Abstract
Calcium homeostasis is essential for maintaining the viability and function of pancreatic β cells and plays a key role in preventing the development of diabetes. Decreased levels of ATPase sarcoplasmic/endoplasmic reticulum Ca2+-transporting 2 (ATP2a2), the main calcium pump in β cells, are often found in individuals with diabetes and in diabetic animal models. However, the regulators of ATP2a2 and the molecular mechanisms responsible for controlling ATP2a2 activity remain unclear. Etoposide-induced protein 2.4 (Ei24) is also down-regulated in β cells of diabetic individuals, whereas the effect of decreased Ei24 level on β-cell function is not clarified. Here, using Cre-LoxP and CRISPR/Cas9-based genomic knockout (KO) approaches to generate pancreatic β cell–specific Ei24 KO mice and pancreatic β-cell lines, we found that Ei24 regulates ATP2a2 activity. Specifically, we observed that Ei24 binds to ATP2a2 through Ei24 residues 293–299, which we named here the ATP2a2-interacting region (AIR). Loss of Ei24 inactivated ATP2a2, disrupted calcium homeostasis, and deactivated the calcium/calmodulin-dependent protein kinase kinase 2 (CAMKK2)–AMP-activated protein kinase (AMPK) pathway. Elevation of calcium concentration in the endoplasmic reticulum or agonist-induced AMPK activation rescued pancreatic β-cell survival and improved glucose tolerance of Ei24 KO mice. Our findings indicate that targeting the Ei24–ATP2a2 interaction to increase ATP2a2 activity can protect pancreatic β cells and improve glucose homeostasis in diabetic models, suggesting that Ei24 could potentially serve as a target to prevent or manage diabetes.
Keywords: diabetes, pancreatic islet, cell metabolism, calcium ATPase, apoptosis, ATPase sarcoplasmic/endoplasmic reticulum Ca2+-transporting 2, etoposide-induced protein 2.4, glucose tolerance
Introduction
Pancreatic β cells are specific endocrine cells with multiple intracellular membrane organelles that synthesize, transport, and secrete a large amount of insulin. The secreted insulin and many intracellular membrane proteins are correctly folded in the ER4 immediately after synthesis in ER-anchored ribosomes. A key factor for the correct folding of secretory proteins is the ER calcium level, which is several orders of magnitude higher than the calcium level in the cytosol. The intraluminal ER calcium pool is largely maintained by pumping Ca2+ from the cytosol to the ER via sarcoendoplasmic reticulum calcium ATPase (SERCA) channels (1), which are chiefly ATP2a2 in mouse pancreatic islets (2). Decreased ATP2a2 levels are often found in type 2 diabetes (T2D) patients and type 1 and type 2 diabetes rodent models (3, 4) and lead to impaired calcium homeostasis, insulin secretion, and β-cell survival (2, 5). However, the mechanism by which ATP2a2 levels and activity are maintained in β cells under physiological conditions remains unclear. Additionally, the downstream regulators that link ATP2a2 levels and β-cell loss in diabetic patients need to be determined.
Etoposide-induced protein 2.4 (Ei24), first identified as a DNA damage response gene induced by the tumor suppressor protein p53, plays a role in preventing the expansion of breast tumors and is frequently lost in aggressive breast cancer (6–8). Similar to its function against tumor cells, Ei24 promotes fibroblast cell death by binding to Bcl2 (7), and Ei24 knockout in mouse fibroblast or human breast cancer cell lines results in increased resistance to etoposide-induced apoptosis (9). In addition to its function in negatively regulating cell growth, Ei24 has also been demonstrated as an essential autophagy gene in Caenorhabditis elegans and mammals (8, 10), as it mediates autophagy signaling pathways and the ubiquitin-proteasome system through its interaction with E3 ligases with a RING domain (11). Ei24 is highly expressed in normal pancreatic β cells but is significantly decreased in pancreatic β cells of T2D patients (12), which indicates that Ei24 is associated with multiple clinical consequences and that the physical and pathophysiological roles of Ei24 in pancreatic β cells should be explored.
Here, we used the Cre-LoxP methodology and CRISPR-Cas9 genomic editing approaches to generate pancreatic β cell–specific Ei24 knockout (KO) mice and an Ei24 KO pancreatic β-cell line, respectively. The results showed that loss of Ei24 leads to glucose intolerance, decreased insulin biosynthesis, impaired β-cell cytosolic calcium homeostasis, reduced ER calcium levels, and increased apoptosis. Using a GFP-trap method, we demonstrated the interaction between Ei24 and ATP2a2 and identified a region of Ei24 involved in the interaction, which was termed the ATP2a2-interacting region (AIR). Moreover, in Ei24 knockout animals, we found a significant improvement in glucose intolerance induced by treatment with CDN1163, a small-molecule activator of ATP2a2, which indicated that Ei24 regulates ATP2a2 activity and is essential for pancreatic β-cell mass.
Results
Ei24 levels are decreased in islets from diabetic models
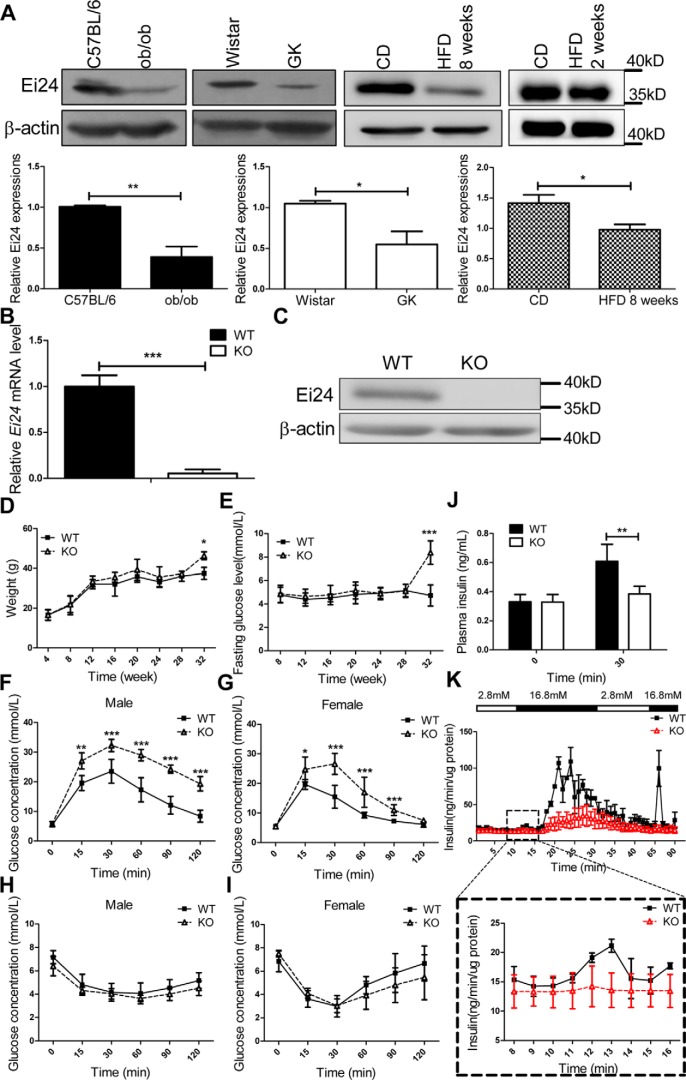
To test the possible function of Ei24 in pancreatic β-cell function under a pathological condition, we first examined the levels of Ei24 in HFD-fed mice, ob/ob mice, and GK rats. Consistent with findings in T2D patients, Ei24 expression was significantly decreased in islets from the diabetic animal models compared with that in the controls. Notably, the level of Ei24 further decreased with prolonged consumption of an HFD, which further indicated the correlation between Ei24 levels and the diabetic process (Fig. 1A).
Figure 1.
Loss of Ei24 impairs glucose homeostasis. A, expression of Ei24 in islets from ob/ob mice, GK rats at 4 months of age, and C57BL/6 mice fed an HFD for 2 weeks and 8 weeks. β-Actin served as the loading control. CD, chow diet. B, total RNA was prepared from islets of WT and KO mice at 8 weeks of age. The transcription levels of Ei24 mRNA are normalized to β-actin mRNA. The results are representative of three individual experiments. C, Western blotting of Ei24 protein from isolated islets (300 islets/group) from WT and KO mice at 12 weeks of age. β-Actin served as the loading control. D, weight curves for WT and KO mice. The mean ± S.D. (error bars) of 15 mice is shown. E, concentration of the basic glucose curve for WT and KO mice. The mean ± S.D. of 10 mice is shown. F and G, glucose tolerance test (GTT) results of 10-week-old male (F) and female mice (G). Solid lines, WT mice (n = 8); dashed lines, KO mice (n = 8). H and I, ITT results of 10-week-old male (H) and female mice (I). Solid lines, WT mice (n = 8); dashed lines, KO mice (n = 8). J, in vivo GSIS detection in WT and KO mice. The results are representative of five replicates for each group. K, in vitro GSIS from isolated islets (70/group) of WT and KO mice by a fast digital perfusion system. The results are representative of three individual experiments. Data are expressed as the mean ± S.D. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Generation and validation of Ei24 KO model
Because homozygous loss of Ei24 is embryonically lethal (13), the Cre-LoxP methodology was used to generate mice with a pancreatic β cell–specific knockout of Ei24. Gene expression of Ei24 in the islets was ∼90% lower in the KO mice than in the WT mice (Fig. 1B). Following this, Ei24 protein abundance was almost fully deleted in KO versus WT mice (Fig. 1C). To exclude the possible impact of LoxP sites on target gene expression (14), we checked the expression of Ei24 in liver, muscle, and fat tissues from Ei24 floxed mice and WT mice. The results showed no significant difference in Ei24 expression in non-β-cell tissues between Ei24 floxed mice and WT mice (Fig. S1).
Loss of Ei24 impairs glucose tolerance but does not affect insulin tolerance
There were no differences in body mass (Fig. 1D) or fasting glucose concentration (Fig. 1E) between the WT and KO mice up to 28 weeks after birth. Considering that the course of diabetes is age-dependent for several diabetes mouse models, we measured these basic metabolic indices of the mice at 32 weeks of age. We found that the fasting glucose concentration and body weight were higher in Ei24 KO mice than in WT mice (Fig. S2), which indicated that with the increase in β-cell apoptosis, the diabetes phenotype becomes more apparent. A glucose tolerance test (GTT) administered at 10 weeks of age showed significant glucose intolerance in both male (Fig. 1F) and female (Fig. 1G) KO mice. In RIP-Cre transgenic mice, the RIP promoter drives CRE expression in both pancreatic β cells and the brain (15, 16). To exclude the influence of neurons expressing CRE activity, we generated proopiomelanocortin (POMC)-specific Ei24 knockout mice and found no difference in glucose tolerance between WT and POMC-specific Ei24 knockout (Ei24Δpomc) mice (Fig. S3). For the insulin-tolerance test (ITT), the insulin sensitivity of KO mice was comparable with that of the control group (Fig. 1, H and I), suggesting a primary defect in β-cell function.
Loss of Ei24 affects glucose-stimulated insulin secretion
The results showed that fasting insulin levels were similar in the KO mice (0.32 ± 0.05 ng/ml) and the WT mice (0.33 ± 0.05 ng/ml) (Fig. 1J). The glucose-stimulated insulin secretion (GSIS) in vivo results showed that the serum insulin levels were increased significantly in the WT mice 30 min (0.61 ± 0.1 ng/ml) after glucose injections, whereas the insulin levels were significantly lower in the KO mice (0.38 ± 0.05 ng/ml) following glucose challenge (Fig. 1J). Similarly, the results from an ex vivo GSIS analysis of isolated islets showed that there was no significant difference in basal insulin secretion (when cultured with 2.8 mm glucose) from size-matched islets from the KO and WT mice. However, in response to the administration of 16.7 mm glucose, insulin secretion from islets isolated from the KO mice was slightly lower during the first phase (0–5 min) and remarkably lower during the second phase than that from islets isolated from control mice (Fig. 1K). To demonstrate that the increased insulin content in the WT group was not due to passive release from the dying cells, the glucose concentration was reverted back to 2.8 mm, and the insulin content was remeasured. The results showed that the insulin release returned to the basal level (Fig. 1K). This suggested that the impaired glucose tolerance in KO mice may be primarily caused by insufficient GSIS.
Loss of Ei24 diminishes pancreatic β-cell mass
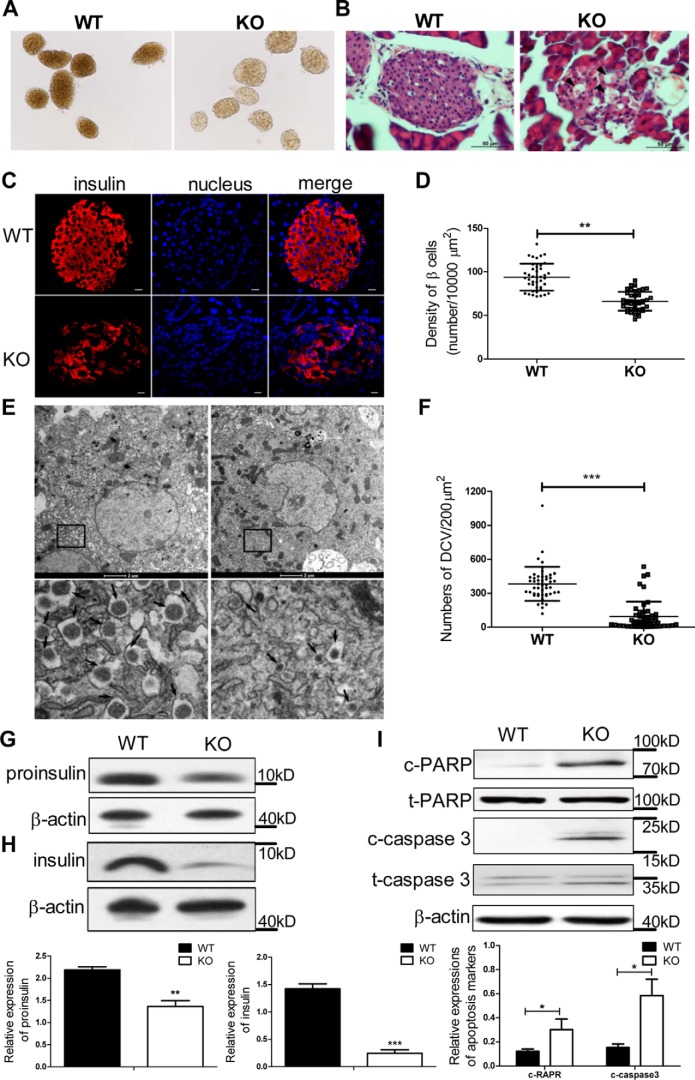
Given that insufficient insulin release may be caused by β-cell abnormalities, we conducted a morphological analysis of the pancreatic islets. In contrast to the dense appearance of the WT islets, the KO islets became uncompact and transparent (Fig. 2A). Microscopic examination after hematoxylin and eosin (H&E) staining showed vacuolated cells in the islets of the KO mice that were not found in control mice (Fig. 2B). We hypothesized that these vacuole-like structures are associated with apoptotic cells. Thus, the relative β-cell mass was estimated by point counting after insulin immunohistochemistry (Fig. 2C), and the results showed lower β-cell mass in the KO mice (65 ± 10/10,000 μm2) than in the WT mice (92 ± 15/10,000 μm2, p < 0.001; Fig. 2D). Insulin granule number was then assessed by quantitative analysis of islet electron micrographic images. Typical mature insulin granules were observed as a dense homogeneous core with a clear halo (Fig. 2E). Fig. 2F shows that the number of mature insulin granules was decreased by ∼75% in the KO islets compared with the WT group. Consistent with the decreased insulin granules and β-cell mass in the KO mice, both proinsulin and insulin protein levels in the islets of the KO mice were significantly lower than those of the WT mice (Fig. 2, G and H). Furthermore, increased protein levels of cleaved poly(ADP-ribose) polymerase (c-PARP) and cleaved caspase-3 (c-caspase-3), which mark apoptotic cascades within cells, were observed in the islets of the KO mice (Fig. 2I). These findings may account for the lack of β-cell mass as well as the decreased insulin production.
Figure 2.
Loss of Ei24 causes apoptosis of pancreatic β cells. A, morphologies of islets. The islets from KO mice became uncompact and transparent compared with the WT islets. B, representative images of H&E staining of islets from WT and KO mice at the age of 12 weeks. The degenerative β cells are indicated by black arrowheads. C, representative sections of islets stained for insulin (red) and nuclei (blue) from WT and KO mice at 12 weeks of age. Scale bars, 10 μm. D, density of β cells determined by counting the number of β cells in islets (n = 40/group) from WT and KO mice. E, EM micrographs of WT and KO pancreatic β cells. The bottom panel in E shows enlargement of the boxed area in the top panel. The arrows indicate the dense core vesicles. F, the number of dense core vesicles (DCV) in pancreatic β cells was counted using Imaris software (cell numbers, n = 48/group). G, Western blotting for proinsulin in the isolated islets (30 islets/group) from WT and KO mice at 8 weeks of age. β-Actin served as the loading control. Data were obtained from three independent experiments. H, Western blotting for insulin in the isolated islets (60 islets/group) from WT and KO mice at 8 weeks of age. β-Actin served as the loading control. Data were obtained from three independent experiments. I, Western blotting for c-PARP, t-PARP, c-caspase-3, and t-caspase-3 in the isolated islets (150 islets/group) from WT and KO mice at 8–10 weeks of age. β-Actin served as the loading control. Data were obtained from five independent experiments. Data are expressed as the mean ± S.D. (error bars). *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Loss of Ei24 disrupts energy sensing of the calcium/calmodulin-dependent protein kinase kinase 2 (CAMKK2)–AMP-activated protein kinase (AMPK) pathway
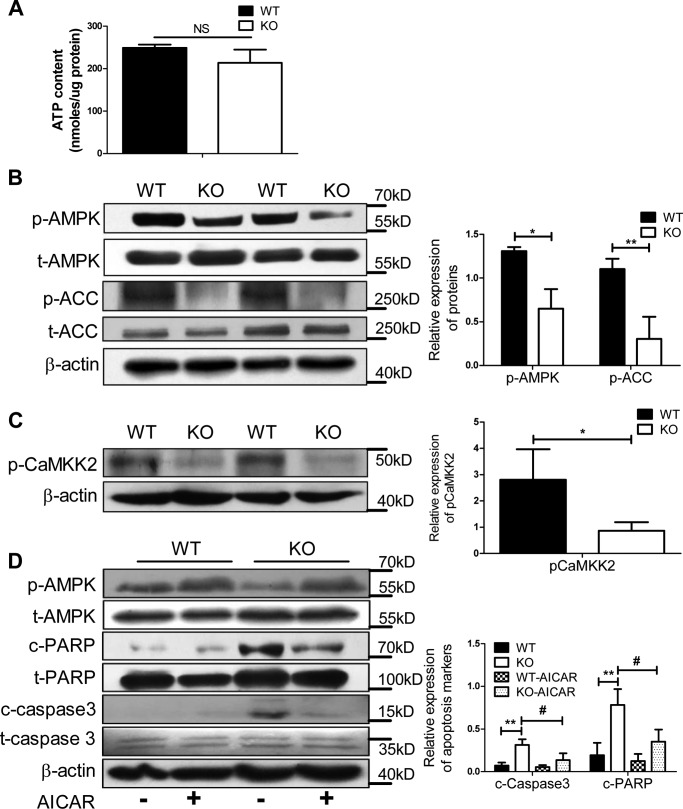
To explore the molecular mechanism of increased apoptosis of pancreatic β cells in the KO mice, we measured the cytosolic ATP content and the activation of AMPK in pancreatic β cells, because β cells require a large amount of energy for insulin secretion and survival. As shown in Fig. 3A, no change in cellular ATP content was observed, but AMPK and its substrate phosphorylated acetyl-CoA carboxylase (p-ACC) were observed to be deactivated in the KO β cells (Fig. 3B). In line with the activation of AMPK, the activity of CAMKK2 was down-regulated in the KO group (Fig. 3C). To evaluate whether the reduced activation of AMPK due to the loss of Ei24 is responsible for the elevated β-cell apoptosis, we treated WT and KO islets with the AMPK activator 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR). Notably, AICAR activated AMPK and rescued the KO β cells from apoptosis (Fig. 3D). Taken together, these data implicated Ei24 as an important regulator of AMPK activation for β-cell survival.
Figure 3.
Loss of Ei24 impairs AMPK activation and induces apoptotic cell death. A, ATP content of islets (40 islets/group), which were cultured in RPMI medium 1640 with 10% FBS from WT and KO mice at 8 weeks of age. Data were normalized to protein content. Data were obtained from three independent experiments. B, Western blotting for p-AMPK, t-AMPK, p-ACC, and t-ACC in the isolated islets (150 islets/group) from WT and KO mice at 8–10 weeks of age. C, Western blotting for p-CAMKK2 in the isolated islets (150 islets/group) from WT and KO mice at 8–10 weeks of age. D, Western blotting for p-AMPK, t-AMPK, c-PARP, t-PARP, c-caspase-3, and t-caspase-3 in the isolated islets (150 islets/group) with or without AICAR treatment from WT and KO mice at 8–10 weeks of age. β-Actin served as the loading control. Data were obtained from three independent experiments. Data are expressed as the mean ± S.D. (error bars): WT versus KO (*, p < 0.05; **, p < 0.01) and KO group without AICAR treatment versus KO with AICAR treatment (#, p < 0.05).
Loss of Ei24 disrupts Ca2+ homeostasis and diminishes the Ca2+ pool
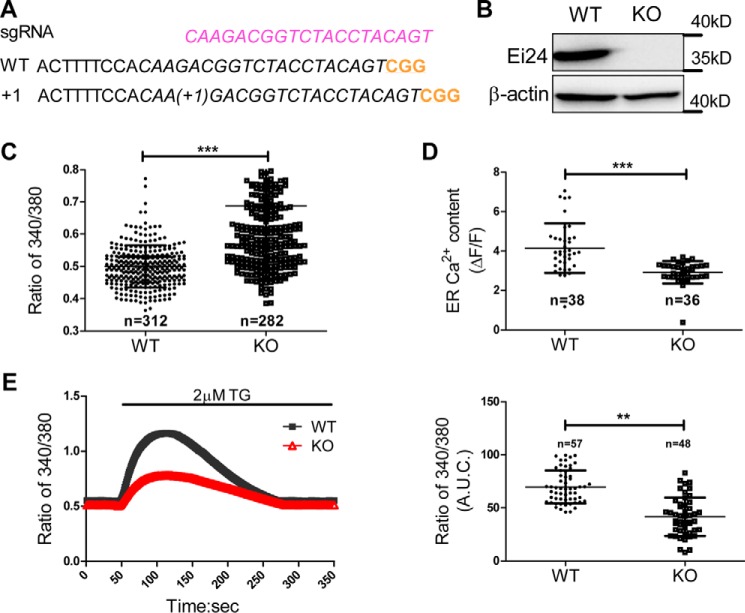
Using CRISPR-Cas9 genomic editing approaches, the Ei24 KO pancreatic β-cell line was generated (Fig. 4, A and B). Considering that CAMKK2 is a Ca2+-related kinase that regulates AMPK activity in other cells (17) and that stimulation by increased intracellular Ca2+ levels increases activation of AMPK, we speculated that loss of Ei24 may decrease the cytosolic calcium concentration. However, the intracellular Ca2+ level was clearly elevated in the KO group (Fig. 4C). We next estimated the ER Ca2+ content by challenging WT and Ei24 KO INS1 cell lines with ER-targeted GCaMP6, a fluorescent calcium indicator (18). Fig. 4D shows that the loss of Ei24 significantly lowered the ER Ca2+ content in the KO cells compared with the WT cells. The filling state of the ER Ca2+ store was further confirmed by the presence of the irreversible ATP2a2 inhibitor thapsigargin (TG) in Ca2+-free medium. Depletion of Ei24 caused a steady-state damage of ER Ca2+ content by ∼30% compared with the WT group (Fig. 4E), which further indicated that loss of Ei24 diminishes the content of the Ca2+ pool.
Figure 4.
Loss of Ei24 disrupts calcium homeostasis. A, mutation in Ei24 generated by CRISPR/Cas9 in INS1 cells. B, Western blotting results demonstrating that Ei24 is effectively depleted in Ei24 KO cells. C, intracellular calcium levels were evaluated in single cells at basal conditions by measuring the 340/380 nm ratio of Fura 2-AM (number of cells analyzed: WT, n = 312; KO, n = 282). D, quantification of ER-GCaMP6 fluorescence peak values in responding WT and Ei24 KO cells (number of cells analyzed: WT, n = 38; KO, n = 36 cells). E, left panel, average curve of Fura 2 in WT and KO INS1 cells stimulated with 2 μm TG (number of cells analyzed: WT, n = 57; KO, n = 48 cells). Right panel, area under the curve (A.U.C.) of calcium entry after depletion of the ER with TG treatment. Experiments were independently repeated three times. Data are represented as the mean ± S.D. (error bars). ***, p < 0.001; **, p < 0.05.
Ei24 is a novel modulator of ATP2a2
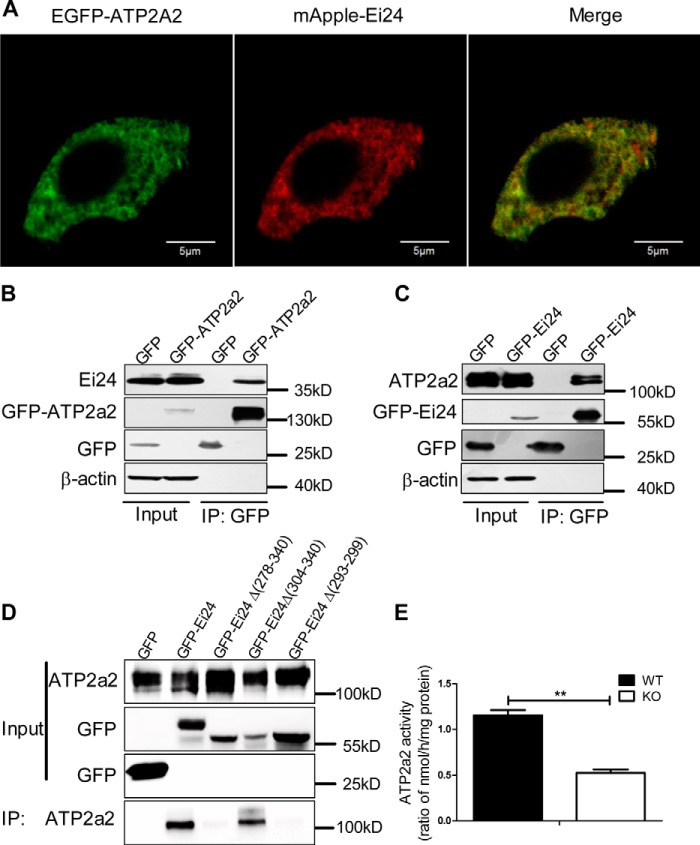
ATP2a2 is a Ca2+ ATPase that transports Ca2+ from the cytosol to the ER lumen and plays an important role in maintaining the ER Ca2+ content. Because loss of Ei24 depletes the ER Ca2+ store, we further studied the relationship between Ei24 and ATP2a2. Laser-scanning confocal imaging showed good co-localization between the EGFP-tagged ATP2a2 and mApple-labeled Ei24 in MIN6 cells (Fig. 5A). Superresolution imaging by structured illumination microscopy (SIM) was employed to analyze the localization of Ei24 and ATP2a2 further. The results showed good co-localization between the EGFP-tagged Ei24 and mCherry-labeled ATP2a2 (Fig. S4). Moreover, we performed coimmunoprecipitation assays and observed that Ei24 specifically bound to ATP2a2 (Fig. 5, B and C). To characterize the interaction between Ei24 and ATP2a2 in more detail, we assessed the binding of ATP2a2 to Ei24 mutants. As shown in Fig. 5D, residues 278–340, which represent the predicted C-terminal domain of Ei24, were essential for binding with ATP2a2. Next, the dichotomy method was applied, and a series of truncation mutants were constructed. As shown in Fig. 5D, Ei24 mutants missing residues 304–340 were dispensable for binding with ATP2a2, suggesting that the critical binding domain resided between residues 278 and 303. The mutants missing residues 293–299 no longer bound at levels equal to that of the control column, which indicated that this region is the critical binding domain of Ei24 for binding with ATP2a2 (Fig. 5D), which we name the AIR domain. The ATP2a2 activity was further studied and was shown to be significantly lower in the microsome fraction purified from the Ei24 KO cells than in microsomes from the WT cells (Fig. 5E), suggesting that Ei24 promotes ATP2a2 activity through direct interaction.
Figure 5.
Ei24 interacts with ATP2a2 to modulate ATP2a2 activity. A, co-localization imaging of EGFP-labeled ATP2a2 and mApple-labeled Ei24 in MIN6 cells by confocal microscopy. Green, ATP2a2; red, Ei24. The presence of the yellow color after merging the images showed that Ei24 co-localizes well with ATP2a2. Scale bars, 5 μm. In GFP-Trap assays, endogenous Ei24 was immunoprecipitated by EGFP-ATP2a2 (B), and endogenous ATP2a2 was immunoprecipitated by EGFP-Ei24 in INS1 cells (C). D, compared with EGFP-Ei24, mutant Ei24 lacking amino acids 293–299 or 278–340 precipitated no endogenous ATP2a2 in the GFP-Trap assays. Lysates from INS1 cells transfected with the indicated plasmids were immunoprecipitated (IP) using GFP-Trap and analyzed by immunoblotting. E, the ATP2a2 activity in Ei24 KO INS1 cells is significantly lower than that in WT cells. Data were obtained from three independent experiments. Data are expressed as the mean ± S.D. (error bars). *, p < 0.05; **, p < 0.01.
Activation of ATP2a2 rescued the diabetic phenotype of Ei24 KO mice
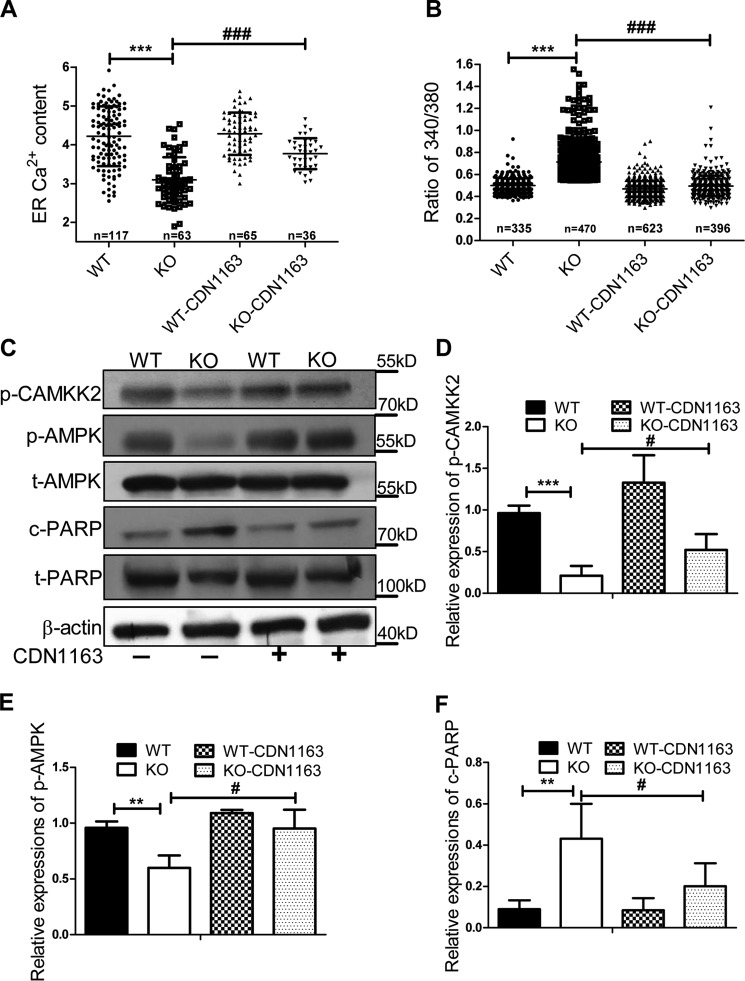
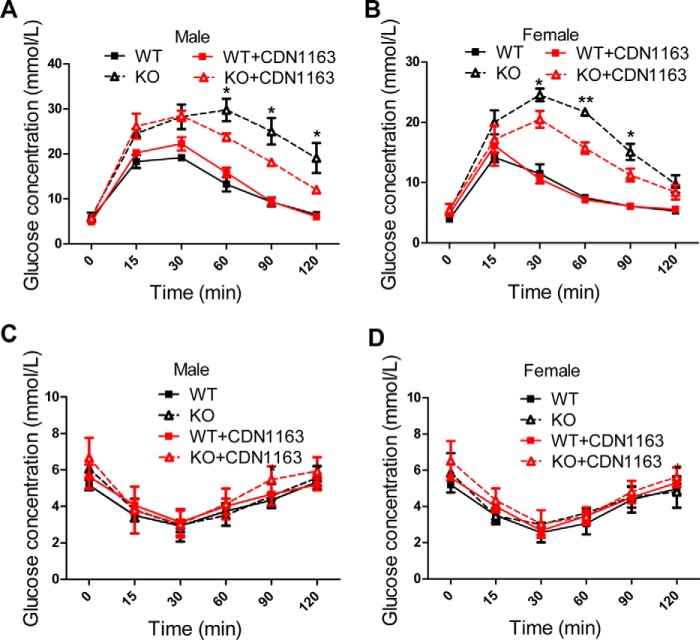
We next examined whether apoptosis of Ei24-depleted primary β cells could be rescued by increasing ATP2a2 activity. CDN1163 is reported as a small molecular activator of ATP2a2 that increases ER Ca2+ content (19). Following CDN1163 treatment, the [Ca2+]ER pool was significantly increased (Fig. 6A), whereas basal [Ca2+]i was decreased in the KO group (Fig. 6B). Moreover, CDN1163 treatment considerably rescued the activation of CAMKK2, which further induced AMPK activation and attenuated the apoptosis of β cells induced by the loss of Ei24 (Fig. 6, C–F). To examine whether the pharmacological activation of ATP2a2 with CDN1163 could improve glucose tolerance in the KO mice, GTT was performed on 10-week-old mice, which were intraperitoneally injected with CDN1163 (50 mg/kg) or a vehicle (5% DMSO, 10% Tween in 0.9% NaCl) once a day for a total of 10 days. The results showed that the CDN1163-treated KO mice exhibited improved glucose tolerance after treatment compared with the vehicle-treated KO mice; however, glucose tolerance in the control WT group was not altered by CDN1163 (Fig. 7, A and B). The insulin tolerance test that was administered on day 15 indicated that the insulin sensitivity did not significantly change (Fig. 7, C and D), further suggesting the protective role of CDN1163 on β cells. These results strongly emphasize a pivotal role for ATP2a2 activity, which is modulated by Ei24 in the maintenance of calcium homeostasis and β-cell functions.
Figure 6.
Increased ATP2a2 activity could rescue calcium homeostasis and cell death in Ei24 KO mice. A, quantification of ER-GCaMP6 fluorescence peak values in responding WT and Ei24 KO cells with or without CDN1163 treatment (number of cells analyzed: WT, n = 117; KO, n = 63 cells; WT with CDN1163, n = 65; KO with CDN1163, n = 36). B, intracellular calcium levels were evaluated in single cells with or without CDN1163 treatment at basal conditions by measuring the 340/380-nm ratio of Fura 2-AM (number of cells analyzed: WT, n = 335; KO, n = 470; WT with CDN1163, n = 623; KO with CDN1163, n = 396). C, Western blotting for p-CAMKK2, p-AMPK, t-AMPK, c-PARP, and t-PARP in the isolated islets (100 islets/group), which were treated with or without 10 μm CDN1163 treatment, from WT and KO mice at 8–10 weeks of age. D–F, quantification analysis of p-CAMKK2 (D), p-AMPK (E), and c-PARP (F) expression normalized to β-actin expression. Data were obtained from three independent experiments. Data are expressed as the mean ± S.D. (error bars): WT versus KO (**, p < 0.01; ***, p < 0.001) and KO without CDN1163 versus KO with CDN163 (#, p < 0.05; ###, p < 0.001).
Figure 7.
Increased ATP2a2 activity could rescue glucose intolerance in Ei24 KO mice. A and B, GTT in 10-week-old male mice (A) and female mice (B) that were intraperitoneally injected with CDN1163 or a vehicle once a day for a total of 10 days. Five days later, ITT was performed on the male (C) and female (D) mice. Black solid lines, WT mice with DMSO treatment (n = 8); black dashed lines, KO mice with DMSO treatment (n = 8); red solid lines, WT mice with CDN1163 treatment (n = 8); red dashed lines, KO mice with CDN1163 treatment (n = 8). *, p < 0.05; **, p < 0.01, KO mice with DMSO treatment versus KO mice with CDN1163 treatment. Error bars, S.D.
Discussion
Ei24 is a target gene of p53 that has been reported as a negative regulator of cell growth (7, 9, 20). Depletion of Ei24 by antisense oligodeoxynucleotides leads to suppression of apoptosis in response to the pro-apoptotic retinoid CD437/AHPN (21). Yuan et al. (22) also showed that microRNA(290–295) clusters prevented apoptosis in mouse embryonic stem cells by targeting Ei24 and inhibiting its expression. However, in pancreatic islets from diabetic patients, which are characterized by severe loss of pancreatic β-cell number, Ei24 expression level was markedly decreased (12). We propose that Ei24 may play a role in pancreatic β cells different from that in other cells. In the current study, we showed strong evidence that the function of Ei24 is essential for β-cell mass and function. First, we found reduced Ei24 protein levels in pancreatic β cells from both diabetic ob/ob mice and GK rats. HFD feeding decreases Ei24 levels in islets; further, prolonged consumption of an HFD suggested a correlation between Ei24 levels and the diabetic process. Next, Ei24 KO β cells demonstrated Ca2+ dyshomeostasis, decreased insulin vesicles, impaired insulin secretion, and impaired β-cell survival. These results suggest that in contrast to the negative function of suppressing cell growth and inducing cell death in cancer (20), Ei24 plays an important protective role in primary pancreatic β cells. Rescue of Ei24 expression in the pancreas in diabetic patients could have clinically beneficial effects on β-cell function and may serve as a novel potential therapeutic treatment.
The course of diabetes is age-dependent for several mouse models of diabetes (23). There are no significant differences in basal glucose concentration and body weight between Ei24 KO mice and WT mice from 4 to 28 weeks. However, as age increases and diabetes develops, the basal glucose concentration and body weight increase sharply at 32 weeks, indicating that Ei24 KO mice are a good model for studying the progression of diabetes.
ATP2a2 has been well-studied in β cells and in other types of cells. It has been reported that decreased ATP2a2 levels are often found in T2D patients and type 1 and type 2 diabetes rodent models (3, 4). However, the molecular mechanisms that regulate ATP2a2 activity remain unclear. Our molecular study showed that Ei24 interacts directly with ATP2a2 through an AIR domain and controls ER calcium levels by regulating ATP2a2 activity, which provides new insight into the regulation of ATP2a2 and the roles of Ei24 in β-cell function and pathophysiology. In addition to directly regulating ATP2a2 activity, Ei24 may also regulate ATP2a2 levels, as Ei24 expression levels were also decreased in pancreatic islets from diabetic patients (12) and ob/ob mice, as shown in Fig. S5A. We demonstrated that CDN1163, which elevates ATP2a2 activity, can improve glucose tolerance in Ei24 KO mice and ob/ob mice in which ATP2a2 and Ei24 levels were significantly decreased (Fig. S5B). CDN1163 could not rescue the blood glucose concentration of KO mice to WT levels. However, KO Ei24 led to 30% β-cell apoptosis, and glucose levels decreased by 43.3, 44.3, and 54.4% at 60, 90, and 120 min, respectively, in Ei24 KO male mice and by 28.8, 41.1, and 42.6% at 30, 60, and 90 min, respectively, in Ei24 KO female mice upon treatment with CDN1163. Therefore, these findings indicate that CDN1163 worked well on the remaining surviving β cells and elevated ATP2a2 activity, which could be a useful strategy for treating Ei24-deficient T2D patients.
We identified AMPK as an important regulator of β-cell survival downstream of the Ei24–ATP2a2 interaction. The mechanism of Ca2+-regulating AMPK activity is complicated. The instantaneous increment in cytosolic Ca2+ by matrix detachment is responsible for rapid AMPK activation (24), whereas chronic calcium exposure decreases AMPK activity through the activation of PP2A (25). In our results, loss of Ei24 led to increased intracellular calcium, which occurred concurrently with the decreased ER calcium, further inhibited CAMKK2 and AMPK activity, and induced β-cell apoptosis. LKB1, CAMKK2, and transforming growth factor β–activated kinase (TAK1) have been reported as the upstream kinases that modulate AMPK activity (17, 26, 27). To date, there is no evidence of AMPK activation by CAMKK2 in pancreatic β cells. In our study, increased CAMKK2 levels by CDN1163 corresponded to induced AMPK activation. Our results demonstrated for the first time that CAMKK2 acts upstream of AMPK in primary pancreatic β cells.
In summary, we provide evidence that Ei24 is a modulator of ATP2a2 that coordinates calcium homeostasis and maintains energy balance. Briefly, Ei24 binds to ATP2a2 via the AIR domain to control ATP2a2 activity and ER calcium homeostasis. In the absence of Ei24, reduced ATP2a2 activity is responsible for ER Ca2+ depletion and intracellular elevation of free Ca2+, which then fails to activate CAMKK2/AMPK and compromises β-cell survival and function. Our data prove that strategies targeting the Ei24–ATP2a2 interaction to enhance ATP2a2 activity are useful for improving glucose homeostasis in diabetic models, and Ei24 could serve as a target to prevent and cure diabetes.
Experimental procedures
Generation of KO mice
All animal procedures were performed in accordance with the guidelines of the Animal Care Committee at the Institute of Biophysics, Chinese Academy of Sciences (approval number SYXK2013-48). To generate KO mice, mice harboring LoxP sites flanking exon 3 of the Ei24 gene (8) (referred to as Ei24flox/flox and kindly provided by Dr. Hong Zhang, Institute of Biophysics, Chinese Academy of Sciences, Beijing, China) were crossed with mice expressing Cre recombinase under the control of the rat insulin promoter (RIP-Cre). The RIP-Cre-Flox strategy was used to delete the Ei24 gene specifically in pancreatic β cells. We crossed RIP-Cre mice (strain J003573) with Ei24flox/+ (where the plus sign refers to a WT allele) mice to generate RIP-Cre:Ei24flox/+ mice. Next, RIP-Cre:Ei24flox/+ mice were crossed with RIP-Cre:Ei24flox/+ mice to generate RIP-Cre:Ei24+/+ (WT) mice as the control group and RIP-Cre:Ei24flox/flox (KO) mice as the experimental group. All animal procedures were performed in accordance with the guidelines of the Animal Care Committee at the Institute of Biophysics, Chinese Academy of Sciences.
Separation and purification of islets
For islet separation and purification, 0.5 mg/ml collagenase (Sigma) in Hanks' buffer (Sigma) was injected into the pancreas through the common bile duct, allowing collagenase to perfuse and access the islets via anatomical structures, and then the pancreas was excised and placed in 2 ml of Hanks' buffer for digestion in a 37 °C water bath for 25 min. The digested pancreas was separated by vigorous shaking, and the islets were isolated and purified with a stereoscopic microscope (DSY5000X, COIC Industrial Co., Ltd., ChongQing, China) three or four times.
Cell lines
INS1 cells (ATCC) were maintained in RPMI 1640 medium (Invitrogen) containing 10% heat-inactivated bovine calf serum (HyClone, Logan, UT), 1 mm sodium pyruvate, 10 mm HEPES, 2 mm glutamine, and 50 μm β-mercaptoethanol in an incubator at 37 °C with 5% CO2. COS7 (ATCC) was maintained in DMEM (Invitrogen) containing 10% heat-inactivated bovine calf serum (HyClone) in an incubator at 37 °C with 5% CO2. MIN6 was maintained in DMEM (Invitrogen) containing 10% heat-inactivated bovine calf serum (HyClone) in an incubator at 37 °C with 5% CO2.
Primary pancreatic β cells were dispersed from the islets using trypsin (Life Technologies, Inc.) digestion and cultured in RPMI 1640 medium (Gibco) containing 10% heat-inactivated bovine calf serum (HyClone) with 1 mm sodium pyruvate, 10 mm HEPES, 2 mm glutamine, and 50 μm β-mercaptoethanol in an incubator at 37 °C with 5% CO2.
For generation of the CRISPR/Cas9 knockout cell line, gRNA-expressing plasmids were constructed using the p × 260 vector. Cells were transiently transfected with the appropriate plasmid by Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's instructions and screened in the presence of 2.5 μg/ml puromycin. Single-knockout clones were verified by immunoblotting and sequencing of the PCR fragment. The following gRNA sequence was used for rat Ei24: ACTGTAGGTAGACCGTCTTG.
Quantitative real-time PCR
Total RNA from the islets was extracted using TRIzol (Invitrogen) and reverse-transcribed using a SuperScript III first-strand kit (Invitrogen). Quantitative PCR was performed using an Eppendorf Mastercycler® ep Realplex 4 with SYBR® Premix Ex TaqTM (TaKaRa, Higa, Japan).
The following primers were used: 5′-ACTTCCCTCTCGCTGTATTTGAT-3′ (F-Ei24) and 5′-TTGCTTCCGCTCTACACTCTG-3′ (R-Ei24); 5′-TGGCGCTTTTGACTCAGGAT-3′ (F-actin) and 5′-GGGATGTTTGCTCCAACCAA-3′ (R-actin).
Glucose tolerance test
Mice were weighed and administered a dose of 20% glucose (calculated based on weight, 2 g/kg) via intraperitoneal injection after 16 h of fasting. Blood samples were collected from the tails at 0, 15, 30, 60, 90, and 120 min after administration. The glucose levels were measured using a glucometer (ACCU-CHEK Active, Roche Applied Science).
Insulin tolerance test
Mice were fasted for 12 h, weighed, and injected intraperitoneally with insulin (0.75 units/kg body weight). Blood was drawn from the tail vein at 0, 15, 30, 60, 90, and 120 min for blood glucose measurements.
GSIS test
For the in vivo study of GSIS, mice were fasted for 16 h, and blood samples of ∼150 μl were obtained from the retro-orbital venous plexus using capillary tubes at the 0- and 30-min time points and kept at 4 °C for 1 h. After centrifuging for 10 min at 3000 rpm, the serum was transferred, aliquoted into small tubes, and stored at −80 °C until use. For the in vitro study, a fast digital perfusion system (VC3-8PG, ALA Scientific Instruments, Farmingdale, NY) with a digital injection pump (RISTRON, Ruichuang Electronic Technology Co., Ltd., Shenzhen, China) was used. The flow rate of the perfusate was 1 ml/min. Islets were separated, purified, seeded in perfusion chamber (consists of an array of small circular wells, 150 mm deep and 500 mm in diameter), and cultured with 2.8 mm glucose in Krebs-Ringer–buffered saline (KRB; 115 mm NaCl, 4.7 mm KCl, 2.5 mm CaCl2, 1.2 mm MgSO4·7H2O, 1.2 mm KH2PO4, 25 mm NaHCO3, and 10 mm HEPES) for 10 min to equilibrate. They were then stimulated by 16.7 mm glucose in KRB for 20 min and then reverted back to 2.8 mm glucose for 30 min, followed by stimulation again with 16.7 mm glucose. The supernatants were collected at the indicated times and stored at −80 °C until assayed. The insulin levels were determined using commercial ELISA kits (Millipore).
Histology and immunohistochemistry
Pancreatic tissue was removed while the mice were under anesthesia and placed in 10% neutral buffered formalin (Sigma). After 24 h, the tissues were dehydrated in a series of ethanol solutions of increasing concentrations up to 100% (75% ethanol for 1 h, 85% ethanol for 1 h, 95% ethanol for 45 min, and 100% ethanol for 20 min, three times). Following dehydration, the tissues were immersed into three different xylene immersions (for 8 min each time) followed by the infiltration of three different paraffin wax immersions (60 °C, 1 h each time). They were then embedded and sectioned at a thickness of 5 μm. For immunohistochemistry, the sections were deparaffinized into three different xylene immersions (for 10 min each time), rehydrated (100% ethanol for 5 min, three times, 90% ethanol for 5 min, 70% ethanol for 5 min, and PBS for 5 min, three times), and antigen-retrieved by heat activation in 0.1 m citrate buffer. Next, the sections were stained with H&E or subjected to immunofluorescence using standard protocols. Images were acquired using an Olympus FV1200 laser-scanning confocal microscope (Olympus, Tokyo, Japan) with a ×60 (numerical aperture = 1.40) oil objective. The images were quantified and analyzed using ImageJ software (National Institutes of Health).
ATP measurement
ATP levels were determined within the linear response of a luciferin-based assay (catalogue no. A-22066, Molecular Probes). Freshly isolated islets were homogenized in a nucleotide-releasing mixture and then frozen. Standard curves were generated with serial dilutions of ATP, and bioluminescence was detected using a luminometer following the manufacturer's instructions.
Microsome isolation
WT and Ei24 KO cells were collected and homogenized using a Dounce tissue grinder (K885300-0002, Thermo Fisher) in homogenized buffer (20 mm HEPES, 250 mm sucrose, 1 mm EDTA, pH 7.4) containing protease inhibitor mixture (Sigma). The homogenates were centrifuged at 1000 × g for 10 min at 4 °C. The supernatant was collected and further centrifuged at 8000 × g for 20 min at 4 °C. The supernatant was then ultracentrifuged at 100,000 × g for 1 h at 4 °C. The obtained pellet was collected and solubilized in homogenized buffer as the microsome fraction.
ATPase activity measurement
ATPase activities of the microsome fractions from the WT and Ei24 KO cells were determined using a colorimetric ATPase assay kit, following the manufacturer's instructions (Innova Biosciences, Cambridge, UK). Protein concentration was measured by the BCA method (Thermo Scientific, Waltham, MA). ATPase activity was normalized by protein content.
SIM superresolution imaging
SIM images were acquired using a DeltaVision OMX V3 system (GE Healthcare, Little Chalfont, UK) with a ×100 (numerical aperture = 1.40) oil objective. The images were reconstructed, aligned, and viewed using softWoRx software (GE Healthcare), and quantitation of the correlation coefficients was performed by ImageJ software.
EM
The islets were fixed in 2.5% glutaraldehyde in 0.1 m cacodylate buffer (pH 7.2) for 1 h at 37 °C and then overnight at 4 °C. After postfixation in 1% osmium tetroxide and 1.5% potassium ferrocyanide for 1 h at room temperature, the islets were washed with distilled water and placed in 1% thiocarbohydrazide for 1 h at room temperature, followed by incubation with 2% osmium tetroxide for 1 h at room temperature. The tissue was then stained with 2% aqueous uranyl acetate for 2 h at room temperature. After washing with double-distilled H2O, the islets were further dehydrated with a graded series of ethanol solutions and embedded in epoxy resin. Ultrathin sections were examined using a 120-kV EM (H-7650B, Hitachi) at 80 kV, and images were captured with an AMT CCD camera (XR-41) using the DigitalMicrograph software.
Plasmid construction
The Ei24 and ATP2a2 cDNAs amplified from a cDNA library of islets were cloned into the BamHI/HindIII sites of pEGFP-C3 (Clontech) and SacI/EcoRI sites of pEGFP-C1 (Clontech) to produce EGFP-Ei24 and EGFP-ATP2a2, respectively. The mCherry cDNA was cloned into the NheI/BglII sites of EGFP-ATP2a2 to replace the EGFP gene and to generate the mCherry-ATP2a2 vector.
Co-immunoprecipitation assays
After transient expression of EGFP-Ei24, EGFP-ATP2a2, or the control EGFP plasmid for 24 h, the cell lysates were incubated for 30 min in lysis buffer (50 mm Tris-HCl, 150 mm NaCl, 1 mm EDTA, 1% Triton X-100, pH 7.4) containing protease inhibitor mixture (Sigma) on ice and centrifuged at 12,000 rpm for 5 min at 4 °C. The supernatants were incubated with GFP-Trap_A beads (ChromoTek, Planegg-Martinsried, Germany) on a rotator for 2 h at 4 °C. Next, the precipitated samples were washed three times with lysis buffer and eluted in 0.1 m glycine (pH 2.0). After neutralization by 1 m Tris base, the immunoprecipitated samples were analyzed by Western blotting.
Protein extraction and Western blotting
The procedure was described in detail previously (28). Briefly, proteins were extracted from the islet samples using radioimmune precipitation assay lysis buffer (Beyotime, Shanghai, China) supplemented with protease inhibitor mixture (Sigma) and incubated for 30 min on ice. Homogenates were centrifuged at 12,000 rpm for 10 min at 4 °C. Supernatants were subjected to SDS-PAGE electrophoresis, and signals were detected using an enhanced chemiluminescent (ECL) kit (Thermo Scientific, Waltham, MA). Antibodies against Ei24 (Sigma), proinsulin (Novus Biologicals), insulin (Abcam), c-PARP, total PARP (t-PARP), c-caspase-3, total caspase 3 (t-caspase-3), phosphorylated AMPK (p-AMPK), total AMPK (t-AMPK), p-ACC, total ACC (t-ACC), phosphorylated CAMKK2 (p-CAMKK2), ATP2a2, and β-actin (Cell Signaling Technology) were used, according to the manufacturer's protocols.
Calcium measurement
The INS1 cells were seeded in poly-l-lysine–precoated dishes. After 48 h, the cells were incubated with a 5 μm concentration of the acetoxymethyl ester form of Fura 2 (Fura 2-AM, Dojindo, Tokyo, Japan) in KRB for 30 min at room temperature and imaged at 340/380 nm excitation on a live cell imaging system (IX81/MicroPoint, Olympus). For [Ca2+]ER measurements, INS1 cells were perfused with Ca2+-free KRB (containing 1 mmol/liter EGTA) for 50 s, which was changed to Ca2+-free KRB containing 2 μm TG (Sigma) by a fast digital perfusion system (VC3-8PG). ER-targeted GCaMP6 plasmids were transfected into the INS1 cells. The ER Ca2+ store, which was indicated by the fluorescent intensity of ΔF/F, was analyzed using ImageJ software.
Statistical analysis
Statistical analyses were performed using Prism version 5 software (GraphPad Software, La Jolla, CA). Unpaired t test, one-way analysis of variance, or two-way one-way analysis of variance was used to determine differences between the WT and KO mice. The values are presented as the means ± S.D. Statistically significant difference was set at p < 0.05.
Author contributions
L. Y., H. W., and Q. L. data curation; L. Y. formal analysis; L. Y. writing-original draft; H. W., Q. L., K. L., and L. C. methodology; Z. W., M. Z., and Y. Z. investigation; T. X. and P. X. supervision; T. X. and P. X. writing-review and editing; P. X. conceptualization.
Supplementary Material
Acknowledgments
We thank Drs. Xiangdong Fu and Hong Zhang for helpful comments on the manuscript.
This work was supported by National Key R&D Program of China Grant 2016YFA0501500; National Basic Research Program Grant 2013CB910103; National Natural Science Foundation of China Grants 31421002, 31401174, and 21778069; Project of the Chinese Academy of Sciences Grants XDA12030201 and XDB08030203; Young Elite Scientist Sponsorship Program by CAST; Beijing Natural Science Foundation Grant 7182063; and Beijing Health System High Level Health Technical Personnel Grant 2014-3-058. The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Figs. S1–S5.
- ER
- endoplasmic reticulum
- T2D
- type 2 diabetes
- KO
- knockout
- AIR
- ATP2a2-interacting region
- HFD
- high-fat diet
- GTT
- glucose tolerance test
- POMC
- proopiomelanocortin
- ITT
- insulin tolerance test
- GSIS
- glucose-stimulated insulin secretion
- H&E
- hematoxylin and eosin
- PARP
- poly(ADP-ribose) polymerase
- c-
- cleaved
- p-
- phosphorylated
- t-
- total
- CAMKK
- calcium/calmodulin-dependent protein kinase kinase 2
- AMPK
- AMP-activated protein kinase
- AICAR
- 5-aminoimidazole-4-carboxamide ribonucleotide
- SIM
- structured illumination microscopy
- gRNA
- guide RNA
- KRB
- Krebs-Ringer–buffered saline
- EGFP
- enhanced green fluorescent protein
- TG
- thapsigargin.
References
- 1. Vandecaetsbeek I., Vangheluwe P., Raeymaekers L., Wuytack F., and Vanoevelen J. (2011) The Ca2+ pumps of the endoplasmic reticulum and Golgi apparatus. Cold Spring Harb. Perspect. Biol. 3, a004184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kono T., Ahn G., Moss D. R., Gann L., Zarain-Herzberg A., Nishiki Y., Fueger P. T., Ogihara T., and Evans-Molina C. (2012) PPAR-γ activation restores pancreatic islet SERCA2 levels and prevents beta-cell dysfunction under conditions of hyperglycemic and cytokine stress. Mol. Endocrinol. 26, 257–271 10.1210/me.2011-1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liang K., Du W., Lu J., Li F., Yang L., Xue Y., Hille B., and Chen L. (2014) Alterations of the Ca2+ signaling pathway in pancreatic β-cells isolated from db/db mice. Protein Cell 5, 783–794 10.1007/s13238-014-0075-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zarain-Herzberg A., García-Rivas G., and Estrada-Avilés R. (2014) Regulation of SERCA pumps expression in diabetes. Cell Calcium 56, 302–310 10.1016/j.ceca.2014.09.005 [DOI] [PubMed] [Google Scholar]
- 5. Johnson J. D., and Luciani D. S. (2010) Mechanisms of pancreatic beta-cell apoptosis in diabetes and its therapies. Adv. Exp. Med. Biol. 654, 447–462 10.1007/978-90-481-3271-3_19 [DOI] [PubMed] [Google Scholar]
- 6. Lehar S. M., Nacht M., Jacks T., Vater C. A., Chittenden T., and Guild B. C. (1996) Identification and cloning of EI24, a gene induced by p53 in etoposide-treated cells. Oncogene 12, 1181–1187 [PubMed] [Google Scholar]
- 7. Zhao X., Ayer R. E., Davis S. L., Ames S. J., Florence B., Torchinsky C., Liou J. S., Shen L., and Spanjaard R. A. (2005) Apoptosis factor EI24/PIG8 is a novel endoplasmic reticulum-localized Bcl-2-binding protein which is associated with suppression of breast cancer invasiveness. Cancer Res. 65, 2125–2129 10.1158/0008-5472.CAN-04-3377 [DOI] [PubMed] [Google Scholar]
- 8. Zhao Y. G., Zhao H., Miao L., Wang L., Sun F., and Zhang H. (2012) The p53-induced gene Ei24 is an essential component of the basal autophagy pathway. J. Biol. Chem. 287, 42053–42063 10.1074/jbc.M112.415968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mork C. N., Faller D. V., and Spanjaard R. A. (2007) Loss of putative tumor suppressor EI24/PIG8 confers resistance to etoposide. FEBS Lett. 581, 5440–5444 10.1016/j.febslet.2007.10.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tian Y., Li Z., Hu W., Ren H., Tian E., Zhao Y., Lu Q., Huang X., Yang P., Li X., Wang X., Kovács A. L., Yu L., and Zhang H. (2010) C. elegans screen identifies autophagy genes specific to multicellular organisms. Cell 141, 1042–1055 10.1016/j.cell.2010.04.034 [DOI] [PubMed] [Google Scholar]
- 11. Devkota S., Jeong H., Kim Y., Ali M., Roh J. I., Hwang D., and Lee H. W. (2016) Functional characterization of EI24-induced autophagy in the degradation of RING-domain E3 ligases. Autophagy 12, 2038–2053 10.1080/15548627.2016.1217371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marselli L., Thorne J., Dahiya S., Sgroi D. C., Sharma A., Bonner-Weir S., Marchetti P., and Weir G. C. (2010) Gene expression profiles of beta-cell enriched tissue obtained by laser capture microdissection from subjects with type 2 diabetes. PLoS One 5, e11499 10.1371/journal.pone.0011499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Devkota S., Sung Y. H., Choi J. M., Lee J., Ha N. Y., Kim H., Cho B. C., Song J., and Lee H. W. (2012) Ei24-deficiency attenuates protein kinase C α signaling and skin carcinogenesis in mice. Int. J. Biochem. Cell Biol. 44, 1887–1896 10.1016/j.biocel.2012.06.034 [DOI] [PubMed] [Google Scholar]
- 14. Abel E. D., Peroni O., Kim J. K., Kim Y. B., Boss O., Hadro E., Minnemann T., Shulman G. I., and Kahn B. B. (2001) Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature 409, 729–733 10.1038/35055575 [DOI] [PubMed] [Google Scholar]
- 15. Wicksteed B., Brissova M., Yan W., Opland D. M., Plank J. L., Reinert R. B., Dickson L. M., Tamarina N. A., Philipson L. H., Shostak A., Bernal-Mizrachi E., Elghazi L., Roe M. W., Labosky P. A., Myers M. G. Jr., Gannon M., Powers A. C., and Dempsey P. J. (2010) Conditional gene targeting in mouse pancreatic ss-Cells: analysis of ectopic Cre transgene expression in the brain. Diabetes 59, 3090–3098 10.2337/db10-0624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Song J., Xu Y., Hu X., Choi B., and Tong Q. (2010) Brain expression of Cre recombinase driven by pancreas-specific promoters. Genesis 48, 628–634 10.1002/dvg.20672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Woods A., Dickerson K., Heath R., Hong S. P., Momcilovic M., Johnstone S. R., Carlson M., and Carling D. (2005) Ca2+/calmodulin-dependent protein kinase kinase-β acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2, 21–33 10.1016/j.cmet.2005.06.005 [DOI] [PubMed] [Google Scholar]
- 18. de Juan-Sanz J., Holt G. T., Schreiter E. R., de Juan F., Kim D. S., and Ryan T. A. (2017) Axonal endoplasmic reticulum Ca2+ content controls release probability in CNS nerve terminals. Neuron 93, 867–881.e6 10.1016/j.neuron.2017.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kang S., Dahl R., Hsieh W., Shin A., Zsebo K. M., Buettner C., Hajjar R. J., and Lebeche D. (2016) Small molecular allosteric activator of the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) attenuates diabetes and metabolic disorders. J. Biol. Chem. 291, 5185–5198 10.1074/jbc.M115.705012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gu Z., Flemington C., Chittenden T., and Zambetti G. P. (2000) ei24, a p53 response gene involved in growth suppression and apoptosis. Mol. Cell. Biol. 20, 233–241 10.1128/MCB.20.1.233-241.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhao X., Demary K., Wong L., Vaziri C., McKenzie A. B., Eberlein T. J., and Spanjaard R. A. (2001) Retinoic acid receptor-independent mechanism of apoptosis of melanoma cells by the retinoid CD437 (AHPN). Cell Death Differ. 8, 878–886 10.1038/sj.cdd.4400894 [DOI] [PubMed] [Google Scholar]
- 22. Yuan K., Ai W. B., Wan L. Y., Tan X., and Wu J. F. (2017) The miR-290–295 cluster as multi-faceted players in mouse embryonic stem cells. Cell Biosci. 7, 38 10.1186/s13578-017-0166-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rees D. A., and Alcolado J. C. (2005) Animal models of diabetes mellitus. Diabetic Med. 22, 359–370 10.1111/j.1464-5491.2005.01499.x [DOI] [PubMed] [Google Scholar]
- 24. Sundararaman A., Amirtham U., and Rangarajan A. (2016) Calcium-oxidant signaling network regulates AMP-activated protein kinase (AMPK) activation upon matrix deprivation. J. Biol. Chem. 291, 14410–14429 10.1074/jbc.M116.731257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Park S., Scheffler T. L., Rossie S. S., and Gerrard D. E. (2013) AMPK activity is regulated by calcium-mediated protein phosphatase 2A activity. Cell Calcium 53, 217–223 10.1016/j.ceca.2012.12.001 [DOI] [PubMed] [Google Scholar]
- 26. Shaw R. J., Lamia K. A., Vasquez D., Koo S. H., Bardeesy N., Depinho R. A., Montminy M., and Cantley L. C. (2005) The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 310, 1642–1646 10.1126/science.1120781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Herrero-Martín G., Hoyer-Hansen M., García-García C., Fumarola C., Farkas T., López-Rivas A., and Jäättelä M. (2009) TAK1 activatesAMPK-dependent cytoprotective autophagy in TRAIL-treated epithelial cells. EMBO J. 28, 677–685 10.1038/emboj.2009.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yuan L., Sakamoto N., Song G., and Sato M. (2013) Low-level shear stress induces human mesenchymal stem cell migration through the SDF-1/CXCR4 axis via MAPK signaling pathways. Stem Cells Dev. 22, 2384–2393 10.1089/scd.2012.0717 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.