Abstract
Reactive oxygen and nitrogen species (ROS/RNS) such as superoxide (O2˙̄), hydrogen peroxide, lipid hydroperoxides, peroxynitrite, and hypochlorous and hypobromous acids play a key role in many pathophysiological processes. Recent studies have focused on mitochondrial ROS as redox signaling species responsible for promoting cell division, modulating and regulating kinases and phosphatases, and activating transcription factors. Many ROS also stimulate cell death and senescence. The extent to which these processes occur is attributed to ROS levels (low or high) in cells. However, the exact nature of ROS remains unknown. Investigators have used redox-active probes that, upon oxidation by ROS, yield products exhibiting fluorescence, chemiluminescence, or bioluminescence. Mitochondria-targeted probes can be used to detect ROS generated in mitochondria. However, because most of these redox-active probes (untargeted and mitochondria-targeted) are oxidized by several ROS species, attributing redox probe oxidation to specific ROS species is difficult. It is conceivable that redox-active probes are oxidized in common one-electron oxidation pathways, resulting in a radical intermediate that either reacts with another oxidant (including oxygen to produce O2˙̄) and forms a stable fluorescent product or reacts with O2˙̄ to form a fluorescent marker product. Here, we propose the use of multiple probes and complementary techniques (HPLC, LC-MS, redox blotting, and EPR) and the measurement of intracellular probe uptake and specific marker products to identify specific ROS generated in cells. The low-temperature EPR technique developed to investigate cellular/mitochondrial oxidants can easily be extended to animal and human tissues.
Keywords: bioenergetics, reactive oxygen species (ROS), electron paramagnetic resonance (EPR), peroxiredoxin, superoxide ion, low-temperature EPR, mitochondrial oxidants, radical scavengers, redox probes, thiol-specific antioxidant enzymes, bioenergetics
Introduction
Reactive oxygen species (ROS)3 is an umbrella term that generally refers to a family of oxidizing species derived from one- or two-electron reduction of molecular oxygen. These include the following: superoxide (O2˙̄ and its protonated form, HO2•); hydrogen peroxide (H2O2) formed from spontaneous or enzyme-catalyzed dismutation of O2˙̄; hydroxyl radical (•OH) formed from H2O2, redox-active metal ions (Fe2+/Fe3+, Cu+/Cu2+, and Cr5+/Cr6+), and metal ion reductants; lipid and protein hydroperoxides; higher oxidizing peroxidase/H2O2-derived species; and singlet oxygen derived from chemical or photochemical mechanisms (1, 2). Added to this list are other species such as: peroxynitrite (ONOO−) formed from O2˙̄ and nitric oxide (•NO); nitrogen dioxide radical (•NO2) formed from ONOO−, •NO/O2, or myeloperoxidase (MPO)/H2O2-dependent oxidation of nitrite anion (NO2−) (also referred to as reactive nitrogen species [RNS]); and hypochlorous acid (HOCl) or hypobromous acid (HOBr) formed from peroxidase (e.g. MPO)-catalyzed oxidation of the chloride anion (Cl−) or bromide anion (Br−) by H2O2. Most of these species are short-lived, react rapidly with low-molecular weight cellular reductants (ascorbate and GSH), and can cause oxidation of critical cellular components (lipid, protein, and DNA). Clearly, the use of multiple probes and methodologies is required for unambiguous detection and characterization of various ROS species (3, 4).
The electron paramagnetic resonance (EPR)/spin-trapping technique is the most unambiguous approach to specifically detect O2˙̄, •OH, and lipid-derived radicals using nitrone or nitroso spin traps in chemical and enzymatic systems (5, 6). However, the EPR-active nitroxide spin adducts derived from the trapping of radicals undergo a facile reduction to EPR-silent hydroxylamines in cells, thus making this technique untenable for intracellular detection of these species. However, EPR at helium-cryogenic temperatures (e.g. 5–40 K) is eminently suitable for detecting and investigating redox-active mitochondrial iron–sulfur proteins (aconitase and mitochondrial respiratory chain complexes) (7–9).
During the last decade, much progress has been made with respect to understanding the mechanisms of ROS-induced oxidation of fluorescent, chemiluminescent, and bioluminescent probes (10, 11). A comprehensive understanding of the kinetics, stoichiometry, and intermediate and product analyses of several ROS probes in various ROS-generating systems makes it possible to investigate these species in cells and tissues (12–15).
Emerging literature provides evidence in support of mitochondria as signaling organelles through their generation of ROS (16–22). Low levels of ROS produced from complex I and/or complex III inhibition in the electron transport chain promote cell division, modulate and regulate mitogen-activated protein kinases (MAPKs) and phosphatases, and activate transcription factors, whereas high levels of ROS can cause DNA damage and stimulate cell death and senescence (23). Although the exact nature of ROS is not specified in most cases, it is likely that the investigators are usually referring to O2˙̄, H2O2, or peroxidase-derived oxidants (24–26). Investigators often use different redox-active probes (Mito-SOX, dichlorodihydrofluorescein (DCFH), or CellROX Deep Red reagent) to imply the detection of different species (O2˙̄ or H2O2) (27–29). For example, the redox probe DCFH has been used to imply intracellular H2O2 and Mito-SOX to indicate mitochondria-derived O2˙̄. However, we and others have shown that intracellular oxidation of DCFH to the green fluorescent product dichlorofluorescein (DCF) is catalyzed by peroxidases or via intracellular iron-dependent mechanisms (30–32). Neither H2O2 nor O2˙̄ appreciably react with DCFH to form DCF (30). In addition, artifactual formation of H2O2 occurs from redox cycling of the DCF radical (33, 34). It is also plausible that DCF formed in the cytosolic compartment could translocate to mitochondria, thereby suggesting that DCFH oxidation occurs in the mitochondria.
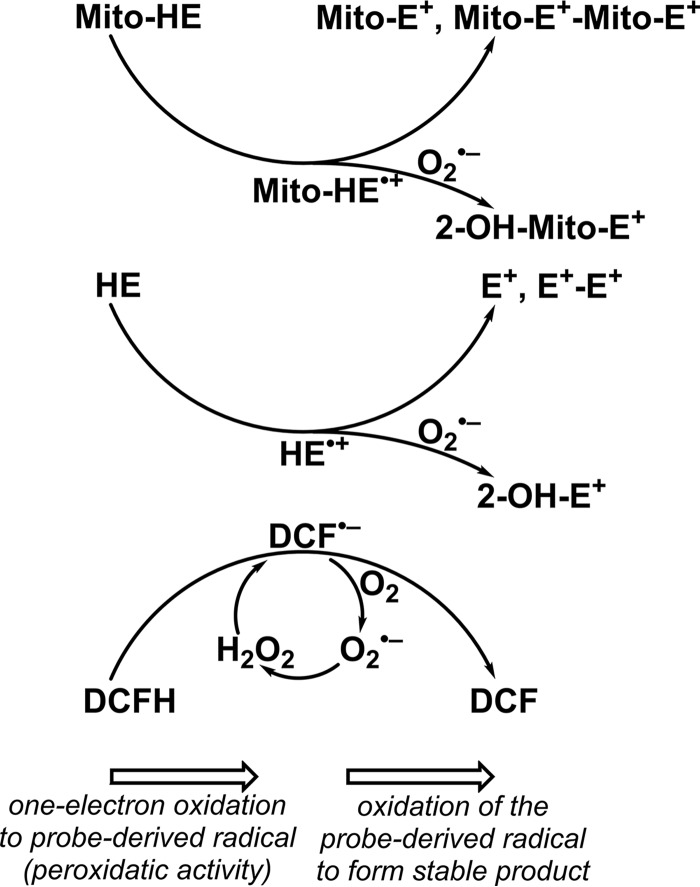
Previously, we reported that the oxidation chemistry of hydroethidine (HE) and its mitochondria-targeted analog, Mito-SOX or Mito-HE, is similar (Fig. S1) (35, 36). Both HE and Mito-SOX form nonspecific two-electron oxidation products that are fluorescent (ethidium [E+] and Mito-E+); nonfluorescent dimers (E+-E+ and Mito-E+–Mito-E+) are also generated in cells. O2˙̄ reacts with HE or HE-derived radical to form a product, 2-hydroxyethidium (2-OH-E+), that is distinctly different from E+ (37, 38). It was proposed that O2˙̄ reacts with HE to form E+ under low oxygen tension (but not at normal oxygen tension) (39). This interpretation was challenged because, irrespective of the O2˙̄ flux, the major specific product of the HE/O2˙̄ reaction was shown to be 2-OH-E+ and not E+ (40).
Both 2-OH-E+ and E+ exhibit overlapping fluorescence spectra as do Mito-E+ and 2-OH-Mito-E+ (41). In addition, the nonspecific two-electron oxidation products E+ or Mito-E+ are formed at much higher levels than 2-OH-E+ or 2-OH-Mito-E+ (42). Thus, the red fluorescence from cells treated with HE and Mito-SOX does not measure mitochondrial O2˙̄ but simply indicates nonspecific oxidation of the probes (43). Clearly, detecting the O2˙̄-specific product (2-OH-E+ or 2-OH-Mito-E+) using HPLC or LC-MS is the only approach that is unequivocal for detecting intracellular and mitochondrial O2˙̄.
The cell-permeable fluorogenic dye, CellROX Deep Red, has also been used as an ROS sensor in cells (27, 28). This probe, whose structure remains unknown, is nonfluorescent in the reduced state, and upon oxidation, it exhibits bright fluorescence in near infrared regions (44). This probe is provided as a kit for measuring oxidative stress. Based on the information provided by the manufacturers, it is clear that this probe can undergo nonspecific oxidation in the presence of various ROS species, and, furthermore, its exact chemical structure, reaction chemistry, kinetics, and products in the presence of various ROS remain unknown. Thus, the potential use of this probe as an ROS sensor in cells is questionable.
Boronates react slowly but stoichiometrically with H2O2 (k ∼1 m−1 s−1) to form phenolic products. Boronate-based fluorogenic probes have been used to measure intracellular H2O2 by monitoring the fluorescent phenolic products in cells (45, 46). Fluorogenic boronates conjugated to a triphenylphosphonium group were also developed for tracking mitochondrial H2O2 (47). A major caveat with the use of boronate probes is the unfavorable reaction kinetics with H2O2 and that other oxidants (ONOO− or HOCl) react with boronates several orders of magnitude (103–106) faster, forming the same phenolic product (48, 49). This makes it difficult to assign the intracellular fluorescence derived from boronate probes to H2O2 (50). However, unlike H2O2, ONOO− and HOCl form specific minor products (nitrated, cyclized, and chlorinated products) upon reaction with boronates. Lack of formation of the specific minor products (Fig. S2) would preclude the intermediacy of these oxidants (51). Mitochondria-targeted boronate (Mito-B) in combination with LC-MS–based analysis has been used to detect mitochondrial H2O2 formed in vitro and in vivo (52).
Mitochondrial membrane potential is one of the key factors controlling the uptake of cationic and fluorescent probes (53, 54). Typically, lipophilic cationic compounds accumulate in the mitochondrial matrix depending on the extent of mitochondrial polarization (55). A more negative or more polarized mitochondrion will enable higher accumulation of cationic dyes (53). Thus, it is important to measure the intracellular uptake and mitochondrial localization of ROS probes to properly interpret the results. To avoid any ambiguity related to changes in intracellular probe concentration, results may also be expressed as the ratio ([ROS product]/[probe]), assuming a linear relationship between the probe concentration and the yield of the oxidation product(s) (56). The cellular uptake of the mitochondria-targeted, triphenylphosphonium cationic moiety (TPP+)-based ROS scavengers is also governed by mitochondrial membrane potential.
Cells rely on mitochondrial and cytosolic reductants (GSH and NADPH) and antioxidant enzymes to mitigate ROS levels in the cytosolic and mitochondrial compartments. O2˙̄ is dismutated catalytically by copper/zinc superoxide dismutase (Cu/Zn-SOD, SOD1) in the cytoplasm and in the mitochondrial intermembrane space. O2˙̄ generated in the mitochondrial matrix is catalytically removed by manganese SOD (MnSOD, SOD2). Peroxiredoxins, GSH peroxidases, and catalase enzymes present in mitochondria and in the cytosol scavenge H2O2 and lipid hydroperoxides. Thioredoxins, thioredoxin reductases, and glutaredoxin in combination with GSH and NADPH repair and protect against excessive oxidation of peroxiredoxins and reactivate these antioxidant enzymes (57). Clearly, the changes in intracellular antioxidant levels that occur during metabolic reprogramming and drug resistance greatly influence the cytosolic and mitochondrial ROS levels. Thus, monitoring changes in the levels or redox state of mitochondrial antioxidant enzymes, by low-temperature EPR, for example (7), could help interpret changes in mitochondrial and cytosolic ROS levels and their role in redox signaling.
Here, we show that it is necessary to determine the cellular uptake of ROS probes (HE and its mitochondria-targeted analog Mito-SOX) and the corresponding hydroxylation and oxidation products, using LC-MS, to authenticate mitochondrial O2˙̄ generation. Similarly, it is essential to determine the cellular uptake of the mitochondria-targeted boronate probe and to measure the major and minor products by LC-MS to implicate the intermediacy of mitochondrial H2O2. Finally, we suggest using complementary “probe-free” compartmentalized redox blotting and low-temperature EPR to further verify mitochondrial oxidant formation. Monitoring the redox changes in intracellular redox-sensing thiol peroxidases, especially peroxiredoxins, should lead to better insights into the results obtained with exogenous probes for H2O2 and its role in redox signaling.
Results
Effect of ROS probes on mitochondrial bioenergetic function
Exposure of neuronal cells to low micromolar levels of Mito-SOX was shown to uncouple neuronal respiration and affect O2˙̄ production (58). Therefore, before proceeding with a complete analyses of ROS detection, the effects of ROS probes on cellular bioenergetics and optimal conditions/concentrations were established. Oxygen consumption rates (OCR) were measured as a readout of mitochondrial function. To determine the mitochondrial inhibitory effects of ROS probes in cells, we used the Seahorse apparatus to measure OCR in cells treated with different concentrations of probes (Fig. 1, A–C). The probes HE, Mito-HE (Mito-SOX), and o-Mito-PBA all dose-dependently inhibited OCR in MiaPaCa-2 pancreatic cancer cells (Fig. 1D). However, at the concentrations chosen for ROS detection, HE 10 μm, Mito-SOX 5 μm, and Mito-PBA 50 μm (Fig. 1), the probes did not affect either basal OCR or the response to mitochondrial modulators (oligomycin, dinitrophenol, and rotenone/antimycin A), indicating a lack of interference with mitochondrial function.
Figure 1.
Effect of HE, Mito-HE (Mito-SOX Red), and o-MitoPBA on mitochondrial function in MiaPaCa-2 cells. MiaPaCa-2 cells (20,000 cells/well) seeded for 24 h in culture plates were treated with HE (A), Mito-HE (B), or o-MitoPBA (C) for 1 h. Next, the cells were washed with unbuffered assay medium. Eight baseline OCR measurements were then taken before injection of oligomycin (Oligo, 1 μg/ml) to inhibit ATP synthase, followed by DNP (50 μm) to uncouple the mitochondria and yield maximal OCR, and rotenone (Rot, 1 μm) and antimycin A (AA, 10 μm) to prevent mitochondrial oxygen consumption through inhibition of complex I and complex III, respectively. The first OCR reading is plotted against the concentration of each ROS probe (D). Dashed lines represent the fitting curves used to determine the IC50 values.
Inhibition of mitochondrial respiration results in increased O2˙̄ and other oxidants
Inhibitors of complex I (NADH-ubiquinone oxidoreductase), complex II (succinate-ubiquinone oxidoreductase), and complex III (cytochrome bcI complex) induce mitochondrial O2˙̄ and H2O2 to various extents (59). We have previously demonstrated that mito-metformin (Mito-Met) inhibits mitochondrial complex I at a 1000-fold lower concentration than metformin. The optimal concentrations of Mito-Met and antimycin A required to inhibit mitochondrial respiration in intact MiaPaCa-2 cells were determined (Fig. 2, A and B). The concentrations to inhibit 50% (IC50) of OCR were determined to be 1.1 μm for Mito-Met (complex I inhibitor) and 2.8 nm for antimycin A (complex III inhibitor) (Fig. 2).
Figure 2.
Concentration-dependent inhibition of mitochondrial respiration in intact MiaPaCa-2 cells by Mito-Met and antimycin A. A, MiaPaCa-2 cells (20,000 cells/well) were seeded in culture plates for 4 h to allow for cell attachment. Cells were treated with Mito-Met for 24 h and washed with unbuffered assay medium as described. After baseline OCR measurements, the response to mitochondrial modulators (oligomycin (Oligo), DNP, and rotenone (Rot)/antimycin A (AA) as described in Fig. 1) was recorded. The last stable baseline OCR reading before oligomycin injection was plotted against the concentration of Mito-Met. The dashed lines represent the fitting curve used to determine the IC50 value. B, cells (20,000 cells/well) were seeded for 24 h before performing the extracellular flux assay. After 1-h baseline OCR measurements, antimycin A was injected. The effects of antimycin A on baseline OCR were monitored for 1 h before injection of rotenone (1 μm). The last OCR reading before injection of rotenone (1-h treatment with antimycin A) is plotted against concentration of antimycin A. The dashed lines represent the fitting curve used to determine the IC50 value.
ROS probe hydroethidine
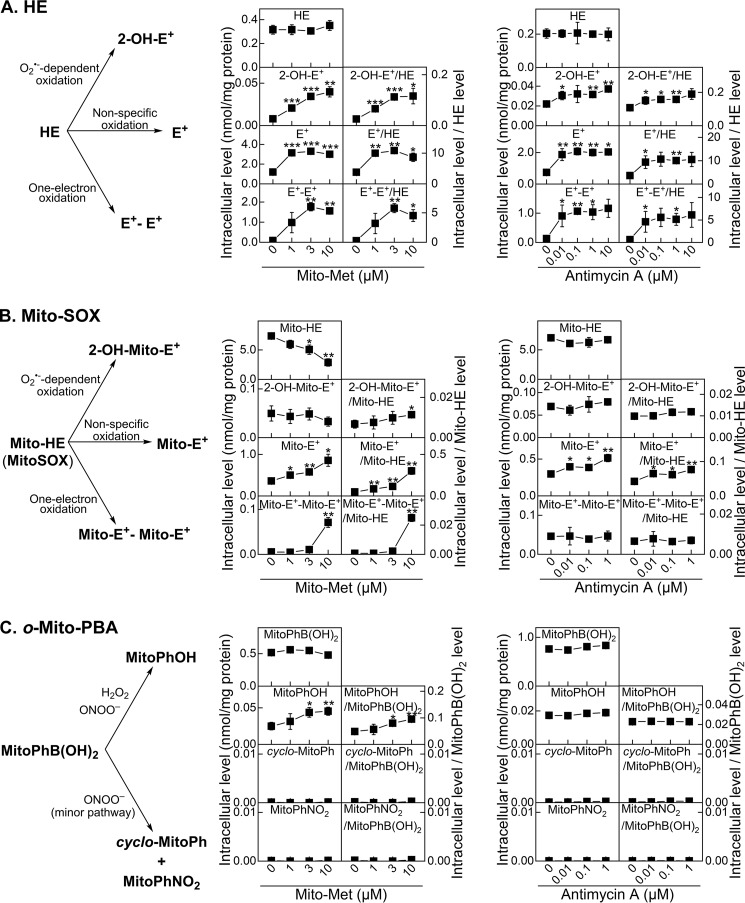
MiaPaCa-2 cells were treated with Mito-Met for 24 h or with antimycin A immediately before the addition of the ROS probe HE. The cells were then incubated for 1 h with HE and collected for analyses of the oxidation products. Cell lysates were analyzed using HPLC (Fig. S3). Fig. 3A shows the amount of HE incorporation into cells, the O2˙̄-specific product 2-OH-E+, the nonspecific two-electron oxidation product E+, and the dimeric product E+-E+ that is formed by the combination of two HE radicals. Formation of dimeric E+-E+ suggests oxidation of HE by a one-electron oxidant to the corresponding radical that subsequently undergoes a radical–radical recombination reaction. The intracellular levels of these products in Mito-Met–treated or antimycin A–treated cells were markedly increased relative to the untreated control cells. Because the intracellular HE level was not significantly affected by the treatments, similar conclusions can be drawn using the absolute intracellular level and the ratios of the product to probe levels.
Figure 3.
Mito-Met- and antimycin A-induced oxidation of ROS probes in MiaPaCa-2 cells. Cells were treated with Mito-Met for 24 h (middle) or antimycin A for 1 h (right), washed free of excess treatments, and treated with the redox probe HE (10 μm, A), Mito-SOX (5 μm, B), or o-Mito-PBA (50 μm, C) for 60 min. Cells were then lysed and analyzed by HPLC (A) or LC-MS (B and C). The graphs show the oxidant-dependent products formed from the probes (left) and quantitative analyses of the probes' uptake and the oxidation products. For each experiment, the data were expressed as the absolute intracellular level (normalized to total cellular protein level, left columns) and the ratio of each product to the detected level of the probe (right columns). t test versus control: *, p < 0.05; **, p < 0.01; ***, p < 0.001.
ROS probe, Mito-SOX
These experiments were performed similarly to those described above for HE, but the samples were analyzed using LC-MS/MS. Fig. 3B shows the intracellular levels of Mito-SOX, the O2˙̄-specific product 2-OH-Mito-E+, the nonspecific oxidation product Mito-E+, and the dimeric product Mito-E+–Mito-E+ formed by the recombination of two Mito-SOX–derived radicals formed from one-electron oxidation of Mito-SOX. Again, formation of Mito-E+–Mito-E+ is indicative of a stronger one-electron oxidant production in mitochondria. Mito-Met or antimycin A treatment induced a marked increase in the oxidation products from Mito-SOX. The increase in the O2˙̄-specific product, 2-OH-Mito-E+, was only apparent when the results were normalized to the intracellular Mito-SOX level and only in the case of the complex I inhibitor Mito-Met. The major oxidation product stimulated by Mito-Met or antimycin A was Mito-E+, both when presented as an absolute intracellular level and when normalized to the probe level. Thus, the increase in confocal fluorescence intensity in cells treated with HE or Mito-SOX does not indicate enhanced intracellular O2˙̄. Increased formation of E+ or Mito-E+ and dimeric products suggests the formation of a one-electron oxidizing species (derived from reaction of H2O2 with redox-active iron complexes, heme proteins, or peroxidases).
ROS probe, Mito-PBA
To detect H2O2 generated in mitochondria in Mito-Met–treated or antimycin A–treated cells, the probe o-MitoPhB(OH)2 (ortho-Mito-PBA) was used (51). Although both the ortho-substituted probe o-MitoPhB(OH)2 and the meta-MitoPhB(OH)2 probe (known as Mito-B) (51) react at similar rates with H2O2 to form the respective phenolic product MitoPhOH, the ortho-substituted probe is preferred because it also reacts with ONOO− to form very specific minor products (∼10%), cyclo-MitoPh and o-MitoPhNO2, in addition to the major product, o-MitoPhOH (∼90%), as shown in Fig. S2. The absence of these minor products definitely rules out the intermediacy of ONOO−.
Experimental conditions using Mito-PBA were identical to those using HE or Mito-SOX probes. Mito-Met treatment of MiaPaCa-2 cells in the presence of Mito-PBA led to an increase in o-MitoPhOH formation (Fig. 3C). Based on the intracellular levels of probe uptake, it is evident that Mito-Met significantly enhanced ROS formation. The absence of detectable ONOO−-specific products suggests that mitochondria-derived H2O2 was responsible for the oxidation of o-MitoPhB(OH)2 to o-MitoPhOH in these cells (Fig. 3C). Interestingly, no increased oxidation of the Mito-PBA probe was detected in cells treated with antimycin A, consistent with the lack of increase of 2-OH-Mito-E+ formation.
Aconitase oxidation: EPR-detectable markers of mitochondrial oxidative stress
Fig. 4 shows EPR data from MiaPaCa-2 pancreatic cancer cells, untreated or treated with Mito-Met or antimycin A. The experimental conditions were identical to those used for ROS probes, except that the ROS probes were not included. The spectra presented were recorded at 12 K. Incubating cells with antimycin A (Fig. 4B) resulted in up to a 6-fold increase in the intensity of a signal at 3350 G (g ∼ 2.02). This signal is due to an iron-containing center of aconitase, previously attributed to O2˙̄-induced oxidative inactivation of the [4Fe-4S]2+ cluster (EPR silent) to a [3Fe-4S]+ cluster (EPR active, shown in Fig. 4 as ox-aconitase) with a concomitant loss of the noncovalent Fea atom of the [4Fe-4S]2+ cluster (60). Changes in the intensity of a second signal at 3530 G, the so-called g = 1.92 signal, due to reduced [2Fe-2S]+ and [4Fe-4S]+ clusters of complexes I and II (61) were either small or not statistically significant. Incubation of cells with Mito-Met (Fig. 4A) also elicited significant increases in the intensity of the aconitase [3Fe-4S]+ signal and, additionally, led to significant diminution of the g = 1.92 FeS signal. The spectral changes observed with both antimycin A and Mito-Met were dose-dependent over the concentration ranges studied (Fig. 4). Table S1 shows the estimated spin concentrations of the oxidatively modified aconitase, [Acn 3Fe-4S]+ cluster, and the composite signal from the reduced [2Fe-2S]+ and [4Fe-4S]+ clusters from mitochondrial complexes I and II.
Figure 4.
Low-temperature EPR detection of the endogenous markers of intracellular oxidants induced by Mito-Met and antimycin A in MiaPaCa-2 cells. MiaPaCa-2 cells were treated with Mito-Met for 24 h (A) or antimycin A for 1 h (B), and cells were collected and stored at −80 °C until EPR measurements. The representative signal at 3350 G labeled Acn is predominantly due to the oxidatively modified [3Fe-4S]+ cluster from aconitase (ox-Aconitase). The representative signal at 3530 G labeled FeS is predominantly due to the “g = 1.92” composite signal due to reduced [2Fe-2S]+ and [4Fe-4S]+ clusters from complexes I and II. Bar graphs (right panels) show the results of quantitative analyses of the EPR signals due to ox-aconitase and complex I/II Fe-S clusters. t test versus control: *, p < 0.05; **, p < 0.01; ***, p < 0.001.
It is important to highlight the following caveats in sample preparation for the EPR experiments. To match the EPR experimental conditions to those used in other experiments (5–10 ml of medium per 106 cells), the volume of the medium was increased by 6-fold such that there was a sufficient amount of inhibitor (Mito-Met or antimycin A) per cell. Under these conditions, Mito-Met or antimycin A treatment yielded a significant formation of ox-aconitase (Fig. 4).
Inhibition of mitochondrial complexes I and III stimulates oxidation of mitochondrial but not cytosolic peroxiredoxin
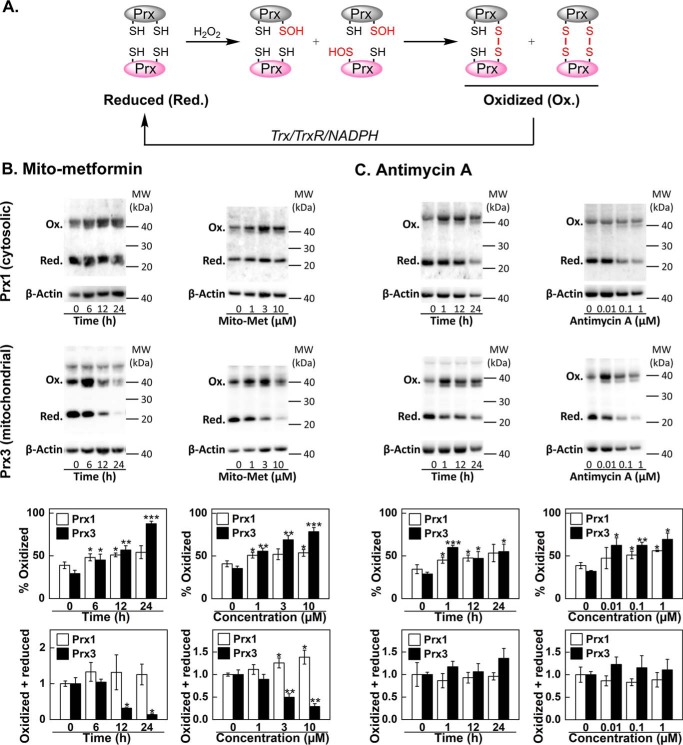
Because peroxiredoxins are endogenous indicators of compartmental redox stress (62), we monitored the oxidation of the predominant mitochondrial peroxiredoxin, Prx3, and the cytosolic peroxiredoxin, Prx1. Prx3 accounts for ∼90% of total mitochondrial peroxide–scavenging activity (k = 2 × 107 m−1 s−1 for H2O2) (63). If Mito-Met and antimycin A induce the generation of mitochondrial oxidant(s), one would expect oxidation of Prx3 cysteinyl sulfhydryl (Prx3-Red) to the corresponding Prx3 disulfide (Prx3-Ox), whereas the oxidation of cytosolic Prx1 would be less likely (Fig. 5A). As shown in Fig. 5, B and C, both Mito-Met and antimycin A caused pronounced oxidation of mitochondrial Prx3 with only moderate effects on cytosolic Prx1, indicating that the enhanced ROS generation occurs primarily within mitochondria. These results also suggest that, under these treatment conditions (e.g. Fig. 3), the mitochondrial capacity to degrade H2O2 was likely overwhelmed in Mito-Met-treated cells. In fact, Mito-Met treatment led to depletion of the total (oxidized + reduced) amount of Prx3 but not Prx1 (Fig. 5B). Under these conditions, using a sensitive analytical technique, it is also possible to detect an increase in the H2O2/mitochondria-targeted boronate reaction product (Fig. 3C). In the case of antimycin A, treatment, the total amount of Prx3 was not decreased (Fig. 5C), which may explain the lack of increase in the boronate oxidation product, o-MitoPhOH (Fig. 3C). Mitochondria-targeted nitroxides (e.g. Mito-CP) can also inhibit the expression of Prx3 and enhance production of mitochondrial oxidants (64). Mitochondrial thioredoxin (Trx2) that acts in concert with Prx3 in detoxifying H2O2 (Fig. 5A) is also inhibited by other mitochondria-targeted compounds (65).
Figure 5.
Mito-Met- and antimycin A-induced oxidation of mitochondrial and cytosolic peroxiredoxins in MiaPaCa-2 cells. A, scheme showing the pathways controlling the redox state and covalent dimer formation of peroxiredoxins. B, cells were treated with increasing concentrations of Mito-Met (1–10 μm for 24 h) or with 10 μm Mito-Met for 6–24 h, and the redox status of cytosolic (Prx1) and mitochondrial (Prx3) peroxiredoxins was determined by redox blotting. C, same as B, but the effect of antimycin A treatment on the redox status of Prx1 and Prx3 was tested using the indicated concentrations (10 nm to 1 μm, for 1 h) or for 1–24 h with 10 nm antimycin A. t test versus control: *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Overlapping fluorescence spectra and lack of distinction between O2˙̄-specific and -nonspecific oxidation products
Fig. 6 shows the fluorescence excitation/emission matrix spectra (3D and 2D) of E+/2-OH-E+ and Mito-E+/2-OH-Mito-E+ in the presence of DNA, as a function of emission and excitation wavelengths. The positions of the excitation and emission maxima for E+ are 525 and 590 nm and for 2-OH-E+ are 500 and 565 nm (Fig. 6, A and B), respectively. Clearly, when using the conventional fluorescence techniques that excite at a single wavelength, it is difficult to distinguish between 2-OH-E+ (O2˙̄-specific marker product) and E+ (nonspecific oxidation product) (38). The same is true for using conventional fluorescence to distinguish Mito-E+ from 2-OH-Mito-E+ (Fig. 6, D and E) (66). Another approach is the use of dual wavelength imaging (67, 68). More specific excitation wavelengths in the range of 385–405 nm can be used for detecting 2-OH-E+ and 2-OH-Mito-E+ and 480–520 nm for detecting E+ and Mito-E+. In fact, when E+ and 2-OH-E+ (or Mito-E+ and 2-OH-Mito-E+) are present in comparable concentrations, the short-wavelength excitation can be used for selective monitoring of the O2˙̄-specific product (Fig. 6, B and E). Being able to achieve live cell imaging of O2˙̄, as suggested (66, 67), is clearly very significant and technically less challenging as compared with HPLC-based assays. However, live cell quantitation of O2˙̄ still remains questionable and ambiguous because the formation of E+ and Mito-E+ in cells is typically at least 5–10 times higher than that of 2-OH-E+ and 2-OH-Mito-E+ (Fig. 3). With an ∼10-fold excess of E+ over 2-OH-E+ or Mito-E+ over 2-OH-Mito-E+, the nonspecific oxidation products still contribute to the total fluorescence intensity, even for the more selective, short excitation wavelengths (Fig. 6, C and F).
Figure 6.
Fluorescence spectral properties of oxidation and hydroxylation products of HE and Mito-SOX. Spectra of the compounds in the presence of DNA (0.2 mg/ml) in aqueous solution of Tris buffer (10 mm, pH 7.4) and EDTA (1 mm) were collected at room temperature. Fluorescence excitation/emission matrices for 2-OH-E+ and E+ (A) or 2-OH-Mito-E+ and Mito-E+ (D) are shown in three-dimensional (upper panels) and two-dimensional (lower panels) spaces. B, excitation and emission spectra of E+ (10 μm) and 2-OH-E+ (7 μm). C, emission spectra of 2-OH-E+ (0.7 μm) and E+ (10 μm) are compared using different excitation wavelengths. E, same as B but for 2-OH-Mito-E+ (10 μm) and Mito-E+ (7.5 μm). F, same as C but for 2-OH-Mito-E+ (1 μm) and Mito-E+ (7.5 μm).
As reported earlier (66), live cell imaging of O2˙̄ allows visualization of the critical sites of O2˙̄ generation and localization of O2˙̄-specific fluorescent products. The limited availability of cells (e.g. primary neurons) is another reason why live cell O2˙̄ imaging is preferred (66). To this end, here we next provide spectral imaging and a proof of concept study to conceivably image overlapping 2-OH-E+ and E+ fluorescence in cells.
Spectral imaging using λ mode confocal microscopy
As discussed (38, 66), conventional fluorescence microscopy cannot distinguish E+ and 2-OH-E+ because of the spectral overlap and the significant excess of E+ in cells over 2-OH-E+. However, the emission maximum of 2-OH-E+ is shifted ∼20 nm relative to the E+ emission maximum. We hypothesized that this difference could be resolved using λ mode confocal microscopy, where emission spectra are recorded for each pixel in a fluorescence micrograph. To illustrate the proof of concept, we treated cells with equimolar levels of E+ or 2-OH-E+ for 20 min in the presence of the plasma membrane permeabilizer (PMP). Cells were imaged by confocal microscopy using excitation at 488 nm and emission collected every 10.7 nm from 506 to 699 nm (Fig. S5). From those images, the pixel-based fluorescence emission spectra were constructed (Fig. 7). It is clear from the results that the spectral shift between 2-OH-E+ and E+ also holds true in cells, regardless of the image pixel chosen. This provides a potential opportunity to use spectral imaging and unmixing to deconvolute the actual spectra into 2-OH-E+ and E+ components. In the actual experimental systems, however, the concentrations of both 2-OH-E+ and E+ are likely to be much lower, and the concentration of E+ is likely to be severalfold higher than that of 2-OH-E+. We presently do not have the ability to perform these experiments using advanced lasers or filters at 405 nm. Nonetheless, we believe that the confocal spectroscopic spectral imaging approach using a super-sensitive confocal microscope or a multiwavelength optical imaging system with time-domain capabilities (39) will help record, in real time, the fluorescence spectra or identify fluorescence lifetimes for both 2-OH-E+ and E+ in cells.
Figure 7.

Fluorescence spectral imaging (λ mode image) of MiaPaCa-2 cells loaded with 2-OH-E+ and E+. Cells were loaded with 2-OH-E+ (A) or E+ (B) by permeabilization and incubation with 2-OH-E+ or E+ (20 μm each) for 20 min. Left panels show the total fluorescence images, with the pixels used for spectral determination indicated by the colored dots. The reconstructed fluorescence emission spectra for the selected pixels (regions 1–3) are shown in right panels.
Discussion
In this study, we have shown that determining redox probe uptake and analyzing the specific oxidation and hydroxylation products are critical for proper characterization of intracellular ROS. Mitochondria-targeted HE (Mito-HE, commercially available as the Mito-SOX Red probe) has routinely been used in previous studies, and the red fluorescence derived from its oxidation product was attributed to mROS or O2˙̄. We show, however, that several products are formed from Mito-SOX as a result of one-electron oxidation and that the levels of the nonspecific, red fluorescent oxidation product (Mito-E+) are much higher than those of the O2˙̄-specific product. The notion that Mito-SOX can be used as an indicator of mitochondrial O2˙̄ should be re-examined. In the following sections, we discuss the mechanism of oxidation of widely used ROS probes, and how the chemistry of the probes impacts the extent of oxidation, the identity of the product(s), and possible interpretation of the results. We have addressed the potential problems in the interpretation of results obtained from mitochondria-targeted agents and N-acetylcysteine as mitochondrial ROS scavengers. We propose that, in addition to monitoring the probe uptake and determining the specific marker products from redox probes, analyzing redox-active iron–sulfur clusters (aconitase oxidation) using EPR at low temperatures, and the redox status of mitochondrial and cytosolic peroxiredoxins, will provide a more complete picture of mitochondrial ROS.
Intracellular oxidation of redox-active probes by one-electron oxidants
In recent years, investigators have used several structurally related and different redox probes to detect different ROS species generated in cells. Clearly, the eternal hope has been that the readout (fluorescent or chemiluminescent) from these probes will indicate both the amount and the identity of different ROS. Unfortunately, this mindset has caused much confusion in the interpretation of results due to a lack of consideration of the chemical reactivity of the probes. Determination of the products formed from ROS probes suggests that one-electron oxidation of ROS probes (e.g. HE, Mito-SOX, DCFH, and dihydrorhodamine (DHR)) is a common mechanistic pathway. Most fluorescent, chemiluminescent, or bioluminescent redox-active ROS probes undergo one-electron oxidation to a probe-derived radical intermediate that subsequently reacts with oxygen and/or O2˙̄. Thus, the second step is the only differentiating feature for the different probes (HE, Mito-SOX, DCFH, DHR, luminol, and L-012 (Fig. S4)) and for lucigenin, which undergoes an initial reduction to form a radical intermediate that reduces oxygen to O2˙̄ and also reacts with O2˙̄ to induce chemiluminescence. Fig. 8 shows the one-electron oxidation of selected ROS probes by oxidizing species such as the hydroxyl radical or a higher oxidation species generated from peroxidase/H2O2 in mitochondria or the cytosol. The intermediate radical derived from DCFH undergoes redox cycling, generating additional O2˙̄ and H2O2 and DCF. This artifactual generation of H2O2 is likely responsible for the nonlinear relationship between fluorescence intensity and ROS levels determined using the DCFH probe. In contrast, radicals (HE+⋅ and Mito-HE+⋅) derived from HE or Mito-SOX form E+ and E+–E+. The intermediate radicals do not appreciably react with oxygen but react rapidly with O2˙̄ to form diagnostic marker products (2-OH-E+ or 2-OH-Mito-E+ (Fig. 8)). Thus, most ROS probes are all initially oxidized by the same one-electron oxidation mechanism in cells, resulting in oxidation products that exhibit fluorescence. Intracellular green fluorescence derived from DCFH or red fluorescence derived from HE or Mito-SOX presumably occurs from the oxidation of the probes by the same one-electron oxidation mechanism. For example, drug-resistant cancer cells that undergo ferroptosis, a nonapoptotic, iron-dependent cell death, are likely to oxidize DCFH and HE/Mito-SOX by a one-electron oxidizing mechanism (mediated by lipid-derived radicals), yielding green fluorescence (from DCF) and red fluorescence (from E+ or Mito-E+) (69). Without the product analyses (for 2-OH-E+ and 2-OH-Mito-E+), one would erroneously conclude that HE and Mito-SOX are oxidized by O2˙̄ generated in the drug-resistant cancer cell phenotype.
Figure 8.
Mechanism of peroxidatic oxidation of HE, Mito-SOX, and DCFH probes and the effect of the presence of O2˙̄ on the identity of the oxidation products. HE and Mito-HE (Mito-SOX Red) are oxidized to their radical cations, which in the absence of O2˙̄ form ethidium- and diethidium-type products. In the presence of O2˙̄, the hydroxylated products (2-OH-E+ or 2-OH-Mito-E+) are formed. DCFH undergoes oxidation to its radical, which in the presence of oxygen undergoes oxidation to DCF, with concomitant formation of O2˙̄. Dismutation of O2˙̄ produces H2O2, which may drive further oxidation of the probe.
The reaction between O2˙̄ and the probe radical forms a hydroxylated product, and this is probably the only major difference between these probes. Mitochondria-derived ROS, usually denoted as “mROS,” are often detected using Mito-SOX. An increase in Mito-SOX–derived red fluorescence is often equated to mitochondrial O2˙̄. However, unless 2-OH-Mito-E+ is isolated and determined (42), attributing increased red fluorescence to mitochondrial O2˙̄ is incorrect. Changes in 2-OH-Mito-E+ may be also due to changes in probe uptake or in the peroxidatic activity. Probe uptake can be determined by monitoring cellular Mito-SOX level (or the sum of Mito-SOX and its oxidation products). Peroxidatic activity results in the formation of dimeric products, including Mito-E+–Mito-E+. Thus, HPLC-based or LC-MS–based profiling of Mito-SOX oxidation products is necessary for proper interpretation of the changes in red fluorescence.
In contrast to phenanthridinium-based fluorescent probes (e.g. HE and Mito-SOX) and the DCFH/DHR family of dyes, the higher oxidation potential of boronate-based probes precludes them from undergoing peroxidatic oxidation. The phenolic products formed from the reaction between ROS and boronate probes can undergo further oxidation with time, forming nonfluorescent dimers. This possibility needs to be experimentally tested, however.
Use of mROS scavengers and interpretation of data
Nitroxides conjugated to TPP+ via various alkyl chain linkers, such as Mito-CP11 and Mito-Tempo(l)10 (Fig. S4), were used to catalytically dismutate mitochondrial O2˙̄ (mitochondrial SOD mimics) and protect against endothelial cell oxidative damage (70). Mito-CP is a mitochondria-targeted nitroxide analog that is synthesized by conjugating a TPP+ group to untargeted CP via an 11-carbon alkyl chain, and Mito-Tempol is synthesized similarly (71). In contrast to the effect observed in normal endothelial cells, these compounds induced antiproliferative effects in cancer cells (72). This was originally attributed to mitigation of O2˙̄-induced redox signaling in cancer cells (19). To unequivocally confirm that the nitroxide group and its SOD mimetic effect are responsible for the observed effects, a proper control (Mito-CP–acetamide) was synthesized, wherein the nitroxide group in Mito-CP was replaced by an acetamido group (73). Mito-CP-Ac does not possess the O2˙̄ dismutating ability, so it was an ideal control molecule. Much to our surprise, we found that Mito-CP-Ac inhibited cancer cell proliferation as effectively as did Mito-CP (73). Thus, we revised our previous interpretation that Mito-CP inhibits cancer cell proliferation by dismutating O2˙̄. We now believe that the antiproliferative effects of Mito-CP and Mito-CP-Ac are linked to their abilities to induce mitochondrial stress and activate other redox-signaling mechanisms.
Mito-Tempo(l) belongs to a group of six-membered nitroxides wherein the nitroxide-containing group, Tempo, is tethered via an alkyl chain to the TPP+ that targets these compounds to mitochondria (74–76). Mito-Tempo, containing a short alkyl chain, is typically used to test the involvement of mitochondrial O2˙̄-induced effects (75). Mito-Tempo was shown to mitigate the red fluorescence originating from Mito-SOX (77). This was attributed to the dismutation of mitochondrial ROS (O2˙̄) by Mito-Tempo. These results need to be evaluated more carefully using the appropriate control compound (Mito-Tempo–acetamide, see Fig. S4) with the nitroxide group substituted by acetamide or a methyl group (78). Mito-Tempo-Ac is similar to Mito-Tempo but devoid of the O2˙̄-dismutating ability. In addition, the fluorescence quenching by the paramagnetic Mito-Tempol should be considered. The commercially available Mito-Tempo contains a short alkyl chain, and Mito-Tempol with a longer alkyl side chain is probably preferred for effective mitochondrial targeting.
Mitochondria-targeted cell-penetrating peptides (Mito-CPPs) based on the Szeto-Schiller peptide sequence (d-Arg-Dmt-Lys-Phe-NH2) have been proposed as site-specific scavengers of mitochondrial ROS (79–81). Mito-CPPs are typically cationic and lipophilic tetrapeptides, consisting of positively charged amino acids and phenylalanine. The antioxidant property of these peptides is attributed to a rapid reaction between ROS and the phenolic group in Dmt, forming the corresponding tyrosine radical that regenerates the phenol by reacting with a reductant or with another radical, forming a dimer and other products (80). This mechanism of the protective effects of Dmt-containing peptides has been challenged, however, as Dmt is known to interact with opioid receptors (82). We have proposed using an analog of Dmt that lacks the hydroxyl group (2,6-dimethylphenylalanine) to distinguish the antioxidant from receptor-binding effects (83). Recently, Mito-CPPs were reported to inhibit mitochondrial superoxide (measured by Mito-SOX fluorescence) in H2O2 and antimycin A-treated cells (80). A decrease in Mito-SOX fluorescence (and O2˙̄ formation) in Mito-CPP–treated cells was attributed to scavenging of mitochondrial O2˙̄ by Mito-CPPs. Based on our present findings (Fig. 3), it is conceivable that Mito-SOX is predominantly oxidized to Mito-E+ (responsible for increased fluorescence) by mitochondrial peroxidatic activity and that Mito-CPPs inhibit Mito-SOX fluorescence by the mechanism(s) unrelated to scavenging of O2˙̄, e.g. acting as peroxidase substrates. Clearly, it is crucial to determine the identity of Mito-SOX–derived products in control and oxidant-treated cells in the presence of Mito-CPPs, and only then will we be able to interpret results concerning reported Mito-CPP antioxidant mechanisms in a meaningful way.
N-Acetylcysteine, reinterpretation of results
N-Acetylcysteine (NAC), a membrane-permeable cysteine precursor, is one of the most frequently used molecules in oxidative redox biology. NAC is often designated as an “ROS scavenger” and a potent antioxidant. Its cytoprotective effects against oxidative damage and the redox-signaling effects in cancer were attributed to its ability to scavenge ROS (O2˙̄, H2O2) (84–86). However, neither O2˙̄ nor H2O2 react at an appreciable rate with NAC (2), calling into question its antioxidant mechanism due to ROS scavenging (87). Other reports suggest the antioxidant mechanism of NAC is due to its ability to enhance intracellular levels of GSH, which in turn increases the functional ability of GSH-dependent enzymatic hydroperoxide-removing systems (e.g. GSH peroxidases) (88). NAC-induced inhibition of Mito-SOX fluorescence could be due to decreased peroxidatic activity in mitochondria, resulting in decreased one-electron oxidation of Mito-SOX. Decreased peroxidatic activity may be due to decreased levels of H2O2 or other peroxides, as a result of the increased activity of hydroperoxide-removing enzymes (peroxiredoxins and/or GSH peroxidases).
Other ROS probes of interest for detecting mitochondrial O2˙̄
A new mitochondria-targeted O2˙̄ probe, MitoNeoD (Fig. S4), was recently developed (89). MitoNeoD contains a reduced phenanthridinium moiety that is modified to prevent DNA intercalation and reacts with O2˙̄ to form the hydroxylated product, MitoNeoOH. MitoNeoD possess a TPP+ and has a carbon–deuterium bond that inhibits nonspecific two-electron oxidation to the corresponding ethidium analog. Because of the deuterium isotope effect and the bulky group attached to the amino group, it is likely that a direct two-electron oxidation of the probe to the corresponding E+ analog is hindered compared with HE or Mito-SOX. These structural modifications are proposed to facilitate the selectivity toward O2˙̄. MitoNeoD was used in vivo to detect the mitochondrial O2˙̄ by LC-MS–based measurements of the O2˙̄-derived product. Additional bioenergetic functional studies are needed to fully explore its potential for detecting mitochondrial ROS in cells.
More recently, new fluorescent probes (e.g. HKSOX-1 and its mitochondria-targeted analog) consisting of a fluorescein-based trifluoromethanesulfonate were synthesized and used to detect O2˙̄ generated in cells (90). This approach involves the nucleophilic addition of O2˙̄ to the activated sulfonate ester yielding a free and stable phenolic fluorophore, a derivative of 2′,4′,5′,7′-tetrafluorofluorescein (Fig. S4). The rate constant for this reaction was estimated to be relatively high (k = 2 × 105 m−1 s−1). It was reported that this reaction is selective for O2˙̄ and that it did not occur in the presence of other ROS and RNS, including ONOO− and HOCl (90). One of the possible drawbacks of this assay is the generation of a potentially oxidizing radical during the reaction, as its toxicological significance is not known. More extensive investigation of the effect of these probes on mitochondrial function in various cells needs to be performed and the results validated in other laboratories. However, this particular probe clearly shows great promise for intracellular imaging of O2˙̄ in real time, taking advantage of the nucleophilic character of O2˙̄.
Recently, more sensitive and noninvasive modalities, such as PET and radionucleotide imaging, were developed for detecting in vivo O2˙̄ (91, 92). In PET detection, an 18F-labeled HE analog was used as a PET tracer. In radionucleotide imaging, the HE analog containing the radiotracer tritium was used. Although these techniques are highly sensitive and clearly make in vivo application in humans possible, these probes share the same chemistry discussed previously. The limitations discussed for fluorescence-based imaging are also applicable to PET imaging and radiolabeled HE tracers. The claims for noninvasive imaging of O2˙̄ using this probe both in vivo and in vitro conditions need to be reexamined and revised.
Detection of mitochondrial oxidant formation in cells and tissues using the low-temperature EPR technique
EPR spectroscopy detects unpaired electrons, including those in free radicals, redox states in transition metal ions, and iron–sulfur clusters. EPR detection of mitochondrial redox centers requires cryogenic temperatures (5–40 K). This technique does not involve the use of an exogenous probe, and low-temperature EPR of flash-frozen intact tissue or cell samples provides a snapshot of the redox status of the various mitochondrial respiratory chain complexes at the time of freezing and reports on the midpoint potentials of the individual redox centers and the integrity of their intramolecular electron transfer pathways. Elevation of the EPR signal due to the oxidatively deactivated aconitase [3Fe-4S]+ is a biochemical marker for the presence of mitochondrial ROS. Diminution of the g = 1.92 signal from reduced iron–sulfur clusters of complexes I and II indicates diminished redox potential (i.e. more oxidized) and compromised thermodynamic potential for ATP generation, and EPR signals from ROS-induced catalase expression indicate chronic exposure to elevated levels of ROS. Signals from the reduced complex I N3 and N4 clusters are exquisitely sensitive to redox status, as they have midpoint potentials close to the NAD+/NADH couple (7, 93). In the cultured MiaPaCa-2 cells, no detectable EPR evidence for catalase expression was observed. Signals from N3 and N4 clusters were complicated by Mn(II) resonances, but both antimycin A and Mito-Met elicited large (up to 6-fold) increases in the aconitase [3Fe-4S]+ signal, and Mito-Met diminished the g = 1.92 signal by a factor of 3 and suppressed the small N4 signal at all but the lowest dose. EPR signals from aconitase arise from the O2˙̄-induced oxidative deactivation (k = 107 m−1 s−1) of the native [4Fe-4S]2+ cluster, through loss of the noncovalent Fea iron, to the EPR-detectable [3Fe-4S]+ species. O2˙̄-oxidized, reversibly inactivated aconitase exhibits a very characteristic [3Fe-4S]+ EPR signal with a maximum at around g = 2.02 (94, 95). Other oxidants (H2O2 and ONOO−) induce inactivation of aconitase, resulting in the EPR signal due to [3Fe-4S]+ species (96, 97). Low-temperature EPR has also been used to characterize the mitochondrial redox status and oxidative biomarkers in tissues isolated from animal experiments (7) and will ultimately be applied to clinically isolated human tissues.
Implications in cancer biology
Understanding the detailed mechanism(s) of oxidation of ROS probes, as discussed above, has significant implications in elucidating the role of ROS signaling in cancer biology. Mitochondrial sirtuin-3, Sirt3, has been shown to regulate the activity of mitochondrial antioxidant enzymes (e.g. MnSOD) and intracellular ROS levels (98, 99). Sirt3, an NAD-dependent deacetylase located primarily in mitochondria, is presumably responsible for maintaining mitochondrial function, dynamics, and redox metabolism (100). In cancer cells, Sirt3 regulates hypoxia-inducible factor and switches metabolic reprogramming from mitochondrial oxidative metabolism to increased glycolysis (101). Sirt3 deficiency reportedly increases ROS, and it has been proposed that Sirt3 functions as a tumor suppressor by inhibiting ROS in various cancers (102). Overexpression of Sirt3 inhibits the growth of xenograft tumors in mice (102). Confocal fluorescence microscopy was used to measure ROS levels in cells with different Sirt3 levels. Either HE, Mito-SOX, or DCFH was used in these experiments.
The overexpression of Sirt3 in cardiomyocytes protects against doxorubicin-induced mitochondrial dysfunction (103). Doxorubicin-induced ROS formation in cardiomyocytes was monitored by following the increase in Mito-SOX red fluorescence, and the overexpression of Sirt3 mitigated Mito-SOX–induced red fluorescence.
Targeted production of ROS to overcome cancer drug resistance was reported using redox-active probes (104). Antitumor drugs like cisplatin initially elicit antitumor activity in lung cancer patients, but with continued use the patients develop resistance to cisplatin and the treatment fails. Studies have implied increased ROS formation and metabolic reprogramming in cisplatin-resistant cells (105, 106). PGC1α, an important transcriptional coactivator promoting mitochondrial biogenesis, was reported to decrease mitochondrial ROS (107). PGC1α-mediated drug resistance in melanoma cells was linked to decreased intracellular ROS levels, increased mitochondrial energy metabolism, and ROS detoxification enzymatic machinery (108). Suppression of PGC1α led to decreased ROS detoxification, enhanced ROS levels, and apoptosis.
We propose that the combined approach described in this study will help obtain a robust and complete picture of oxidants and redox changes in these systems.
Concluding remarks
We have described the combined use of several redox-active ROS probes and analytical methods for detecting intracellular and mitochondrial ROS. Most of the redox-active probes (e.g. HE, Mito-SOX, DCFH, DHR, CellROX, L-012, luminol, and related analogs) undergo one-electron oxidation, forming a probe-derived radical intermediate that may reduce oxygen to O2˙̄ or disproportionate to form stable products exhibiting fluorescence, chemiluminescence, or bioluminescence. In some cases, the probe-derived radical reacts with O2˙̄ to form a specific hydroxylated product that also exhibits fluorescence. The O2˙̄-specific product can be isolated and detected by HPLC or LC-MS. The enhanced intracellular fluorescence observed using the redox-active probes (e.g. HE, Mito-SOX, DCFH, DHR, and related analogs) indicates one-electron oxidation of the probes induced by higher oxidation state peroxidase (or other heme protein), hydroxyl radical, or iron-oxo intermediate. At present, the HPLC or LC-MS is the only viable approach to unambiguously detect intracellular O2˙̄ using ROS probes, although extraction of TPP+-containing probes and products from mitochondria is cumbersome and suboptimal. The spectral imaging technique currently lacks the sensitivity to distinguish the fluorescence from the nonspecific oxidized product (E+) and the O2˙̄-specific, hydroxylated product (2-OH-E+). However, the spectral imaging approach combined with spectral unmixing is a promising technique that requires a more-sensitive high-resolution confocal microscope equipped with multiple filters for detection and differentiation of oxidized species with overlapping fluorescence spectra. Boronate probes (e.g. Mito-B) react slowly with mitochondrial H2O2 to form specific products that can be detected by LC-MS. Boronate-based probes, however, are ideal for detecting intracellular ONOO−. Using low-temperature EPR, evidence for mitochondrial ROS can be obtained by monitoring the oxidative inactivation of aconitase, compromised reduction potential of the electron transport chain, and markers for chronic exposure to ROS (e.g. catalase), in cells and tissues. The oxidized aconitase ([3Fe-4S]+ form) can be used as an EPR-detectable biomarker of mitochondrial oxidants in cells and tissues.
Finally, we conclude that no single probe or technique can adequately assess and detect mitochondrial ROS formation, and we propose the use of multiple probes and analyses of products and paramagnetic intermediates using several analytical techniques including “probe-free” EPR at cryogenic temperatures. Other endogenous redox indicators (e.g. peroxiredoxins) are an additional means to gain further insights into the cellular redox environment and oxidative stress.
Materials and methods
Cell culture and treatment conditions
The MiaPaCa-2 cell line was obtained from the American Type Culture Collection (Manassas, VA), where cells are regularly authenticated. All cells were obtained over the last 5 years, stored in liquid nitrogen, and used within 20 passages after thawing. Cells were grown at 37 °C in 5% CO2. MiaPaCa-2 cells were maintained in DMEM (catalog no. 11965, Invitrogen) containing 10% (v/v) fetal bovine serum, penicillin (100 units/ml), and streptomycin (100 μg/ml).
ROS probes
HE and Mito-SOX Red were from Invitrogen. Stock solutions (20 and 5 mm, respectively) were prepared in deoxygenated DMSO and stored at −80 °C before use. The ortho-MitoPhB(OH)2 was synthesized, as described recently (109) and stored as a 0.1 m stock solution in DMSO at −20 °C.
Preparation of HPLC standards
Ethidium cation (bromide salt) was purchased from Sigma. Mito-E+ was prepared by reacting Mito-SOX with chloranil (35, 36, 110, 111). The hydroxylated oxidation products from HE and Mito-SOX (2-OH-E+ and 2-OH-Mito-E+) were prepared by reacting the probes with Fremy's salt as described in detail previously (36, 110). The dimeric products were prepared by reacting the probes with excess potassium ferricyanide, as detailed elsewhere (35, 111). A detailed description of the synthesis of the Mito-E+–Mito-E+ dimer and its structural characterization is provided in the supporting information. Synthesis of the oxidation and nitration products from ortho-MitoPhB(OH)2 probe was described elsewhere (51, 109). Synthesized standards of all oxidation products of HE and Mito-SOX were purified by HPLC.
Preparation of cell lysates for HPLC and LC-MS analyses
Frozen cell pellets were placed on ice and lysed in 150 μl of DPBS containing 0.1% (v/v) Triton X-100 and 1 μm internal standards. The internal standards were 3,8-diamino-6-phenylphenanthridine (DAPP) for the HE assay, (2-methylbenzyl)triphenylphosphonium cation (MBTPP) for the Mito-SOX assay, and a mixture of MBTPP, ortho-MitoPhB(OH)2-d15, ortho-MitoPhOH-d15, and ortho-MitoPhNO2-d15 for the ortho-MitoPhB(OH)2 assay (51). Immediately after lysis, the lysate (100 μl) was mixed (1:1) with acetonitrile (MeCN) containing 0.1% (v/v) formic acid, vortexed for 10 s, and left on ice for 30 min. The protein concentration in the remaining lysate was determined using the Bradford assay. Next, samples were centrifuged (30 min at 20,000 × g, 4 °C), and the supernatant (100 μl) was mixed (1:1) with water containing 0.1% (v/v) formic acid. Samples were centrifuged again (15 min at 20,000 × g, 4 °C), and the supernatant (150 μl) was transferred into HPLC vials equipped with conical inserts, and the samples were analyzed by HPLC or LC-MS. During sample collection, and preparation for and during analysis, all samples were kept on ice or at 4 °C and shielded from light.
LC-MS and HPLC
HPLC-based measurements of HE and its oxidation products
HPLC analyses were performed using an Agilent 1100 system (North Billerica, MA) equipped with absorption and fluorescence detectors and refrigerated autosampler (4 °C). The samples (50 μl) were injected into a reverse-phase column (Phenomenex, Kinetex C18, 100 × 4.6 mm, 2.6 μm) equilibrated with 20% (v/v) MeCN, 80% (v/v) water containing 0.1% (v/v) TFA. The compounds were eluted by increasing the content of MeCN (v/v) from 20 to 56% over 4.5 min at a flow rate of 1.5 ml/min. The absorption detector was used to measure DAPP (at 290 nm; retention time 2.5 min), HE-E+ (at 290 nm; retention time 4.1 min), E+-E+ (at 290 nm; retention time 4.3 min), and HE (at 370 nm, retention time 1.6 min). A fluorescence detector (initial excitation at 358 nm and emission at 440 nm) was used to monitor HE (when present at low concentrations). At 2.3 min after injection, the fluorescence parameters were changed (excitation at 490 nm and emission at 567 nm) for sensitive detection of 2-OH-E+ (retention time 3.2 min) and E+ (retention time 3.3 min).
LC-MS/MS-based measurements of HE and Mito-SOX and their oxidation products
Alternatively, HE, Mito-SOX, and their oxidation products were analyzed using a Shimadzu LC-MS8030 triple quadrupole system (Columbia, MD) equipped with an electrospray source, refrigerated autosampler (4 °C), and diverter valve in the thermostated column compartment (kept at 40 °C). The samples were injected into a reverse-phase column (Supelco, Titan C18, 100 × 2.1 mm, 1.9 μm) equilibrated with 20% (v/v) MeCN in water containing 0.1% (v/v) formic acid. The compounds were eluted by increasing the content (v/v) of MeCN from 20 to 50% over 7 min at the flow rate of 0.5 ml/min. The multiple reaction monitoring (MRM) mode was used to detect the compounds of interest as follows: HE (316.1 > 287.1, retention time 2.2 min); 2-OH-E+ (330.1 > 300.0, retention time 2.9 min); E+ (314.1 > 284.0, retention time 3.1 min); E+-E+ (313.1 > 298.9, retention time 3.8 min); Mito-SOX (632.0 > 262.0, retention time 5.5 min); 2-OH-Mito-E+ (323.7 > 182.9, retention time 4.7 min); Mito-E+ (315.5 > 284.1, retention time 4.8 min); Mito-E+–Mito-E+ (315.2 > 183.0 retention time, 4.7 min); DAPP (286.0 > 208.0, retention time 2.2 min); and MBTPP (367.0 > 105.1, retention time 6.1 min).
LC-MS/MS-based measurements of ortho-MitoPhB(OH)2 and its oxidation products
Analyses of o-MitoPhB(OH)2 oxidation products were performed using the same LC-MS method as described above for HE and Mito-SOX but using different MRM transitions, as described below. Alternatively, a different column and gradient were used to shorten the time of analysis (51, 109). Briefly, samples were injected into a reverse-phase column (Phenomenex, phenyl-hexyl, 50 × 2.1 mm, 1.7 μm) and equilibrated with 25% (v/v) MeCN in water containing 0.1% (v/v) formic acid. Compounds were eluted by raising the MeCN concentration (v/v) from 25 to 31.7% over 2 min followed by an increase to 100% from 2 to 4 min at the flow rate of 0.5 ml/min. The MRM mode was used for detecting the compounds of interest as follows: o-MitoPhB(OH)2 (397.0 > 135.0, retention time 1.6 min); o-MitoPhB(OH)2-d15 (412.2 > 117.1, retention time 1.55 min); ortho-MitoPhOH (369.0 > 107.1, retention time 1.9 min); ortho-MitoPhOH-d15 (384.1 > 278.1, retention time 1.85 min); ortho-MitoPhNO2 (397.9 > 262.1, retention time 2.1 min); ortho-MitoPhNO2-d15 (413.1 > 277.2, retention time 2.0 min); cyclo-ortho-MitoPh (351.1 > 183.1, retention time 2.1 min); MitoPhH (353.0 > 91.0, retention time 2.4 min); and MBTPP (367.0 > 105.1, retention time 2.7 min).
Extracellular flux assay
The bioenergetic function of MiaPaCa-2 cells in response to ROS probes was determined using a Seahorse Bioscience XF96 Extracellular Flux Analyzer (Agilent Seahorse Bioscience; North Billerica, MA). MiaPaCa-2 cells were seeded for 24 h in specialized V3-PS 96-well Seahorse tissue culture plates. Cells were treated with ROS probes, Mito-Met, or antimycin A as indicated. Prior to the start of the bioenergetic function assay, cells were washed and changed to unbuffered assay medium adjusted to pH 7.4, final volume 180 μl (DMEM, Gibco, catalog no. 12100-038) per well. Eight baseline OCR measurements were then taken before the injection of oligomycin (1 μg/ml) to inhibit ATP synthase, 2,4-dinitrophenol (DNP, 50 μm) to uncouple the mitochondria and yield maximal OCR, and rotenone (1 μm) and antimycin A (10 μm) to prevent mitochondrial oxygen consumption through inhibition of complex I and complex III, respectively. From these measurements, indices of mitochondrial function were determined as described previously (112, 113).
Low-temperature EPR spectroscopy
MiaPaCa-2 cells (0.5–2 × 106 cells/dish) were grown on 15-cm dishes until reaching 70% confluency. After the treatments indicated, the medium was removed from the cells, and the cells were washed once with ice-cold DPBS buffer. The cells were then scraped in 2 ml of ice-cold DPBS. Next, the cells were pelleted by centrifugation (2 min at 250 × g at 4 °C), and the supernatant was carefully removed by aspiration. The pellet was quickly transferred into a quartz tube (Wilmad 707-SQ-250M) using a long-tipped Pasteur pipette. The EPR tube was deep-frozen by slowly plunging it into liquid nitrogen contained in a Dewar flask. Samples were then stored either at −80 °C or at liquid nitrogen temperature. In general, cells combined from three to four 15-cm dishes can adequately fill the active region of an EPR tube. The two key criteria for harvesting cell samples for EPR are as follows: 1) cell samples should be deep frozen as quickly as possible, and 2) frozen samples should never be thawed and refrozen for subsequent EPR studies.
EPR spectroscopy was carried out at 9.5 GHz on a Bruker EMX-TDU/L E4002501 spectrometer equipped with E532LX digital acquisition, an ER4112 Super-High-Q resonator, a ColdEdge/Bruker ER4112HV-S5-L Stinger cryogen-free 5–100 K closed cycle helium cryocooler, an HP 5350B microwave counter, and an Oxford Instruments ESR900 cryostat and MercuryITC temperature controller. Spectra were typically recorded at 12 K, 5.2 milliwatts of microwave power, 12 G (1.2 millitesla) magnetic field modulation amplitude at 100 kHz, and 1.2 G (0.12 millitesla) digital field resolution. Additional measurements were carried out at 40 K to discriminate between overlapping signals with differing temperature dependences (7). Other acquisition parameters were chosen such that the spectral resolution was limited by the modulation amplitude.
Spin concentrations of the aconitase [3Fe-4S]+ cluster and of the composite “g = 1.92” signal due to the overlapping lines of [2Fe-2S]+ and [4Fe-4S]+ clusters from complexes I and II were determined by double-integration of spectra from which a background signal recorded on water was subtracted. Spectral regions containing the aconitase signal and the composite feature at g = 1.92 were each windowed for integration (Bruker Xenon); the integration windows were conserved across the study except for shifts due to precise frequency differences. This approach was preferred to computer fitting to a library, which was previously used in the case of tissue samples (7), because of the interfering signal from Mn(II) that is associated with cultured cells. Because the g = 1.92 region contains only the contributions from overlapping individual g2 or g2,3 (g⊥) lines of distinct multiline rhombic or axial signals, the raw integral was multiplied by a factor of 7.41. This factor was derived from comparison of the integrated intensities of the g‖ line and the entire spectrum of a rhombic S = ½ Fe(III) signal from nitrile hydratase, with a spectral envelope of 450 G, that showed that 6% of the total spin density was reflected in the double integral of the single g‖ line (114). The conversion factor was then scaled for the ratios of the squares of the spectral envelopes of the nitrile hydratase signal (450 G) and an averaged envelope for the FeS clusters (300 G) that contribute to the g = 1.92 region (7). The raw (aconitase) and corrected (FeS) integrals were then compared with that from a 1.7 mm Cu(II)-imidazole standard recorded under nonsaturating conditions (0.5 milliwatt at 50 K). Corrections for temperature and (microwave power)1/2 were applied to estimate the spin concentrations of the iron signals.
Redox blotting
The redox status of cytosolic and mitochondrial peroxiredoxins (Prx1 and Prx3, respectively) was determined by redox Western blotting, adapted from Cox et al. (115) as described previously (116, 117). Briefly, after treatment, cells were washed quickly with Hanks' balanced salt solution and overlaid with a thiol blocking buffer containing 0.1 m N-ethylmaleimide, 50 mm NaCl, 40 mm HEPES, pH 7.4, 1 mm phenylmethylsulfonyl fluoride, 1 mm EDTA, 1 mm EGTA, and 10 μg/ml catalase for 1–2 min. The cells were then scraped into the thiol blocking buffer, and incubation was continued at room temperature for a total of 15 min. Then, the cells were pelleted (5 min, 800 × g), and the pellet was lysed in the small volume of the thiol blocking buffer supplemented with 1% (v/v) CHAPS. Lysates were stored at −80 °C until analysis. On the day of analysis, the lysates were thawed on ice and then centrifuged for 5 min (8000 × g, 4 °C). The supernatants were run on nonreducing SDS-PAGE. The blots were probed with anti-Prx1 and anti-Prx3 antibodies, followed by horseradish peroxidase-conjugated secondary antibodies. After the addition of chemiluminescence reagent, measurements were taken using the luminescence imager, and the blots were stripped and probed for β-actin (indicator of protein load).
Confocal microscopy (fluorescence imaging)
Cells were seeded overnight into four-chamber NuncTM chambered coverglass slides at 1 × 105 cells per chamber. Intact cells were permeabilized using 1 nm PMP (Seahorse Bioscience) in phenol red-free DMEM with 10% (v/v) FBS. All the indicated treatments were made in PMP containing solution. Cells were imaged on a Carl Zeiss LSM510 META detection system (Jena, Germany). Images were collected with a Plan apochromat ×20/0.8NA objective and image acquisition software (Aim 4.2). Excitation of the fluorophores was performed using a multiline argon laser controlled with the acousto-optic tunable filter, and the 488 nm line was utilized. The emitted light was collected in the META detector every 10.7 nm from 506 to 699 nm.
Author contributions
G. C., B. D., B. B., A. M. G., L. D. D. M., J. Z., and B. K. conceptualization; G. C., B. B., A. M. G., L. D. D. M., J. Z., and B. K. data curation; G. C., B. B., A. M. G., L. D. D. M., J. Z., and B. K. formal analysis; G. C., M. Z., B. B., A. M. G., L. D. D. M., and J. Z. investigation; G. C., S. N. K., C. R. M., B. B., A. M. G., L. D. D. M., D. T., O. O., M. H., and J. Z. methodology; G. C., B. B., J. Z., and B. K. writing-original draft; G. C., M. Z., B. D., S. N. K., C. R. M., B. B., A. M. G., L. D. D. M., D. T., O. O., M. H., J. Z., and B. K. writing-review and editing; B. B., D. T., O. O., and M. H. resources; B. B. and B. K. funding acquisition; B. K. supervision; B. K. project administration.
Supplementary Material
Acknowledgment
EPR was supported by National Science Foundation Major Research Instrumentation Award CHE-1532168 (to B. B.) and by Bruker BioSpin.
Note added in proof
In the version of this article that was published as a Paper in Press on May 8, 2018, Fig. 1D was a duplicate of Fig. 1C. This error has now been corrected.
This work was supported by National Institutes of Health Grants U01 CA178960 and R01 CA208648 from the NCI, Grant ANR-16-CE07-0023-01 from the Agence Nationale de la Recherche (ANR), and the Quadracci Endowment. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Figs. S1–S5 and Table S1.
- ROS
- reactive oxygen species
- mROS
- mitochondria-derived ROS
- 2-OH-E+
- 2-hydroxyethidium
- DNP
- 2,4-dinitrophenol
- Dmt
- 2,6-dimethyltyrosine
- DAPP
- 3,8-diamino-6-phenylphenanthridine
- DCF
- dichlorofluorescein
- DCFH
- dichlorodihydrofluorescein
- DHR
- dihydrorhodamine
- E+
- ethidium
- HE
- hydroethidine
- MeCN
- acetonitrile
- MRM
- multiple reaction monitoring
- MPO
- myeloperoxidase
- NAC
- N-acetylcysteine
- OCR
- oxygen consumption rate
- PMP
- plasma membrane permeabilizer
- RNS
- reactive nitrogen species
- Prx1
- peroxiredoxin-1
- Prx3
- peroxiredoxin-3
- TPP+
- triphenylphosphonium cationic moiety
- Mito-Met
- mito-metformin
- Mito-HE
- mitochondria-targeted HE
- Mito-B
- mitochondria-targeted boronate
- Ox
- oxidized
- Red
- reduced
- DMEM
- Dulbecco's modified Eagle's medium
- CP
- carboxyl proxy
- Mito-CP-Ac
- Mito-CP-acetamide
- Mito-CPP
- mitochondria-targeted cell-penetrating peptide
- PET
- positron emission tomography
- MBTPP
- (2-methylbenzyl)triphenylphosphonium
- SOD
- superoxide dismutase
- PBA
- phenylboronic acid.
References
- 1. Kalyanaraman B., Darley-Usmar V., Davies K. J., Dennery P. A., Forman H. J., Grisham M. B., Mann G. E., Moore K., Roberts L. J. 2nd, and Ischiropoulos H. (2012) Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic. Biol. Med. 52, 1–6 10.1016/j.freeradbiomed.2011.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Murphy M. P., Holmgren A., Larsson N. G., Halliwell B., Chang C. J., Kalyanaraman B., Rhee S. G., Thornalley P. J., Partridge L., Gems D., Nyström T., Belousov V., Schumacker P. T., and Winterbourn C. C. (2011) Unraveling the biological roles of reactive oxygen species. Cell Metab. 13, 361–366 10.1016/j.cmet.2011.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kalyanaraman B., Dranka B. P., Hardy M., Michalski R., and Zielonka J. (2014) HPLC-based monitoring of products formed from hydroethidine-based fluorogenic probes–the ultimate approach for intra- and extracellular superoxide detection. Biochim. Biophys. Acta 1840, 739–744 10.1016/j.bbagen.2013.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dikalov S. I., and Harrison D. G. (2014) Methods for detection of mitochondrial and cellular reactive oxygen species. Antioxid. Redox Signal. 20, 372–382 10.1089/ars.2012.4886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davies M. J. (2016) Detection and characterisation of radicals using electron paramagnetic resonance (EPR) spin trapping and related methods. Methods 109, 21–30 10.1016/j.ymeth.2016.05.013 [DOI] [PubMed] [Google Scholar]
- 6. Janzen E. G. (1984) Spin trapping. Methods Enzymol. 105, 188–198 10.1016/S0076-6879(84)05025-4 [DOI] [PubMed] [Google Scholar]
- 7. Bennett B., Helbling D., Meng H., Jarzembowski J., Geurts A. M., Friederich M. W., Van Hove J. L. K., Lawlor M. W., and Dimmock D. P. (2016) Potentially diagnostic electron paramagnetic resonance spectra elucidate the underlying mechanism of mitochondrial dysfunction in the deoxyguanosine kinase deficient rat model of a genetic mitochondrial DNA depletion syndrome. Free Radic. Biol. Med. 92, 141–151 10.1016/j.freeradbiomed.2016.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chandran K., Aggarwal D., Migrino R. Q., Joseph J., McAllister D., Konorev E. A., Antholine W. E., Zielonka J., Srinivasan S., Avadhani N. G., and Kalyanaraman B. (2009) Doxorubicin inactivates myocardial cytochrome c oxidase in rats: cardioprotection by Mito-Q. Biophys. J. 96, 1388–1398 10.1016/j.bpj.2008.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Myers C. R., Antholine W. E., and Myers J. M. (2010) The pro-oxidant chromium(VI) inhibits mitochondrial complex I, complex II, and aconitase in the bronchial epithelium: EPR markers for Fe-S proteins. Free Radic. Biol. Med. 49, 1903–1915 10.1016/j.freeradbiomed.2010.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zielonka J., Lambeth J. D., and Kalyanaraman B. (2013) On the use of L-012, a luminol-based chemiluminescent probe, for detecting superoxide and identifying inhibitors of NADPH oxidase: a reevaluation. Free Radic. Biol. Med. 65, 1310–1314 10.1016/j.freeradbiomed.2013.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zielonka J., Podsiadl̸y R., Zielonka M., Hardy M., and Kalyanaraman B. (2016) On the use of peroxy-caged luciferin (PCL-1) probe for bioluminescent detection of inflammatory oxidants in vitro and in vivo-Identification of reaction intermediates and oxidant-specific minor products. Free Radic. Biol. Med. 99, 32–42 10.1016/j.freeradbiomed.2016.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Smulik R., Dębski D., Zielonka J., Michal̸owski B., Adamus J., Marcinek A., Kalyanaraman B., and Sikora A. (2014) Nitroxyl (HNO) reacts with molecular oxygen and forms peroxynitrite at physiological pH. Biological Implications. J. Biol. Chem. 289, 35570–35581 10.1074/jbc.M114.597740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zielonka J., Joseph J., Sikora A., and Kalyanaraman B. (2013) Real-time monitoring of reactive oxygen and nitrogen species in a multiwell plate using the diagnostic marker products of specific probes. Methods Enzymol. 526, 145–157 10.1016/B978-0-12-405883-5.00009-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sikora A., Zielonka J., Adamus J., Debski D., Dybala-Defratyka A., Michalowski B., Joseph J., Hartley R. C., Murphy M. P., and Kalyanaraman B. (2013) Reaction between peroxynitrite and triphenylphosphonium-substituted arylboronic acid isomers: identification of diagnostic marker products and biological implications. Chem. Res. Toxicol. 26, 856–867 10.1021/tx300499c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Michalski R., Zielonka J., Gapys E., Marcinek A., Joseph J., and Kalyanaraman B. (2014) Real-time measurements of amino acid and protein hydroperoxides using coumarin boronic acid. J. Biol. Chem. 289, 22536–22553 10.1074/jbc.M114.553727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Handy D. E., and Loscalzo J. (2012) Redox regulation of mitochondrial function. Antioxid. Redox Signal. 16, 1323–1367 10.1089/ars.2011.4123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vyas S., Zaganjor E., and Haigis M. C. (2016) Mitochondria and cancer. Cell 166, 555–566 10.1016/j.cell.2016.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Finkel T. (2012) Signal transduction by mitochondrial oxidants. J. Biol. Chem. 287, 4434–4440 10.1074/jbc.R111.271999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weinberg F., Hamanaka R., Wheaton W. W., Weinberg S., Joseph J., Lopez M., Kalyanaraman B., Mutlu G. M., Budinger G. R., and Chandel N. S. (2010) Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc. Natl. Acad. Sci. U.S.A. 107, 8788–8793 10.1073/pnas.1003428107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brand M. D. (2016) Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic. Biol. Med. 100, 14–31 10.1016/j.freeradbiomed.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 21. Shadel G. S., and Horvath T. L. (2015) Mitochondrial ROS signaling in organismal homeostasis. Cell 163, 560–569 10.1016/j.cell.2015.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Idelchik M. D. P. S., Begley U., Begley T. J., and Melendez J. A. (2017) Mitochondrial ROS control of cancer. Semin. Cancer Biol. 47, 57–66 10.1016/j.semcancer.2017.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ogrunc M., Di Micco R., Liontos M., Bombardelli L., Mione M., Fumagalli M., Gorgoulis V. G., and d'Adda di Fagagna F. (2014) Oncogene-induced reactive oxygen species fuel hyperproliferation and DNA damage response activation. Cell Death Differ. 21, 998–1012 10.1038/cdd.2014.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li P., Zhang D., Shen L., Dong K., Wu M., Ou Z., and Shi D. (2016) Redox homeostasis protects mitochondria through accelerating ROS conversion to enhance hypoxia resistance in cancer cells. Sci. Rep. 6, 22831 10.1038/srep22831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ge C., Cao B., Feng D., Zhou F., Zhang J., Yang N., Feng S., Wang G., and Aa J. (2017) The down-regulation of SLC7A11 enhances ROS induced P-gp over-expression and drug resistance in MCF-7 breast cancer cells. Sci. Rep. 7, 3791 10.1038/s41598-017-03881-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chan S. H., Wu K. L., Chang A. Y., Tai M.-H., and Chan J. Y. (2009) Oxidative impairment of mitochondrial electron transport chain complexes in rostral ventrolateral medulla contributes to neurogenic hypertension. Hypertension 53, 217–227 10.1161/HYPERTENSIONAHA.108.116905 [DOI] [PubMed] [Google Scholar]
- 27. Ahn H. Y., Fairfull-Smith K. E., Morrow B. J., Lussini V., Kim B., Bondar M. V., Bottle S. E., and Belfield K. D. (2012) Two-photon fluorescence microscopy imaging of cellular oxidative stress using profluorescent nitroxides. J. Am. Chem. Soc. 134, 4721–4730 10.1021/ja210315x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee E., Choi J., and Lee H. S. (2017) Palmitate induces mitochondrial superoxide generation and activates AMPK in podocytes. J. Cell. Physiol. 232, 3209–3217 10.1002/jcp.25867 [DOI] [PubMed] [Google Scholar]
- 29. Esterberg R., Linbo T., Pickett S. B., Wu P., Ou H. C., Rubel E. W., and Raible D. W. (2016) Mitochondrial calcium uptake underlies ROS generation during aminoglycoside-induced hair cell death. J. Clin. Invest. 126, 3556–3566 10.1172/JCI84939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tampo Y., Kotamraju S., Chitambar C. R., Kalivendi S. V., Keszler A., Joseph J., and Kalyanaraman B. (2003) Oxidative stress-induced iron signaling is responsible for peroxide-dependent oxidation of dichlorodihydrofluorescein in endothelial cells: role of transferrin receptor-dependent iron uptake in apoptosis. Circ. Res. 92, 56–63 10.1161/01.RES.0000048195.15637.AC [DOI] [PubMed] [Google Scholar]
- 31. Kotamraju S., Kalivendi S. V., Konorev E., Chitambar C. R., Joseph J., and Kalyanaraman B. (2004) Oxidant-induced iron signaling in doxorubicin-mediated apoptosis. Methods Enzymol. 378, 362–382 10.1016/S0076-6879(04)78026-X [DOI] [PubMed] [Google Scholar]
- 32. Karlsson M., Kurz T., Brunk U. T., Nilsson S. E., and Frennesson C. I. (2010) What does the commonly used DCF test for oxidative stress really show? Biochem. J. 428, 183–190 10.1042/BJ20100208 [DOI] [PubMed] [Google Scholar]
- 33. Rota C., Chignell C. F., and Mason R. P. (1999) Evidence for free radical formation during the oxidation of 2′-7′-dichlorofluorescin to the fluorescent dye 2′-7′-dichlorofluorescein by horseradish peroxidase: possible implications for oxidative stress measurements. Free Radic. Biol. Med. 27, 873–881 10.1016/S0891-5849(99)00137-9 [DOI] [PubMed] [Google Scholar]
- 34. Bonini M. G., Rota C., Tomasi A., and Mason R. P. (2006) The oxidation of 2′,7′-dichlorofluorescin to reactive oxygen species: a self-fulfilling prophesy? Free Radic. Biol. Med. 40, 968–975 10.1016/j.freeradbiomed.2005.10.042 [DOI] [PubMed] [Google Scholar]
- 35. Zielonka J., Srinivasan S., Hardy M., Ouari O., Lopez M., Vasquez-Vivar J., Avadhani N. G., and Kalyanaraman B. (2008) Cytochrome c-mediated oxidation of hydroethidine and mito-hydroethidine in mitochondria: identification of homo- and heterodimers. Free Radic. Biol. Med. 44, 835–846 10.1016/j.freeradbiomed.2007.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zielonka J., Vasquez-Vivar J., and Kalyanaraman B. (2008) Detection of 2-hydroxyethidium in cellular systems: a unique marker product of superoxide and hydroethidine. Nat. Protoc. 3, 8–21 10.1038/nprot.2007.473 [DOI] [PubMed] [Google Scholar]
- 37. Zhao H., Kalivendi S., Zhang H., Joseph J., Nithipatikom K., Vásquez-Vivar J., and Kalyanaraman B. (2003) Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic. Biol. Med. 34, 1359–1368 10.1016/S0891-5849(03)00142-4 [DOI] [PubMed] [Google Scholar]
- 38. Zhao H., Joseph J., Fales H. M., Sokoloski E. A., Levine R. L., Vasquez-Vivar J., and Kalyanaraman B. (2005) Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc. Natl. Acad. Sci. U.S.A. 102, 5727–5732 10.1073/pnas.0501719102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hall D. J., Han S. H., Chepetan A., Inui E. G., Rogers M., and Dugan L. L. (2012) Dynamic optical imaging of metabolic and NADPH oxidase-derived superoxide in live mouse brain using fluorescence lifetime unmixing. J. Cereb. Blood Flow Metab. 32, 23–32 10.1038/jcbfm.2011.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Michalski R., Michalowski B., Sikora A., Zielonka J., and Kalyanaraman B. (2014) On the use of fluorescence lifetime imaging and dihydroethidium to detect superoxide in intact animals and ex vivo tissues: a reassessment. Free Radic. Biol. Med. 67, 278–284 10.1016/j.freeradbiomed.2013.10.816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zielonka J., and Kalyanaraman B. (2010) Hydroethidine- and MitoSOX-derived red fluorescence is not a reliable indicator of intracellular superoxide formation: another inconvenient truth. Free Radic. Biol. Med. 48, 983–1001 10.1016/j.freeradbiomed.2010.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kalyanaraman B., Hardy M., Podsiadly R., Cheng G., and Zielonka J. (2017) Recent developments in detection of superoxide radical anion and hydrogen peroxide: opportunities, challenges, and implications in redox signaling. Arch. Biochem. Biophys. 617, 38–47 10.1016/j.abb.2016.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kalyanaraman B., Cheng G., Hardy M., Ouari O., Bennett B., and Zielonka J. (2018) Teaching the basics of reactive oxygen species and their relevance to cancer biology: mitochondrial reactive oxygen species detection, redox signaling, and targeted therapies. Redox Biol. 15, 347–362 10.1016/j.redox.2017.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Plaza Davila M., Martin Muñoz P., Tapia J. A., Ortega Ferrusola C., Balao da Silva C. C., and Peña F. J. (2015) Inhibition of mitochondrial complex I leads to decreased motility and membrane integrity related to increased hydrogen peroxide and reduced ATP production, while the inhibition of glycolysis has less impact on sperm motility. PLoS ONE 10, e0138777 10.1371/journal.pone.0138777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dickinson B. C., and Chang C. J. (2008) A targetable fluorescent probe for imaging hydrogen peroxide in the mitochondria of living cells. J. Am. Chem. Soc. 130, 9638–9639 10.1021/ja802355u [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dickinson B. C., Tang Y., Chang Z., and Chang C. J. (2011) A nuclear-localized fluorescent hydrogen peroxide probe for monitoring sirtuin-mediated oxidative stress responses in vivo. Chem. Biol. 18, 943–948 10.1016/j.chembiol.2011.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xu J., Zhang Y., Yu H., Gao X., and Shao S. (2016) Mitochondria-targeted fluorescent probe for imaging hydrogen peroxide in living cells. Anal. Chem. 88, 1455–1461 10.1021/acs.analchem.5b04424 [DOI] [PubMed] [Google Scholar]
- 48. Sikora A., Zielonka J., Lopez M., Joseph J., and Kalyanaraman B. (2009) Direct oxidation of boronates by peroxynitrite: mechanism and implications in fluorescence imaging of peroxynitrite. Free Radic. Biol. Med. 47, 1401–1407 10.1016/j.freeradbiomed.2009.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zielonka J., Sikora A., Hardy M., Joseph J., Dranka B. P., and Kalyanaraman B. (2012) Boronate probes as diagnostic tools for real time monitoring of peroxynitrite and hydroperoxides. Chem. Res. Toxicol. 25, 1793–1799 10.1021/tx300164j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zielonka J., Sikora A., Joseph J., and Kalyanaraman B. (2010) Peroxynitrite is the major species formed from different flux ratios of co-generated nitric oxide and superoxide: direct reaction with boronate-based fluorescent probe. J. Biol. Chem. 285, 14210–14216 10.1074/jbc.M110.110080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zielonka J., Zielonka M., VerPlank L., Cheng G., Hardy M., Ouari O., Ayhan M. M., Podsiadl̸y R., Sikora A., Lambeth J. D., and Kalyanaraman B. (2016) Mitigation of NADPH oxidase 2 activity as a strategy to inhibit peroxynitrite formation. J. Biol. Chem. 291, 7029–7044 10.1074/jbc.M115.702787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cochemé H. M., Quin C., McQuaker S. J., Cabreiro F., Logan A., Prime T. A., Abakumova I., Patel J. V., Fearnley I. M., James A. M., Porteous C. M., Smith R. A., Saeed S., Carré J. E., Singer M., et al. (2011) Measurement of H2O2 within living Drosophila during aging using a ratiometric mass spectrometry probe targeted to the mitochondrial matrix. Cell Metab. 13, 340–350 10.1016/j.cmet.2011.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Logan A., Pell V. R., Shaffer K. J., Evans C., Stanley N. J., Robb E. L., Prime T. A., Chouchani E. T., Cochemé H. M., Fearnley I. M., Vidoni S., James A. M., Porteous C. M., Partridge L., Krieg T., et al. (2016) Assessing the mitochondrial membrane potential in cells and in vivo using targeted click chemistry and mass spectrometry. Cell Metab. 23, 379–385 10.1016/j.cmet.2015.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Perry S. W., Norman J. P., Barbieri J., Brown E. B., and Gelbard H. A. (2011) Mitochondrial membrane potential probes and the proton gradient: a practical usage guide. BioTechniques 50, 98–115 10.2144/000113610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Smith R. A., Porteous C. M., Gane A. M., and Murphy M. P. (2003) Delivery of bioactive molecules to mitochondria in vivo. Proc. Natl. Acad. Sci. U.S.A. 100, 5407–5412 10.1073/pnas.0931245100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Maghzal G. J., and Stocker R. (2007) Improved analysis of hydroethidine and 2-hydroxyethidium by HPLC and electrochemical detection. Free Radic. Biol. Med. 43, 1095–1096 10.1016/j.freeradbiomed.2007.06.023 [DOI] [PubMed] [Google Scholar]
- 57. Dey S., Sidor A., and O'Rourke B. (2016) Compartment-specific control of reactive oxygen species scavenging by antioxidant pathway enzymes. J. Biol. Chem. 291, 11185–11197 10.1074/jbc.M116.726968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Roelofs B. A., Ge S. X., Studlack P. E., and Polster B. M. (2015) Low micromolar concentrations of the superoxide probe MitoSOX uncouple neural mitochondria and inhibit complex IV. Free Radic. Biol. Med. 86, 250–258 10.1016/j.freeradbiomed.2015.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Quinlan C. L., Perevoschikova I. V., Goncalves R. L., Hey-Mogensen M., and Brand M. D. (2013) The determination and analysis of site-specific rates of mitochondrial reactive oxygen species production. Methods Enzymol. 526, 189–217 10.1016/B978-0-12-405883-5.00012-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kennedy M. C., Antholine W. E., and Beinert H. (1997) An EPR investigation of the products of the reaction of cytosolic and mitochondrial aconitases with nitric oxide. J. Biol. Chem. 272, 20340–20347 10.1074/jbc.272.33.20340 [DOI] [PubMed] [Google Scholar]
- 61. Beinert H. (1978) EPR spectroscopy of components of the mitochondrial electron-transfer system. Methods Enzymol. 54, 133–150 10.1016/S0076-6879(78)54014-7 [DOI] [PubMed] [Google Scholar]
- 62. Winterbourn C. C. (2017) Biological production, detection, and fate of hydrogen peroxide. Antioxid. Redox Signal. 2017, 10.1089/ars.2017.7425 [DOI] [PubMed] [Google Scholar]
- 63. Andreyev A. Y., Kushnareva Y. E., Murphy A. N., and Starkov A. A. (2015) Mitochondrial ROS metabolism: 10 years later. Biochemistry 80, 517–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cunniff B., Benson K., Stumpff J., Newick K., Held P., Taatjes D., Joseph J., Kalyanaraman B., and Heintz N. H. (2013) Mitochondrial-targeted nitroxides disrupt mitochondrial architecture and inhibit expression of peroxiredoxin 3 and FOXM1 in malignant mesothelioma cells. J. Cell. Physiol. 228, 835–845 10.1002/jcp.24232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jayakumar S., Patwardhan R. S., Pal D., Singh B., Sharma D., Kutala V. K., and Sandur S. K. (2017) Mitochondrial targeted curcumin exhibits anticancer effects through disruption of mitochondrial redox and modulation of TrxR2 activity. Free Radic. Biol. Med. 113, 530–538 10.1016/j.freeradbiomed.2017.10.378 [DOI] [PubMed] [Google Scholar]
- 66. Robinson K. M., Janes M. S., and Beckman J. S. (2008) The selective detection of mitochondrial superoxide by live cell imaging. Nat. Protoc. 3, 941–947 10.1038/nprot.2008.56 [DOI] [PubMed] [Google Scholar]
- 67. Robinson K. M., Janes M. S., Pehar M., Monette J. S., Ross M. F., Hagen T. M., Murphy M. P., and Beckman J. S. (2006) Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc. Natl. Acad. Sci. U.S.A. 103, 15038–15043 10.1073/pnas.0601945103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pearson T., Kabayo T., Ng R., Chamberlain J., McArdle A., and Jackson M. J. (2014) Skeletal muscle contractions induce acute changes in cytosolic superoxide, but slower responses in mitochondrial superoxide and cellular hydrogen peroxide. PLoS ONE 9, e96378 10.1371/journal.pone.0096378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hangauer M. J., Viswanathan V. S., Ryan M. J., Bole D., Eaton J. K., Matov A., Galeas J., Dhruv H. D., Berens M. E., Schreiber S. L., McCormick F., and McManus M. T. (2017) Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature 551, 247–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dhanasekaran A., Kotamraju S., Karunakaran C., Kalivendi S. V., Thomas S., Joseph J., and Kalyanaraman B. (2005) Mitochondria superoxide dismutase mimetic inhibits peroxide-induced oxidative damage and apoptosis: role of mitochondrial superoxide. Free Radic. Biol. Med. 39, 567–583 10.1016/j.freeradbiomed.2005.04.016 [DOI] [PubMed] [Google Scholar]
- 71. Dickey J. S., Gonzalez Y., Aryal B., Mog S., Nakamura A. J., Redon C. E., Baxa U., Rosen E., Cheng G., Zielonka J., Parekh P., Mason K. P., Joseph J., Kalyanaraman B., Bonner W., Herman E., Shacter E., and Rao V. A. (2013) Mito-tempol and dexrazoxane exhibit cardioprotective and chemotherapeutic effects through specific protein oxidation and autophagy in a syngeneic breast tumor preclinical model. PLoS ONE 8, e70575 10.1371/journal.pone.0070575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cheng G., Zielonka J., Dranka B. P., McAllister D., Mackinnon A. C. Jr., Joseph J., and Kalyanaraman B. (2012) Mitochondria-targeted drugs synergize with 2-deoxyglucose to trigger breast cancer cell death. Cancer Res. 72, 2634–2644 10.1158/0008-5472.CAN-11-3928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cheng G., Zielonka J., McAllister D., Hardy M., Ouari O., Joseph J., Dwinell M. B., and Kalyanaraman B. (2015) Antiproliferative effects of mitochondria-targeted cationic antioxidants and analogs: role of mitochondrial bioenergetics and energy-sensing mechanism. Cancer Lett. 365, 96–106 10.1016/j.canlet.2015.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zou L., Linck V., Zhai Y. J., Galarza-Paez L., Li L., Yue Q., Al-Khalili O., Bao H. F., Ma H. P., Thai T. L., Jiao J., and Eaton D. C. (2018) Knockout of mitochondrial voltage-dependent anion channel type 3 increases reactive oxygen species (ROS) levels and alters renal sodium transport. J. Biol. Chem. 293, 1666–1675 10.1074/jbc.M117.798645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ni R., Cao T., Xiong S., Ma J., Fan G. C., Lacefield J. C., Lu Y., Le Tissier S., and Peng T. (2016) Therapeutic inhibition of mitochondrial reactive oxygen species with mito-TEMPO reduces diabetic cardiomyopathy. Free Radic. Biol. Med. 90, 12–23 10.1016/j.freeradbiomed.2015.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dikalova A. E., Bikineyeva A. T., Budzyn K., Nazarewicz R. R., McCann L., Lewis W., Harrison D. G., and Dikalov S. I. (2010) Therapeutic targeting of mitochondrial superoxide in hypertension. Circ. Res. 107, 106–116 10.1161/CIRCRESAHA.109.214601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. McCarthy C., and Kenny L. C. (2016) Therapeutically targeting mitochondrial redox signalling alleviates endothelial dysfunction in preeclampsia. Sci. Rep. 6, 32683 10.1038/srep32683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Yasui H., Yamamoto K., Suzuki M., Sakai Y., Bo T., Nagane M., Nishimura E., Yamamori T., Yamasaki T., Yamada K.-I., and Inanami O. (2017) Lipophilic triphenylphosphonium derivatives enhance radiation-induced cell killing via inhibition of mitochondrial energy metabolism in tumor cells. Cancer Lett. 390, 160–167 10.1016/j.canlet.2017.01.006 [DOI] [PubMed] [Google Scholar]
- 79. Szeto H. H. (2006) Cell-permeable, mitochondrial-targeted, peptide antioxidants. AAPS J. 8, E277–E283 10.1208/aapsj080232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zhao K., Zhao G. M., Wu D., Soong Y., Birk A. V., Schiller P. W., and Szeto H. H. (2004) Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J. Biol. Chem. 279, 34682–34690 10.1074/jbc.M402999200 [DOI] [PubMed] [Google Scholar]
- 81. Cerrato C. P., Pirisinu M., Vlachos E. N., and Langel Ü. (2015) Novel cell-penetrating peptide targeting mitochondria. FASEB J. 29, 4589–4599 10.1096/fj.14-269225 [DOI] [PubMed] [Google Scholar]
- 82. Skulachev V. P. (2005) How to clean the dirtiest place in the cell: cationic antioxidants as intramitochondrial ROS scavengers. IUBMB Life 57, 305–310 10.1080/15216540500092161 [DOI] [PubMed] [Google Scholar]
- 83. Zielonka J., Joseph J., Sikora A., Hardy M., Ouari O., Vasquez-Vivar J., Cheng G., Lopez M., and Kalyanaraman B. (2017) Mitochondria-targeted triphenylphosphonium-based compounds: syntheses, mechanisms of action, and therapeutic and diagnostic applications. Chem. Rev. 117, 10043–10120 10.1021/acs.chemrev.7b00042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Tung J. N., Lin P. L., Wang Y. C., Wu D. W., Chen C. Y., and Lee H. (2018) PD-L1 confers resistance to EGFR mutation-independent tyrosine kinase inhibitors in non-small cell lung cancer via upregulation of YAP1 expression. Oncotarget 9, 4637–4646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Oien D. B., Garay T., Eckstein S., and Chien J. (2017) Cisplatin and pemetrexed activate AXL and AXL inhibitor BGB324 enhances mesothelioma cell death from chemotherapy. Front. Pharmacol. 8, 970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ma L., Fu Q., Xu B., Zhou H., Gao J., Shao X., Xiong J., Gu Q., Wen S., Li F., Shen L., Chen G., Fang H., and Lyu J. (2018) Breast cancer-associated mitochondrial DNA haplogroup promotes neoplastic growth via ROS-mediated AKT activation. Int. J. Cancer 142, 1786–1796 [DOI] [PubMed] [Google Scholar]
- 87. Samuni Y., Goldstein S., Dean O. M., and Berk M. (2013) The chemistry and biological activities of N-acetylcysteine. Biochim. Biophys. Acta 1830, 4117–4129 10.1016/j.bbagen.2013.04.016 [DOI] [PubMed] [Google Scholar]
- 88. Beatty A., Fink L. S., Singh T., Strigun A., Peter E., Ferrer C. M., Nicolas E., Cai K. Q., Moran T. P., Reginato M. J., Rennefahrt U., and Peterson J. R. (2018) Metabolite profiling reveals the glutathione biosynthetic pathway as a therapeutic target in triple-negative breast cancer. Mol. Cancer Ther. 17, 264–275 10.1158/1535-7163.MCT-17-0407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Shchepinova M. M., Cairns A. G., Prime T. A., Logan A., James A. M., Hall A. R., Vidoni S., Arndt S., Caldwell S. T., Prag H. A., Pell V. R., Krieg T., Mulvey J. F., Yadav P., Cobley J. N., et al. (2017) MitoNeoD: A mitochondria-targeted superoxide probe. Cell Chem. Biol. 24, 1285–1298 10.1016/j.chembiol.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hu J. J., Wong N. K., Ye S., Chen X., Lu M. Y., Zhao A. Q., Guo Y., Ma A. C., Leung A. Y., Shen J., and Yang D. (2015) Fluorescent probe HKSOX-1 for imaging and detection of endogenous superoxide in live cells and in vivo. J. Am. Chem. Soc. 137, 6837–6843 10.1021/jacs.5b01881 [DOI] [PubMed] [Google Scholar]
- 91. Abe K., Takai N., Fukumoto K., Imamoto N., Tonomura M., Ito M., Kanegawa N., Sakai K., Morimoto K., Todoroki K., and Inoue O. (2014) In vivo imaging of reactive oxygen species in mouse brain by using [3H]hydromethidine as a potential radical trapping radiotracer. J. Cereb. Blood Flow Metab. 34, 1907–1913 10.1038/jcbfm.2014.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Chu W., Chepetan A., Zhou D., Shoghi K. I., Xu J., Dugan L. L., Gropler R. J., Mintun M. A., and Mach R. H. (2014) Development of a PET radiotracer for noninvasive imaging of the reactive oxygen species, superoxide, in vivo. Org. Biomol. Chem. 12, 4421–4431 10.1039/C3OB42379D [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Medvedev E. S., Couch V. A., and Stuchebrukhov A. A. (2010) Determination of the intrinsic redox potentials of FeS centers of respiratory complex I from experimental titration curves. Biochim. Biophys. Acta 1797, 1665–1671 10.1016/j.bbabio.2010.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hausladen A., and Fridovich I. (1994) Superoxide and peroxynitrite inactivate aconitases, but nitric oxide does not. J. Biol. Chem. 269, 29405–29408 [PubMed] [Google Scholar]
- 95. Vasquez-Vivar J., Kalyanaraman B., and Kennedy M. C. (2000) Mitochondrial aconitase is a source of hydroxyl radical. An electron spin resonance investigation. J. Biol. Chem. 275, 14064–14069 10.1074/jbc.275.19.14064 [DOI] [PubMed] [Google Scholar]
- 96. Tórtora V., Quijano C., Freeman B., Radi R., and Castro L. (2007) Mitochondrial aconitase reaction with nitric oxide, S-nitrosoglutathione, and peroxynitrite: mechanisms and relative contributions to aconitase inactivation. Free Radic. Biol. Med. 42, 1075–1088 10.1016/j.freeradbiomed.2007.01.007 [DOI] [PubMed] [Google Scholar]
- 97. Bulteau A. L., Ikeda-Saito M., and Szweda L. I. (2003) Redox-dependent modulation of aconitase activity in intact mitochondria. Biochemistry 42, 14846–14855 10.1021/bi0353979 [DOI] [PubMed] [Google Scholar]
- 98. Ren T., Zhang H., Wang J., Zhu J., Jin M., Wu Y., Guo X., Ji L., Huang Q., Zhang H., Yang H., and Xing J. (2017) MCU-dependent mitochondrial Ca2+ inhibits NAD+/SIRT3/SOD2 pathway to promote ROS production and metastasis of HCC cells. Oncogene 36, 5897–5909 10.1038/onc.2017.167 [DOI] [PubMed] [Google Scholar]
- 99. Tao R., Coleman M. C., Pennington J. D., Ozden O., Park S. H., Jiang H., Kim H. S., Flynn C. R., Hill S., Hayes McDonald W., Olivier A. K., Spitz D. R., and Gius D. (2010) Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol. Cell 40, 893–904 10.1016/j.molcel.2010.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Yu W., Denu R. A., Krautkramer K. A., Grindle K. M., Yang D. T., Asimakopoulos F., Hematti P., and Denu J. M. (2016) Loss of SIRT3 provides growth advantage for B cell malignancies. J. Biol. Chem. 291, 3268–3279 10.1074/jbc.M115.702076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Torrens-Mas M., Oliver J., Roca P., and Sastre-Serra J. (2017) SIRT3: Oncogene and tumor suppressor in cancer. Cancers 9, 90 10.3390/cancers9070090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Bell E. L., Emerling B. M., Ricoult S. J., and Guarente L. (2011) SirT3 suppresses hypoxia inducible factor 1α and tumor growth by inhibiting mitochondrial ROS production. Oncogene 30, 2986–2996 10.1038/onc.2011.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Pillai V. B., Kanwal A., Fang Y. H., Sharp W. W., Samant S., Arbiser J., and Gupta M. P. (2017) Honokiol, an activator of Sirtuin-3 (SIRT3) preserves mitochondria and protects the heart from doxorubicin-induced cardiomyopathy in mice. Oncotarget 8, 34082–34098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wang H., Gao Z., Liu X., Agarwal P., Zhao S., Conroy D. W., Ji G., Yu J., Jaroniec C. P., Liu Z., Lu X., Li X., and He X. (2018) Targeted production of reactive oxygen species in mitochondria to overcome cancer drug resistance. Nat. Commun. 9, 562 10.1038/s41467-018-02915-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wangpaichitr M., Kandemir H., Li Y. Y., Wu C., Nguyen D., Feun L. G., Kuo M. T., and Savaraj N. (2017) Relationship of metabolic alterations and PD-L1 expression in cisplatin resistant lung cancer. Cell Dev. Biol. 6, 183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Wangpaichitr M., Wu C., Li Y. Y., Nguyen D. J. M., Kandemir H., Shah S., Chen S., Feun L. G., Prince J. S., Kuo M. T., and Savaraj N. (2017) Exploiting ROS and metabolic differences to kill cisplatin resistant lung cancer. Oncotarget 8, 49275–49292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. St-Pierre J., Drori S., Uldry M., Silvaggi J. M., Rhee J., Jäger S., Handschin C., Zheng K., Lin J., Yang W., Simon D. K., Bachoo R., and Spiegelman B. M. (2006) Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 127, 397–408 10.1016/j.cell.2006.09.024 [DOI] [PubMed] [Google Scholar]
- 108. Vazquez F., Lim J. H., Chim H., Bhalla K., Girnun G., Pierce K., Clish C. B., Granter S. R., Widlund H. R., Spiegelman B. M., and Puigserver P. (2013) PGC1α expression defines a subset of human melanoma tumors with increased mitochondrial capacity and resistance to oxidative stress. Cancer Cell 23, 287–301 10.1016/j.ccr.2012.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zielonka J., Sikora A., Adamus J., and Kalyanaraman B. (2015) Detection and differentiation between peroxynitrite and hydroperoxides using mitochondria-targeted arylboronic acid. Methods Mol. Biol. 1264, 171–181 10.1007/978-1-4939-2257-4_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Zielonka J., Zhao H., Xu Y., and Kalyanaraman B. (2005) Mechanistic similarities between oxidation of hydroethidine by Fremy's salt and superoxide: stopped-flow optical and EPR studies. Free Radic. Biol. Med. 39, 853–863 10.1016/j.freeradbiomed.2005.05.001 [DOI] [PubMed] [Google Scholar]
- 111. Zielonka J., Hardy M., and Kalyanaraman B. (2009) HPLC study of oxidation products of hydroethidine in chemical and biological systems: Ramifications in superoxide measurements. Free Radic. Biol. Med. 46, 329–338 10.1016/j.freeradbiomed.2008.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Cheng G., Zielonka J., McAllister D., Tsai S., Dwinell M. B., and Kalyanaraman B. (2014) Profiling and targeting of cellular bioenergetics: Inhibition of pancreatic cancer cell proliferation. Br. J. Cancer 111, 85–93 10.1038/bjc.2014.272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Nicholls D. G., Darley-Usmar V. M., Wu M., Jensen P. B., Rogers G. W., and Ferrick D. A. (2010) Bioenergetic profile experiment using C2C12 myoblast cells. J. Vis. Exp. 2010, 2511 10.3791/2511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Stein N., Gumataotao N., Hajnas N., Wu R., Lankathilaka K. P. W., Bornscheuer U. T., Liu D., Fiedler A. T., Holz R. C., and Bennett B. (2017) Multiple states of nitrile hydratase from Rhodococcus equi TG328–2: structural and mechanistic insights from electron paramagnetic resonance and density functional theory studies. Biochemistry 56, 3068–3077 10.1021/acs.biochem.6b00876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Cox A. G., Pullar J. M., Hughes G., Ledgerwood E. C., and Hampton M. B. (2008) Oxidation of mitochondrial peroxiredoxin 3 during the initiation of receptor-mediated apoptosis. Free Radic. Biol. Med. 44, 1001–1009 10.1016/j.freeradbiomed.2007.11.017 [DOI] [PubMed] [Google Scholar]
- 116. Myers C. R. (2016) Enhanced targeting of mitochondrial peroxide defense by the combined use of thiosemicarbazones and inhibitors of thioredoxin reductase. Free Radic. Biol. Med. 91, 81–92 10.1016/j.freeradbiomed.2015.12.008 [DOI] [PubMed] [Google Scholar]
- 117. Myers J. M., and Myers C. R. (2009) The effects of hexavalent chromium on thioredoxin reductase and peroxiredoxins in human bronchial epithelial cells. Free Radic. Biol. Med. 47, 1477–1485 10.1016/j.freeradbiomed.2009.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.