging? A study with healthy volunteers. Radiology. 2001;220:81–9. doi: 10.1148/radiology.220.1.r01jn1481. [DOI] [PubMed] [Google Scholar]
- 105.Albuquerque A, Macedo G. Idiopathic internal anal sphincter degeneration: how common is it? Does size really matter? Colorectal Disease. 2017;19:396–397. doi: 10.1111/codi.13621. [DOI] [PubMed] [Google Scholar]
- 106.Halligan S, Sultan A, Rottenberg G, et al. Endosonography of the anal sphincters in solitary rectal ulcer syndrome. International Journal of Colorectal Disease. 1995;10:79–82. doi: 10.1007/BF00341201. [DOI] [PubMed] [Google Scholar]
- 107.Dvorkin LS, Chan CL, Knowles CH, et al. Anal sphincter morphology in patients with full-thickness rectal prolapse. Diseases of the Colon & Rectum. 2004;47:198–203. doi: 10.1007/s10350-003-0035-4. [DOI] [PubMed] [Google Scholar]
- 108.Dvorkin LS, Chan CL, Knowles CH, et al. Anal sphincter morphology in patients with full-thickness rectal prolapse. Dis Colon Rectum. 2004;47:198–203. doi: 10.1007/s10350-003-0035-4. [DOI] [PubMed] [Google Scholar]
- 109.Marshall M, Halligan S, Fotheringham T, et al. Predictive value of internal anal sphincter thickness for diagnosis of rectal intussusception in patients with solitary rectal ulcer syndrome. British journal of surgery. 2002;89:1281–1285. doi: 10.1046/j.1365-2168.2002.02197.x. [DOI] [PubMed] [Google Scholar]
- 110.Sultan AH, Kamm MA, Hudson CN, et al. Anal-sphincter disruption during vaginal delivery. N Engl J Med. 1993;329:1905–11. doi: 10.1056/NEJM199312233292601. [DOI] [PubMed] [Google Scholar]
- 111.Mahony R, Behan M, Daly L, et al. Internal anal sphincter defect influences continence outcome following obstetric anal sphincter injury. American journal of obstetrics and gynecology. 2007;196:217e1–217.e5. doi: 10.1016/j.ajog.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 112.Titi M, Jenkins J, Urie A, et al. Correlation between anal manometry and endosonography in females with faecal incontinence. Colorectal Disease. 2008;10:131–137. doi: 10.1111/j.1463-1318.2007.01312.x. [DOI] [PubMed] [Google Scholar]
- 113.Pinsk I, Brown J, Phang P. Assessment of sonographic quality of anal sphincter muscles in patients with faecal incontinence. Colorectal Disease. 2009;11:933–940. doi: 10.1111/j.1463-1318.2008.01730.x. [DOI] [PubMed] [Google Scholar]
- 114.Townsend D, Carrington E, Grossi U, et al. Pathophysiology of fecal incontinence differs between men and women: a case - matched study in 200 patients. Neurogastroenterology & Motility. 2016 doi: 10.1111/nmo.12858. [DOI] [PubMed] [Google Scholar]
- 115.Valsky DV, Cohen SM, Lipschuetz M, et al. Third - or Fourth - Degree Intrapartum Anal Sphincter Tears Are Associated With Levator Ani Avulsion in Primiparas. Journal of Ultrasound in Medicine. 2016;35:709–715. doi: 10.7863/ultra.15.04032. [DOI] [PubMed] [Google Scholar]
- 116.Shek K, Guzman - Rojas R, Dietz H. Residual defects of the external anal sphincter following primary repair: an observational study using transperineal ultrasound. Ultrasound in Obstetrics & Gynecology. 2014;44:704–709. doi: 10.1002/uog.13368. [DOI] [PubMed] [Google Scholar]
- 117.Bharucha AE, Fletcher JG, Harper CM, et al. Relationship between symptoms and disordered continence mechanisms in women with idiopathic fecal incontinence. Gut. 2005;54:546–55. doi: 10.1136/gut.2004.047696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bharucha AE, Fletcher JG, Melton LJ, 3rd, et al. Obstetric Trauma, Pelvic Floor Injury And Fecal Incontinence: A Population-Based Case-Control Study. American Journal of Gastroenterology. 2012;107:902–11. doi: 10.1038/ajg.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bharucha AE. Update of tests of colon and rectal structure and function. Journal of Clinical Gastroenterology. 2006;40:96–103. doi: 10.1097/01.mcg.0000196190.42296.a9. [DOI] [PubMed] [Google Scholar]
- 120.Gladman MA, Dvorkin LS, Lunniss PJ, et al. Rectal hyposensitivity: a disorder of the rectal wall or the afferent pathway? An assessment using the barostat. Am J Gastroenterol. 2005;100:106–14. doi: 10.1111/j.1572-0241.2005.40021.x. [DOI] [PubMed] [Google Scholar]
- 121.Whitehead WE, Delvaux M. Standardization of barostat procedures for testing smooth muscle tone and sensory thresholds in the gastrointestinal tract. The Working Team of Glaxo-Wellcome Research. UK Dig Dis Sci. 1997;42:223–41. doi: 10.1023/a:1018885028501. [DOI] [PubMed] [Google Scholar]
- 122.Hammer HF, Phillips SF, Camilleri M, et al. Rectal tone, distensibility, and perception: reproducibility and response to different distensions. American Journal of Physiology. 1998;274:G584–90. doi: 10.1152/ajpgi.1998.274.3.G584. [DOI] [PubMed] [Google Scholar]
- 123.Fox M, Thumshirn M, Fruhauf H, et al. Determinants of fecal continence in healthy, continent subjects: a comprehensive analysis by anal manometry, rectal barostat and a stool substitute retention test. Digestion. 2011;83:46–53. doi: 10.1159/000314588. [DOI] [PubMed] [Google Scholar]
- 124.Cremonini F, Houghton LA, Camilleri M, et al. Barostat testing of rectal sensation and compliance in humans: comparison of results across two centres and overall reproducibility. Neurogastroenterol Motil. 2005;17:810–20. doi: 10.1111/j.1365-2982.2005.00709.x. [DOI] [PubMed] [Google Scholar]
- 125.Gladman MA, Lunniss PJ, Scott SM, et al. Rectal hyposensitivity. American Journal of Gastroenterology. 2006;101:1140–51. doi: 10.1111/j.1572-0241.2006.00604.x. [DOI] [PubMed] [Google Scholar]
- 126.Simren M, Tornblom H, Palsson OS, et al. Visceral hypersensitivity is associated with GI symptom severity in functional GI disorders: consistent findings from five different patient cohorts. Gut. 2017 doi: 10.1136/gutjnl-2016-312361. [DOI] [PubMed] [Google Scholar]
- 127.Chan CL, Scott SM, Williams NS, et al. Rectal hypersensitivity worsens stool frequency, urgency, and lifestyle in patients with urge fecal incontinence. Dis Colon Rectum. 2005;48:134–40. doi: 10.1007/s10350-004-0774-x. [DOI] [PubMed] [Google Scholar]
- 128.Chan CL, Scott SM, Williams NS, et al. Rectal hypersensitivity worsens stool frequency, urgency, and lifestyle in patients with urge fecal incontinence. Diseases of the Colon & Rectum. 2005;48:134–40. doi: 10.1007/s10350-004-0774-x. [DOI] [PubMed] [Google Scholar]
- 129.Rao GN, Drew PJ, Lee PW, et al. Anterior resection syndrome is secondary to sympathetic denervation. Int J Colorectal Dis. 1996;11:250–8. doi: 10.1007/s003840050056. [DOI] [PubMed] [Google Scholar]
- 130.Chan CL, Lunniss PJ, Wang D, et al. Rectal sensorimotor dysfunction in patients with urge faecal incontinence: evidence from prolonged manometric studies. Gut. 2005;54:1263–72. doi: 10.1136/gut.2005.071613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Houghton LA, Calvert E, Jackson N, et al. Visceral sensation and emotion: a study using hypnosis. Gut. 2002;51:701–704. doi: 10.1136/gut.51.5.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Houghton LA, Fell C, Whorwell PJ, et al. Effect of a second-generation α 2 δ ligand (pregabalin) on visceral sensation in hypersensitive patients with irritable bowel syndrome. Gut. 2007;56:1218–1225. doi: 10.1136/gut.2006.110858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Algladi T, Harris M, Whorwell PJ, et al. Modulation of human visceral sensitivity by noninvasive magnetoelectrical neural stimulation in health and irritable bowel syndrome. Pain. 2015;156:1348–1356. doi: 10.1097/j.pain.0000000000000187. [DOI] [PubMed] [Google Scholar]
- 134.Harraf F, Schmulson M, Saba L, et al. Subtypes of constipation predominant irritable bowel syndrome based on rectal perception. Gut. 1998;43:388–94. doi: 10.1136/gut.43.3.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gladman MA, Lunniss PJ, Scott SM, et al. Rectal hyposensitivity. Am J Gastroenterol. 2006;101:1140–51. doi: 10.1111/j.1572-0241.2006.00604.x. [DOI] [PubMed] [Google Scholar]
- 136.Burgell RE, Scott SM. Rectal hyposensitivity. J Neurogastroenterol Motil. 2012;18:373–84. doi: 10.5056/jnm.2012.18.4.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gladman MA, Aziz Q, Scott SM, et al. Rectal hyposensitivity: pathophysiological mechanisms. Neurogastroenterol Motil. 2009;21:508–16. e4–5. doi: 10.1111/j.1365-2982.2008.01216.x. [DOI] [PubMed] [Google Scholar]
- 138.Sun WM, Read NW, Miner PB. Relation between rectal sensation and anal function in normal subjects and patients with faecal incontinence. Gut. 1990;31:1056–61. doi: 10.1136/gut.31.9.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wald A, Tunuguntla AK. Anorectal sensorimotor dysfunction in fecal incontinence and diabetes mellitus. Modification with biofeedback therapy. New England Journal of Medicine. 1984;310:1282–7. doi: 10.1056/NEJM198405173102003. [DOI] [PubMed] [Google Scholar]
- 140.Buser WD, Miner PB., Jr Delayed rectal sensation with fecal incontinence. Successful treatment using anorectal manometry. Gastroenterology. 1986;91:1186–91. doi: 10.1016/s0016-5085(86)80015-4. [DOI] [PubMed] [Google Scholar]
- 141.Rhee P-L, Choi MS, Kim YH, et al. An increased rectal maximum tolerable volume and long anal canal are associated with poor short-term response to biofeedback therapy for patients with anismus with decreased bowel frequency and normal colonic transit time. Diseases of the colon & rectum. 2000;43:1405–1411. doi: 10.1007/BF02236637. [DOI] [PubMed] [Google Scholar]
- 142.Knowles CH, Scott M, Lunniss PJ. Outcome of colectomy for slow transit constipation. Ann Surg. 1999;230:627–38. doi: 10.1097/00000658-199911000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chiarioni G, Whitehead WE, Pezza V, et al. Biofeedback is superior to laxatives for normal transit constipation due to pelvic floor dyssynergia. Gastroenterology. 2006;130:657–664. doi: 10.1053/j.gastro.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 144.Rao SS, Welcher KD, Pelsang RE. Effects of biofeedback therapy on anorectal function in obstructive defecation. Digestive diseases and sciences. 1997;42:2197–2205. doi: 10.1023/a:1018846113210. [DOI] [PubMed] [Google Scholar]
- 145.Lindberg G, Hamid SS, Malfertheiner P, et al. World Gastroenterology Organisation global guideline: Constipation--a global perspective. J Clin Gastroenterol. 2011;45:483–7. doi: 10.1097/MCG.0b013e31820fb914. [DOI] [PubMed] [Google Scholar]
- 146.Wald A, Bharucha AE, Cosman BC, et al. ACG clinical guideline: management of benign anorectal disorders. Am J Gastroenterol. 2014;109:1141–57. doi: 10.1038/ajg.2014.190. (Quiz) 1058. [DOI] [PubMed] [Google Scholar]
- 147.Sauter M, Heinrich H, Fox M, et al. Toward more accurate measurements of anorectal motor and sensory function in routine clinical practice: validation of high-resolution anorectal manometry and Rapid Barostat Bag measurements of rectal function. Neurogastroenterology and Motility. 2014;26:685–95. doi: 10.1111/nmo.12317. [DOI] [PubMed] [Google Scholar]
- 148.Sun WM, Read NW, Prior A, et al. Sensory and motor responses to rectal distention vary according to rate and pattern of balloon inflation. Gastroenterology. 1990;99:1008–15. doi: 10.1016/0016-5085(90)90620-g. [DOI] [PubMed] [Google Scholar]
- 149.Fox JC, Fletcher JG, Zinsmeister AR, et al. Effect of aging on anorectal and pelvic floor functions in females. Diseases of the Colon & Rectum. 2006;49:1726–35. doi: 10.1007/s10350-006-0657-4. [DOI] [PubMed] [Google Scholar]
- 150.Chiarioni G, Kim SM, Vantini I, et al. Validation of the balloon evacuation test: reproducibility and agreement with findings from anorectal manometry and electromyography. Clin Gastroenterol Hepatol. 2014;12:2049–54. doi: 10.1016/j.cgh.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 151.Minguez M, Herreros B, Sanchiz V, et al. Predictive value of the balloon expulsion test for excluding the diagnosis of pelvic floor dyssynergia in constipation. Gastroenterology. 2004;126:57–62. doi: 10.1053/j.gastro.2003.10.044. [DOI] [PubMed] [Google Scholar]
- 152.Rao SS, Ozturk R, Laine L. Clinical utility of diagnostic tests for constipation in adults: a systematic review. Am J Gastroenterol. 2005;100:1605–15. doi: 10.1111/j.1572-0241.2005.41845.x. [DOI] [PubMed] [Google Scholar]
- 153.Palit S, Thin N, Knowles CH, et al. Diagnostic disagreement between tests of evacuatory function: a prospective study of 100 constipated patients. Neurogastroenterol Motil. 2016;28:1589–98. doi: 10.1111/nmo.12859. [DOI] [PubMed] [Google Scholar]
- 154.Chiarioni G, Salandini L, Whitehead WE. Biofeedback benefits only patients with outlet dysfunction, not patients with isolated slow transit constipation. Gastroenterology. 2005;129:86–97. doi: 10.1053/j.gastro.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 155.Rao SS, Seaton K, Miller M, et al. Randomized controlled trial of biofeedback, sham feedback, and standard therapy for dyssynergic defecation. Clin Gastroenterol Hepatol. 2007;5:331–8. doi: 10.1016/j.cgh.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 156.Shim L, Jones M, Prott G, et al. Predictors of outcome of anorectal biofeedback therapy in patients with constipation. Alimentary pharmacology & therapeutics. 2011;33:1245–1251. doi: 10.1111/j.1365-2036.2011.04653.x. [DOI] [PubMed] [Google Scholar]
- 157.Lee J, Hong KS, Kim JS, et al. Balloon expulsion test does not seem to be useful for screening or exclusion of dyssynergic defecation as a single test. Journal of neurogastroenterology and motility. 2017;23:446. doi: 10.5056/jnm16158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dedeli O, Turan I, Ozturk R, et al. Normative values of the balloon expulsion test in healthy adults. Turk J Gastroenterol. 2007;18:177–81. [PubMed] [Google Scholar]
- 159.Mahieu P, Pringot J, Bodart P. Defecography: I. Description of a new procedure and results in normal patients. Gastrointest Radiol. 1984;9:247–51. doi: 10.1007/BF01887845. [DOI] [PubMed] [Google Scholar]
- 160.Lunniss PJ, Gladman MA, Benninga MA, et al. Pathophysiology of evacuation disorders. Neurogastroenterol Motil. 2009;21(Suppl 2):31–40. doi: 10.1111/j.1365-2982.2009.01402.x. [DOI] [PubMed] [Google Scholar]
- 161.Roos JE, Weishaupt D, Wildermuth S, et al. Experience of 4 years with open MR defecography: pictorial review of anorectal anatomy and disease. Radiographics. 2002;22:817–32. doi: 10.1148/radiographics.22.4.g02jl02817. [DOI] [PubMed] [Google Scholar]
- 162.Fletcher JG, Busse RF, Riederer SJ, et al. Magnetic resonance imaging of anatomic and dynamic defects of the pelvic floor in defecatory disorders. Am J Gastroenterol. 2003;98:399–411. doi: 10.1111/j.1572-0241.2003.07235.x. [DOI] [PubMed] [Google Scholar]
- 163.Mortele KJ, Fairhurst J. Dynamic MR defecography of the posterior compartment: Indications, techniques and MRI features. Eur J Radiol. 2007;61:462–72. doi: 10.1016/j.ejrad.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 164.Bharucha AE. Update of tests of colon and rectal structure and function. J Clin Gastroenterol. 2006;40:96–103. doi: 10.1097/01.mcg.0000196190.42296.a9. [DOI] [PubMed] [Google Scholar]
- 165.Bharucha AE, Zinsmeister AR, Schleck CD, et al. Bowel disturbances are the most important risk factors for late onset fecal incontinence: a population-based case-control study in women. Gastroenterology. 2010;139:1559–1566. doi: 10.1053/j.gastro.2010.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Van Dam J, Ginai A, Gosselink M, et al. Role of defecography in predicting clinical outcome of rectocele repair. Diseases of the colon & rectum. 1997;40:201–207. doi: 10.1007/BF02054989. [DOI] [PubMed] [Google Scholar]
- 167.Hubner M, Hetzer F, Weishaupt D, et al. A prospective comparison between clinical outcome and open - configuration magnetic resonance defecography findings before and after surgery for symptomatic rectocele. Colorectal Disease. 2006;8:605–611. doi: 10.1111/j.1463-1318.2006.01026.x. [DOI] [PubMed] [Google Scholar]
- 168.Palit S, Bhan C, Lunniss P, et al. Evacuation proctography: a reappraisal of normal variability. Colorectal Disease. 2014;16:538–546. doi: 10.1111/codi.12595. [DOI] [PubMed] [Google Scholar]
- 169.Chan CL, Scott SM, Knowles CH, et al. Exaggerated rectal adaptation - another cause of outlet obstruction. Colorectal Dis. 2001;3:141–2. doi: 10.1046/j.1463-1318.2001.00222.x. [DOI] [PubMed] [Google Scholar]
- 170.Shorvon P, McHugh S, Diamant N, et al. Defecography in normal volunteers: results and implications. Gut. 1989;30:1737–1749. doi: 10.1136/gut.30.12.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Collinson R, Cunningham C, D’Costa H, et al. Rectal intussusception and unexplained faecal incontinence: findings of a proctographic study. Colorectal Disease. 2009;11:77–83. doi: 10.1111/j.1463-1318.2008.01539.x. [DOI] [PubMed] [Google Scholar]
- 172.Morandi C, Martellucci J, Talento P, et al. Role of enterocele in the obstructed defecation syndrome (ODS): a new radiological point of view. Colorectal Disease. 2010;12:810–816. doi: 10.1111/j.1463-1318.2009.02050.x. [DOI] [PubMed] [Google Scholar]
- 173.Piloni V, Tosi P, Vernelli M. MR-defecography in obstructed defecation syndrome (ODS): technique, diagnostic criteria and grading. Techniques in coloproctology. 2013;17:501–510. doi: 10.1007/s10151-013-0993-z. [DOI] [PubMed] [Google Scholar]
- 174.Goh V, Halligan S, Kaplan G, et al. Dynamic MR imaging of the pelvic floor in asymptomatic subjects. AJR Am J Roentgenol. 2000;174:661–6. doi: 10.2214/ajr.174.3.1740661. [DOI] [PubMed] [Google Scholar]
- 175.Pilkington S, Nugent K, Brenner J, et al. Barium proctography vs magnetic resonance proctography for pelvic floor disorders: a comparative study. Colorectal Disease. 2012;14:1224–1230. doi: 10.1111/j.1463-1318.2012.02945.x. [DOI] [PubMed] [Google Scholar]
- 176.Dvorkin LS, Hetzer F, Scott SM, et al. Open-magnet MR defaecography compared with evacuation proctography in the diagnosis and management of patients with rectal intussusception. Colorectal Dis. 2004;6:45–53. doi: 10.1111/j.1463-1318.2004.00577.x. [DOI] [PubMed] [Google Scholar]
- 177.van Iersel J, Formijne JH, Verheijen P, et al. Comparison of dynamic magnetic resonance defaecography with rectal contrast and conventional defaecography for posterior pelvic floor compartment prolapse. Colorectal disease: the official journal of the Association of Coloproctology of Great Britain and Ireland. 2017;19:O46–O53. doi: 10.1111/codi.13563. [DOI] [PubMed] [Google Scholar]
- 178.Zafar A, Seretis C, Feretis M, et al. Comparative study of Magnetic Resonance Defaecography and Evacuation Proctography in the evaluation of obstructed defaecation. Colorectal Disease. 2017 doi: 10.1111/codi.13657. [DOI] [PubMed] [Google Scholar]
- 179.Bertschinger KM, Hetzer FH, Roos JE, et al. Dynamic MR Imaging of the Pelvic Floor Performed with Patient Sitting in an Open-Magnet Unit versus with Patient Supine in a Closed-Magnet Unit 1. Radiology. 2002;223:501–508. doi: 10.1148/radiol.2232010665. [DOI] [PubMed] [Google Scholar]
- 180.Schouten W, Briel J, Auwerda J, et al. Anismus: fact or fiction? Diseases of the colon & rectum. 1997;40:1033–1041. doi: 10.1007/BF02050925. [DOI] [PubMed] [Google Scholar]
- 181.Palit S, Thin N, Knowles C, et al. Diagnostic disagreement between tests of evacuatory function: a prospective study of 100 constipated patients. Neurogastroenterology & Motility. 2016;28:1589–1598. doi: 10.1111/nmo.12859. [DOI] [PubMed] [Google Scholar]
- 182.Lindberg G, Hamid SS, Malfertheiner P, et al. World Gastroenterology Organisation global guideline: constipation a global perspective. Journal of clinical gastroenterology. 2011;45:483–487. doi: 10.1097/MCG.0b013e31820fb914. [DOI] [PubMed] [Google Scholar]
- 183.Bove A, Pucciani F, Bellini M, et al. Consensus statement AIGO/SICCR: diagnosis and treatment of chronic constipation and obstructed defecation (part I: diagnosis) World J Gastroenterol. 2012;18:1555–1564. doi: 10.3748/wjg.v18.i14.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wald A, Bharucha AE, Cosman BC, et al. ACG clinical guideline: management of benign anorectal disorders. The American journal of gastroenterology. 2014;109:1141–1157. doi: 10.1038/ajg.2014.190. [DOI] [PubMed] [Google Scholar]
- 185.Duthie G, Bartolo D. Anismus: the cause of constipation? Results of investigation and treatment. World journal of surgery. 1992;16:831–835. doi: 10.1007/BF02066978. [DOI] [PubMed] [Google Scholar]
- 186.Videlock E, Lembo A, Cremonini F. Diagnostic testing for dyssynergic defecation in chronic constipation: meta - analysis. Neurogastroenterology & Motility. 2013;25:509–e370. doi: 10.1111/nmo.12096. [DOI] [PubMed] [Google Scholar]
- 187.Luft F, Fynne L, Gregersen H, et al. Functional luminal imaging probe: a new technique for dynamic evaluation of mechanical properties of the anal canal. Techniques in coloproctology. 2012;16:451–457. doi: 10.1007/s10151-012-0871-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Remes-Troche JM, Tantiphlachiva K, Attaluri A, et al. A bi-directional assessment of the human brain-anorectal axis. Neurogastroenterol Motil. 2011;23:240–8. e117–8. doi: 10.1111/j.1365-2982.2010.01619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Paris G, Chastan N, Gourcerol G, et al. Evoked pressure curves from the external anal sphincter following transcranial magnetic stimulation in healthy volunteers and patients with faecal incontinence. Colorectal Disease. 2013;15:e732–e740. doi: 10.1111/codi.12386. [DOI] [PubMed] [Google Scholar]
- 190.Tantiphlachiva K, Attaluri A, Valestin J, et al. Translumbar and transsacral motor-evoked potentials: a novel test for spino-anorectal neuropathy in spinal cord injury. The American journal of gastroenterology. 2011;106:907–914. doi: 10.1038/ajg.2010.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mittal RK, Bhargava V, Sheean G, et al. Purse-string morphology of external anal sphincter revealed by novel imaging techniques. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2014;306:G505–G514. doi: 10.1152/ajpgi.00338.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Burgell RE, Lelic D, Carrington EV, et al. Assessment of rectal afferent neuronal function and brain activity in patients with constipation and rectal hyposensitivity. Neurogastroenterol Motil. 2013;25:260–9. doi: 10.1111/nmo.12047. [DOI] [PubMed] [Google Scholar]
- 193.Delechenault P, Leroi AM, Bruna T, et al. Cerebral potentials evoked by electrical stimulation of the anal canal. Dis Colon Rectum. 1993;36:55–60. doi: 10.1007/BF02050302. [DOI] [PubMed] [Google Scholar]
- 194.Haas S, Brock C, Krogh K, et al. Abnormal neuronal response to rectal and anal stimuli in patients with idiopathic fecal incontinence. Neurogastroenterology & Motility. 2015;27:954–962. doi: 10.1111/nmo.12567. [DOI] [PubMed] [Google Scholar]
- 195.Harmston C, Jones O, Cunningham C, et al. The relationship between internal rectal prolapse and internal anal sphincter function. Colorectal Disease. 2011;13:791–795. doi: 10.1111/j.1463-1318.2010.02266.x. [DOI] [PubMed] [Google Scholar]
- 196.Damon H, Henry L, Roman S, et al. Influence of rectal prolapse on the asymmetry of the anal sphincter in patients with anal incontinence. BMC gastroenterology. 2003;3:23. doi: 10.1186/1471-230X-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Prior A, Maxton D, Whorwell P. Anorectal manometry in irritable bowel syndrome: differences between diarrhoea and constipation predominant subjects. Gut. 1990;31:458–462. doi: 10.1136/gut.31.4.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Salvioli B, Bharucha AE, Rath-Harvey D, et al. Rectal compliance, capacity, and rectoanal sensation in fecal incontinence. The American journal of gastroenterology. 2001;96:2158–2168. doi: 10.1111/j.1572-0241.2001.03954.x. [DOI] [PubMed] [Google Scholar]
- 199.Basilisco G, De Marco E, Tomba C, et al. Bowel urgency in patients with irritable bowel syndrome. Gastroenterology. 2007;132:38–44. doi: 10.1053/j.gastro.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 200.Johanson JF, Sonnenberg A, Koch TR. Clinical epidemiology of chronic constipation. J Clin Gastroenterol. 1989;11:525–36. doi: 10.1097/00004836-198910000-00008. [DOI] [PubMed] [Google Scholar]
- 201.Dinning PG, Smith TK, Scott SM. Pathophysiology of colonic causes of chronic constipation. Neurogastroenterol Motil. 2009;21(Suppl 2):20–30. doi: 10.1111/j.1365-2982.2009.01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Halverson AL, Orkin BA. Which physiologic tests are useful in patients with constipation? Diseases of the colon & rectum. 1998;41:735–739. doi: 10.1007/BF02236261. [DOI] [PubMed] [Google Scholar]
- 203.Kusters JG, van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev. 2006;19:449–90. doi: 10.1128/CMR.00054-05. [DOI] [PMC free article] [PubMed] [Google Scholar]