Abstract
Background:
Dermoscopy is used increasingly in dermatological practice. Although dermoscopic findings of alopecia areata (AA) are described in the literature, studies are limited.
Aim:
Our aim was to evaluate dermoscopic findings of Iranian patients with AA and correlate them with disease activity and severity.
Subjects and Methods:
Totally 126 patients were examined using a Dermlite II multispectral dermoscope. Severity, activity, pull test, nail changes, treatments, and dermoscopic findings were recorded.
Statistical Analysis Used:
Statistical analysis was done by SPSS version 22, using appropriate statistical tools.
Results:
The most common dermoscopic findings were yellow dots (84.1%), vellus hairs (62.6%), black dots (48.4%), exclamation mark (30.9%), and broken hair (9.5%), in decreasing order. Furthermore, the most common dermoscopic findings in patients on diphencyprone were vellus hairs and yellow dots. Yellow dots and vellus hairs were most common in patients with alopecia universalis. However, broken hairs and exclamation mark hairs were mostly observed in patchy multiple AA patients. Yellow dots and exclamation mark hairs were also significantly more common in patients with positive pull test. Furthermore, vellus hairs were more common in patients with remitting disease pattern. With regard to scalp severity, yellow dots related positively, while vellus hairs, broken hairs, and exclamation mark hairs related negatively with severity of disease.
Conclusions:
Dermoscopic findings differ in various stages of activity and severity of AA. Dermoscopy is a valuable tool for the dermatologist for the diagnosis, follow-up, and evaluation of response to treatment.
Key words: Alopecia areata, dermoscopy, trichoscopy
INTRODUCTION
Alopecia areata (AA) is a common, clinically heterogeneous, nonscarring autoimmune hair loss.[1,2,3]
Dermoscopy is a noninvasive diagnostic procedure used increasingly in dermatological practice not only for evaluation of pigmented skin lesions but also for hair disorders.[4,5] Although dermoscopic findings of AA are described in the literature, studies are limited. Recently, trichoscopy has been suggested to name the use of dermoscopy in evaluating hair disorders.[6] Several studies have reported that various trichoscopic characteristics, such as black dots, “exclamation-mark” hairs, broken hairs, yellow dots, and clustered short vellus hairs, are useful clinical indicators for AA in areas of hair loss.[7,8,9,10,11] Using trichoscopy, we have evaluated 126 Iranian patients with AA and reported common dermoscopic findings and correlated them with disease activity and severity.
SUBJECTS AND METHODS
One hundred and twenty-six patients (males = 78; females = 48) with AA were examined by dermoscopy in the General Dermatology Clinic and Diphencyprone (DPCP) Clinic at Razi Hospital, Tehran University of Medical Sciences, from December 2012 to March 2014. The diagnosis of AA was clinically established; suspicious cases and patients with coexistent androgenetic alopecia were excluded from the study. Demographic data, family history, underlying disease, type of disease and its duration, disease management, and signs of nail changes were assessed.
The disease activity of AA (based on subjective assessment) was estimated as follows:.
Progressive AA: An increase in total hair loss of more than 5%
Stable: A change in total hair loss of <5%
Remitting AA: A decrease in total hair loss of more than 5% over the month before the dermoscopic examination.
The severity of AA at the time of dermoscopic examination was evaluated in each patient based on severity of alopecia tool (SALT) scoring.
Severity of alopecia tool scoring
The scalp is divided into the following four parts:
Vertex: 40% (0.4) of scalp surface area
Right profile of scalp: 18% (0.18) of scalp surface area
Left profile of scalp: 18% (0.18) of scalp surface area
Posterior aspect of scalp: 24% (0.24) of scalp surface area.
Percentage of hair loss in each area is the percentage hair loss multiplied by percentage surface area of the scalp in that area. Summing the products of each area will give the SALT score.[12]
The patients were divided into five groups according to disease severity:
S0, no hair loss; S1,< 25% hair loss; S2, 26%–50% hair loss; S3, 51%–75% hair loss; S4, 76%–99% hair loss; and S5, total scalp hair loss.
Pull test
Pull test is a simple test to evaluating hair disease activity. It is applied by a gentle traction on a group of hairs (approximately 40–60 hairs) from proximal to distal end, in three different areas of the scalp. The grasp is made with help of thumb, index finger, and middle finger. Normally less than three hairs come out with each pull from each area, and if six or more hairs come out with every pull, the pull test is considered positive.[13,14]
Dermoscopic examination
Dermoscopic examination of the hair loss areas was performed using a DermLite® II Multispectral (3 Gen, San Juan Capistrano, CA, USA), which contains polarizing filters.
Dermoscopic features of AA include:
Yellow dots: Round or polycyclic yellow to yellow-pink dots that represent distended follicular infundibula filled with sebum and keratin remnants
Black dots: Remnant of broken hair shafts inside follicular ostia
Exclamation mark hairs: Broken hairs that tapered toward follicles
Short vellus hairs: Thin, nonpigmented hairs with length ≤10 mm may demonstrate early disease remission
Broken hairs: Due to fracture of dystrophic hair shafts or rapid regrowth of hairs that formerly manifested as black dots.[15]
Statistical analysis
Descriptive data have been demonstrated by frequency and percentage. Correlations between the incidence of each dermoscopic finding and the severity of disease or disease activity were analyzed using the Spearman's rank-order correlation coefficient by rank test. The difference in the frequency of the specific dermoscopic features among the AA subtypes was examined by Chi-square test and Fisher's exact probability test. Statistical analysis was done by SPSS version 22 (Released 2013. IBM SPSS Statistics for Windows, Armonk, NY: IBM Corp). Statistically significance was set at P < 0.05.
RESULTS
Our study included 126 patients with AA. The age varied from 4 to 61 years, with a median age of 26.32 ± 11.92 years. Demographic, baseline, clinical, and dermoscopic characteristics are shown in Table 1.
Table 1.
Demographic, baseline, and clinical characteristics of study population
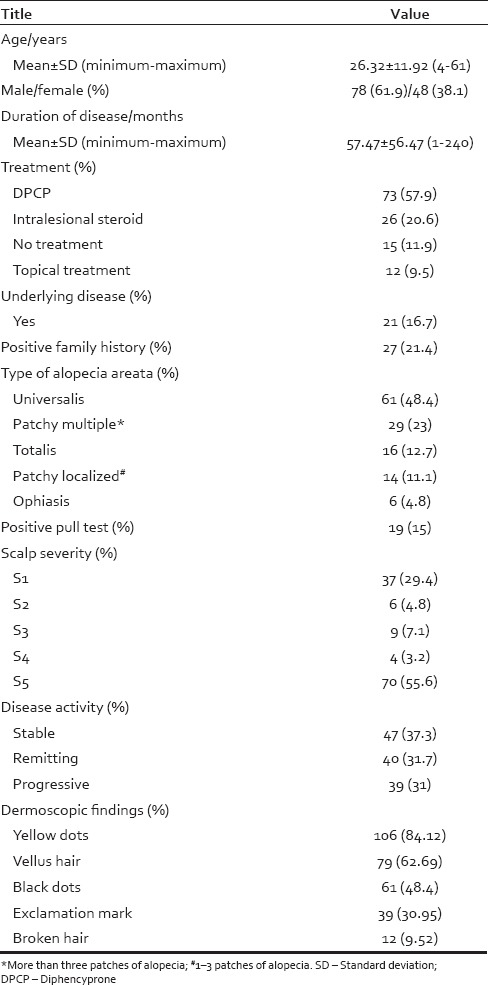
Regarding underlying diseases, 8 (6%) patients had hypothyroidism, 3 (2%) patients had psychiatric problems, 2 (1.5%) patients had diabetes mellitus, 2 (1.5%) patients had herpes, 2 (1.5%) patients had rheumatoid arthritis, 2 (1.5%) patients had gout, and 2 (1.5%) patients had mitral valve prolapse.
Nail involvement was present in 56 (44.4%) patients, and the most common presentations were pitting (n = 42 [33.3%]), ridging (n = 6 [4.8%]), and koilonychias (n = 6 [4.8%]).
Dermoscopic findings were yellow dots (n = 106 [84.13%]), vellus hair (n = 79 [62.7%]), black dots (n = 61 [48.4%]), exclamation mark (n = 39 [30.95%]), and broken hair (n = 12 [9.52%]).
The correlation between dermoscopic findings and different treatments of AA is shown in Table 2.
Table 2.
Dermoscopic features seen in patients with each type of alopecia areata treatment

As shown in Table 2, the most common dermoscopic findings in patients treated with DPCP were vellus hairs and yellow dots; the latter differed significantly among treatment groups. The distributions of other dermoscopic findings in treatment groups were not significant.
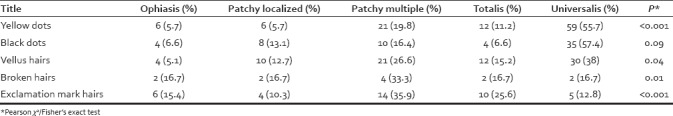
As shown in Table 3, there was a significant association between AA types and dermoscopic findings. Yellow dots and vellus hairs were the most common in patients with universalis subtype. However, broken hairs and exclamation mark hairs were mostly observed in patchy multiple subtypes.
Table 3.
Dermoscopic features seen in patients with each type of alopecia areata
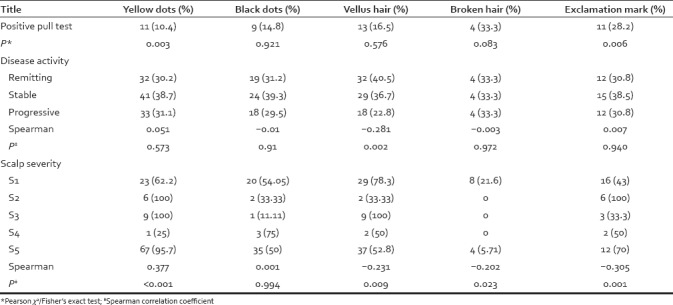
Disease activity, scalp severity, body severity, and pull test had significant difference in based on dermoscopic findings [Table 4]. Yellow dot and exclamation mark were significantly more common in positive pull test group. Furthermore, vellus hairs were more common in patients with remitting disease pattern. With regard to scalp severity, yellow dots related positively, while vellus hairs, broken hairs, and exclamation mark hairs related negatively with scalp severity.
Table 4.
Dermoscopic features seen in patients with alopecia areata based on disease activity, scalp severity, and pull test results
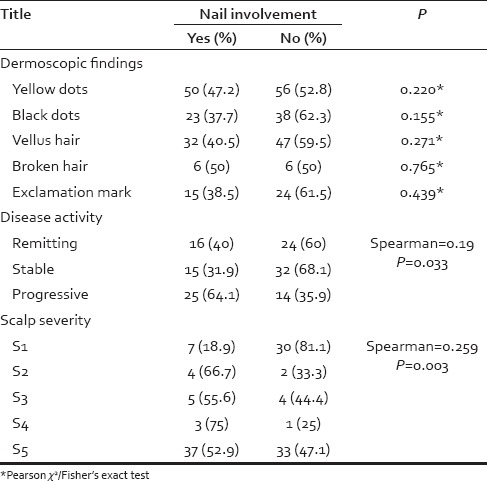
The association of nail involvement and dermoscopic findings, disease activity, and scalp severity was analyzed [Table 5]. There was not any significant association between dermoscopic findings and nail involvement. We found a positive correlation between nail involvement and disease activity or scalp severity.
Table 5.
Nail involvement association with dermoscopic findings, disease activity, and scalp severity
DISCUSSION
AA is an autoimmune hair loss which frequently starts in childhood.[2,3] Its presentation had an extreme variability not only in the time of initial onset but also in the duration, extent, and pattern of hair loss during any given episode of active loss.[1,16,17] Moreover, the course of disease is unpredictable, with spontaneous regrowth of hair occurring in 80% of patients within the 1st year, and sudden relapse at any given time.[18,19] Due to the clinical variability and unpredictable nature of spontaneous regrowth, diagnosis and management may be difficult and challenging.
Nowadays, dermoscopy is used increasingly for the evaluation of hair loss either scarring or nonscarring. Dermoscopic findings of different forms of hair loss help clinicians in their routine practice and obviate unnecessary biopsies.[7,11]
In this study, we evaluated trichoscopic findings in 126 patients with AA and correlated clinical and dermoscopic findings.
There was a male predominance in our study, and 61.9% of patients were male.
The mean age of participants was 26.32 years which is comparable with Rudnicka et al.'s study,[8] Karain's study (25 years), and also in Karadağ Köse and Güleç's study (25.15 years).[20]
The most common disease pattern in our study was universalis (48.4%), followed by patchy multiple (23%), totalis (12.7%), patchy localized (11.1%), and ophiasis (4.8%). In Bapu et al.'s study,[21] patchy localized (65.5%), patchy multiple (22.41%), and ophiasis (4.31%) types were most common. In Inui et al.'s study,[22] patchy multiple (38.3%), diffuse (19%), and totalis (17%) comprised the majority of AA subtypes. Our patients had more severe presentations compared with other previous studies; many of our patients were selected from the DPCP clinic leading to higher frequency of universalis and severe subtypes.
The proportion of different patterns of disease activity was almost equal, 37.3% were stable, 31.7% have remitting, and 31% have progressive disease. However, in Bapu et al.'s study,[21] 88.8% patients had progressive disease versus 11.2% who were nonprogressive.
Most of our patients had S5 (53.3%) and S1 (31.7%) grade of scalp severity, whereas in Bapu et al.'s study,[21] S1 (72.4%), S2 (10.3%), and S4 (10.3%) were the most common. In other words, the scalp involvement was more severe in our study population.
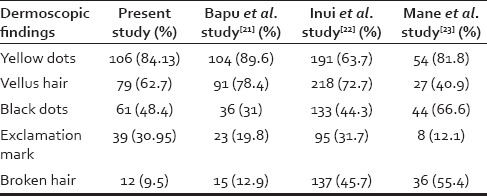
The most common dermoscopic finding based on our results was yellow dots (82.5%), and the least one was broken hair (9.5%). Similarly, in Bapu et al.'s study,[21] the most common finding was yellow dots (89.6%) and the most uncommon one was broken hair (12.9%). However, in Inui et al.'s study,[22] vellus hair (72.7%) was the most common finding, and the exclamation mark (31.7%) has the lowest prevalence [Table 6]. According to previous studies and our results, the most common finding is yellow dots, except in Inui et al.'s study,[22] in which probably due to yellow skin color of patients, yellow dots were difficult to see.
Table 6.
Comparison of dermoscopic findings in several studies
With regard to scalp severity, yellow dots related positively; however, vellus hairs, broken hairs, and exclamation mark hairs related negatively [Table 4].
Although exclamation mark seems to be a prevalent finding in acute phase of AA at the first glance, a probable explanation for the observed negative relationship between scalp severity and exclamation marks might be that many of our cases were of severe universalis subtype and were totally bald, so there was no hair available in the scalp to detect exclamation mark finding.
In Bapu et al.'s study,[21] yellow dots had also significant association with scalp severity. In Inui et al.'s study,[22] there was a positive correlation between black dots and yellow dots with scalp severity but a negative correlation between vellus hair and scalp severity.
In view of disease activity, there was just a negative correlation between vellus hair and disease activity [Table 4]. Since vellus hairs could be indicative of hair regrowth in early disease remission, this finding is somehow justified. In Bapu et al.'s study,[21] yellow dots prevalence did not have any association with disease activity. According to Inui et al.,[22] there was a positive and significant correlation between disease activity and black dots, exclamation mark, and broken hair findings. There was also negative correlation between disease activity and vellus hair findings.
As shown in Table 4, yellow dots and exclamation mark were more common in patients who had positive pull test on examination, which seems utterly logical.
Another remarkable finding in our study was the definition of different dermoscopic findings in different AA subtypes [Table 3]. Yellow dots and vellus hair findings were mostly common in patients with universalis subtype. Because yellow dots are related with scalp severity, this significant association between yellow dots and universalis type is logical. On the other hand, since the presence of vellus hairs is usually indicative of hair regrowth, the association between vellus hair and alopecia universalis is somewhat controversial at first glance. However, considering the significant correlation between vellus hair and treated cases of alopecia universalis, one can conclude that this significant correlation between vellus hair and universalis type could be due to treatment implementation. Besides, broken hair and exclamation mark were mostly observed in patchy multiple patients. According to previous similar studies, in Bapu et al.'s study,[21] yellow dots were most common in universalis and ophiasis subtypes and least common in patchy alopecia. In Mane et al.'s study,[23] there was not any association between dermoscopic findings and type of AA, similar in Inui et al.'s study.[22]
Regarding the distribution of dermoscopic findings in treatment groups [Table 2], yellow dots were significantly more common in patients who received DPCP. Simultaneously, yellow dots were also correlated with the severity of scalp alopecia [Table 4]. The high prevalence of yellow dots in DPCP users is somehow logical. First, DPCP is specifically used in severe cases of AA. Second, DPCP is a contact sensitizer that causes inflammation and scaling in the treated area, and yellow dots are dilated infundibular orifices plugged with degenerated follicular keratinocyte. Hence, the accumulation of scales in the follicular orifice might result in yellow dots formation. Our results also confirmed that there was a positive correlation between disease activity or scalp severity, and nail involvement in AA [Table 5].
CONCLUSIONS
Dermoscopic findings can be a useful in differentiating various stages of activity and severity of AA. Dermoscopy may also be a valuable tool for the dermatologist for the diagnosis, follow-up, and evaluation of response to treatment in AA. Hence, it is recommended to use dermoscopy in the routine examination of AA patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Spano F, Donovan JC. Alopecia areata: Part 1: Pathogenesis, diagnosis, and prognosis. Can Fam Physician. 2015;61:751–5. [PMC free article] [PubMed] [Google Scholar]
- 2.D'Ovidio R. Alopecia areata: News on diagnosis, pathogenesis and treatment. G Ital Dermatol Venereol. 2014;149:25–45. [PubMed] [Google Scholar]
- 3.Bertolino AP. Alopecia areata. A clinical overview. Postgrad Med. 2000;107:81. doi: 10.3810/pgm.2000.06.1111. [DOI] [PubMed] [Google Scholar]
- 4.Russo T, Piccolo V, Lallas A, Argenziano G. Recent advances in dermoscopy. F1000Res. 2016;5:F1000. doi: 10.12688/f1000research.7597.1. Faculty Rev-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Errichetti E, Stinco G. The practical usefulness of dermoscopy in general dermatology. G Ital Dermatol Venereol. 2015;150:533–46. [PubMed] [Google Scholar]
- 6.Lacarrubba F, Micali G, Tosti A. Scalp dermoscopy or trichoscopy. Curr Probl Dermatol. 2015;47:21–32. doi: 10.1159/000369402. [DOI] [PubMed] [Google Scholar]
- 7.Jain N, Doshi B, Khopkar U. Trichoscopy in alopecias: Diagnosis simplified. Int J Trichology. 2013;5:170–8. doi: 10.4103/0974-7753.130385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rudnicka L, Olszewska M, Rakowska A, Slowinska M. Trichoscopy update 2011. J Dermatol Case Rep. 2011;5:82–8. doi: 10.3315/jdcr.2011.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torres F, Tosti A. Trichoscopy: An update. G Ital Dermatol Venereol. 2014;149:83–91. [PubMed] [Google Scholar]
- 10.Kowalska-Oledzka E, Slowinska M, Rakowska A. Sensitivity and specificity of the trichoscopy. Indian J Dermatol Venereol Leprol. 2012;78:636–7. doi: 10.4103/0378-6323.100591. [DOI] [PubMed] [Google Scholar]
- 11.Inui S. Trichoscopy for common hair loss diseases: Algorithmic method for diagnosis. J Dermatol. 2011;38:71–5. doi: 10.1111/j.1346-8138.2010.01119.x. [DOI] [PubMed] [Google Scholar]
- 12.Olsen EA, Hordinsky MK, Price VH, Roberts JL, Shapiro J, Canfield D, et al. Alopecia areata investigational assessment guidelines – Part II. National Alopecia Areata Foundation. J Am Acad Dermatol. 2004;51:440–7. doi: 10.1016/j.jaad.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 13.Gordon KA, Tosti A. Alopecia: Evaluation and treatment. Clin Cosmet Investig Dermatol. 2011;4:101–6. doi: 10.2147/CCID.S10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hillmann K, Blume-Peytavi U. Diagnosis of hair disorders. Semin Cutan Med Surg. 2009;28:33–8. doi: 10.1016/j.sder.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Miteva M, Tosti A. Hair and scalp dermatoscopy. J Am Acad Dermatol. 2012;67:1040–8. doi: 10.1016/j.jaad.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Mounsey AL, Reed SW. Diagnosing and treating hair loss. Am Fam Physician. 2009;80:356–62. [PubMed] [Google Scholar]
- 17.Finner AM. Alopecia areata: Clinical presentation, diagnosis, and unusual cases. Dermatol Ther. 2011;24:348–54. doi: 10.1111/j.1529-8019.2011.01413.x. [DOI] [PubMed] [Google Scholar]
- 18.Ito T. Recent advances in the pathogenesis of autoimmune hair loss disease alopecia areata. Clin Dev Immunol 2013. 2013:348546. doi: 10.1155/2013/348546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lew BL, Shin MK, Sim WY. Acute diffuse and total alopecia: A new subtype of alopecia areata with a favorable prognosis. J Am Acad Dermatol. 2009;60:85–93. doi: 10.1016/j.jaad.2008.08.045. [DOI] [PubMed] [Google Scholar]
- 20.Karadağ Köse Ö, Güleç AT. Clinical evaluation of alopecias using a handheld dermatoscope. J Am Acad Dermatol. 2012;67:206–14. doi: 10.1016/j.jaad.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 21.Bapu NG, Chandrashekar L, Munisamy M, Thappa DM, Mohanan S. Dermoscopic findings of alopecia areata in dark skinned individuals: An analysis of 116 cases. Int J Trichology. 2014;6:156–9. doi: 10.4103/0974-7753.142853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inui S, Nakajima T, Nakagawa K, Itami S. Clinical significance of dermoscopy in alopecia areata: Analysis of 300 cases. Int J Dermatol. 2008;47:688–93. doi: 10.1111/j.1365-4632.2008.03692.x. [DOI] [PubMed] [Google Scholar]
- 23.Mane M, Nath AK, Thappa DM. Utility of dermoscopy in alopecia areata. Indian J Dermatol. 2011;56:407–11. doi: 10.4103/0019-5154.84768. [DOI] [PMC free article] [PubMed] [Google Scholar]


