ABSTRACT
Quorum sensing (QS) is a form of bacterial chemical communication that regulates cellular phenotypes, including certain cooperative behaviors, in response to environmental and demographic changes. Despite the existence of proposed mechanisms that stabilize QS against defector exploitation, it is unclear if or how QS cooperators can proliferate in some model systems in populations mostly consisting of defectors. We predicted that growth in fragmented subpopulations could allow QS cooperators to invade a QS defector population. This could occur despite cooperators having lower relative fitnesses than defectors due to favored weighting of genotypes that produce larger populations of bacteria. Mixed metapopulations of Vibrio QS-proficient or unconditional cooperators and QS defectors were diluted and fragmented into isolated subpopulations in an environment that requires QS-regulated public good production to achieve larger population yields. Under these conditions, we observed global invasions of both cooperator genotypes into populations composed of primarily defectors. This spatially dependent increase in cooperator frequency was replicated for QS cooperators when mixed populations were competed in soft agar motility plates under conditions that allowed cooperators to disperse and outcompete defectors at the population edge, despite being less motile in isolation than defectors. These competition results show that the coordinated growth and dispersal of QS cooperators to additional resources is heavily favored in comparison to unconditional cooperation, and that dispersal of cooperators by motility into new environments, examined here in laboratory populations, constitutes a key mechanism for maintaining QS-regulated cooperation in the face of defection.
IMPORTANCE Behaviors that are cooperative in nature are at risk of exploitation by cheating and are thus difficult to maintain by natural selection alone. While bacterial cell-cell communication, known as quorum sensing (QS), can stabilize microbial cooperative behaviors and is widespread in Vibrio species, it is unclear how QS can increase the frequency of cooperative strains in the presence of defectors without additional mechanisms. In this study, we demonstrate under multiple conditions that QS-mediated cooperation can increase in populations of Vibrio strains when cells experience narrow population bottlenecks or disperse from defectors. This occurred for both conditional cooperation mediated by QS and for unconditional cooperation, although conditional cooperators were better able to stabilize cooperation over a much wider range of conditions. Thus, we observed that population structuring allowed for assortment of competing genotypes and promoted cooperation via kin selection in microbes in a QS-dependent manner.
KEYWORDS: Vibrio harveyi, quorum sensing, cooperation, evolution, communication, Simpson's paradox, Vibrio
INTRODUCTION
Explaining the ongoing persistence of cooperative behavior has been a long-standing challenge for evolutionary biology (1–3). Cooperative behaviors are not expected to be evolutionarily stable by natural selection alone and thus require additional mechanisms to explain their existence and persistence in nature (4, 5). Nonetheless, cooperation is widely found at all levels of biological organization, from subcellular molecular systems to multispecies communities. One well-studied class of cooperative behaviors is the production of public goods, costly products that increase individual fitness but whose resulting benefits can be shared between all members of a population, including nonproducers. Public good production provides an opportunity for under- or nonproducing defectors to cheat contributing cooperators in mixed populations, increasing their frequency while simultaneously destabilizing productivity of the whole population. This can in turn lead to a tragedy of the commons undermining the stability of the cooperative behavior (6–8).
An available mechanism contributing to the limitation of defectors is facultative production of public goods when the benefit to do so is increased or the cost is reduced (9, 10). Bacteria often regulate public good production in a condition-dependent manner by using a common form of chemical communication known as quorum sensing (QS). In members of the Vibrionaceae bacteria, including the marine bacterium Vibrio harveyi and the human pathogen Vibrio cholerae, the QS circuit consists of a phosphorelay pathway that is controlled by exogenous chemical signals known as autoinducers (11, 12). At low cell densities, when autoinducer concentrations are low, membrane-bound kinases funnel phosphate through this pathway, leading to phosphorylation of the response regulator LuxO, which represses expression of the high-cell-density master regulators LuxR for V. harveyi and HapR for V. cholerae via the induction of regulatory small RNAs (sRNAs) (11, 12). At high cell densities, autoinducer molecules rise to sufficient concentrations to be bound by their cognate receptors and thereby activate these receptors' phosphatase activity, leading to dephosphorylation of LuxO and expression of LuxR or HapR, which induces expression of many genes (12, 13). The Vibrio QS circuit is a powerful system to study the role of QS in coordinating cooperation because multiple mutant phenotypes can be readily generated. An unconditional cooperator (UC) genotype can be engineered through null mutations to luxO, which locks the cells into a high-density state, while a defector (D) genotype that is locked into a low-density, noncooperative state can be generated by null mutations to the luxR or hapR master regulator gene (Fig. 1). We have shown that in V. harveyi, the cooperative production of extracellular proteases, which are public goods susceptible to cheating by nonproducing defectors, can be stabilized even in well-mixed conditions by facultative expression via QS regulation (14). However, the unregulated protease production of the UC strain is exploited, resulting in a tragedy of the commons.
FIG 1.
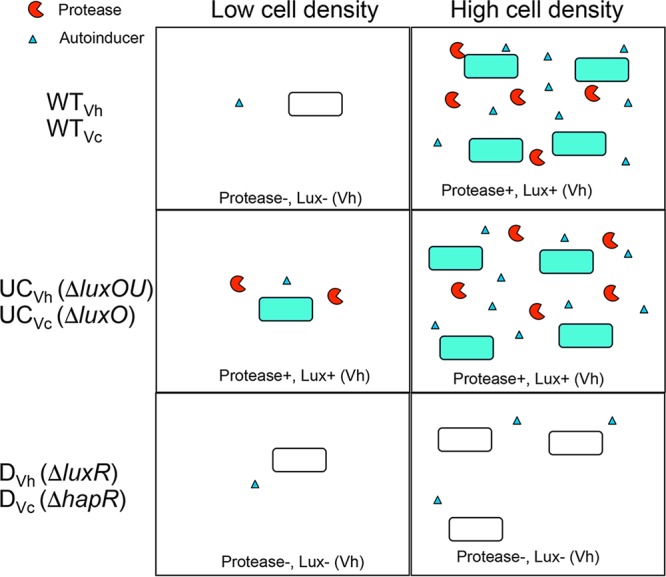
Phenotypes of strains used in the study. WT, wild type; UC, unconditional cooperator; D, defector; subscript letters refer to the species (Vh, V. harveyi; Vc, V. cholerae). Blue coloration of cells depicts the activation of QS-regulated lux gene expression that leads to bioluminescence.
Although we previously showed that QS regulation stabilizes protease production, even the facultative QS cooperator genotype of V. harveyi was unable to invade a population of mostly defectors in an environment that required proteolytic activity for maximum productivity (14). Yet, QS-proficient Vibrio strains are common in natural environments, and multiple V. cholerae QS mutant strains that are QS defective or even strains locked in high-cell-density states have also been isolated (15). While different explanations, including stress response regulation, metabolic constraint, metabolic prudence, policing, and spatial structure, have been noted to promote cooperation in other bacterial species, primarily Pseudomonas aeruginosa (16–23), none of these strategies have been described for Vibrio species. Therefore, the question of how Vibrio QS is so prevalent in natural environments and whether conditions exist that enable Vibrio QS cooperators to successfully invade in populations composed mostly of defectors remains.
In this study, we demonstrate that QS-proficient Vibrio strains can invade a population predominantly composed of defectors in structured environments that promote population bottlenecks and/or dispersal, including experimental metapopulations and expanding motile colonies. One way this could occur is via Simpson's paradox (24, 25), a statistical phenomenon in which a given trend is evident in multiple independent subgroups (i.e., A > B), but the reverse trend is observed when the outcomes of all the groups are combined (i.e., B > A). For cooperative traits, Simpson's paradox (originally termed “trait group selection” in this context [26]) describes a situation wherein defectors outperform cooperators on local scales in subpopulations due to higher relative fitnesses (defined as a change in genotype frequency due to differences in realized growth rates), but cooperators ultimately outperform defectors on a global scale due to higher absolute fitnesses afforded by the cooperative trait (defined as a change in genotype abundance due to higher potential carrying capacities). We show that this weighting leads to increased growth of subpopulations that possess a higher proportion of cooperators driven by active dispersal events, such as bacterial motility that promote kin selection, by selecting for higher growth yields driven by cooperative traits. This can occur even when those genotypes possess relatively lower growth rates than defectors. Although Simpson's paradox has been demonstrated to increase cooperator frequency in simulated or engineered microbial systems (27, 28), we demonstrate here that this phenomenon occurs in a nonsynthetic phenotypic system in which cooperators and defectors differ primarily by growth yield rather than by growth rates. Our results offer a mechanism by which structured environments can promote maintenance of QS in Vibrio populations.
RESULTS
Cooperators and defectors of both Vibrio species examined exhibit similar patterns of growth in M9-casein medium.
For the duration of the paper, we will use “WT” to refer to the wild-type facultative cooperating wild-type strains V. harveyi BB120 (WTVh) and V. cholerae C6706str2 (WTVc), “UC” to refer to the unconditional ΔluxO cooperator strains (UCVh or UCVc), and “D” to refer to the ΔluxR or ΔhapR defector strains (DVh or DVc, respectively) (Fig. 1). We have previously shown that when V. harveyi WTVh and DVh are competed in large populations of bacteria under well-mixed conditions where growth is dependent upon the induction of extracellular protease production by the QS system, the WTVh strain exhibits equivalent fitness to the DVh strain at all starting frequencies examined (14). Alternatively, a population of the UCVh strain suffers from a prisoner's dilemma and is invaded by both WTVh and DVh strains at all starting frequencies. This finding showed that QS could stabilize public goods in V. harveyi. Importantly, however, neither cooperating strain was able to invade populations of DVh cells, regardless of their introduced abundance. For V. cholerae, a previous study had shown that the DVc mutant was able to invade the WTVc in minimal casein medium (29). Therefore, it was unclear how such functional QS systems in Vibrio species maintain their widespread abundance in the natural world.
To extend our previous study, we first increased the duration of growth in a minimal medium containing casein as the sole carbon source (M9-casein) and compared cooperator and defector growth in monoculture. The V. harveyi cooperator strains that can express the high-cell-density QS state (WTVh and UCVh) that results in upregulated extracellular protease production are able to grow to higher densities in this environment (Fig. 2). This is due to the QS-regulated production of extracellular proteases, which can digest the extracellular casein and liberate nutrients for growth. Alternatively, the DVh mutant is unable to grow to equivalent high densities in M9-casein, due to an inability to produce high levels of extracellular proteases, even at 48 and 72 h, compared to the levels produced by WTVh and UCVh (Fig. 2, left). However, low levels of DVh growth do occur due to small amounts of already liberated nutrients that do not require enzyme-mediated proteolysis (14). This analysis was next extended to analogous QS strains of the human pathogen V. cholerae. We observed that, similar to V. harveyi strains, WTVc and UCVc cooperator strains that could activate the high-density QS state exhibited higher absolute yields (Fig. 2, right). However, one difference we noted between the two Vibrio species was that DVc grew to higher cell densities by 48 and 72 h than DVh, although it still did not reach the densities of the cooperator strains.
FIG 2.
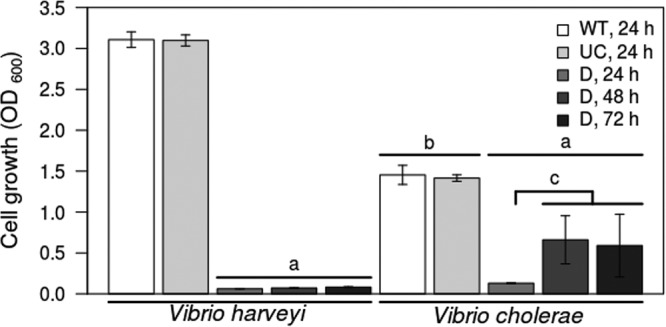
Growth of cooperator and defector strains in M9-casein medium. Growth was measured by observing the optical density at 600 nm (OD600). Data for defectors is reported at 24, 48, and 72 h of growth to demonstrate differences between V. harveyi and V. cholerae defectors. Measures for cooperator strains did not vary between the days tested, so only the 24-hour measures are displayed here. Error bars are 95% confidence intervals on 5 biological replicates. WT, wild type; UC, unconditional cooperator; D; defector; and subscript letters refer to the species. Significance labels depicted represent (a) defector strains significantly less than both cooperator strains of both species, P < 0.001; (b) cooperator growth significantly differ between Vibrio species, P < 0.001; and (c) V. cholerae defector strain growth significantly differs between first and later days postinoculation, P < 0.05.
We determined the growth rate of the three V. harveyi and three V. cholerae strains during low cell density (see Table S1 in the supplemental material). An analysis of growth during the first 6 h of the culture by quantifying CFU shows that the growth rates of the WTVh and DVh strains are statistically indistinguishable from one another but 74% and 66% greater, respectively, than that of the UCVh (Table S1). V. cholerae exhibited more rapid growth in this medium. The UCVc strain exhibited the lowest growth rate, although none of the three strains were statistically indistinguishable from each other, even though the DVc has been previously shown to rapidly invade WTVc in this medium (29). This suggests that an additional mechanism other than the growth rate may promote invasion of DVc during dual strain competition.
Cycles of population bottlenecks, fragmentation, and regrowth increase the frequency of Vibrio cooperator strains via Simpson's paradox, mediated by kin selection.
Because QS cooperators can maximize population growth yield in M9-casein (Fig. 2), we hypothesized that environments that select for growth yield rather than growth rate could provide a mechanism by which the examined cooperative behaviors are enriched. To test this hypothesis, we initiated cycles of global cell mixing as a metapopulation (30), fragmentation into subpopulations with strong bottlenecks and regrowth, followed by recombining the subpopulations into a metapopulation and repeating the cycle. We hypothesized that defectors should be favored over cooperators on a local scale within mixed subpopulations due to higher growth rates, but subpopulations that by chance had more cooperators present would be favorably weighted once the metapopulation was regenerated, due to higher growth yields (26–28). In essence, we asked if growth rate or growth yield was favored under these conditions. This situation could constitute a Simpson's paradox, in which cooperators could increase in frequency in mixed populations without ever having relative fitnesses higher than those of their conspecific defectors if cells more frequently interact with their own genotype, a situation that would promote kin selection (31).
We initiated this experiment by mixing either the WT or UC strains into a predominantly (∼90%) D population in M9-casein medium, followed by dilution and division into subpopulations in 96-well plates to promote assortment and inhibit gene flow between subpopulations. We fitted the number of wells with no growth to a Poisson distribution to estimate that each well received on average approximately 2 bacteria (see Fig. S1 in the supplemental material). In support of our hypothesis, we observed that both WTVh and WTVc strains invaded defectors in metapopulations when passaged through these cycles of population bottlenecks, fragmentation, and regrowth (Fig. 3, black lines). More surprisingly, both the UCVh and UCVc strains were also able to invade defectors (Fig. 3, gray lines), despite having lower growth rates and consequently lower relative fitnesses that led them to be driven to extinction by defectors in larger well-mixed population conditions (Table S1) (14). The invasion of V. cholerae cooperator genotypes into populations of the DVc defector contrasts with results of previous experiments performed in well-mixed liquid cultures with large populations (29) and demonstrates that this result is robust across different Vibrio species.
FIG 3.
Simpson's paradox results in increasing fractions of cooperators in metapopulations of Vibrio bacteria. Populations initiated with 10% cooperators (WT or UC) were serially passaged through eight cycles of stringent bottlenecks and regrowth in partitioned subpopulations. All subpopulations were remixed to begin each subsequent experimental cycle. (A) For V. harveyi, the growth time between transfers is 3 days, and (B) for V. cholerae it is 2 days. The data points represent the mean from the 3 metapopulations after recombining all subpopulations at each cycle, and the lines show the cooperation frequency of each individual replicate metapopulation.
To more closely examine the dynamics of V. harveyi cooperator invasion, we determined final QS activity (measured by bioluminescence) and growth (measured by optical density at 600 nm [OD600]) of all subpopulations (Fig. 4). After the first growth cycle, only a small fraction of subpopulations exhibited bioluminescence (14.1% in WTVh metapopulations and 23.7% in UCVh metapopulations produced normalized bioluminescence of >106 relative light units [RLU]) because the populations were initiated with ∼90% DVh cells, which do not produce observable bioluminescence (Fig. 4A). However, following the final experimental cycle, the vast majority of subpopulations for both cooperator strain treatments exhibited significant bioluminescence (>106 RLU), suggesting that they contained large numbers of cooperator cells (Fig. 4B). The WTVh treatment subpopulations produced homogeneous OD600 values among subpopulations by the completion of the last cycle, with the vast majority of subpopulations reaching both higher densities (96.6% at an OD600 of >0.5) and high bioluminescence (98.9% of subpopulations with normalized bioluminescence of >106 RLU), with few subpopulations present at intermediate states of either variable (Fig. 4B). Alternatively, the UCVh populations exhibited a wide range of OD600 values. This outcome is likely a result of the WTVh strain having a higher growth rate than the UCVh (Table S1) and the UCvh strain not reaching the maximum yield during the conditions of this experiment. As expected, the UCVh strain produces more bioluminescence at lower cell densities, as can be seen when all transfers are plotted on the same graph (see Fig. S2 in the supplemental material), consistent with constitutive expression of high-cell-density behaviors that delay growth (14). Moreover, there was a positive correlation between total cell density in the metapopulation and cooperator frequency, demonstrating that for either genotype, increasing public good-producing cooperator numbers improved overall productivity in a manner expected of Simpson's paradox (see Fig. S3 in the supplemental material).
FIG 4.
Growth and luminescence of V. harveyi subpopulations. Phenotypic profiles of individual subpopulations of V. harveyi over the course of metapopulation experiments were determined by measuring QS activity (measured in proxy by bioluminescence normalized to cell growth [RLU/OD600]) and growth (measured by OD600) at the completion of the (A) first and (B) final growth cycle of the experiment. Each data point depicts a single well subpopulation.
Swimming motility promotes cooperation for facultative cooperators in competitions against defectors.
The observed increases of cooperator frequencies in our metapopulation experiments demonstrated that the cooperator strains can invade defector-majority populations via a Simpson's paradox. This is dependent upon sufficient separation from defectors to colonize new environments that allow maximization of growth yields. We hypothesized that swimming motility could also provide such a mechanism. Previous studies have demonstrated interconnections between swimming motility and quorum sensing, although these studies were not performed on M9-casein medium (32, 33). To test this hypothesis, we first compared the swimming capabilities of Vibrio strains in isolation by inoculating monocultures into M9-casein motility plates and measuring the total dispersal area. We observed in both species that defector strains dispersed more than either of the cooperator strains (P < 0.05; Fig. 5). However, the area that the defector strains, particularly strain DVh, colonized was not as dense as the colonies produced by either cooperator strain (see Fig. S4B in the supplemental material), consistent with the idea that the defectors were not able to utilize the casein carbon source as effectively (Fig. 2).
FIG 5.

Vibrio strains exhibit a colonization-dispersal trade-off in M9-casein medium. Strains were seeded on motility (0.3%) agar plates with 1/1,000 defector strain carrying capacity initiating cells (∼105 to 106 cells). Motility was calculated by measuring the total area of the region over which cells had traversed from their initial inoculation spot, determined by the fringe of visible cell growth. Statistically significant differences are labeled as such: (a) D strain produced a significantly larger dispersal area than both conspecific cooperator strains, P < 0.001; (b) UC strain produced a significantly smaller dispersal area than both conspecific WT and D strains, P < 0.01; (c) mixed colony produced a significantly smaller dispersal area than the contained defector strain in monoculture, P < 0.001; (d) mixed colony produced a significantly larger dispersal area than the contained cooperator strain in monoculture, P < 0.001; (e) UC versus D dispersal areas significantly differ between species, P < 0.05.
To test if swimming motility could promote cooperator dispersal and an overall enrichment of cooperators via Simpson's paradox, we mixed populations of cooperator and defector strains in equal proportions and allowed them to compete in the M9-casein motility plates. Different regions of these colonies were sampled for cooperator frequency during growth. Because much larger seeding populations were used in these experiments (around 106 cells) than in the metapopulation experiments, this experiment served as a much more stringent test of the potential for Simpson's paradox to drive an increase of cooperators. In mixed populations, the WT strains constituted the majorities of the cell populations on the colony edges at day 2, whereas they comprised relatively equivalent frequencies as the defector strains in the colony centers (Fig. 6 and Fig. S4). Alternatively, the UC strains were largely undetectable at the colony exterior and were outcompeted in the center (Fig. 6). This result is not due to the WT strains being generally more motile, as the D strains exhibited greater motility in monoculture (Fig. 5). The prevalence of the WTVh strain at the colony exterior through the first 2 days is also visibly evident, as the edge of the motile colony is highly bioluminescent (Fig. S4). This was not evolutionarily stable under these conditions, as DVh nonluminescent strains ultimately take over the population. However, as we argue in the Discussion, the conditions encountered by Vibrio strains in natural environments are likely more complex than the laboratory conditions tested here.
FIG 6.

Motility plate competitions. Each cooperator strain of both V. harveyi and V. cholerae were competed against their conspecific defector strain in M9-casein motility plates. During growth, samples were taken from the center and edge of colony growth, diluted, and plated to determine the composition of these populations. At the completion of the experiment, the entire colony was harvested, homogenized, diluted, and plated to determine the composition of these populations. The frequency of the included cooperator strain is measured on the y axis. Error bars represent 95% confidence intervals for 4 biological replicates per strain mix.
Unlike the metapopulation experiments, the UCVh and UCVc strains were outcompeted by the defector in all regions of the motile population at day 2 (Fig. 6). The low relative fitness of the ΔluxOU strain in these competitions is evident by visualizing bioluminescence of V. harveyi competitors, as the entire colony is dim or completely nonluminescent (Fig. S4). Thus, while the Vibrio WT and UC strains performed similarly well when passaged through population bottlenecks and were grown in isolated subpopulations, stark differences appeared when they were competed against defectors in motility plates.
Harvesting of entire dispersed colonies from motility plates on day 3 revealed that it is possible for the V. cholerae wild-type strain to significantly increase in frequency in the presence of DVc (P < 0.05, Fig. 6). The WTVh strain also showed no significant drop from its starting frequency of 50% throughout the dispersed colonies. However, the UC mutants of both Vibrio species exhibited low fitnesses, leading to strong drops in their frequencies following competitions against defectors (Fig. 6). The relative fitness of the competing strains versus defectors under all conditions tested is shown in Fig. S5 in the supplemental material.
As an additional control, we performed similar experiments with a nonmotile ΔflrA mutant of V. cholerae (34). Even though the nonmotile mutant can activate QS, in the absence of motility it was outcompeted by the DVc strain on M9-casein motility agar plates, showing that active dispersal is required for increased frequency of the WTVc strain on the colony edge and throughout the dispersed colony (see Fig. S6 in the supplemental material). Together, these experiments provide evidence that a functional QS system, combined with dispersal by flagellar-based motility, renders the QS cooperators of either Vibrio species more competitive against defectors, even allowing the V. cholerae WT strain to increase in frequency in large, premixed populations with defectors.
DISCUSSION
The results of this study provide evidence that cooperative genotypes can persist and even increase in frequency in the presence of defector genotypes when provided with appropriate environmental conditions. For Vibrio strains, this appears to be driven by both the growth advantages conveyed by QS regulation, as well as by the ability to disperse via flagellar motility. While previously published results demonstrated that cooperator strains of V. harveyi could persist in the presence of defectors in well-mixed conditions, cooperators did not overtake defectors under these conditions (14). Alternatively, V. cholerae defectors were able to outcompete the facultative cooperators in well-mixed M9-casein (29). We predicted that providing a means of assortment between competing strains could allow cooperators to invade defectors. Both the experimental metapopulation and motility competition results were implemented under conditions that fostered increased numbers of local interactions with neighboring cells, which are more likely to be kin than cells encountered in well-mixed populations, and these examples demonstrate kin selection in operation. Structure and limited dispersal serve as proxies for increased relatedness (1, 35, 36), but our results suggest that dispersal by cooperator strains was a necessary component of being competitive in the presence of defectors. This is reinforced by the observation that mutant strains incapable of producing flagella for swimming dispersal were not capable of invading defectors, while those that retained this functionality could (Fig. 6 and Fig. S6). While it seems counterintuitive to suggest that the ability to disperse should foster assortment of strains, it makes sense when considering that dispersal allows cells to navigate their environment to locate uncolonized patches of untapped resources and then increase their numbers via clonal reproduction, and differences in dispersal abilities between competing strains may exacerbate this effect.
In this work, we have demonstrated that Simpson's paradox can increase the frequency of cooperator Vibrio genotypes with functional QS systems in metapopulations of mixed cooperating and defecting genotypes when the ability to cooperate provides an advantage for population growth yields. Unlike previous studies demonstrating this paradox, our study examined a natural QS system regulating production of a public good important for fitness, rather than an engineered system consisting of only signal molecule production as a cooperative behavior (27) or simulated systems not concerning other cellular constraints (28). While these other studies have pursued the use of simplified systems that avoid possible associated pleiotropic effects, here we explore a system that embraces all associated complexities and layers of pleiotropy that may have evolved between signaling, cooperation, and other coregulated behaviors. Another important distinction is that while these earlier examples primarily relied upon differences in growth rates between cooperators and defectors, here the key difference was that cooperators could achieve higher growth yields.
We predicted that growth under conditions of fragmented populations without gene flow between subpopulations would result in favored weighting of cooperator strains, mediated by Simpson's paradox, which can act to promote increases in cooperation due to differences in carrying capacities, and this is indeed what we observed (Fig. 3). These results are ecologically relevant because natural environments are frequently fragmented or structurally discontinuous, and differences in growth yields are likely to commonly occur among genotypes that elicit cooperative behaviors to differing degrees (37). Namely, it is relatively common for cooperative behaviors to positively influence population growth, and thus more cooperative genotypes should be able to achieve larger population sizes in environments where a cooperative behavior impacts an important trait like nutrient supply or accessibility.
To provide a more stringent test of Simpson's paradox promoting cooperation in Vibrio strains, we used larger starting populations of cooperators and defectors and allowed dispersal through the environment to be actively driven by the bacteria themselves using flagellar-based swimming, unlike previous studies examining competitions of cooperators and defectors during colonization and dispersal (38–42). Moreover, swimming motility is an individual behavior that cannot be cheated by nonproducers, unlike swarming motility, which has been extensively studied as a cooperative behavior (18). And unlike those results, cooperator strains are not completely dominated by defectors in the colony centers, or “homeland” (Fig. 6 and Fig. S4) (42), likely due to the pleiotropic effects that result from regulating other traits in addition to cooperative behavior with QS. Furthermore, colonies do not display the distinct stochastic sectoring patterns shown in these previous studies, but rather exhibit strongly uniform colony edges dominated by WT cooperators (Fig. S4).
Based on the results of previous studies in well-mixed conditions that did not include dispersal, we would expect DVh strains to outcompete UCVh strains and be equivalent with WTVh strains (14), while DVc strains should outcompete both cooperators (29). Interestingly, WT cooperator strains were capable of gaining large advantages on colony edges, despite being significantly less motile than defector strains when grown in monocultures, suggesting that the success of the WT strains resulted in part from the ability to more fully utilize resources rather than just from cooperator dispersal (Fig. 5 and 6 and Fig. S5). However, this advantage was not indefinitely maintained (Fig. S4), suggesting this could lead to altering cycles of invasion between cooperators and defectors. Although the laboratory conditions that we implement here are highly useful for inferring competitive dynamics, they likely do not fully mimic growth in the environment in several ways, including the abundance of resources, lack of spatial heterogeneity, and the time scale of competitions. Therefore, we expect that in the natural marine environment where Vibrio bacteria reside, resources are highly heterogeneous and populations sizes are much lower, and thus more reflective of the earlier time points of our assay.
Previous population genetic results for production of the public good siderophores in Vibrio strains demonstrated coexistence between producers and nonproducers (43), and our results similarly show mixed populations at the colony frontiers after several days of colony growth (Fig. 6 and Fig. S4). While trade-offs between colonization and dispersal in Vibrio strains have been reported, the flexibility in gene regulation and resulting physiological growth responses afforded to the WT strains by QS could help mediate this type of trade-off and provide strong competitiveness under a variety of selective pressures (40, 44). It is even possible that QS regulation could provide a more heterogeneous population-level response, in which subpopulations of cells invest more heavily in dispersal or growth (45–47).
Examining outcomes in motility plates confirmed our predictions that while UC strains performed quite well in metapopulation experiments that had strong bottlenecks and diminished mixing of cooperators and defectors, they were less fit than defectors in more mixed populations (Fig. S5). Alternatively, for both V. harveyi and V. cholerae, the WT strains were more fit relative to defectors than UC strains were in the colony center, where the populations were larger and more well-mixed, and this improvement in competitive growth impacted its ability to reach and succeed at the colony edges (Fig. 6). Indeed, for both species, the WT strain was highly successful at the edges of the dispersed colonies, where it exhibited a majority throughout 2 days of competition (Fig. S6). This result is consistent with observations in other systems that cooperators can outpace defectors at the leading edge of range expansions (42, 48); however, those previous studies focused solely on growth effects in the absence of motility, while here, cooperator success depends upon both growth parameters and functional motility.
The composition of motility agar medium used in this study constitutes a uniform, resource-rich environment. A patchier and more varied environment, such as particles encountered in natural aquatic environments, could select for both the ability to disperse rapidly to reach new patches and also to grow maximally on patches of limited resource once colonized (49–52). In such environments, the defector strains could act as a “fugitive species,” while the cooperator strains would represent a strategy that possesses superior colonizing capabilities, resulting in their increased carrying capacities. Our results suggest that WT strains harness quorum sensing to best utilize both dispersal and growth, which would diminish the potential trade-off between them (40, 53, 54). Indeed, the quorum sensing system of Vibrio bacteria negatively regulates biofilm formation (55), and computational modeling predicts this regulatory network is designed to promote dispersal from a biofilm at the appropriate time (39). Fugitives and colonizers could also coexist in more complex environments capable of supporting multiple niches, the fugitive strains by dispersing more effectively to open patches or those occupied by populations of exploitable cooperators and the cooperator strains by being more fecund in this environment.
This complex scenario would be expected for aquatic Vibrio strain that are known to cycle between motile, planktonic lifestyles and formation of biofilms on chitin surfaces. Once attached to the chitin, the bacteria secrete extracellular chitinases, another form of public good that degrades the polymer, releasing N-acetylglucosamine monomers and oligomers for growth (56). Expression of chitinases is also regulated by quorum sensing, like the proteases that are studied here, although in the case of the former, the regulation is negative (57). Our results suggest that the patchy resource environment in which Vibrio bacteria often exist is ideal for maintaining cooperative traits, especially when coregulated with other traits, which could explain the prevalence and stability of the complex QS systems that have evolved in these bacteria. Our results also explain the observation that both locked low- and high-cell-density Vibrio mutants can be isolated from natural sources (15).
Together, we have demonstrated that cooperation can be favored in strains utilizing QS when assortment is sufficient to allow differences in growth and motility to be realized. One way in which this was observed was that competing strains underwent population bottlenecks and assortment before regrowth, allowing Simpson's paradox to occur for both WT and UC cooperators. While bottlenecks have been appreciated as potentially important for maintaining cooperative behaviors in populations (58, 59), our findings show that even in large populations, cooperation can be promoted if there is sufficient assortment between cooperators and noncooperators through mechanisms such as bacterial motility.
MATERIALS AND METHODS
Strains and media.
V. harveyi strain BB120 (wild type [WT], ATCC BAA-1116, recently reassigned as Vibrio campbellii, a sister taxon in the Harveyi clade, and to which we refer with the common-use designation V. harveyi in this study [60, 61]) and derivative strains JAF78 (ΔluxOU [62]) and KM669 (ΔluxR [63]) were grown at 30°C as indicated. V. cholerae strain C6706str2 (wild type [WT]) and derivative strains ΔluxO (64), ΔhapR (65), and ΔflrA (34) were grown at 35°C. Strains were grown in M9-casein, a liquid medium composed of M9 salts (Sigma-Aldrich or Beckton, Dickinson, and Company) supplemented with sodium chloride (Macron) to 2% (wt/vol) and sodium caseinate (Sigma-Aldrich) as the sole carbon source at a concentration of 0.5% (wt/vol). We have previously found that noncooperator strains lacking protease production can grow to low levels in this medium, suggesting the casein contains nutrients that do not require public good proteolysis for utilization (14). Strains were streaked from frozen stocks, and individual colonies were inoculated into and passaged for 24 h in LB before growth in M9-casein medium. With the exception of motility plate competitions, all growth was conducted in liquid media. Viable cell counts were conducted on LB agar plates.
Metapopulation growth.
Strains were passaged in monoculture in M9-casein medium for 24 h before mixing. During initial mixing, 6 biological replicates each of competing strains were washed, diluted to equivalent densities, and then combined to form 3 replicate cultures. Upon mixing, cells were diluted and divided into subpopulations in 96-well plates and incubated under shaking conditions for growth. Dilution estimates were made based upon OD600 measurements, such that the target density was approximately 2 cells per subpopulation. Estimates of average starting subpopulation size were additionally corroborated by counting the number of empty wells (determined as wells with no significant increase in OD600 over the medium control at the completion of the growth cycle) present and fitting that number to a Poisson distribution (as in reference 27; Fig. S1). Plates were grown for 3 days at 30°C for V. harveyi strains and for 2 days at 35°C for V. cholerae strains between transfers, to allow subpopulations to reach saturating densities. Plates were incubated on orbital shakers to facilitate even mixing within subpopulations. After the incubation period, growth (for both V. harveyi and V. cholerae) and bioluminescence (for V. harveyi only) of all subpopulations were measured with an Envision plate reader (PerkinElmer). Bioluminescence data are reported as normalized bioluminescence (RLU/OD600). All subpopulations were combined into a metapopulation, and overall growth was measured using viable cell counts on LB agar plates. The frequency of cooperators versus defectors was determined by quantifying bioluminescent and nonluminescent colonies for V. harveyi, while the frequency of V. cholerae cooperators/defectors was determined by quantifying rugose colonies (indicative phenotype of the ΔhapR strain) and smooth colonies, indicative of the cooperator strains. This cycle was completed a total of 8 times.
Motility plates.
Prior to plating, strains were grown for 16 h in LB medium and then transferred and subsequently grown in M9-casein medium for 24 h for acclimation. Competing strains were mixed in a 1:1 ratio, and approximately 106 total cells were plated on the surface of M9-casein petri plates containing 0.3% agar. Cells were spotted in the center of the plate and incubated to allow populations to grow and disperse (34). V. harveyi plates were incubated at 30°C, and V. cholerae plates were incubated at 35°C. Plates were imaged daily with an AlphaImager HP imaging system (ProteinSimple). Center samples were picked from the inoculation point on the motility plate, and edge samples were obtained from the visible boundary of the expanding colonies, sampled daily over the course of the experiment. When whole colonies were sampled on day 3, the entire dispersed colony was excised from the plate, suspended in 10 ml M9-casein liquid medium, vortexed until homogenized, and then diluted and plated to assess colony composition. Data presented in Fig. 5 were normalized to the maximum dispersal area from the inoculation point for a given species on a given day and then standardized to the dispersal fraction obtained by the WT strain of that species.
Statistical comparisons.
All analyses were performed using R version 3.0.2. In all graphs containing them, error bars represent 95% confidence intervals. One-way analysis of variance (ANOVA) was used for comparisons between strains or competition treatments (Fig. 6 and Fig. S6). Statistical comparisons in Fig. 2 and 5 were completed by two-way ANOVA with Tukey's honestly significant difference (HSD) test for multiple comparisons. Comparisons between productivity and cooperator frequency were conducted via linear regression (Fig. S3).
Supplementary Material
ACKNOWLEDGMENTS
E.L.B. and C.M.W. designed the experiments, E.L.B. conducted the experiments, E.L.B. analyzed the data, and E.L.B. and C.M.W. wrote the paper.
We declare no conflict of interest.
This study was supported by grants NIH GM109259, GM110444, AI130554, and NSF MCB-1253684 to C.M.W., Frank Peabody and Dr. Marvin Hensley fellowships to E.L.B., and the BEACON Center for the Study of Evolution in Action (NSF Cooperative Agreement DBI-0939454).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.00402-18.
REFERENCES
- 1.Hamilton WD. 1964. The genetical evolution of social behaviour. I. J Theor Biol 7:1–16. [DOI] [PubMed] [Google Scholar]
- 2.Hamilton WD. 1964. The genetical evolution of social behaviour II. J Theor Biol 7:17–52. [DOI] [PubMed] [Google Scholar]
- 3.Axelrod R, Hamilton WD. 1981. The evolution of cooperation. Science 211:1390–1396. doi: 10.1126/science.7466396. [DOI] [PubMed] [Google Scholar]
- 4.Nowak MA. 2006. Five rules for the evolution of cooperation. Science 314:1560–1563. doi: 10.1126/science.1133755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruger E, Waters C. 2015. Sharing the sandbox: evolutionary mechanisms that maintain bacterial cooperation. F1000Research 4:1504. doi: 10.12688/f1000research.7363.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hardin G. 1968. The tragedy of the commons. Science 162:1243–1248. doi: 10.1126/science.162.3859.1243. [DOI] [PubMed] [Google Scholar]
- 7.Rankin DJ, Bargum K, Kokko H. 2007. The tragedy of the commons in evolutionary biology. Trends Ecol Evol 22:643–651. doi: 10.1016/j.tree.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 8.MacLean RC. 2008. The tragedy of the commons in microbial populations: insights from theoretical, comparative and experimental studies. Heredity (Edinb) 100:233–239. doi: 10.1038/sj.hdy.6801073. [DOI] [PubMed] [Google Scholar]
- 9.Foster KR. 2004. Diminishing returns in social evolution: the not-so-tragic commons. J Evol Biol 17:1058–1072. doi: 10.1111/j.1420-9101.2004.00747.x. [DOI] [PubMed] [Google Scholar]
- 10.Heilmann S, Krishna S, Kerr B. 2015. Why do bacteria regulate public goods by quorum sensing?—How the shapes of cost and benefit functions determine the form of optimal regulation. Front Microbiol 6:767. doi: 10.3389/fmicb.2015.00767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waters CM, Bassler BL. 2005. Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol 21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 12.Ng WL, Bassler BL. 2009. Bacterial quorum-sensing network architectures. Annu Rev Genet 43:197–222. doi: 10.1146/annurev-genet-102108-134304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Kessel JC, Rutherford ST, Shao Y, Utria AF, Bassler BL. 2013. Individual and combined roles of the master regulators AphA and LuxR in control of the Vibrio harveyi quorum-sensing regulon. J Bacteriol 195:436–443. doi: 10.1128/JB.01998-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruger EL, Waters CM. 2016. Bacterial quorum sensing stabilizes cooperation by optimizing growth strategies. Appl Environ Microbiol 82:6498–6506. doi: 10.1128/AEM.01945-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Wang H, Cui Z, Chen H, Zhong Z, Kan B, Zhu J. 2011. The prevalence of functional quorum-sensing systems in recently emerged Vibrio cholerae toxigenic strains. Environ Microbiol Rep 3:218–222. doi: 10.1111/j.1758-2229.2010.00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griffin AS, West SA, Buckling A. 2004. Cooperation and competition in pathogenic bacteria. Nature 430:1024–1027. doi: 10.1038/nature02744. [DOI] [PubMed] [Google Scholar]
- 17.Kreft JU. 2004. Biofilms promote altruism. Microbiology 150:2751–2760. doi: 10.1099/mic.0.26829-0. [DOI] [PubMed] [Google Scholar]
- 18.Xavier JB, Kim W, Foster KR. 2011. A molecular mechanism that stabilizes cooperative secretions in Pseudomonas aeruginosa. Mol Microbiol 79:166–179. doi: 10.1111/j.1365-2958.2010.07436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dandekar AA, Chugani S, Greenberg EP. 2012. Bacterial quorum sensing and metabolic incentives to cooperate. Science 338:264–266. doi: 10.1126/science.1227289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Popat R, Crusz SA, Messina M, Williams P, West SA, Diggle SP. 2012. Quorum-sensing and cheating in bacterial biofilms. Proc Biol Sci 279:4765–4771. doi: 10.1098/rspb.2012.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.García-Contreras R, Nunez-Lopez L, Jasso-Chávez R, Kwan BW, Belmont JA, Rangel-Vega A, Maeda T, Wood TK. 2015. Quorum sensing enhancement of the stress response promotes resistance to quorum quenching and prevents social cheating. ISME J 9:115–125. doi: 10.1038/ismej.2014.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang M, Schaefer AL, Dandekar AA, Greenberg EP. 2015. Quorum sensing and policing of Pseudomonas aeruginosa social cheaters. Proc Natl Acad Sci U S A 112:2187–2191. doi: 10.1073/pnas.1500704112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steenackers HP, Parijs I, Foster KR, Vanderleyden J. 2016. Experimental evolution in biofilm populations. FEMS Microbiol Rev 40:373–397. doi: 10.1093/femsre/fuw002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simpson EH. 1951. The interpretation of interaction in contingency tables. J R Stat Soc Series B Stat Methodol 13:238–241. [Google Scholar]
- 25.Blyth CR. 1972. On Simpson's paradox and the sure-thing principle. J Am Stat Assoc 67:364–366. doi: 10.1080/01621459.1972.10482387. [DOI] [Google Scholar]
- 26.Wilson DS. 1975. A theory of group selection. Proc Natl Acad Sci U S A 72:143–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chuang JS, Rivoire O, Leibler S. 2009. Simpson's paradox in a synthetic microbial system. Science 323:272–275. doi: 10.1126/science.1166739. [DOI] [PubMed] [Google Scholar]
- 28.Cremer J, Melbinger A, Frey E. 2012. Growth dynamics and the evolution of cooperation in microbial populations. Sci Rep 2:281. doi: 10.1038/srep00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katzianer DS, Wang H, Carey RM, Zhu J. 2015. “Quorum non-sensing”: social cheating and deception in Vibrio cholerae. Appl Environ Microbiol 81:3856–3862. doi: 10.1128/AEM.00586-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levins R. 1969. Some demographic and genetic consequences of environmental heterogeneity for biological control. Bull Entomol Soc Am 15:237–240. [Google Scholar]
- 31.Nadell CD, Foster KR, Xavier JB. 2010. Emergence of spatial structure in cell groups and the evolution of cooperation. PLoS Comput Biol 6:e1000716. doi: 10.1371/journal.pcbi.1000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Srivastava D, Waters CM. 2012. A tangled web: regulatory connections between quorum sensing and cyclic di-GMP. J Bacteriol 194:4485–4493. doi: 10.1128/JB.00379-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Q, Defoirdt T. 2015. Quorum sensing positively regulates flagellar motility in pathogenic Vibrio harveyi. Environ Microbiol 17:960–968. doi: 10.1111/1462-2920.12420. [DOI] [PubMed] [Google Scholar]
- 34.Srivastava D, Hsieh ML, Khataokar A, Neiditch MB, Waters CM. 2013. Cyclic di-GMP inhibits Vibrio cholerae motility by repressing induction of transcription and inducing extracellular polysaccharide production. Mol Microbiol 90:1262–1276. doi: 10.1111/mmi.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Queller DC. 1992. Does population viscosity promote kin selection? Trends in Ecol Evol 7:322–324. doi: 10.1016/0169-5347(92)90120-Z. [DOI] [PubMed] [Google Scholar]
- 36.Queller DC. 1994. Genetic relatedness in viscous populations. Evol Ecol 8:70–73. doi: 10.1007/BF01237667. [DOI] [Google Scholar]
- 37.Foster KR, Parkinson K, Thompson CR. 2007. What can microbial genetics teach sociobiology? Trends Genet 23:74–80. doi: 10.1016/j.tig.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xavier JB, Foster KR. 2007. Cooperation and conflict in microbial biofilms. Proc Natl Acad Sci U S A 104:876–881. doi: 10.1073/pnas.0607651104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nadell CD, Xavier JB, Levin SA, Foster KR. 2008. The evolution of quorum sensing in bacterial biofilms. PLoS Biol 6:e14. doi: 10.1371/journal.pbio.0060014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nadell CD, Bassler BL. 2011. A fitness trade-off between local competition and dispersal in Vibrio cholerae biofilms. Proc Natl Acad Sci U S A 108:14181–14185. doi: 10.1073/pnas.1111147108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Penn AS, Conibear TC, Watson RA, Kraaijeveld AR, Webb JS. 2012. Can Simpson's paradox explain co-operation in Pseudomonas aeruginosa biofilms? FEMS Immunol Med Microbiol 65:226–235. doi: 10.1111/j.1574-695X.2012.00970.x. [DOI] [PubMed] [Google Scholar]
- 42.Van Dyken JD, Müller MJ, Mack KM, Desai MM. 2013. Spatial population expansion promotes the evolution of cooperation in an experimental prisoner's dilemma. Curr Biol 23:919–923. doi: 10.1016/j.cub.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cordero OX, Ventouras LA, DeLong EF, Polz MF. 2012. Public good dynamics drive evolution of iron acquisition strategies in natural bacterioplankton populations. Proc Natl Acad Sci U S A 109:20059–20064. doi: 10.1073/pnas.1213344109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yawata Y, Cordero OX, Menolascina F, Hehemann JH, Polz MF, Stocker R. 2014. Competition–dispersal tradeoff ecologically differentiates recently speciated marine bacterioplankton populations. Proc Natl Acad Sci U S A 111:5622–5627. doi: 10.1073/pnas.1318943111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anetzberger C, Pirch T, Jung K. 2009. Heterogeneity in quorum sensing-regulated bioluminescence of Vibrio harveyi. Mol Microbiol 73:267–277. doi: 10.1111/j.1365-2958.2009.06768.x. [DOI] [PubMed] [Google Scholar]
- 46.Anetzberger C, Schell U, Jung K. 2012. Single cell analysis of Vibrio harveyi uncovers functional heterogeneity in response to quorum sensing signals. BMC Microbiol 12:209. doi: 10.1186/1471-2180-12-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Gestel J, Vlamakis H, Kolter R. 2015. Division of labor in biofilms: the ecology of cell differentiation. Microbiol Spectr 3:MB-0002. doi: 10.1128/microbiolspec.MB-0002-2014. [DOI] [PubMed] [Google Scholar]
- 48.Datta MS, Korolev KS, Cvijovic I, Dudley C, Gore J. 2013. Range expansion promotes cooperation in an experimental microbial metapopulation. Proc Natl Acad Sci U S A 110:7354–7359. doi: 10.1073/pnas.1217517110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu DW, Wilson HB, Pierce NE. 2001. An empirical model of species coexistence in a spatially structured environment. Ecology 82:1761–1771. doi: 10.1890/0012-9658(2001)082[1761:AEMOSC]2.0.CO;2. [DOI] [Google Scholar]
- 50.Cadotte MW, Fortner AM, Fukami T. 2006. The effects of resource enrichment, dispersal, and predation on local and metacommunity structure. Oecologia 149:150–157. doi: 10.1007/s00442-006-0426-z. [DOI] [PubMed] [Google Scholar]
- 51.Muller-Landau HC. 2010. The tolerance–fecundity trade-off and the maintenance of diversity in seed size. Proc Natl Acad Sci U S A 107:4242–4247. doi: 10.1073/pnas.0911637107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Datta MS, Sliwerska E, Gore J, Polz MF, Cordero OX. 2016. Microbial interactions lead to rapid micro-scale successions on model marine particles. Nat Comm 7:11965. doi: 10.1038/ncomms11965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hutchinson GE. 1951. Copepodology for the ornithologist. Ecology 32:571–577. doi: 10.2307/1931746. [DOI] [Google Scholar]
- 54.Yi X, Dean AM. 2016. Phenotypic plasticity as an adaptation to a functional trade-off. Elife 5:e19307. doi: 10.7554/eLife.19307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Waters CM, Lu W, Rabinowitz JD, Bassler BL. 2008. Quorum sensing controls biofilm formation in Vibrio cholerae through modulation of cyclic di-GMP levels and repression of vpsT. J Bacteriol 190:2527–2536. doi: 10.1128/JB.01756-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Drescher K, Nadell CD, Stone HA, Wingreen NS, Bassler BL. 2014. Solutions to the public goods dilemma in bacterial biofilms. Curr Biol 24:50–55. doi: 10.1016/j.cub.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Defoirdt T, Darshanee Ruwandeepika HA, Karunasagar I, Boon N, Bossier P. 2010. Quorum sensing negatively regulates chitinase in Vibrio harveyi. Environ Microbiol Rep 2:44–49. doi: 10.1111/j.1758-2229.2009.00043.x. [DOI] [PubMed] [Google Scholar]
- 58.Brockhurst MA. 2007. Population bottlenecks promote cooperation in bacterial biofilms. PLoS One 2:e634. doi: 10.1371/journal.pone.0000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Waite AJ, Cannistra C, Shou W. 2015. Defectors can create conditions that rescue cooperation. PLoS Comput Biol 11:e1004645. doi: 10.1371/journal.pcbi.1004645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bassler BL, Greenberg EP, Stevens AM. 1997. Cross-species induction of luminescence in the quorum sensing bacterium Vibrio harveyi. J Bacteriol 179:4043–4045. doi: 10.1128/jb.179.12.4043-4045.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lin B, Wang Z, Malanoski AP, O'Grady EA, Wimpee CF, Vuddhakul V, Alves N Jr, Thompson FL, Gomez-Gil B, Vora GJ. 2010. Comparative genomic analyses identify the Vibrio harveyi genome sequenced strains BAA-1116 and HY01 as Vibrio campbellii. Environ Microbiol Rep 2:81–89. doi: 10.1111/j.1758-2229.2009.00100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Freeman JA, Bassler BL. 1999. A genetic analysis of the function of LuxO, a two-component response regulator involved in quorum sensing in Vibrio harveyi. Mol Microbiol 31:665–677. doi: 10.1046/j.1365-2958.1999.01208.x. [DOI] [PubMed] [Google Scholar]
- 63.Pompeani AJ, Irgon JJ, Berger MF, Bulyk ML, Wingreen NS, Bassler BL. 2008. The Vibrio harveyi master quorum-sensing regulator, LuxR, a TetR-type protein is both an activator and a repressor: DNA recognition and binding specificity at target promoters. Mol Microbiol 70:76–88. doi: 10.1111/j.1365-2958.2008.06389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miller MB, Skorupski K, Lenz DH, Taylor RK, Bassler BL. 2002. Parallel quorum sensing systems converge to regulate virulence in Vibrio cholerae. Cell 110:303–314. doi: 10.1016/S0092-8674(02)00829-2. [DOI] [PubMed] [Google Scholar]
- 65.Hammer BK, Bassler BL. 2009. Distinct sensory pathways in Vibrio cholerae El Tor and classical biotypes modulate cyclic dimeric GMP levels to control biofilm formation. J Bacteriol 191:169–177. doi: 10.1128/JB.01307-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




