Abstract
Background and Aim:
Interaction of hair with water is common. This study was conducted to compare changes in baseline strength of hair after treating it with hard water and deionized water.
Material and Methods:
Hardness level of water samples collected from 10 districts of KP, Pakistan was determined, and that with maximum hardness was considered our sample hard water. Hair samples of 70 male individuals, from district with minimum hardness levels, were collected. Each hair sample was divided into three equal parts, and three groups of hair were established, each group containing 70 hairs. Group A was considered control. Group B was treated with deionized water and Group C was treated with hard water. Tensile strength of all three groups was measured using the universal testing machine and compared using paired t-test.
Results:
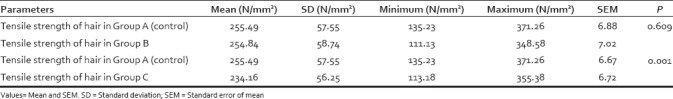
The mean age of all 70 participants were 23.87 ± 3. The mean values of tensile strength for hairs of Groups A, B, and C were 255.49, 254.84, and 234.16 with a standard deviation of 57.55, 58.74, and 56.25, respectively. Results were significant in case of hard water (P = 0.001) as compared to deionized water (P = 0.609).
Conclusion:
Hard water decreases strength of hair and thus increases breakage.
Key words: Calcium carbonate, hair, hard water, magnesium sulfate, tensile strength
INTRODUCTION
Hair are valued as a most important part of human body as they portray an individual's personality and confidence. Therefore, a great care is taken in handling hair to prevent hair problems.
However, hair problems are usually observed in all communities and commonly hair loss (16%–96%) and breakage.[1] It is a well-established fact that there are underlying genetic causes of hair loss,[2] but hair breakage, on the other hand, is mostly thought to be due to the use of hard water.[3,4]
Hardness represents salts of calcium carbonate and magnesium sulfate in water that results in temporary and permanent hardness, respectively, and is expressed as equivalent of calcium carbonate.[5] Although hard water is thought to have negative effect on hair, it is found to have positive effects on health as it serves as a fine source of magnesium and calcium.[6,7] The United States Geological Survey (USGS) classifies water into four main types based on its water hardness contents [Table 1].[8]
Table 1.
Hard water classification[8]

The main constituent of hair is a very stable protein, which is rich in cysteine and is resistant to proteolytic enzyme activity, known as keratin.[9] The stability is due to the existence of different kind of bonds, i.e., covalent bonds, ionic bonds, and hydrogen bonds, which may also act as a site for chemical process, thus making hair reactive as well.[10,11] A property of hair to swell up in a medium with pH >5.5, thus allowing entry of the metal ions from the medium into the hair structure and a pH below 5.5 stops this process.[12] A chemical reaction results in oxidation of the disulfide bonds in hair that further results in the creation of sulfonic acid and sulfonate and in turn causes the ionization (de-protonation) of the side chains of hair proteins forming a negatively charged resin that assist in dragging the cations from the solution in to the hair structure (e.g., as in hair dyeing).[11] Similarly hair interact with hard water and the cations i.e. magnesiumand calcium, present in hard water are absorbed by the negatively charged resin and results in oxidation of hair.[11,13] The more exposure to the cations may result in oxidative damage (similar to oxidative damage in hair dyeing), which is directly proportional to both levels of hardness and pH of the medium.[11,13]
In our previous study, we compared strengths of hair after treating it with deionized water and hard water and observed that strength of hair decreases when treated with hard water as compared to deionized water.[3] The question then raised was what if both deionized and hard water had changed the strength of the hair. To get a clear idea of what actually happened, we designed this study to first get the baseline strength of hair and then compare it with hair treated with hard water and deionized water.
MATERIALS AND METHODS
Two types of samples were used in this experimental study, i.e., hard water and hair. This study included a purposive, nonprobability sampling technique and randomized control trials. This study was approved by the institutional review board and advanced studies and research board (AS and RB), Khyber Medical University, Peshawar, Pakistan.
Hard water sample
From 10 main districts, of Khyber Pakhtunkhwa (KP), Pakistan, tap water samples were collected from 10 different places in each district. Three samples per station, one each day for consecutively 3 days, were collected leading to a total of 30 samples per districts. pH values of all the samples were calculated using pH meter, and their relative hardness was evaluated with help of ethylendiaminetetraacetate assay, according to the International Organization for Standardization standards,[14,15] in term of standard unit for hardness, i.e., mg/L of CaCO3. Average hardness was calculated from all the 30 samples per district [Table 2].
Table 2.
Average hardness of tap water samples from different districts of Khyber Pakhtunkhwa and their pH values
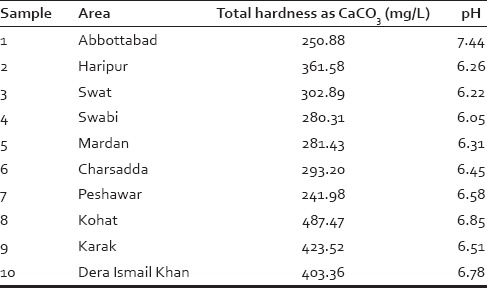
From Table 1, we concluded that average maximum water hardness is found in district Kohat and is considered as our sample hard water that will be used in the experiment. The purpose of finding maximum hardness was to establish the amount of maximum hardness; a hair can encounter in KP, Pakistan. Table 2 gives us complete profile of average hardness and pH values of water sample from district Kohat [Table 1].
Hair samples
Hair samples were collected from Peshawar, Pakistan, the area with minimum average hardness to see changes in its strength after being exposed to water with more hardness, i.e., water from Kohat with almost double the hardness as that in Peshawar [Table 1].
After a well-informed consent, 70 young and healthy male individuals, aged 20–30, and at least 24 cm long, straight hair with no history of cardiac disease,[16] skin infection (fungal),[17] no hair loss patterns, autoimmune disease (alopecia), chemotherapy, smoking [18] and diabetics,[19] etc., were selected. Hair achieves maximum diameter during age 20–30 years (thus has maximum strength at this age), and the contents of hair are mostly proteins, whereas straight hair has a uniform diameter.[12]
The minimum length was kept 24 cm as each hair had to be divided into three equal halves. Thus, each hair of the 70 samples was divided into three equal parts, and thus, three groups were made, namely, Group A, Group B, and Group C, with 70 hairs in each group. Each hair of Group B and Group C was tied to a glass rod [Figure 1], labeled for the sake of identification, and stored in a controlled temperature room at 22°C ± 3°C.
Figure 1.

Hair tied up to glass rods
Hairs in Group A were left as such to keep it as a control group for baseline strength of hair. Hairs of Group B were treated with deionized water whereas hairs of Group C were treated with hard water from district Kohat, for 10 min on alternate days and for 3 months. The experiment was designed to give hairs same exposure to hard water and deionized water as it gets during bathing, washing, etc. The whole experiment was performed at 22°C ± 3°C, and fresh samples of both deionized and hard water were provided.
Tensile strength of each hair, of all three groups, was then measured with the help of universal testing machine (UTM) (M500-1000KN, United Kingdom), available at the Centralized Resources Laboratory (CRL), Department of Physics, Peshawar University, Pakistan, and results were stored in a preformed pro forma [Figure 2].
Figure 2.

Universal testing machine (left), hair in clamps of universal testing machine (right)
Tensile strength is the measure of tensile stress applied to a material. In UTM, each hair was stretched at a speed of 1 mm/s.
Tensile strengths of hairs in Group A represented actual strength of hair where those of Group B and Group C represented tensile strengths of hairs after being treated with hard water and deionized water, respectively. To find whether strength of hair is altered because of the application of deionized water and hard water, first tensile strengths of hairs in Group A and Group B were compared, and then, tensile strengths of Group A and Group C were compared. This comparison for matched samples was done by Students' paired t-test using the Statistical Package for the Social Sciences (SPSS, Version 20, Inc., Chicago, IL, USA). P <0.05 was regarded to be statistically significant. Results were tabulated as means and ± standard error of mean.
RESULTS
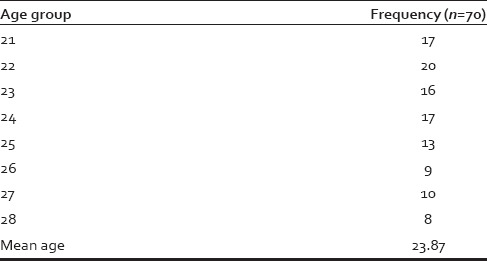
Seventy young and healthy male individuals, aged 20-30 years, were included in the study. Mean age and frequency of individuals with different age groups are shown in Table 3.
Table 3.
Age groups with frequency
USGS classifies water into four main types based on its water hardness contents [Table 1].[8]
Hardness level and pH values of water samples, collected from 10 different main districts of KP, Pakistan, were measured [Table 2]. The unit used was hardness as CaCO3. The chemical analysis of water from district Kohat is shown in Table 4.
Table 4.
Total hardness level of water sample collected from Kohat

Strength of hair was compared using paired t-test [Table 5]. First Group A and Group B were compared (P = 0.609), and then, Group A and Group C were compared (P = 0.001).
Table 5.
Mean tensile strength of Group A (control), compared with Group B, and Group C using Student's paired t-test (n=70)
DISCUSSION
The importance of hairs cannot be denied in an individual's life and extra care is taken in their handling to avoid any damage to them. The use of oils, conditioners, and shampoos is in routine practices. Interaction of hair with water is also very common, for example, during bathing and washing, etc., but the contents of water can have a negative effect on hair. This study was conducted to evaluate changes in baseline strength of hair after treating them with deionized water and hard water. It was observed that baseline strength of hair decreased significantly after treating it with hard water as compare to deionized water which is similar to the results in a previous study by Luqman et al.[3] in which hair was divided into two groups; one group was treated with deionized water and other was treated with hard water. In this recent study, hair was divided into three groups, and baseline tensile strength of hair (Group A) was compared with tensile strength of hairs (Group B) treated with deionized water and tensile strength of hair (Group C) treated with hard water (from district Kohat). A similar study was carried out by Srinivasan et al.[4] and Evans et al.[13] and revealed no significance of hard water on strength of hair. The reason perhaps is that Srinivasan collected hair samples from women, and in our study, hairs were taken only from male individuals, pointing toward the role of gender. Besides, the hardness level of hard water used in both Srinivasan (212.71 mg/L of CaCO3) and Evans (272 mg/L of CaCO3) study was almost half that of hard water used in our study (i.e., 486.7 mg/L of CaCO3. Furthermore, the hair samples in our study were exposed for a longer period of time, and the sample size was much larger than either study. Furthermore, the use of deionized water (which is more pure form of water) in our study rather than distilled water may have played a role in different results. There was no specific relation or pattern observed when age and strength of hair were compared.
Normally, we observe a frequent interaction between water and hair, but the damage is not that evident. It is because the process metal uptake by hair (oxidation) and thus hair damage (oxidative damage) is slowed down by the use of different chelants in form of shampoos, hair conditioners, etc. The chelants lead to the removal of metal ions from water by surrounding them [11] and thus decrease the chances of interaction between hair and those metal ions.
The addition of acids, for example, citric acid into shampoos and conditioners and the also the use of topical application of yogurt and lemon (containing lactic acid and ascorbic acids, respectively) in some areas helps decrease the action of metal ions on hair. The acidic environment (especially pH <5.5) has a number of implications that include desorption of metal ions, side chain reprotonation, and shrinking of hair scales; thus, prevents the entry of metal ions into the hair structure [11] and thus minimizing the hair damage.
In our study, we observed that a significant statistical decrease in strength of hair in men is observed after treating them with hard water. However, different results may be observed in females. The results may also vary with different levels of water hardness and different sample size. The mechanism, of how this happens, is much debatable and needs further comprehensive studies, especially on molecular levels.
CONCLUSIONS
Our study showed that there is a significant decrease in strength of hair when baseline strength of hair was compared with strength of hair treated with hard water as compared to strength of hair with deionized water. This also gives us an idea that the use of hard water may result in an increase in hair breakage as well.
Financial support and sponsorship
The study was partially supported by Khyber Medical University, Peshawar, Pakistan.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
All authors present immense gratitude to Khyber Medical University, Institute of Basic Medical Sciences, Peshawar, Pakistan, CRL, Physics Department, University of Peshawar, Pakistan, and Pakistan Council of Scientific and Industrial Research Laboratories, Peshawar, Pakistan, and its staff for their help and support in carrying out different experimental procedures involved in our study.
REFERENCES
- 1.Ellis JA, Sinclair RD. Male pattern baldness: Current treatments, future prospects. Drug Discov Today. 2008;13:791–7. doi: 10.1016/j.drudis.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Kiso M, Tanaka S, Saba R, Matsuda S, Shimizu A, Ohyama M, et al. The disruption of sox21-mediated hair shaft cuticle differentiation causes cyclic alopecia in mice. Proc Natl Acad Sci U S A. 2009;106:9292–7. doi: 10.1073/pnas.0808324106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luqman MW, Ali R, Khan Z, Ramzan MH, Hanan F, Javaid U, et al. Effect of topical application of hard water in weakening of hair in men. J Pak Med Assoc. 2016;66:1132–6. [PubMed] [Google Scholar]
- 4.Srinivasan G, Srinivas CR, Mathew AC, Duraiswami D. Effects of hard water on hair. Int J Trichology. 2013;5:137–9. doi: 10.4103/0974-7753.125609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Calcium and Magnesium in Drinking-Water: Public Health Significance. Geneva, Switzerland: World Health Organization; 2009. [Google Scholar]
- 6.Momeni M, Gharedaghi Z, Amin MM, Poursafa P, Mansourian M. Does water hardness have preventive effect on cardiovascular disease? Int J Prev Med. 2014;5:159–63. [PMC free article] [PubMed] [Google Scholar]
- 7.Ghanaat M. Types of hair loss and treatment options, including the novel low-level light therapy and its proposed mechanism. South Med J. 2010;103:917–21. doi: 10.1097/SMJ.0b013e3181ebcf71. [DOI] [PubMed] [Google Scholar]
- 8.Briggs JC, Ficke JF. Quality of Rivers of the United States, 1975 Water Year; Based on the National Stream Quality Accounting Network (NASQAN) USA: 1977. [Google Scholar]
- 9.Tobin DJ. Hair in Toxicology: An Important Bio-Monitor. London, UK: Royal Society of Chemistry; 2005. [Google Scholar]
- 10.Quadflieg JM. Fundamental Properties of Afro-American Hair as Related to Their Straightening/Relaxing Behaviour: Bibliothek der RWTH Aachen. 2003 [Google Scholar]
- 11.Evans AO, Marsh JM, Wickett RR. The uptake of water hardness metals by human hair. J Cosmet Sci. 2011;62:383–91. [PubMed] [Google Scholar]
- 12.Robbins CR. Chemical and Physical Behavior of Human Hair. New York, USA: Springer; 2012. [Google Scholar]
- 13.Evans AO. Investigation of the Interaction Between Water Hardness Metals and Human Hair. Cincinnati, Ohio, USA: University of Cincinnati; 2011. [Google Scholar]
- 14.Flaschka HA. EDTA Titrations: An Introduction to Theory and Practice. Atlanta, Georgia: Elsevier; 2013. [Google Scholar]
- 15.World Health Organization. Determination of Hardness of Water. Geneva, Switzerland: World Health Organization; 1999. [Last accessed on 2016 Dec 10]. Available from: http://www.who.int/whopes/quality/en/MethodM26.pdf . [Google Scholar]
- 16.Yamada T, Hara K, Umematsu H, Kadowaki T. Male pattern baldness and its association with coronary heart disease: A meta-analysis. BMJ Open. 2013;3:e002537. doi: 10.1136/bmjopen-2012-002537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pappas PG, Kauffman CA, Perfect J, Johnson PC, McKinsey DS, Bamberger DM, et al. Alopecia associated with fluconazole therapy. Ann Intern Med. 1995;123:354–7. doi: 10.7326/0003-4819-123-5-199509010-00006. [DOI] [PubMed] [Google Scholar]
- 18.Su LH, Chen TH. Association of androgenetic alopecia with smoking and its prevalence among asian men: A community-based survey. Arch Dermatol. 2007;143:1401–6. doi: 10.1001/archderm.143.11.1401. [DOI] [PubMed] [Google Scholar]
- 19.Iwasaki H. Vitiligo and alopecia areata as early signs preceding type 1 diabetes mellitus. Gen Med. 2015;16:47–9. [Google Scholar]


