Abstract
Introduction:
Minoxidil has been used topically to stimulate hair growth for male androgenetic alopecia (AGA) for more than 3 decades. It is currently being used for female AGA and alopecia areata (AA) as well. Although much time has passed since its first use, our understanding of its mechanism of action is highly limited. Therefore, we examined the inflammatory properties of AGA and AA, two entities in which minoxidil is being used as a therapeutic agent. We investigated the in vitro expression levels of cytokine interleukin-1 alpha (IL-1α), a potent inhibitor of hair growth, in minoxidil-treated human keratinocyte (HaCaT) cells to determine whether this molecule exerts anti-inflammatory effects.
Materials and Methods:
Cellular proliferation was examined using the Cell Proliferation Kit II (XTT) reagent. After determining a noncytotoxic concentration, HaCaT cells were treated with minoxidil. RNA was isolated from both untreated and treated cells with TRI Reagent®. Expression of the IL-1α gene was determined by reverse transcription quantitative polymerase chain reaction analysis and is reported relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH), which served as a control.
Results:
Results are presented as IL-1α/GAPDH fold change. Minoxidil treatment downregulated IL-1α expression by 0.3433-fold compared with untreated cells (P = 0.001).
Conclusion:
This anti-inflammatory effect of minoxidil, as evidenced by significant downregulation of IL-1α gene expression in HaCaT cells, may represent one of its mechanisms of action in alopecia.
Key words: Androgenetic alopecia, HaCaT cells, interleukin-1 alpha, minoxidil
INTRODUCTION
Oral minoxidil has been used to treat hypertension since the 1960s. Hypertrichosis as a consequence of minoxidil treatment was observed shortly thereafter. These observations led to the development of topical minoxidil as a treatment for hair loss. Although it was approved by the Food and Drug Administration for the treatment of male androgenetic alopecia (AGA) in 1984,[1] our understanding of its mechanism of action on the hair follicle remains highly limited.[2]
Orally administered minoxidil lowers blood pressure by relaxing vascular smooth muscles through the action of its sulfated metabolite, as an opener of sarcolemmal adenosine triphosphate-sensitive potassium channels (KATP); it is postulated that the stimulatory effect of topical minoxidil on hair growth is due to the opening of KATP by minoxidil sulfate.[2,3,4,5] One hypothesis of the mechanism of action of minoxidil concerns its vasodilatory properties. Cutaneous blood flow increases 10–15 min after application of topical minoxidil.[6] Several in vitro effects of minoxidil have been described in monocultures of various skin and hair follicle cell types, including increased cell proliferation, decreased senescence of keratinocytes, inhibition of collagen synthesis, stimulation of vascular endothelial growth factor (VEGF), and prostaglandin synthesis.[2,7,8,9,10] Some or all of these effects may be relevant to hair growth, but application of cell culture studies to the complex biology of the hair follicle is uncertain.[2] It is also known that human follicles respond biologically to KATP channel regulators in culture and proteins for two KATP channels, sulfonylurea receptors (SUR) 1 and SUR2B, whereas minoxidil only stimulates SUR2 channels. Thus, Shorter et al. indicated that novel drugs designed specifically for these channels could treat hair disorders.[11]
Since the mechanism of action of minoxidil remains unclear, we examined a common feature observed in the pathogenesis of diseases, in which minoxidil is used for treatment. The two most widely used indications of topical minoxidil are AGA and alopecia areata (AA). The inflammatory process is an integral part of the pathogenesis of AA, which is already described as an inflammatory disease.[12,13,14,15,16,17] However, polygenic heredity and testosterone are assumed to be the main causes in AG, inflammation plays an important role. Lymphocytic microfolliculitis targeting the bulge epithelium along with deposits of epithelial basement membrane zone immunoreactants were found as frequent findings in AGA.[18,19,20,21,22] It is reported a sustained microscopic follicular inflammation with connective tissue remodeling in AGA.[19] Follicular microinflammation in early cases resulted in marked perifollicular fibrosis over time.[21] Furthermore, a significant association was depicted between early-onset AGA and inflammation which was detected by the measurement of high-sensitivitiy C-reactive protein in blood.[20] Thus, immunological changes have been suggested as a triggering factor in AGA.[18,19,20,21,22] Among the various cytokines related to inflammation in alopecia, interleukin-1 alpha (IL-1α) has a direct growth inhibitory effect on hair follicles and plays an active role in both AGA and AA.[13,14,16,18]
Due to its active role in diseases that are treated with topical minoxidil, we investigated the in vitro expression levels of cytokine IL-1α in minoxidil-treated human keratinocyte (HaCaT) cells to determine whether this molecule exerts anti-inflammatory effects.
MATERIALS AND METHODS
HaCaT-cells
Hair follicles consist of mesenchymal and ectodermal components. The ectodermal part is formed by an invagination of epidermis into the dermis. The ectodermal hair bulb at the bottom of follicle contains the hair matrix which produces the hair shaft. The mesenchymal component is dermal papilla, a small collection of specialized fibroblasts that is totally surrounded by ectodermal structures of the bulb. Although the mesenchyme-derived papilla regulates the epithelial follicle in many aspects, intrinsic dermal-epidermal interactions are central to the development and growth of hair. Besides the inductive powers of dermal papilla cells, germinative epidermal cells of the lower follicle also can stimulate hair growth.[23,24] HaCaT cells are the spontaneously immortalized human keratinocyte cell line that has been used for studies of the epidermal homeostasis and its pathophysiology.[25] HaCaT cell line has a high differentiation potential in cell culture based on the expression of various epidermal differentiation markers. Cell doubling time is approximately 24 h that provides to calculate dilutions for splitting cells.[26] Here, we studied with HaCaT cells both because the ectodermal keratinocytes are one of the functional components of the follicle structure and are also the major producers of IL-1α.[9,22,25,26]
Cell culture
HaCaT cells were cultured in Dulbecco's modified eagle's medium with high glucose, supplemented with 10% heat-inactivated fetal bovine serum and 100 U/mL gentamicin. Cells were maintained at 37°C in a humidified atmosphere at 5% CO2 in a New Brunswick™ (Innova®) incubator. All supplements and media were purchased from Sigma-Aldrich.
Preparation of the minoxidil solution
Minoxidil (522.5 mg) was dissolved in a 25-mL distilled water/25-mL ethanol mixture to obtain a 5 mM minoxidil solution. This solution was used as 100%; other concentrations (0.2, 1, 3, 5, and 10%) of the solution were prepared by dilution with distilled water. We chose the concentration of drug at the value which is nearest to 80% cell proliferation ratio. In general, to take this ratio higher, causes us to study with a very low concentration which may be inappropriate for the experiment. Hence, we used 1% concentration of minoxidil in this study.
Cell proliferation assay
HaCaT cells were seeded onto 96-well plates (1 × 104 cells/well) and treated with the indicated concentrations of minoxidil solution to assess cell proliferation. XTT and activator reagents (Roche) were added to the plates after a 72-h incubation period, according to the manufacturer's instructions. Cells were then incubated at 37°C for 4 h to ensure that the XTT reagent was reduced to the formazan compound. The optical density of the soluble formazan compound was measured at 495 nm on a microplate reader (Bio-Rad Laboratories).
RNA isolation and reverse transcription
Total RNA was extracted from cells treated with minoxidil solution and from untreated cells with TRI Reagent® according to the manufacturer's instructions (Sigma-Aldrich). The concentration and purity of the isolated RNA samples were determined by measuring the optical density at 260 nm and 280 nm on a BioSpec-nano UV-vis spectrophotometer [Shimadzu®]. The Transcriptor First-strand cDNA Synthesis Kit (Roche) was used for reverse transcription (RT). cDNA synthesis was performed with 500 ng total RNA, 2 μM of each gene-specific primer for IL-1α and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Integrated DNA Technologies), 10 U Transcriptor Reverse Transcriptase, 20 U Protector RNase Inhibitor, 1 mM each deoxynucleotide triphosphates, and 5× Transcriptor RT Buffer, according to the manufacturer's instructions (Roche).
Real-time quantitative polymerase chain reaction
RT-quantitative polymerase chain reaction (qPCR) was carried out in a LightCycler® 96 Real-Time PCR System (Roche). The amplification of products was examined through intercalation of the fluorescent dye SYBR® Green (FastStart DNA Green Master Kit; Roche). The reaction mixture contained 10 μL 2× SYBR® Green Master Mix, 0.5 μM of each reverse and forward primer, 2.5 ng cDNA, and the appropriate amount of nuclease-free water to bring the final volume to 20 μL. All samples were run in triplicate. A nontemplate control and 4 standards (1:1, 1:10, 1:100, and 1:1,000) were also included. The PCR conditions were determined separately for each target gene according to the melting and annealing temperatures of the primers. Each parameter included a preincubation step for 10 min at 95°C followed by 45 cycles of 3 amplification and melting steps. A melting curve analysis was performed to verify primer specificity. For the quantitation of RT-qPCR results, the ΔΔCt method was employed (2−ΔΔCt).
Statistical analysis
All data are representative of three replicates (n = 3) and are expressed as the mean value. Statistical analysis was performed by an unpaired t-test using GraphPad Prism® 5 Software (GraphPad Software, Inc. San Diego, CA, USA). The P < 0.05 was considered statistically significant.
RESULTS
Cytotoxicity analysis (cell proliferation assay)
The cytotoxicity of minoxidil (at the indicated concentrations) was determined based on the cell proliferation ratio of minoxidil-treated cells to untreated cells; higher concentrations were toxic to HaCaT cells. The highest concentration that was not toxic was 1%. HaCaT cells were incubated with 1% minoxidil solution before total RNA isolation [Figure 1].
Figure 1.
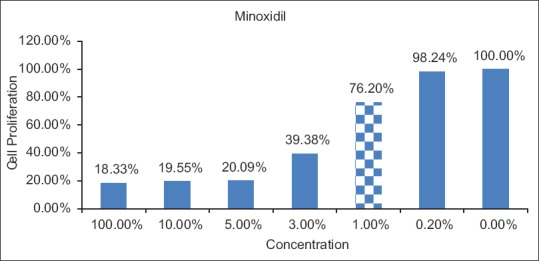
Cytotoxicity analysis of minoxidil treatment. The dashed bar represents the nontoxic concentration chosen for subsequent experiments
Gene expression analysis (real-time qPCR)
Results are presented as the target gene/GAPDH fold change. The gene expression analysis through RT-qPCR revealed that minoxidil treatment caused a significant downregulation of IL-1α expression (P = 0.001) compared with untreated cells. Minoxidil treatment downregulated IL-1α expression by 0.3433-fold compared with untreated cells [Figure 2].
Figure 2.
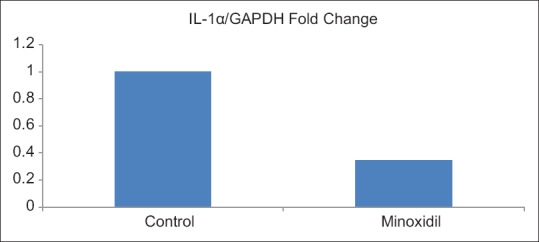
Gene expression levels of interleukin-1α after minoxidil treatment relative to control (untreated) cells (P=0.001). All data are representative of three replicates
DISCUSSION
The pilosebaceous unit is the site of numerous cell and tissue interactions. It involves epithelial cells, the sebaceous gland, fibroblasts, dermal papilla, melanocytes, endothelial cells, and Langerhans cells of the immune system. The histology and structure of the follicle is well-known. The development of in vitro and in vivo models has provided insights on the role of growth factors, such as VEGF, insulin-like growth factor-1, and transforming growth factor alpha, as well as androgens, such as dihydrotestosterone and cytokines, such as IL-1. However, we are still far from understanding the diverse signals resulting from the harmonious development of the hair follicle and the hair cycle.[9,27]
The two clinical entities AGA and AA both involve hair follicles, have a genetic predisposition, and consist of inflammatory mechanisms in their pathogenesis.[15,16,19,21,22,28] In AGA, scalp biopsies taken from both men and women have revealed follicular microinflammation and lymphocytic folliculitis targeting an immunologically driven trigger.[21,22] This sustained microscopic follicular inflammation associated with connective tissue remodeling, eventually resulting in permanent hair loss, is considered as a possible cofactor in the complex etiology of AGA.[19] AA is a T-cell-driven autoimmune disease of the hair follicle characterized by focal inflammatory lesions with perifollicular T-cell infiltrates.[16,17]
The normal human epidermis is a rich source of biologically active IL-1α. Keratinocytes both synthesize this cytokine and respond to it through cell surface receptors, suggesting that the IL-1 system may play an important role in epidermal physiology and inflammation.[29,30,31] IL-1α contributes to cell growth and repair functions in various epithelial cells and keratinocytes. During inflammation, injury, immunological challenge, or infection, it is overproduced and because of its multiple biological properties it contributes to disease.[32] Although IL-1α is prominent in skin wounding and inflammatory responses, it is downregulated during anagen phase of hair cycle.[33,34] Higher concentration of IL-1α exerts a rapid antiproliferative effect on hair follicles and is a potent inhibitor of hair follicle growth that causes inhibition as a secondary response.[12,13,18,30,35] The serum levels of IL-1α are significantly elevated in patients with the localized form of AA.[14] Despite data suggesting the inflammatory mechanism and the role of cytokines in both AGA and AA, the mechanism of action of minoxidil, an agent used for the treatment of both entities, on the inflammatory process is still unknown. Although the suppressive effects of minoxidil on lymphocyte-mediated immunologic phenomena have been reported,[36] the details and the role of cytokines remain obscure. Among the various cytokines in the IL-1 family, IL-1α is the main inhibitor of hair growth.[12,13,14,16,18]
In our study, minoxidil caused significant downregulation of IL-1α gene expression in HaCaT cells compared with untreated cells. Considering the role of the inflammatory process in both AGA and AA and the clinical efficacy of minoxidil in both entities, as well as the results of our study (i.e., the inhibition of IL-1α expression by minoxidil), this inhibitory effect may be one mechanism of action of minoxidil.
Minoxidil sulfate is the active form of minoxidil and sulfation is catalyzed by minoxidil sulfotransferase in a number of tissues including skin. Among the skin structures, minoxidil sulfotransferase activity is condensed in epithelial cells such as proliferating keratinocytes and outer root sheath of the follicle, rather than mesenchymal cells.[37,38] Some authors also propose that sulfotransferase activity in hair follicles predicts a patient's response to topical minoxidil therapy and even it is reported the clinical utility of a sulfotransferase test in ruling out a significant amount of nonresponders to topical minoxidil for the treatment of AGA.[39] Here, instead of minoxidil sulfate we preferred to study with minoxidil in order not to intervene the natural interaction between the molecule and the cell. Due to the inhibitory role of IL-1α on human hair growth, it was previously suggested that identifying the “inflammatory alopecic individual” may be one of clinical interest to determine the individuals for whom anti-IL-1 strategies could be therapeutically significant in AGA.[18] Considering the anti-IL-1α effect of minoxidil demonstrated in our study, this strategy may be another convenient method to exclude nonresponders to minoxidil therapy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Rathnayake D, Sinclair R. Male androgenetic alopecia. Expert Opin Pharmacother. 2010;11:1295–304. doi: 10.1517/14656561003752730. [DOI] [PubMed] [Google Scholar]
- 2.Messenger AG, Rundegren J. Minoxidil: Mechanisms of action on hair growth. Br J Dermatol. 2004;150:186–94. doi: 10.1111/j.1365-2133.2004.05785.x. [DOI] [PubMed] [Google Scholar]
- 3.Buhl AE, Waldon DJ, Conrad SJ, Mulholland MJ, Shull KL, Kubicek MF, et al. Potassium channel conductance: A mechanism affecting hair growth both in vitro and in vivo. J Invest Dermatol. 1992;98:315–9. doi: 10.1111/1523-1747.ep12499788. [DOI] [PubMed] [Google Scholar]
- 4.Buhl AE, Conrad SJ, Waldon DJ, Brunden MN. Potassium channel conductance as a control mechanism in hair follicles. J Invest Dermatol. 1993;101:148S–52S. doi: 10.1111/1523-1747.ep12363290. [DOI] [PubMed] [Google Scholar]
- 5.Davies GC, Thornton MJ, Jenner TJ, Chen YJ, Hansen JB, Carr RD, et al. Novel and established potassium channel openers stimulate hair growth in vitro: Implications for their modes of action in hair follicles. J Invest Dermatol. 2005;124:686–94. doi: 10.1111/j.0022-202X.2005.23643.x. [DOI] [PubMed] [Google Scholar]
- 6.Wester RC, Maibach HI, Guy RH, Novak E. Minoxidil stimulates cutaneous blood flow in human balding scalps: Pharmacodynamics measured by laser doppler velocimetry and photopulse plethysmography. J Invest Dermatol. 1984;82:515–7. doi: 10.1111/1523-1747.ep12261084. [DOI] [PubMed] [Google Scholar]
- 7.Baden HP, Kubilus J. Effect of minoxidil on cultured keratinocytes. J Invest Dermatol. 1983;81:558–60. doi: 10.1111/1523-1747.ep12523220. [DOI] [PubMed] [Google Scholar]
- 8.Cohen RL, Alves ME, Weiss VC, West DP, Chambers DA. Direct effects of minoxidil on epidermal cells in culture. J Invest Dermatol. 1984;82:90–3. doi: 10.1111/1523-1747.ep12259181. [DOI] [PubMed] [Google Scholar]
- 9.Lachgar S, Charveron M, Gall Y, Bonafe JL. Minoxidil upregulates the expression of vascular endothelial growth factor in human hair dermal papilla cells. Br J Dermatol. 1998;138:407–11. doi: 10.1046/j.1365-2133.1998.02115.x. [DOI] [PubMed] [Google Scholar]
- 10.Yoo HG, Chang IY, Pyo HK, Kang YJ, Lee SH, Kwon OS, et al. The additive effects of minoxidil and retinol on human hair growth in vitro. Biol Pharm Bull. 2007;30:21–6. doi: 10.1248/bpb.30.21. [DOI] [PubMed] [Google Scholar]
- 11.Shorter K, Farjo NP, Picksley SM, Randall VA. Human hair follicles contain two forms of ATP-sensitive potassium channels, only one of which is sensitive to minoxidil. FASEB J. 2008;22:1725–36. doi: 10.1096/fj.07-099424. [DOI] [PubMed] [Google Scholar]
- 12.Harmon CS, Nevins TD. IL-1 alpha inhibits human hair follicle growth and hair fiber production in whole-organ cultures. Lymphokine Cytokine Res. 1993;12:197–203. [PubMed] [Google Scholar]
- 13.Philpott MP, Sanders DA, Bowen J, Kealey T. Effects of interleukins, colony-stimulating factor and tumour necrosis factor on human hair follicle growth in vitro: A possible role for interleukin-1 and tumour necrosis factor-alpha in alopecia areata. Br J Dermatol. 1996;135:942–8. doi: 10.1046/j.1365-2133.1996.d01-1099.x. [DOI] [PubMed] [Google Scholar]
- 14.Teraki Y, Imanishi K, Shiohara T. Cytokines in alopecia areata: Contrasting cytokine profiles in localized form and extensive form (alopecia universalis) Acta Derm Venereol. 1996;76:421–3. doi: 10.2340/0001555576421423. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann R, Eicheler W, Huth A, Wenzel E, Happle R. Cytokines and growth factors influence hair growth in vitro. Possible implications for the pathogenesis and treatment of alopecia areata. Arch Dermatol Res. 1996;288:153–6. doi: 10.1007/BF02505825. [DOI] [PubMed] [Google Scholar]
- 16.Tazi-Ahnini R, McDonagh AJ, Cox A, Messenger AG, Britton JE, Ward SJ, et al. Association analysis of IL1A and IL1B variants in alopecia areata. Heredity (Edinb) 2001;87:215–9. doi: 10.1046/j.1365-2540.2001.00916.x. [DOI] [PubMed] [Google Scholar]
- 17.Garzorz N, Alsisi M, Todorova A, Atenhan A, Thomas J, Lauffer F, et al. Dissecting susceptibility from exogenous triggers: The model of alopecia areata and associated inflammatory skin diseases. J Eur Acad Dermatol Venereol. 2015;29:2429–35. doi: 10.1111/jdv.13325. [DOI] [PubMed] [Google Scholar]
- 18.Mahé YF, Buan B, Billoni N, Loussouarn G, Michelet JF, Gautier B, et al. Pro-inflammatory cytokine cascade in human plucked hair. Skin Pharmacol. 1996;9:366–75. doi: 10.1159/000211447. [DOI] [PubMed] [Google Scholar]
- 19.Trüeb RM. Molecular mechanisms of androgenetic alopecia. Exp Gerontol. 2002;37:981–90. doi: 10.1016/s0531-5565(02)00093-1. [DOI] [PubMed] [Google Scholar]
- 20.Hirsso P, Rajala U, Hiltunen L, Jokelainen J, Keinänen-Kiukaanniemi S, Näyhä S, et al. Obesity and low-grade inflammation among young Finnish men with early-onset alopecia. Dermatology. 2007;214:125–9. doi: 10.1159/000098570. [DOI] [PubMed] [Google Scholar]
- 21.El-Domyati M, Attia S, Saleh F, Abdel-Wahab H. Androgenetic alopecia in males: A histopathological and ultrastructural study. J Cosmet Dermatol. 2009;8:83–91. doi: 10.1111/j.1473-2165.2009.00439.x. [DOI] [PubMed] [Google Scholar]
- 22.Magro CM, Rossi A, Poe J, Manhas-Bhutani S, Sadick N. The role of inflammation and immunity in the pathogenesis of androgenetic alopecia. J Drugs Dermatol. 2011;10:1404–11. [PubMed] [Google Scholar]
- 23.Jahoda CA, Reynolds AJ. Dermal-epidermal interactions. Adult follicle-derived cell populations and hair growth. Dermatol Clin. 1996;14:573–83. doi: 10.1016/s0733-8635(05)70385-5. [DOI] [PubMed] [Google Scholar]
- 24.Cranwell W, Sinclair R. Male androgenetic alopecia. In: De Groot LJ, Chrousos G, Dungan K, et al., editors. Endotext. South Dartmouth, MA: MDText.com Inc; 2000. [Last updated on 2016 Feb 29]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK278957/ [Google Scholar]
- 25.Seo MD, Kang TJ, Lee CH, Lee AY, Noh M. HaCaT Keratinocytes and Primary Epidermal Keratinocytes Have Different Transcriptional Profiles of Cornified Envelope-Associated Genes to T Helper Cell Cytokines. Biomol Ther (Seoul) 2012;20:171–6. doi: 10.4062/biomolther.2012.20.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson VG. Growth and differentiation of haCaT keratinocytes. Methods Mol Biol. 2014;1195:33–41. doi: 10.1007/7651_2013_42. [DOI] [PubMed] [Google Scholar]
- 27.Bernard BA. Molecular approach of hair biology. C R Seances Soc Biol Fil. 1994;188:223–33. [PubMed] [Google Scholar]
- 28.Bakry OA, Elshazly RM, Shoeib MA, Gooda A. Oxidative stress in alopecia areata: A case-control study. Am J Clin Dermatol. 2014;15:57–64. doi: 10.1007/s40257-013-0036-6. [DOI] [PubMed] [Google Scholar]
- 29.Groves RW, Sherman L, Mizutani H, Dower SK, Kupper TS. Detection of interleukin-1 receptors in human epidermis. Induction of the type II receptor after organ culture and in psoriasis. Am J Pathol. 1994;145:1048–56. [PMC free article] [PubMed] [Google Scholar]
- 30.Groves RW, Mizutani H, Kieffer JD, Kupper TS. Inflammatory skin disease in transgenic mice that express high levels of interleukin 1 alpha in basal epidermis. Proc Natl Acad Sci U S A. 1995;92:11874–8. doi: 10.1073/pnas.92.25.11874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoffmann R, Eicheler W, Wenzel E, Happle R. Interleukin-1beta-induced inhibition of hair growth in vitro is mediated by cyclic AMP. J Invest Dermatol. 1997;108:40–2. doi: 10.1111/1523-1747.ep12285625. [DOI] [PubMed] [Google Scholar]
- 32.Singh M, Nuutila K, Sinha I, Eriksson E. Endotoxin-induced inflammation in a rodent model up-regulates IL-1a expression and CD45+ leukocyte recruitment and increases the rate of reepithelialization and wound closure. Wound Repair Regen. 2016;24:820–8. doi: 10.1111/wrr.12461. [DOI] [PubMed] [Google Scholar]
- 33.Boivin WA, Jiang H, Utting OB, Hunt DW. Influence of interleukin-1alpha on androgen receptor expression and cytokine secretion by cultured human dermal papilla cells. Exp Dermatol. 2006;15:784–93. doi: 10.1111/j.1600-0625.2006.00462.x. [DOI] [PubMed] [Google Scholar]
- 34.Hamada K, Hirotsu S, Uchiwa H, Yamazaki S, Suzuki K. Pro-inflammatory cytokine interleukin-1alpha is downregulated during anagen phase of hair cycle in vivo. J Dermatol Sci. 2003;33:195–8. doi: 10.1016/j.jdermsci.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Rückert R, Lindner G, Bulfone-Paus S, Paus R. High-dose proinflammatory cytokines induce apoptosis of hair bulb keratinocytes in vivo. Br J Dermatol. 2000;143:1036–9. doi: 10.1046/j.1365-2133.2000.03784.x. [DOI] [PubMed] [Google Scholar]
- 36.Fiedler-Weiss VC. Potential mechanisms of minoxidil-induced hair growth in alopecia areata. J Am Acad Dermatol. 1987;16:653–6. doi: 10.1016/s0190-9622(87)70083-8. [DOI] [PubMed] [Google Scholar]
- 37.Olsen EA, Whiting D, Bergfeld W, Miller J, Hordinsky M, Wanser R, et al. A multicenter, randomized, placebo-controlled, double-blind clinical trial of a novel formulation of 5% minoxidil topical foam versus placebo in the treatment of androgenetic alopecia in men. J Am Acad Dermatol. 2007;57:767–74. doi: 10.1016/j.jaad.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 38.Buhl AE, Waldon DJ, Baker CA, Johnson GA. Minoxidil sulfate is the active metabolite that stimulates hair follicles. J Invest Dermatol. 1990;95:553–7. doi: 10.1111/1523-1747.ep12504905. [DOI] [PubMed] [Google Scholar]
- 39.Goren A, Castano JA, McCoy J, Bermudez F, Lotti T. Novel enzymatic assay predicts minoxidil response in the treatment of androgenetic alopecia. Dermatol Ther. 2014;27:171–3. doi: 10.1111/dth.12111. [DOI] [PubMed] [Google Scholar]


