Abstract
Background
Usutu virus (USUV) is an emerging zoonotic virus originally from sub-Saharan Africa. It has been introduced into Europe on multiple occasions, causing substantial mortality within the Eurasian blackbird (Turdus merula) population. It is transmitted by the mosquito species Culex pipiens in Europe and Africa. Vector competence studies indicate that European strains of USUV are readily transmitted by indigenous Cx. pipiens. However, there is limited information on the ability of an African strain to infect European mosquitoes.
Methods
We evaluated the ability of African strain SAAR-1776 to infect two lines of Cx. pipiens colonised within the United Kingdom (UK). Mosquitoes were fed blood meals containing this virus and maintained at 25 °C for up to 21 days. Individual mosquitoes were tested for the presence of virus in the body, legs and an expectorate saliva sample. Changes to the consensus of the virus genome were monitored in samples derived from infected mosquitoes using amplicon based next generation sequencing.
Results
Infection, dissemination and the presence of virus in saliva in one mosquito line was observed, but no evidence for dissemination in the second mosquito line. This suggests a strong barrier to infection in UK Cx. pipiens for this strain of USUV. When comparing the genome of input virus within the blood meal with USUV recovered from an infected mosquito, we observed limited changes in the consensus genome sequence.
Conclusions
The evaluation of vector competence of UK populations of Cx. pipiens for Usutu virus suggests a limited susceptibility to infection with USUV strain SAAR-1776 of African origin. However, within a single mosquito there was complete dissemination and expectoration of USUV, indicating that infection, and potentially transmission, is possible. Sequence changes were observed that may represent early adaption to the mosquito host and could reflect the early events of USUV establishment in European mosquito populations.
Electronic supplementary material
The online version of this article (10.1186/s13071-018-2959-5) contains supplementary material, which is available to authorized users.
Keywords: Culex pipiens, Usutu virus, Vector competence, UK, Mosquito
Background
Usutu virus (USUV) is an emerging, mosquito-borne flavivirus that belongs to the Japanese encephalitis virus (JEV) antigenic complex [1, 2]. Like the closely related West Nile virus (WNV), USUV is maintained and transmitted primarily by members of the mosquito genus Culex with birds acting as amplifying hosts. Human infection is common [3–6], but disease is rarely reported, usually associated with immunocompromised individuals [7]. The virus was first isolated in 1959 from Culex neavei collected near the Usutu River, Natal, South Africa [8]. Subsequently, it has been recorded in birds and other mosquito species including Cx. perfuscus, Cx. univitattus and Cx. quinquefasciatus, throughout sub-Saharan countries [9]. Migratory birds are considered responsible for short and long-distance dispersal of USUV [9]. The first documented evidence of USUV introduction to Europe was during a retrospective study of dead Eurasian blackbirds (Turdus merula) in 1996, in Italy [10]. It was next recorded in Austria in 2001, where it again caused significant mortality among Eurasian blackbirds [11]. The virus appeared to overwinter causing further deaths amongst avian species. Since then, USUV has spread to many European countries including the Czech Republic, Germany, Hungary, Spain and Switzerland [2, 9, 12]. In 2016, the virus was recorded in the Netherlands causing widespread die-offs of blackbirds and captive great grey owls (Strix nebulosa) [13].
Surveillance of European mosquito species [14] has detected USUV predominantly in the northern house mosquito, Culex pipiens (s.l.), one of the most abundant species in the northern hemisphere. In addition, USUV has been isolated from Cx. modestus [15] and Aedes albopictus [16]. Phylogenetic studies indicated there have been multiple introductions with onward transmission of USUV in Europe, and that these viruses are distinct from those circulating in Africa [9]. Genetic variation is indicative of local adaptation of USUV to European populations of Cx. pipiens following its introduction. The vector competence of Cx. pipiens (s.l.) colonised in the Netherlands has been investigated and demonstrated that this species is highly susceptible to infection with a European-derived strain of USUV [17]. In this study, 69% of infected mosquitoes could expectorate USUV in saliva suggesting that they could efficiently transmit virus. However, it is not clear that an African strain is equally infectious in European Cx. pipiens mosquitoes. This would be an early event in the introduction of African mosquito-borne virus in Europe, but one that has presumably occurred on multiple occasions.
In the United Kingdom there has been no evidence for autochthonous vector transmission of a mosquito-borne arbovirus since the 19th century [18], although Buckley et al. [19] reported seropositivity in sentinel chickens for West Nile virus, Usutu virus, and Sindbis virus in the UK. Targeted surveillance in locations where migratory birds and mosquitoes are abundant has found no evidence for the presence of arboviruses [20]. However, recent studies have shown that under experimental conditions, UK-derived Aedes (Ochlerotatus) detritus is a potential vector for JEV at 23 °C and 28 °C [21] and WNV [22]. We assessed the ability of an African strain of USUV to infect colonised strains of Cx. pipiens derived from the UK. In addition, we monitored the genomic sequence of the infecting virus to evaluate virus adaptation.
Methods
Colonization of mosquitoes
Culex pipiens species has two ecological forms, Cx. pipiens form pipiens, and Cx. pipiens form molestus, which are morphologically indistinguishable. They are found sympatrically and can hybridize along their distribution range [23], with hybrids said to have a greater vector competence for certain arboviruses [24, 25]. Both forms and a hybrid are present in the UK. Two colonised lines: a Cx. pipiens typical form (Caldbeck: CBK) and Cx. pipiens hybrid form (Brookwood: BKW) were obtained from The Pirbright Institute [26]. Details of mosquito maintenance are provided in Additional file 1: Text S1.
Cells and viruses
The USUV strain SAAR-1776 originally isolated from Cx. neavi in South Africa (provided by Professor E. Gould, Centre for Hydrology and Ecology), was passaged three times in Vero C1008 cells to a titre of 4.0 × 106 PFU/ml. Vero cells were maintained in Dulbecco Modified Eagle’s Medium (DMEM) (Sigma-Aldrich, United Kingdom) containing 10% heat-inactivated Foetal Calf Serum (FCS), 2 mM L-glutamine and 50 μg/ml Penicillin/Streptomycin (Sigma-Aldrich).
Assessment of vector competence of UK Cx. pipiens mosquitoes
Both UK lines of Culex pipiens (CBK and BKW) were tested for their vector competence for the SAAR-1776 strain of USUV at 25°C. Mosquitoes were provided an infectious blood meal composed of defibrinated horse blood, adenosine 5’-triphosphate (final concentration 0.02 mM) and virus stock to give a final virus concentration of 1.0 × 106 PFU/ml using a Hemotek membrane feeding system (Hemotek Ltd Accrington, Lancashire, UK). Five to ten day-old adult female mosquitoes were allowed to feed for a minimum of 16 h at room temperature. Blood-fed and non-blood-fed specimens were anaesthetized with Triethylamine (TEA) FlyNap® (Blades Biological Limited, Edenbridge, UK) and separated in groups of 10–20 mosquitoes, which were placed in microhabitat pots of 118 × 73 mm in dimension (www.bugzarre.co.uk). Once fully recovered, the mosquitoes were maintained at 25 °C at a relative humidity of between 55–65% with a light cycle of 12:12 light:dark cycle. At 0, 7, 14 and 21 days post-infection (dpi) mosquito groups were processed to provide body, legs, head and saliva samples. Further details of mosquito infections are provided in Additional file 2: Text S2.
To estimate vector competence, we used previous methods [27–30]. Infection rate were calculated by the number of USUV positive mosquitoes with an infected body per number tested at each time point. Dissemination efficiency was calculated as the number of USUV positive mosquitoes with an infected leg per number tested at each point. Transmission efficiency was calculated as the number of mosquitoes with USUV detected in their saliva per number of mosquitoes with an infected leg at each time point.
Processing of samples for reverse transcription (RT) PCR
Legs/wings, head, and body (thorax/abdomen) of female Cx. pipiens were homogenized individually in flat-cap homogenization tubes (Qiagen, Mancherster, United Kingdom a) containing 300 μl of EMEM media containing 10% foetal bovine serum (FBS), penicillin/streptomycin 1% and amphotericin-B 1% and one 3 mm stainless steel bead; beads were not added to the tubes containing the saliva. Homogenisation was undertaken using a Qiagen Tissue Lyser (model Retsch MM301) at 25 MHz for 3 min. All tubes were centrifuged for 5 min at 14,000× rpm and 30 μl of supernatant was collected for virus isolation assays. The remainder of the homogenate was used for RNA extraction using TriZol (see www.tools.thermofisher.com). The RNA pellet was eluted in 20 μl of nuclease-free water.
USUV RNA was detected using the primers and probe targeting a 91 base pair region of the NS1 as previously published [31]. The real time reverse transcription PCR was performed on the MxPro 3005P thermal cycler (Stratagene,Thermofisher, United Kingdom) in a 25 μl reaction containing: RNase-free water (5.25 μl); 2× QuantiTect RT-PCR Master mix (12.50 μl); Jöst USUV Primer mix (10 μM primer, 1.25 μM probe) (2 μl); QuantiTect RT mix (Qiagen) (0.25 μl), and 5 μl of RNA template. The conditions were as follows: reverse transcription 50 °C for 30 min; reverse transcriptase inactivation 95 °C for 15 min; and PCR amplification and detection 50 cycles consisting of 95 °C for 15 s, 55 °C for 30 s, and final extension 72 °C for 30 s.
Virus isolation and titration
Plaque assays were performed on those samples positive by PCR. A ten-fold dilution series of supernatant of the homogenized body parts or salivary secretions (30 μl) was prepared, and dispensed onto a confluent monolayer of Vero cells on a 12-well plate (Costar®, Corning Life Sciences, Massachusetts, , USA). The plates were incubated at 37 °C in an atmosphere of 5% CO2 for 3 h. After incubation, 1–1.5 ml overlay of warmed 3% carboxymethylcellulose (CMC) in 250 ml 2× EMEM mix (Deionised water, 7.5% sodium bicarbonate, HEPES 1M, L-glutamine 200 mM, FBS, antibiotics (penicillin and streptomycin), and 10× MEME-E) were added to each well. After 7 days incubation, 1 ml of 10% neutral buffered formalin solution was added to each well and the plates left for least 3 h to complete virus inactivation. Wells were stained with 200 μl of 2.3% crystal violet solution.
Generation of USUV genome consensus sequence
Multiplex PCR that generates amplicons spanning the whole genome in two reactions was achieved following the methodology of Quick and co-workers [32]. A set of 35 oligonucleotide primer pairs were designed using a multiple alignment of European and African USUV sequences (Additional file 3: Table S1) using the Primal Scheme design tool [see 33 for details]. Two reaction tubes were prepared for each cDNA (one for primer Pool A and the other for primer Pool B), and the reactions carried out in a final volume of 25.5 μl [32]: 5 μl of Q5 Reaction buffer (5×,), 0.5 μl of dNTPs (10mM), 0.25 μl of Q5 DNA Polymerase (BioLabs, Massachusetts, USA), 1 μl of respective primer Pool at 10 μM (Primer Pool A or primer Pool B), 2.5 μl of cDNA and 16.25 μl of nuclease-free water. The conditions used were: 45 cycles of 95 °C for 30 s, 95 °C for 15 s and 65 °C for 5 min.
Amplified products were sequenced using the MiSeq (Illumina, San Diego, CA, USA) sequencing platform at the Animal and Plant Health Sequencing Unit. After excluding primer regions using Trimmomatic (see [33] for details), reads were mapped employing an iterative process described previously [33] using a complete USUV genome as the reference sequence (KC754958).
Statistical analysis
Survival curves for each group of specimens with infected or uninfected blood meals were compared using Kaplan Meier curve analysis and groups compared by Log-Rank test using Graphpad Prism 5 software.
Results
Vector competence of colonised UK mosquitoes for USUV
Two Cx. pipiens lines were used to investigate the vector competence of UK mosquitoes for an African strain of USUV, both colonised from natural populations. A total of 120 female Cx. pipiens typical form (CBK) and 100 female Cx. pipiens hybrid (BKW) were offered an infectious blood meal. In addition, 70 females for the CBK were used as non-infected controls. Examination of specimens showed that over 95% took a full blood meal. The exposure of engorged specimens to FlyNap for immobilization did not have a deleterious effect for their recovery as shown at dpi 0 and 3. However, a slightly higher mortality was seen after dpi 14 (Fig. 1). Survival of CBK did not differ significantly from uninfected controls (P = 0.0874) or BKW (P = 0.9962) (log-rank test).
Fig. 1.
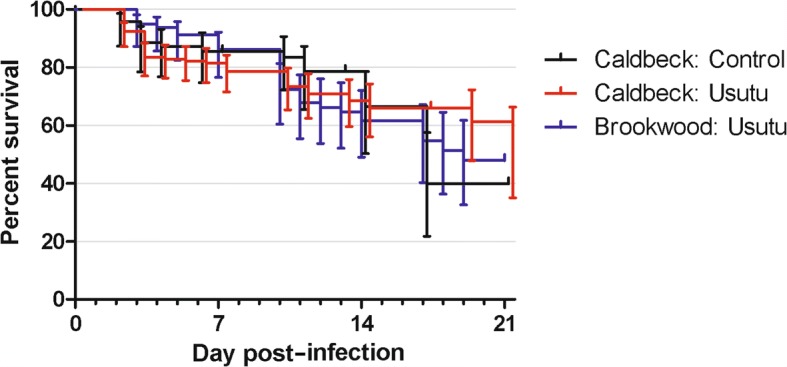
Comparison of survival curves for UK Caldbeck and Brookwood Lines of female Cx. pipiens (s.l.) infected with USUV, versus uninfected females following immobilization with FlyNap
In both mosquito lines, USUV was detected by PCR in the blood meal (abdomen) at dpi 0. However, the infection rates and transmission efficiency were low, when examined at later time points. Only one CBK specimen had detectable USUV RNA in the body at dpi 14 and one at dpi 21 (Infection rate: 5 % and 14.2%, respectively), while a single specimen from BKW was positive at dpi 21 (Infection rate: 5.5 %) (Table 1). Of the two mosquitoes susceptible to infection in this study, only one specimen in the CBK line had USUV present in the saliva at dpi 14 (100%). No USUV RNA was detected in the saliva in the BKW line.
Table 1.
Infection (Body), dissemination (Legs) and transmission (Saliva) rates of UK Caldbeck and Brookwood lines of Cx. pipiens infected with Usutu virus at 25 °C
| Dpi | Culex pipiens (Caldbeck line) | Culex pipiens (Brookwood line) | |
|---|---|---|---|
| Body | 7 | 0/20 (0%) | 0/14 (0%) |
| 14 | 1/20 (5%) | 0/18 (0%) | |
| 21 | 1/7 (14.2%) | 1/18 (5.5%) | |
| Legs | 7 | 3/20 | 0/14 (0%) |
| 14 | 1/20 | 0/18 (0%) | |
| 21 | 0/6 (0%) | 0/10 (0%) | |
| Saliva | 7 | 0/20 (0%) | 0/14 (0%) |
| 14 | 1/1 (100%) | 0/18 (0%) | |
| 21 | 0/1 (0%) | 0/18 (0%) |
Although the titre of the frozen virus was estimated to be 4 × 106 PFU/ml, and the freshly harvested viral stock (working stock) was originally estimated at 6.3 × 106 PFU/ml, the assessment of virus in the blood meal before and after the blood-feeding yielded a titre of 2 × 104 PFU/ml, equating to a 2-log drop in viral titre. Infectious viral titres (in PFU/ml) in the CBK specimen with positive saliva were: saliva 4.0 × 101, leg/wings 8.3 × 103, head 8.0 × 104, and abdomen 6.8 × 105. All other USUV RNA positive samples detected by RT PCR did not induce cytopathic effect to the Vero cell monolayer.
Impact of replication in Cx. pipiens on the USUV genome of African origin
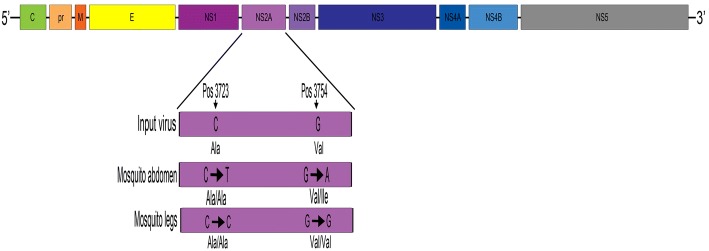
USUV sequence was obtained covering > 97% of the genome from the input virus (10,758 nucleotides) as well as the abdomen (10,758) of the USUV positive CBK mosquito at day 14 post-infection (see Table 2). Two nucleotide substitutions, C3723T and G3754A, were detected in the non-structural protein 2A (NS2A) gene retrieved from the abdomen of the mosquito (Fig. 2, Table 2). These substitutions at consensus level between the input virus and the virus recovered from the mosquito were only observed in the body sample. The nucleation variation at each position is as follows: 3723 - T (99.83%), C (0.08%), G (0.07%), A (0.01%); 3754 - A (99.72%), C (0.12%), G (0.09%), T (0.07%). The USUV recovered from the leg was identical to the input virus albeit with a low depth coverage (Table 2). Despite numerous attempts, no amplicons were obtained from the saliva sample for comparison. These nucleotides changes led to one synonymous amino acid change (A1220A) and one non-synonymous amino acid change (V1209I).
Table 2.
Consensus sequence changes detected in the abdomen from one Culex pipiens CBK mosquito found positive for USUV at day 14
| Genome positiona | Region | Input codon | Depth coverage | Abdomen codon | Depth coverage | Leg codon | Depth coverage | AA change |
|---|---|---|---|---|---|---|---|---|
| 3723 | NS2a | CGC | 9232 | CGT | 10,708 | CGC | 22 | A1220A |
| 3754 | NS2a | CGG | 9448 | CGA | 10,862 | CGG | 24 | V1209I |
aRelative to accession KC754958
Abbreviations: NS2a non-structural protein, AA amino acid
Fig. 2.
Amino acid substitutions in the polyprotein NS2A of USUV strain SAAR1776 in the abdomen and legs of UK CBK line of Cx. pipiens at day post-infection 14
Discussion
A range of factors have heightened the need to investigate the capacity of UK mosquito species to act as vectors of arboviruses. These include: the detection of virus specific neutralizing antibodies to USUV, WNV and Sindbis virus (SINV) in birds in the UK [19, 34], the recent emergence and spread of exotic mosquito-borne viruses in Europe [35], and the ongoing expansion of invasive species such as Ae. aegypti, Ae. albopictus and Ae. japonicus [35–37]. USUV has repeatedly been introduced into Europe since the 1990s [9, 38]. All USUVs isolated and characterised in Europe are grouped into three lineages based on their geographical location, Europe 1, 2 and 3, and are distinct from viruses isolated in Africa. This suggests that there has been USUV genome evolution following its introduction into European mosquito populations. The co-circulation of WNV and USUV in Culex species in Europe have been demonstrated [15, 16, 39], and the vector competence of European population of Cx. pipiens for USUV has recently been assessed [17]. The authors concluded that Cx. pipiens of European origin is highly competent for both viruses, with a significant increase in vector competence for USUV at higher temperatures. In order to assess the ability of USUV of African origin, we have attempted infection of two lines of UK Cx. pipiens with strain SAAR-1776, isolated in South Africa. This is also the first study to investigate the vector competence of UK populations of Cx. pipiens for USUV.
In contrast to high infection/transmission rates from experiments with a European USUV isolate, our results showed that only a single specimen of UK CBK Cx. pipiens form pipiens was positive for USUV RNA in the saliva 14 days after taking a blood meal. A range of factors could influence the competency of a particular mosquito to vector a virus. These include the genetic variability of the virus strains, virus titres used during oral infection, the chosen temperature and selective pressures during laboratory colonization that may change susceptibility to infection [23, 40–42]. This study was conducted with these factors in mind, therefore temperature, viral titre and procedures were all carefully selected to minimise these effects.
The effect of two nucleotide substitutions in the non-structural protein NS2A on the phenotype of the progeny virus is unknown, although it is interesting to note that a change with a deep coverage was only observed in the mosquitoes’ abdomen, where bottlenecks and selective pressures are said to occur while the virus overcome the midgut barrier (Table 2) [43, 44]. The nucleotide substitutions detected in the leg cannot be fully explained because of the low depth retrieved from the analysis (Table 2). Engel et al. [9] proposed that migratory birds can act as potential long-distance dispersal vehicles, the African lineages of USUV have been driven by extensive gene flow, and that the European lineages have been shaped by in situ evolution with host-specific mutations also being detected. Their observed mutations at the amino acid level in the NS2A protein (V91A) was suggested to be involved in the formation of the USUV European lineages 1 to 3. Nonetheless, it was concluded that the pathogenicity of specific USUV lineages has been poorly studied and more work is needed to determine the biological characteristics in each lineage.
The previous study investigating USUV infection in mosquitoes infected mosquitoes with the Bologna 09 strain at 4 × 107 PFU/ml and experimentally fed the females for 1 h. Here, we used the USUV isolate SAAR-1776 from South Africa at a final titre of 1.0 × 106 PFU/ml. Furthermore, preliminary infection trials resulted in low blood-feeding success; therefore mosquitoes were fed overnight to increase the number of engorged females. Overnight feeding resulted in large numbers of blood-fed females, and virus isolation confirmed that USUV was still viable in the blood at the end of the feeding period. However, a 2-log drop in virus concentration was observed in the infectious blood meal post-feeding, likely due to the longer feeding period. Numerous authors have proposed there is a clear relationship between virus titer in the blood sample and the infection rate [40] and the low vector competency observed in this study could be related to lower levels of virus in the blood meal, particularly if mosquitoes took a blood meal towards the end of the feeding period. The range of viraemias detected in USUV infected birds is not known, but high, medium and low titres in experimentally infected wild birds with closely related WNV are > 106, 104, 106 and ≤ 104 PFU/ml, suggesting that the titer used in our study was appropriate [45].
Conclusions
In this study, two UK strains of Cx. pipiens challenged with USUV showed reduced competency for an African strain of USUV, although infection, dissemination and virus expectoration can occur. As a result, further vector competence experiments employing higher titres of the USUV at temperatures representative of the UK climate, or using recently isolated European USUV strain, are required to test the competency of indigenous mosquitoes for mosquito-borne viruses.
Additional files
Text S1. Mosquito maintenance. (DOCX 15 kb)
Text S2. Membrane-based infection studies in mosquitoes. (DOCX 17 kb)
Table S1. Primers used to amplify USUV genomes. (DOCX 22 kb)
Acknowledgements
We thank colleagues at The Pirbright Institute for providing material to establish the colony of Cx. pipiens at the Animal and Plant Health Agency. LMHT would like to thank colleagues at Evolution Biotechnologies, Cambridge, UK; Public Health England, the London School of Tropical Medicine and Hygiene, the Liverpool School of Tropical Medicine, and the Natural History Museum for discussions on vector competence studies, mosquito ecology and insectary maintenance.
Funding
Funding was provided by the Department for Environment Food and Rural Affairs (DEFRA), Scottish Government and Welsh Government through grants SV3045, SE4112, and SE4113, and the EU Framework Horizon 2020 Innovation Grant (EVAg, No. 653316).
Availability of data and materials
All data that this paper relies upon is presented within this article.
Abbreviations
- BKW
Brookwood
- CBK
Caldbeck
- JEV
Japanese encephalitis virus
- SIV
Sindbis virus
- USUV
Usutu virus
- WNV
West Nile virus
Authors’ contributions
LMHT, MF and NJ designed the study. LMHT, KLM and LT cultured and titrated the virus. DM, SL and MF performed PCR and analysis for NGS. SL obtained survival curves. ARF and NJ obtained funding. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s13071-018-2959-5) contains supplementary material, which is available to authorized users.
Contributor Information
Luis M. Hernández-Triana, Email: luis.hernandez-triana@apha.gsi.gov.uk
Maria Fernández de Marco, Email: mar.fernandez@apha.gsi.gov.uk.
Karen L. Mansfield, Email: karen.mansfield@apha.gsi.gov.uk
Leigh Thorne, Email: leigh.thorne@apha.gsi.gov.uk.
Sarah Lumley, Email: sarah.lumley@phe.gov.uk.
Denise Marston, Email: denise.marston@apha.gsi.gov.uk.
Anthony A. Fooks, Email: tony.fooks@apha.gsi.gov.uk
Nick Johnson, Email: luis.hernandez-triana@apha.gsi.gov.uk.
References
- 1.Nikolay B, Diallo M, Faye O, Boye CS, Sall AA. Vector competence of Culex neavei (Diptera: Culicidae) for Usutu virus. Am J Trop Med Hyg. 2012;86:993–996. doi: 10.4269/ajtmh.2012.11-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashraf U, Ye J, Ruan X, Wan S, Zhu B, Cao S. Usutu virus: an emerging flavivirus in Europe. Viruses. 2015;7:219–238. doi: 10.3390/v7010219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakonyi T, Gould EA, Kolodziejek J, Weissenbök H, Nowotny N. Complete genome analysis and molecular characterization of Usutu virus that emerged in Austria in 2001. Comparison with the South African Strain SAAR-1776 and other flaviviruses. Virology. 2004;328:301–310. doi: 10.1016/j.virol.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Bakonyi T, Jungbauer C, Aberle SW, Kolodzieje D, Stiasny K, Allerberger F, et al. Usutu virus infections among blood donors, Austria, July and Ausgut 2017 - Raising awareness for diagnostic challenges. Euro Surveill. 2017;22: 10.2807/1560-7917.ES.2017. 22.41.17-00644. [DOI] [PMC free article] [PubMed]
- 5.Cavrini F, Gaibani P, Longo G, Pierro AM, Rossini G, Bonilauri P, et al. Usutu virus infection in a patient who underwent orthotropic liver transplantation, Italy, August-September 2009. Euro Surveill. 2009;14:19448. [PubMed] [Google Scholar]
- 6.Santini M, Vilibic-Cavlek T, Barsic B, Barsic L, Savic V, Stevanovic V, et al. First cases of human Usutu virus neuroinvasive infection in Croatia, August-September 2013: clinical and laboratory features. J Neurovirol. 2015;21:92–97. doi: 10.1007/s13365-014-0300-4. [DOI] [PubMed] [Google Scholar]
- 7.Cadar D, Becker N, Campos R de M, Börstler J, Jöst H, Schmidt-Chanasit J. Usutu virus in bats, Germany, 2013. Emerg Infect Dis. 2014;20:1771–1773. doi: 10.3201/eid2010.140909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams MC, Simpson DIH, Haddow AJ, Knight EM. The isolation of West Nile virus from mand and of Usutu virus from the bird-biting mosquito Mansonia aurities (Theobald) in the Entebbe area of Uganda. Ann Trop Med Parasitol. 1964;58:367–374. doi: 10.1080/00034983.1964.11686258. [DOI] [PubMed] [Google Scholar]
- 9.Engel D, Jöst H, Wink M, Börstler J, Bosch S, Garigliany M, et al. Reconstruction of the evolutionary history and dispersal of Usutu virus, a neglected emerging arbovirus in Europe and Africa. mBio. 2016;7:e01938–e01915. doi: 10.1128/mBio.01938-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mani P, Rossi G, Perruci S, Bertini S. Mortality of Turdus merula in Tuscany. Sel Vet. 1998;8:749–753. [Google Scholar]
- 11.Weissenböck H, Kolodziejek J, Fragner K, Kuhn R, Pfeffer M, Nowotny N. Usutu virus activity in Austria, 2001–2002. Microbes Infect. 2003;5:1132–1136. doi: 10.1016/S1286-4579(03)00204-1. [DOI] [PubMed] [Google Scholar]
- 12.Busquets N, Alba A, Allepuz A, Aranda C, Núñez JI. Usutu virus sequences in Culex pipiens (Diptera: Culicidae) in Spain. Emerg Infect Dis. 2008;14:861–2. [DOI] [PMC free article] [PubMed]
- 13.Rijks JM, Kik ML, Slaterus R, Foppen RPB, Stroo A, IJzer J, et al. Widespread Usutu virus outbreak in birds in the Netherlands, 2016. Euro Surveill. 2016;21:pii=30391. [DOI] [PMC free article] [PubMed]
- 14.Engler O, Savini G, Papa A, Figuerola J, Groschup MH, Kampen H, et al. European surveillance for West Nile virus in mosquito populations. Int J Environ Res Public Health. 2013;10:4869–4895. doi: 10.3390/ijerph10104869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rudolf I, Bakonyi T, Šebesta O, Mendel J, Peško J, Betášová L, et al. Co-circulation of Usutu virus and West Nile virus in a reed bed ecosystem. Parasit Vectors. 2015;8:520. doi: 10.1186/s13071-015-1139-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calzolari M, Gaibani P, Bellini R, Defilippo F, Pierro A, Albieri A, et al. Mosquito, bird and human surveillance of West Nile and Usutu viruses in Emilia-Romagna region (Italy) in 2010. PLoS One. 2012;7:e38058. doi: 10.1371/journal.pone.0038058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fros JJ, Miesen P, Vogels CB, Gaibani P, Sambri V, Martina BE, et al. Comparative Usutu and West Nile virus transmission potential by local Culex pipiens mosquitoes in north-western Europe. One Health. 2015:31–6. [DOI] [PMC free article] [PubMed]
- 18.Ramsdale CD, Gunn N. History of and prospects for mosquito-borne disease in Britain. Europ Mosq Bull. 2005;20:15–31. [Google Scholar]
- 19.Buckley A, Dawson A, Gould EA. Detection of seroconversion to West Nile virus, Usutu virus and Sindbis virus in UK sentinel chickens. Virol J. 2006;3:71. doi: 10.1186/1743-422X-3-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaux AGC, Medlock JM. Current status of invasive mosquito surveillance in the UK. Parasit Vectors. 2015;8:351. doi: 10.1186/s13071-015-0936-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mackenzie-Impoinvil L, Impoinvil DE, Galbraith SE, Dillon RJ, Ranson H, Johnson N, et al. Evaluation of a temperate climate mosquito, Ochlerotatus detritus (Aedes detritus), as a potential vector of Japanese encephalitis virus. Med Vet Entomol. 2015;29:1–9. doi: 10.1111/mve.12083. [DOI] [PubMed] [Google Scholar]
- 22.Blagrove MSC, Sherlock K, Chapman GE, Impoinvil DE, McCall PJ, Medlock JM, et al. Evaluation of the vector competence of a native UK mosquito Ochlerotatus detritus (Aedes detritus) for dengue chikungunya and West Nile viruses. Parasit Vectors. 2017;9:452. doi: 10.1186/s13071-016-1739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fonseca DM, Atkinson CT, Fleischer RC. Microsatellite primers for Culex pipiens quinquefasciatus, the vector of avian malaria in Hawaii. Mol Ecol. 1998;7:1617–1619. [PubMed] [Google Scholar]
- 24.Vogels CBF, Fros JJ, Göertz GP, Pijlman GP, Koenraadt CJM. Vector competence of northern European Culex pipiens biotypes and hybrids for West Nile virus is differentially affected by temperature. Parasit Vectors. 2016;9:393. doi: 10.1186/s13071-016-1677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vogels CBF, Hartemink N, Koenraadt CJM. Modelling West Nile virus transmission risk in Europe: effect of temperature and mosquito biotypes on the basic reproduction number. Sci Rep. 2017;7:5022. doi: 10.1038/s41598-017-05185-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manley R, Harrup LE, Veronesi E, Stubbins F, Stoner J, Gubbins S, et al. Testing of UK populations of Culex pipiens L. for Schmallenberg virus vector competence and their colonization. PLoS One. 2015;10:0134453. doi: 10.1371/journal.pone.0134453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Guinn M, Turell M. Effect of Triethylamine on the recovery of selected South American alphaviruses, flaviviruses, and bunyaviruses from mosquito (Diptera: Culicidae) pools. J Med Entomol. 2002;39:806–808. doi: 10.1603/0022-2585-39.5.806. [DOI] [PubMed] [Google Scholar]
- 28.Amraoui F, Atyame-Nten C, Vega-Rúa A, Lourenço-de-Oliveira R, Vazeille M, Failloux AB. Culex mosquitoes are experimentally unable to transmit Zika virus. Euro Surveill. 2016;21:30333. doi: 10.2807/1560-7917.ES.2016.21.35.30333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Luca M, Severini F, Toma L, Boccolini D, Romi R, Remoli ME, et al. Experimental studies of susceptibility of Italian Aedes albopictus to Zika virus. Euro Surveill. 2016;21:30223. doi: 10.2807/1560-7917.ES.2016.21.18.30223. [DOI] [PubMed] [Google Scholar]
- 30.Heitmann A, Jansen S, Lühken R, Leggewie M, Badusche M, Pluskota B, et al. Experimental transmission of Zika virus by mosquitoes from central Europe. Euro Surveill. 2017;22:30437. doi: 10.2807/1560-7917.ES.2017.22.2.30437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jöst H, Bialonski A, Schmetz C, Günther S, Becker N, Schmidt-Chanasit J. Isolation and phylogenetic analysis of Batai virus Germany. Am J Trop Med Hyg. 2011;84:241–243. doi: 10.4269/ajtmh.2011.10-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quick J, Grubaugh ND, Pullan ST, Claro IM, Smith AD, Gangavarapu K, et al. Multiplex PCR method for MinION and Illumina sequencing of Zika and other virus genomes directly from clinical samples. Nat Protoc. 2017;12:1261–1276. doi: 10.1038/nprot.2017.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marston DA, Wise EL, Ellis RJ, McElhinney LM, Banyard AC, Johnson N, et al. Complete genomic sequence of rabies virus from an ethiopian wolf. Genome Announc. 2015;3:e00157–e00115. doi: 10.1128/genomeA.00157-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buckley A, Dawson A, Moss SR, Hinsley SA, Bellamy PE, Gould EA. Serological evidence of West Nile virus, Usutu virus and Sindbis virus infection of birds in the UK. J Gen Virol. 2003;84:2807–2817. doi: 10.1099/vir.0.19341-0. [DOI] [PubMed] [Google Scholar]
- 35.Hubálek Z. Mosquito-borne viruses in Europe. Parasitol Res. 2008;103(Suppl. 1):29–43. doi: 10.1007/s00436-008-1064-7. [DOI] [PubMed] [Google Scholar]
- 36.Dallimore T, Hunter T, Medloch M, Vaux AGV, Harbach RE, Strode C. Discovery of a single male Aedes aegypti (L.) in Merseyside, England. Parasit Vectors. 2017;10:309. doi: 10.1186/s13071-017-2251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Medlock JM, Vaux AGC, Cull B, Schaffner F, Gillingham E, Pfluger V, et al. Detection of the invasive mosquito species Aedes albopictus in southern England. Lancet Infect Dis. 2016;2:140. [DOI] [PubMed]
- 38.Vaux AGC, Gibson G, Hernandez-Triana LM, Cheke RA, McCracken F, Jeffries CL, et al. Enhanced West Nile virus surveillance in the North Kent marshes. UK. Parasit Vectors. 2015;8:91. doi: 10.1186/s13071-015-0705-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saiz JC, Blásquez AB. Usutu virus: current knowledge and future perspectives. Virus Adapt Treat. 2017;9:27–40. doi: 10.2147/VAAT.S123619. [DOI] [Google Scholar]
- 40.Nikolay B. A review of West Nile and Usutu virus co-circulation in Europe: how much do transmission cycles overlap? Trans R Soc Trop Med Hyg. 2015;109:609–618. doi: 10.1093/trstmh/trv066. [DOI] [PubMed] [Google Scholar]
- 41.Chouin-Carneiro T, Vega-Rua A, Vazeille M, Yebakima A, Girod R, Goindin D, et al. Differential susceptibilities of Aedes aegypti and Aedes albopictus from the Americas to Zika virus. PLoS Negl Trop Dis. 2016;10:e0004543. doi: 10.1371/journal.pntd.0004543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pérez-Ramírez E, Llorente F, Jiménez-Clavero MA. Experimental infections of wild birds with West Nile virus. Viruses. 2014;6:752–781. doi: 10.3390/v6020752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Forrester NL, Coffey LL, Weaver SC. Arboviral bottlenecks and challenges to maintaining diversity and fitness during mosquito transmission. Viruses. 2014;6:3991–4004. doi: 10.3390/v6103991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grubaugh ND, Fauver JR, Rückert C, Weger-Lucarelli J, Garcia-Luna S, Murrieta RA, et al. Mosquitoes transmit unique West Nile Virus populations during each feeding episode. Cell Rep. 2017;9:709–718. doi: 10.1016/j.celrep.2017.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ndiaye EH, Fal G, Gaye A, Bob NS, Talla C, Diagne CT, et al. Vector competence of Aedes vexans (Meigen), Culex poicilipes (Theobald) and Cx. quinquefasciatus Say from Senegal for West and East African lineages of Rift Valley fever virus. Parasit Vectors. 2016;9:94. doi: 10.1186/s13071-016-1383-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Text S1. Mosquito maintenance. (DOCX 15 kb)
Text S2. Membrane-based infection studies in mosquitoes. (DOCX 17 kb)
Table S1. Primers used to amplify USUV genomes. (DOCX 22 kb)
Data Availability Statement
All data that this paper relies upon is presented within this article.