ABSTRACT
Microbial mutualistic cross-feeding interactions are ubiquitous and can drive important community functions. Engaging in cross-feeding undoubtedly affects the physiology and metabolism of individual species involved. However, the nature in which an individual species' physiology is influenced by cross-feeding and the importance of those physiological changes for the mutualism have received little attention. We previously developed a genetically tractable coculture to study bacterial mutualisms. The coculture consists of fermentative Escherichia coli and phototrophic Rhodopseudomonas palustris. In this coculture, E. coli anaerobically ferments sugars into excreted organic acids as a carbon source for R. palustris. In return, a genetically engineered R. palustris strain constitutively converts N2 into NH4+, providing E. coli with essential nitrogen. Using transcriptome sequencing (RNA-seq) and proteomics, we identified transcript and protein levels that differ in each partner when grown in coculture versus monoculture. When in coculture with R. palustris, E. coli gene expression changes resembled a nitrogen starvation response under the control of the transcriptional regulator NtrC. By genetically disrupting E. coli NtrC, we determined that a nitrogen starvation response is important for a stable coexistence, especially at low R. palustris NH4+ excretion levels. Destabilization of the nitrogen starvation regulatory network resulted in variable growth trends and, in some cases, extinction. Our results highlight that alternative physiological states can be important for survival within cooperative cross-feeding relationships.
IMPORTANCE Mutualistic cross-feeding between microbes within multispecies communities is widespread. Studying how mutualistic interactions influence the physiology of each species involved is important for understanding how mutualisms function and persist in both natural and applied settings. Using a bacterial mutualism consisting of Rhodopseudomonas palustris and Escherichia coli growing cooperatively through bidirectional nutrient exchange, we determined that an E. coli nitrogen starvation response is important for maintaining a stable coexistence. The lack of an E. coli nitrogen starvation response ultimately destabilized the mutualism and, in some cases, led to community collapse after serial transfers. Our findings thus inform on the potential necessity of an alternative physiological state for mutualistic coexistence with another species compared to the physiology of species grown in isolation.
KEYWORDS: Escherichia coli, NtrC, Rhodopseudomonas, coculture, cross-feeding, fermentation, mutualism, nitrogen fixation, nitrogen starvation, photoheterotrophy
INTRODUCTION
Within diverse microbial communities, species engage in nutrient cross-feeding with reciprocating partners as a survival strategy (1). In cases where species are not obligate mutualists, transitioning from a free-living lifestyle to one based on cross-feeding can change the physiological state of the cells involved, the extent to which depends on the nature of the cross-feeding relationship. Cross-feeding can promote physiological changes that increase virulence in pathogens, for example, by increasing the fitness of pathogenic subpopulations (2) or by supporting the establishment of polymicrobial infections (3). In addition, cross-feeding interactions can drastically alter cellular metabolism (4), in some cases allowing for lifestyles that are possible only during mutualistic growth with a partner (4–7). For example, microbial lifestyles that are normally thermodynamically infeasible, such as the fermentation of ethanol to acetate and H2 gas, can become feasible when a microbe is paired with a cooperative partner that consumes the fermentation products, essentially pulling the metabolism of its partner (5, 7). Aside from these examples, relatively little is known about how cell physiology is influenced by mutualistic cross-feeding, despite the prevalence of cross-feeding in microbial communities.
Synthetic communities, or cocultures, are ideally suited for studying the physiological responses to cooperative cross-feeding given their tractability (8, 9). We previously developed a bacterial coculture that consists of fermentative Escherichia coli and the N2-fixing photoheterotroph Rhodopseudomonas palustris (Fig. 1) (10). In this coculture, E. coli anaerobically ferments glucose into organic acids, providing R. palustris with essential carbon. In return, a genetically engineered R. palustris strain (Nx) constitutively fixes N2 gas, resulting in NH4+ excretion that provides E. coli with essential nitrogen. The result is an obligate mutualism that maintains a stable coexistence and reproducible growth trends (10) as long as bidirectional nutrient cross-feeding levels are maintained within a defined range (11, 12).
FIG 1.
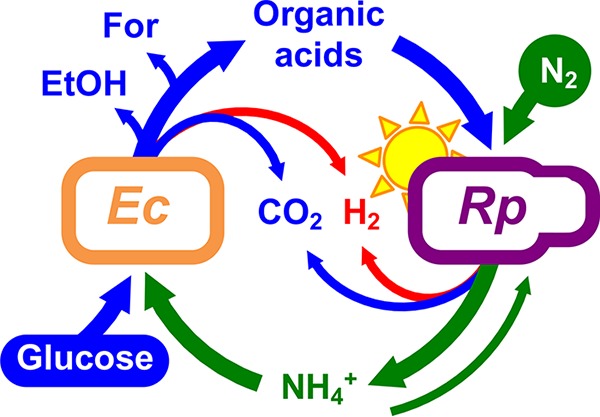
Bidirectional cross-feeding of carbon and nitrogen in an anaerobic bacterial mutualism between fermentative Escherichia coli (Ec) and phototrophic Rhodopseudomonas palustris (Rp). E. coli ferments glucose into excreted organic acids that R. palustris Nx consumes (acetate, lactate, and succinate) and other products that R. palustris Nx does not consume (formate [For] and ethanol [EtOH]). In return, R. palustris Nx constitutively fixes N2 gas and excretes NH4+, supplying E. coli with essential nitrogen. R. palustris Nx grows photoheterotrophically, wherein organic compounds are used for carbon and electrons and light is used for energy.
Here we determined how nutrient cross-feeding between E. coli and R. palustris Nx alters the physiological state of each partner population. Using transcriptome sequencing (RNA-seq) and proteomic analyses, we identified genes in both species that were differentially expressed in coculture compared to monoculture, with E. coli exhibiting more overall changes in gene expression than R. palustris Nx. Specifically, E. coli gene expression patterns resembled that of nitrogen-deprived cells, as many upregulated genes were within the nitrogen starvation response regulon, controlled by the master transcriptional regulator NtrC. Genetic disruption of E. coli ntrC resulted in variable growth trends at low R. palustris NH4+ excretion levels and prevented long-term mutualistic coexistence with R. palustris across serial transfers. Our results highlight the fact that cross-feeding relationships can stimulate alternative physiological states for at least one of the partners involved and that adjusting cell physiology to these alternative states can be critical for maintaining coexistence.
RESULTS
Engaging in an obligate mutualism alters the physiology of cooperating partners.
In our coculture, E. coli and R. palustris Nx carry out complementary anaerobic metabolic processes whose products serve as essential nutrients for the respective partner. Specifically, E. coli ferments glucose into acetate, lactate, and succinate, which serve as carbon sources for R. palustris Nx, while other fermentation products, such as formate and ethanol, accumulate; in return, R. palustris Nx fixes N2 and excretes NH4+ as the nitrogen source for E. coli (Fig. 1). We demonstrated previously that our coculture supports a stable coexistence and exhibits reproducible growth and metabolic trends when started from a wide range of starting species ratios, including single colonies (10). However, we hypothesized that coculture conditions would affect the physiology of each species, particularly E. coli, based on the following observations. First, as growth is coupled in our coculture, E. coli is forced to grow 4.6 times slower in coculture with R. palustris Nx than it does in monoculture with abundant NH4+ due to slow NH4+ cross-feeding from R. palustris Nx (10). In contrast, R. palustris Nx grows at a rate in coculture that is comparable to that in monoculture (12), consuming excreted organic acids from E. coli. Second, coculturing pulls E. coli fermentation forward due to the removal of inhibitory end products by R. palustris. For example, we observed higher yields of formate, an E. coli fermentation product that R. palustris does not consume, in cocultures than in E. coli monocultures (10).
To determine changes in gene expression patterns imposed by coculturing, we performed RNA-seq and comparative proteomic analyses (13) on exponential-phase cocultures and monocultures of E. coli and R. palustris Nx. To make direct comparisons, all cultures were grown in the same basal anaerobic minimal medium, and monocultures were supplemented with the required carbon or nitrogen sources to permit growth for each species. Cocultures and E. coli monocultures were provided glucose as a sole carbon source, whereas a mixture of organic acids and bicarbonate was provided to R. palustris Nx monocultures, as R. palustris does not consume glucose. For a nitrogen source, all cultures were grown under a N2 headspace, and E. coli monocultures were further supplemented with NH4Cl, as E. coli is incapable of using N2. We identified several differentially expressed genes between monoculture and coculture conditions in both species, with more differences being observed for E. coli than for R. palustris Nx, in agreement with our initial hypothesis (Fig. 2). For E. coli, out of 4,377 open reading frames (ORFs), 55 were upregulated and 68 were downregulated (Table 1) (log2 value cutoff of 2). Out of 4,836 ORFs in R. palustris Nx, 14 were upregulated and 20 were downregulated (Table 1) (log2 value cutoff of 2). We also considered that due to the lower E. coli abundance in coculture, the apparently larger E. coli gene response may be due partly to decreased resolution and, thus, increased error variance. Reassuringly, many of the genes identified as being differentially expressed by RNA-seq were in agreement with the proteomic results (Table 2). Both RNA-seq and proteomic analyses identified the E. coli ammonium transporter AmtB as an important, upregulated gene in coculture, corroborating our previous findings that E. coli AmtB activity is important for stable coexistence with R. palustris (12). Many E. coli genes involved in amino acid and purine biosynthesis were downregulated in coculture (Tables 1 and 2), consistent with the lower observed growth rate. Additionally, many E. coli flagellar and chemotaxis proteins were downregulated in coculture (Tables 1 and 2), perhaps suggesting that motility is not important for coculture growth. Alternatively, lower flagellar and chemotaxis transcript levels could be part of a general stress response (14), perhaps associated with nitrogen limitation in coculture. Whereas many of the differentially expressed E. coli genes have been characterized in the literature, the R. palustris genes showing the largest differential expression were uncharacterized genes encoding upregulated putative alcohol/aldehyde dehydrogenases and a downregulated putative TonB-dependent receptor/siderophore (Tables 1 and 2). Together, these data sets provide insight into how engaging in obligate cross-feeding changes the lifestyle of each partner.
FIG 2.
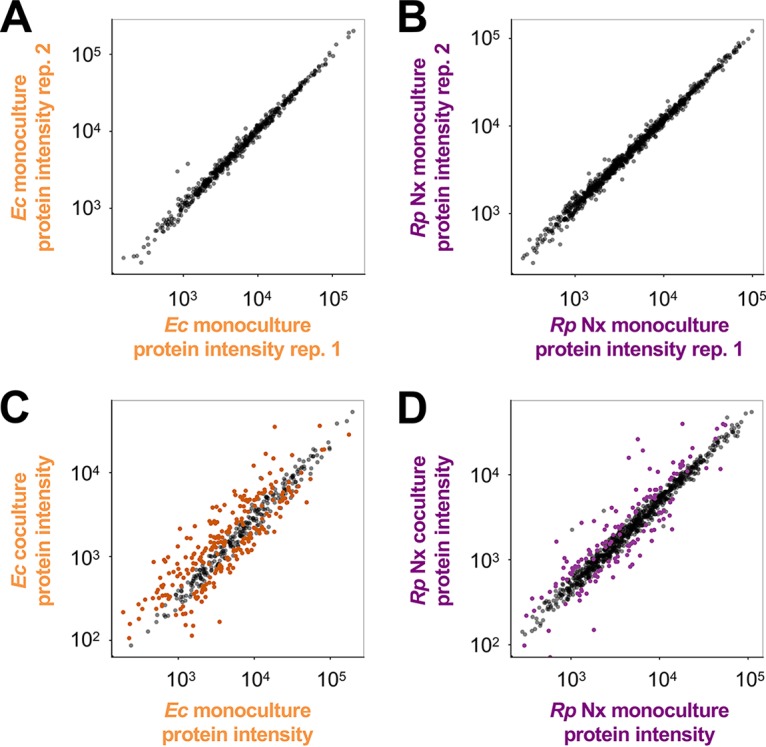
Coculture conditions result in altered protein expression patterns in both species, with more differences in WT E. coli than in R. palustris Nx. Protein expression (estimated by the LC-MS/MS sum of peptide intensities) of WT E. coli (left) (A and C) and R. palustris Nx (right) (B and D) comparing protein expression patterns between monoculture biological replicates (replicate 1 versus replicate 2) (A and B) and monoculture (average of data from monoculture replicates) versus coculture (C and D). Colored spots indicate significant differences in protein expression levels determined by using the median of normalized peptide ratios rather than summed peptide ratios. Significant changes in protein expression levels under coculture conditions at a false discovery rate of 1% were computed by determining the ratio, r, at which 99% of genes have a ratio less than r when comparing biological-replicate monocultures. Total protein sums are shown. Thus, gray spots that appear far from the center diagonal reflect proteins that did not show a significant change compared using the median of normalized peptide ratios.
TABLE 1.
Selected differentially expressed transcripts in cocultures of E. coli and R. palustris compared to monoculturesd
| Species | Gene | Gene description or general role |
R. palustris Nx + WT E. coli |
R. palustris NxΔAmtB + WT E. coli |
R. palustris ΔAmtB + WT E. coli |
|||
|---|---|---|---|---|---|---|---|---|
| Mean fold change ± SDc | FDR-adjusted P value | Mean fold change ± SD | FDR-adjusted P value | Mean fold change ± SD | FDR-adjusted P value | |||
| E. coli | rutAb | Pyrimidine degradation | 114.5 ± 0.0 | 0.09 | 108.0 ± 0.0 | 0.09 | 118.0 ± 0.1 | 0.09 |
| rutCb | Pyrimidine degradation | 60.7 ± 0.1 | 0.01 | 58.0 ± 0.1 | 0.01 | 60.9 ± 0.1 | 0.01 | |
| ddpXa,b | d-Ala dipeptidase | 58.3 ± 0.1 | 0.01 | 59.9 ± 0.1 | 0.01 | 50.1 ± 0.0 | 0.01 | |
| rutDb | Pyrimidine degradation | 56.9 ± 0.0 | 0.01 | 52.9 ± 0.1 | 0.01 | 56.6 ± 0.1 | 0.01 | |
| rutEb | Pyrimidine degradation | 48.8 ± 0.1 | 0.01 | 44.4 ± 0.1 | 0.01 | 48.2 ± 0.1 | 0.01 | |
| rutFb | Pyrimidine degradation | 45.2 ± 0.1 | 0.01 | 40.3 ± 0.1 | 0.01 | 45.5 ± 0.1 | 0.01 | |
| patAa,b | Putrescine aminotransferase | 36.3 ± 0.1 | 0.01 | 33.6 ± 0.1 | 0.01 | 34.4 ± 0.0 | 0.01 | |
| argTa,b | Lysine/arginine/ornithine binding protein | 35.1 ± 0.3 | 0.01 | 38.9 ± 0.3 | 0.01 | 35.3 ± 0.3 | 0.01 | |
| rutGb | Pyrimidine degradation | 28.5 ± 0.0 | 0.01 | 26.9 ± 0.0 | 0.01 | 29.0 ± 0.1 | 0.01 | |
| ddpAa,b | Dipeptide binding periplasmic protein | 23.7 ± 0.0 | 0.01 | 26.8 ± 0.0 | 0.01 | 21.0 ± 0.0 | 0.01 | |
| amtBa,b | Ammonium transporter | 21.3 ± 0.2 | 0.02 | 25.0 ± 0.2 | 0.01 | 24.1 ± 0.2 | 0.01 | |
| yeaGb | Eukaryote-like serine/threonine kinase | 13.6 ± 0.0 | 0.08 | 15.2 ± 0.0 | 0.04 | 14.5 ± 0.1 | 0.06 | |
| yeaHb | Unknown | 12.8 ± 0.0 | 0.06 | 14.2 ± 0.1 | 0.05 | 14.0 ± 0.1 | 0.06 | |
| metE | Methionine biosynthesis | −16.2 ± 0.1 | 0.03 | −23.6 ± 0.6 | 0.03 | −22.8 ± 0.5 | 0.02 | |
| fimF | Fimbria regulatory protein | −16.3 ± 0.0 | 0.01 | −18.4 ± 0.0 | 0.01 | −20.3 ± 0.1 | 0.01 | |
| tar | Methyl-accepting chemotaxis protein II | −16.3 ± 0.2 | 0.01 | −15.8 ± 0.2 | 0.02 | −15.4 ± 0.2 | 0.01 | |
| purLa | Purine biosynthesis | −16.8 ± 0.0 | 0.03 | −20.4 ± 0.1 | 0.02 | −18.8 ± 0.0 | 0.02 | |
| flgD | Flagellar basal body rod modification | −17.1 ± 0.1 | 0.02 | −16.9 ± 0.0 | 0.01 | −17.4 ± 0.1 | 0.01 | |
| ilvLa | Branched-chain amino acid biosynthesis | −17.4 ± 0.7 | 0.02 | −14.9 ± 0.4 | 0.02 | −14.2 ± 0.5 | 0.02 | |
| pgaB | Glucosamine deacetylase | −17.9 ± 0.0 | 0.02 | −18.8 ± 0.0 | 0.03 | −17.3 ± 0.0 | 0.04 | |
| ilvCa | Branched-chain amino acid biosynthesis | −18.0 ± 0.2 | 0.03 | −17.1 ± 0.2 | 0.04 | −17.6 ± 0.2 | 0.03 | |
| metK | Methionine biosynthesis | −19.2 ± 0.1 | 0.03 | −17.5 ± 0.1 | 0.03 | −17.4 ± 0.1 | 0.04 | |
| tap | Methyl-accepting chemotaxis protein IV | −19.7 ± 0.3 | 0.01 | −22.0 ± 0.2 | 0.01 | −22.1 ± 0.2 | 0.01 | |
| flgC | Flagellar basal body rod protein | −20.1 ± 0.1 | 0.05 | |||||
| purKa | Purine biosynthesis | −20.7 ± 0.1 | 0.03 | −25.1 ± 0.1 | 0.01 | −21.0 ± 0.1 | 0.03 | |
| metA | Methionine biosynthesis | −21.0 ± 0.1 | 0.02 | −20.6 ± 0.1 | 0.02 | −20.8 ± 0.2 | 0.02 | |
| ilvGa | Branched-chain amino acid biosynthesis | −22.1 ± 0.1 | 0.01 | −19.3 ± 0.1 | 0.03 | −22.1 ± 0.1 | 0.01 | |
| metF | Methionine biosynthesis | −23.3 ± 0.1 | 0.01 | −22.5 ± 0.1 | 0.01 | −17.6 ± 0.4 | 0.03 | |
| nadB | Aspartate oxidase | −24.3 ± 0.0 | 0.08 | −29.1 ± 0.1 | 0.05 | −23.7 ± 0.0 | 0.07 | |
| R. palustris | RPA1206a | Aldehyde dehydrogenase | 36.0 ± 0.9 | 0.02 | 62.4 ± 0.4 | 0.01 | ||
| RPA1205a | Putative alcohol dehydrogenase | 32.8 ± 0.5 | 0.02 | 28.6 ± 0.4 | 0.01 | |||
| RPA0538 | Putative porin | 31.6 ± 2.3 | 0.03 | |||||
| RPA1009a | Possible cytochrome P450 | 10.4 ± 0.8 | 0.03 | |||||
| RPA3101a | Unknown | 9.4 ± 0.3 | 0.03 | 10.3 ± 0.3 | 0.04 | |||
| RPA4045a | Putative amino acid ABC transport | 8.8 ± 0.4 | 0.02 | |||||
| RPA3100 | Unknown | 7.8 ± 0.2 | 0.02 | |||||
| RPA1010 | Beta-lactamase-like | 7.7 ± 0.4 | 0.04 | |||||
| RPA4020a | Putative amino acid ABC transport | 7.7 ± 0.2 | 0.02 | |||||
| RPA1204 | Unknown | 7.4 ± 0.1 | 0.02 | 7.4 ± 0.1 | 0.03 | |||
| RPA2376 | Unknown | −6.9 ± 0.1 | 0.04 | −15.4 ± 0.2 | 0.04 | −9.0 ± 0.2 | 0.03 | |
| RPA2142 | Putative fatty acid CoA ligase | −7.3 ± 0.1 | 0.03 | |||||
| RPA2377 | Unknown | −8.4 ± 0.2 | 0.02 | −16.4 ± 0.6 | 0.05 | −7.3 ± 0.1 | 0.02 | |
| RPA2379 | Probable acetyltransferase | −8.5 ± 0.3 | 0.02 | |||||
| RPA2390 | Possible rhizobactin siderophore biosynthesis | −9.6 ± 0.2 | 0.06 | −22.8 ± 0.2 | 0.05 | −16.8 ± 0.5 | 0.03 | |
| RPA1260a | Universal stress protein | −10.5 ± 0.0 | 0.02 | −7.2 ± 0.0 | 0.07 | |||
| RPA2380 | Possible TonB-dependent iron siderophore | −11.4 ± 0.6 | 0.03 | −17.1 ± 0.1 | 0.06 | −18.4 ± 0.2 | 0.01 | |
| RPA1259 | Putative cation-transporting P-type ATPase | −11.6 ± 0.4 | 0.02 | −10.6 ± 0.0 | 0.06 | |||
| RPA2378a | Putative TonB-dependent receptor | −13.1 ± 0.1 | 0.03 | −24.1 ± 0.3 | 0.06 | −17.5 ± 0.3 | 0.02 | |
Genes were also identified as differentially expressed proteins in coculture (Table 2 and supplemental material).
The gene is transcriptionally activated by E. coli NtrC during nitrogen limitation.
Fold change values represent means ± standard deviations. Positive values indicate that the gene was upregulated in coculture. Negative values indicate that the gene was downregulated in coculture. The initial cutoff was set to a log2 value of 2 in at least 2 of 3 biological replicates. For a complete list of all differentially regulated transcripts, see the supplemental material. Differential expression was determined with the Cufflinks tool Cuffdiff (v.2.2.0) (46).
The genes shown here are directly or indirectly mentioned in the text. For a full list of differentially expressed genes, see the supplemental material.
TABLE 2.
Selected differentially expressed proteins in cocultures of E. coli and R. palustris compared to monoculturese
| Species | Gene | Gene description or general role | Normalized relative protein intensity |
||
|---|---|---|---|---|---|
| R. palustris Nx + WT E. colic | R. palustris NxΔAmtB + WT E. colid | R. palustris ΔAmtB + WT E. colid | |||
| E. coli | argTa,b | Lysine/arginine/ornithine binding protein | 10.9 | 11.1 | 10.3 |
| ddpAa,b | d-Ala dipeptide permease | 5.8 | 7.2 | 6.8 | |
| gss | Bifunctional glutathionylspermidine synthetase/amidase | 4.5 | 4.7 | 4.0 | |
| tktB | Transketolase | 4.1 | 5.5 | 3.8 | |
| potFa,b | Putrescine binding periplasmic protein | 3.8 | 4.2 | 4.1 | |
| modA | Molybdate binding periplasmic protein | 3.8 | 4.0 | 4.9 | |
| gabDa,b | Succinate-semialdehyde dehydrogenase | 3.7 | 4.8 | 3.5 | |
| dapB | 4-Hydroxy-tetrahydrodipicolinate reductase | 3.6 | 2.8 | 2.7 | |
| talA | Transaldolase A | 3.6 | 4.2 | 4.4 | |
| amtBa,b | Ammonium transporter | 3.5 | 3.5 | 3.5 | |
| asnS | Asparagine biosynthesis | −2.1 | −1.9 | −1.9 | |
| serA | Serine biosynthesis | −2.1 | −2.5 | −2.3 | |
| secE | Protein translocase subunit | −2.1 | −1.8 | −2.3 | |
| glf | Lipopolysaccharide biosynthesis | −2.1 | −1.9 | −1.9 | |
| yjiM | Putative dehydratase | −2.2 | −1.9 | −1.8 | |
| sstT | Serine/threonine transporter | −2.2 | −2.4 | −2.4 | |
| rmlA1 | Carbohydrate biosynthesis | −2.3 | −2.1 | −2.4 | |
| ompF | Outer membrane porin | −2.3 | −2.3 | −2.6 | |
| ribE | Riboflavin biosynthesis | −2.3 | −1.7 | −1.9 | |
| secY | Protein translocase subunit | −2.6 | −2.0 | −2.0 | |
| glyA | Glycine biosynthesis | −3.2 | −3.0 | −3.4 | |
| purEa | Purine biosynthesis | −3.3 | −3.6 | −3.5 | |
| yqjI | Transcriptional regulator | −3.6 | −3.0 | −3.4 | |
| asnA | Aspartate-ammonia ligase | −6.4 | −3.8 | −3.7 | |
| R. palustris | RPA1206a | Aldehyde dehydrogenase | 10.0 | 3.3 | |
| RPA1205a | Putative alcohol dehydrogenase | 7.8 | 1.2 | 3.9 | |
| RPA3101a | Unknown | 7.1 | 1.5 | 2.6 | |
| RPA3093 | ABC transporter urea/short-chain binding protein | 4.8 | 1.6 | 3.4 | |
| RPA3297 | ABC transporter urea/short-chain binding protein | 4.7 | 1.5 | 3.2 | |
| RPA4019 | Putative amino acid ABC transport | 3.9 | 1.4 | 2.5 | |
| RPA4045a | Putative amino acid ABC transport | 3.3 | 1.4 | 2.1 | |
| RPA1009a | Possible cytochrome P450 | 3.2 | 1.3 | 2.1 | |
| RPA1748 | Putative branched-chain-amino-acid transport | −2.1 | −1.4 | −2.5 | |
| RPA2378a | Putative TonB-dependent receptor protein | −2.1 | −1.2 | −1.1 | |
| RPA2124 | TonB-dependent iron siderophore receptor | −2.3 | −1.5 | −1.7 | |
| RPA1260a | Universal stress protein | −2.5 | −1.5 | −1.8 | |
| RPA2050 | Unknown | −2.7 | −1.6 | −2.6 | |
| RPA3669 | Putative urea short-chain amide or branched-chain amino acid ABC transport | −2.8 | −1.1 | −1.3 | |
| RPA2120 | Periplasmic binding protein | −6.0 | −1.6 | −1.7 | |
Genes were also identified as differentially expressed transcripts in coculture (Table 1 and supplemental material).
The gene is transcriptionally activated by E. coli NtrC.
Values represent mean normalized relative protein intensities for two biological replicates. Positive values indicate that the gene was upregulated in coculture. Negative values indicate that the gene was downregulated in coculture.
Values represent mean normalized relative protein intensity for one biological replicate. Positive values indicate that the gene was upregulated in coculture. Negative values indicate that the gene was downregulated in coculture.
Proteins shown here are directly or indirectly mentioned in the text. For a full list of differentially expressed proteins, see the supplemental material.
An E. coli nitrogen starvation response is important for mutualistic growth with R. palustris.
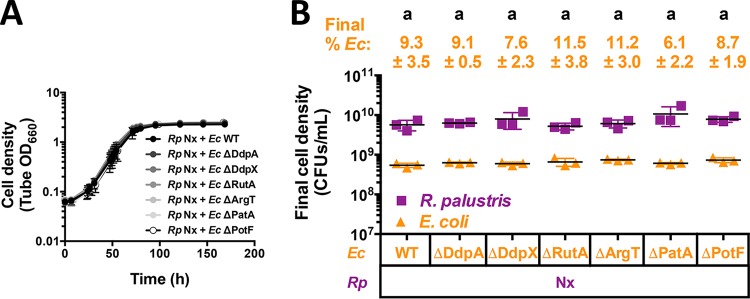
We chose to further examine differential gene expression patterns in E. coli, as its growth rate and fermentation profile are drastically affected by coculturing, whereas the R. palustris Nx growth rate is similar to that in monoculture. We identified several E. coli genes and proteins that were upregulated in coculture with R. palustris Nx compared to monoculture growth (Tables 1 and 2). We hypothesized that the deletion of highly upregulated E. coli genes would negatively affect E. coli growth in coculture. We made deletions in E. coli genes that were identified in both RNA-seq and proteome data sets as well as the most highly upregulated E. coli transcript (rutA). We did not examine the effect of the deletion of amtB in this case, as we determined previously that it was important for coculture growth (12). These selected E. coli genes were all previously known to be involved in the metabolism of alternative nitrogen sources, such as d-Ala–d-Ala dipeptides (ddpX and ddpA) (15), pyrimidines (rutA) (16), amino acids (argT) (17), and polyamines (patA and potF) (18). In monocultures with 15 mM NH4Cl, there were negligible differences in growth or fermentation profiles between wild-type (WT) E. coli and any of the single deletion mutants (see Fig. S1 in the supplemental material). These results are consistent with findings by others, as these genes are important only when scavenging nitrogen sources that are not present in our defined medium. We next tested these E. coli mutants in coculture with R. palustris Nx. All cocultures using the E. coli mutants paired with R. palustris Nx exhibited growth and population trends similar to those of cocultures with WT E. coli (Fig. 3). Additionally, there were no significant differences in the growth rates, growth yields, or product yields from cocultures containing the E. coli mutants (Fig. S2). These data suggest that none of these highly expressed E. coli genes are solely important for coculture growth. While we cannot rule out rapid evolution of compensatory mutations, the emergence of such mutations is unlikely, given that mutations would be expected to arise at different times in each replicate coculture and lead to variable trends, whereas we observed little variability between biological replicates (Fig. 3). It is also possible that the synergistic expression of these genes is important for E. coli growth in coculture. However, the nitrogen sources that E. coli could access by expressing these genes are absent in the defined medium. Thus, unless E. coli gains access to alternative nitrogen sources that we are unaware of in coculture with R. palustris Nx (e.g., through unknown secondary cross-feeding mechanisms), the synergistic expression of these genes likely provides little to no benefit.
FIG 3.
Single deletions of upregulated E. coli genes do not impair mutualistic growth with R. palustris Nx. Shown are growth curves (A) and final cell densities (B) from cocultures pairing E. coli (Ec) mutants with deletions in highly upregulated genes with R. palustris (Rp) Nx. Final cell densities in panel B were taken at the final time point in panel A. Cocultures were started with a 1% inoculum of stationary-phase starter cocultures grown from single colonies. Error bars indicate standard deviations (n = 3). Different letters indicate statistical differences (P < 0.05), determined by one-way analysis of variance with Tukey's multiple-comparisons posttest.
Even though individual deletions of the E. coli genes showing high expression levels in coculture had no effect on coculture trends, we noted that they were all involved in nitrogen scavenging and fell within the regulon of the transcription factor NtrC, which controls the nitrogen starvation response (19). During nitrogen limitation, the sensor kinase NtrB phosphorylates the response regulator NtrC (19). Phosphorylated NtrC then binds to DNA and activates the expression of ∼45 genes (20), including those that we tested genetically as described above and amtB, which we previously determined to be important for coculture growth (12). To examine the importance of the E. coli nitrogen starvation response in coculture, we deleted ntrC. We first checked for any general defects of the resulting ΔNtrC mutant in monoculture with 15 mM NH4Cl and found that it exhibited growth and metabolic trends similar to those of WT E. coli (Fig. S3). We then paired E. coli ΔNtrC with R. palustris Nx in coculture. Compared to cocultures using WT E. coli, cocultures with E. coli ΔNtrC exhibited lower growth rates, longer lag periods (Fig. 4A), and lower final E. coli cell densities (Fig. 4D). The long lag phase was less prominent in cocultures inoculated from single colonies (Fig. S4A) than in cocultures inoculated with a 1% dilution of stationary cocultures (Fig. 4A). This result suggests that starting E. coli ΔNtrC cocultures from single colonies stimulated early growth, perhaps by increasing the E. coli frequency to be similar to that of R. palustris when cultures were started with colonies of similar sizes rather than a dilution of stationary cocultures wherein the E. coli frequency was low (∼0.1%) (Fig. 4D). A higher initial E. coli frequency might help E. coli acquire excreted NH4+ before it is taken back up by R. palustris cells and thereby promote reciprocal cross-feeding, similar to what we observed previously in cocultures with E. coli ΔAmtB mutants that were defective for NH4+ uptake (12).
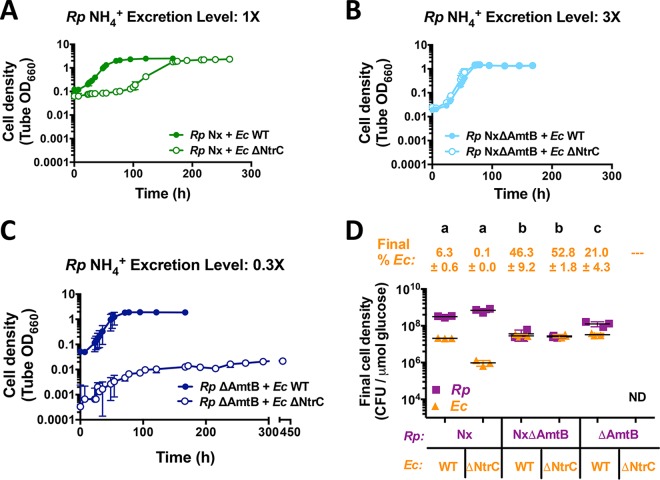
FIG 4.
R. palustris NH4+ excretion level affects growth and population trends in cocultures with E. coli NtrC. Shown are growth curves (A to C) and final cell densities normalized to glucose consumption (D) from cocultures pairing WT E. coli (Ec) or the ΔNtrC mutant with R. palustris (Rp) strains with different NH4+ excretion levels. Final cell densities in panel D were taken at the final time points in the respective growth curves in panels A to C, except for cocultures pairing R. palustris ΔAmtB with E. coli ΔNtrC, which were sampled at 260 h. Cell densities were normalized to the levels of glucose consumed to account for incomplete glucose consumption in cocultures containing E. coli ΔNtrC. Cocultures were started with a 1% inoculum of stationary-phase starter cocultures grown from single colonies. Error bars indicate standard deviations (n = 3). Different letters indicate statistical differences (P < 0.05), determined by one-way analysis of variance with Tukey's multiple-comparisons posttest. ND, not determined.
The overall coculture metabolism was also altered when E. coli ΔNtrC was paired with R. palustris Nx. In cocultures pairing WT E. coli with R. palustris Nx, glucose is typically fully consumed within 5 days, coinciding with the accumulation of formate and ethanol (10). Cocultures pairing E. coli ΔNtrC with R. palustris Nx left ∼40% of the glucose unconsumed after 10 days and exhibited little to no formate and ethanol accumulation (Fig. S4B). Even despite the lower rate of glucose consumption, the final R. palustris cell density of cocultures pairing R. palustris Nx with E. coli ΔNtrC was similar to that with WT E. coli. This unexpectedly high cell density could be explained by the consumption of formate and ethanol by R. palustris Nx, although we have never observed the consumption of formate by R. palustris Nx in monoculture. Alternatively, a lack of formate and/or ethanol production by E. coli could explain the high cell density if the fermentation profile were shifted toward organic acids that R. palustris normally consumes, namely, acetate, lactate, and succinate. Together, these data indicate that the misregulation of the nitrogen starvation response affected coculture growth and metabolism.
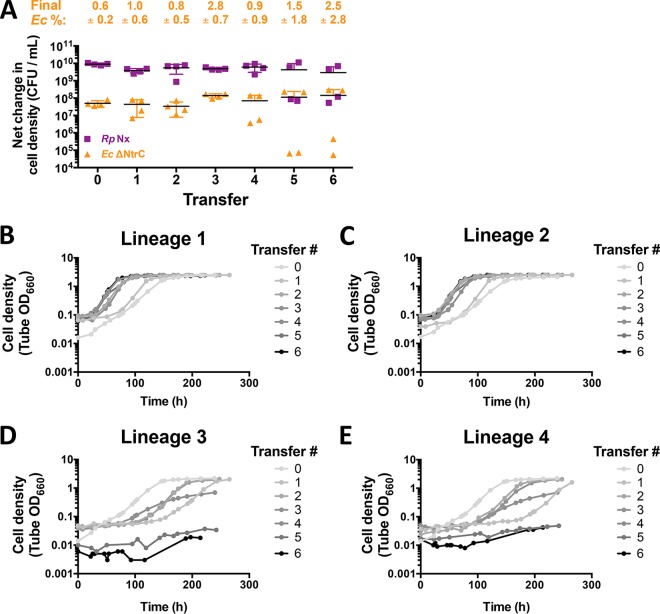
As noted above, the small E. coli ΔNtrC population and decreased coculture growth rate when paired with R. palustris Nx resembled trends from cocultures that contained E. coli ΔAmtB mutants (12). We found previously that the E. coli NH4+ transporter AmtB was required for coexistence with R. palustris Nx across serial transfers, as the transporter gives E. coli a competitive advantage in acquiring the transiently available NH4+ before it can be reclaimed by the R. palustris population (12). To determine if E. coli ΔNtrC was capable of maintaining a stable coexistence in coculture, we inoculated cocultures pairing E. coli ΔNtrC with R. palustris Nx at equivalent CFU and performed serial transfers every 10 days. While average final E. coli frequencies were consistently between 0.6 and 2.8% (Fig. 5A), the values became variable over serial transfers, as did coculture growth rates, lag periods, and net changes in both E. coli and R. palustris cell densities (Fig. 5). This variability was due to 2 of the 4 lineages exhibiting improved coculture growth over successive transfers (Fig. 5B and C), perhaps due to the emergence of compensatory mutations, while the other two lineages showed declining-growth trends (Fig. 5D and E). Indeed, by transfers 5 and 6, there was little to no coculture growth in the slower-growing lineages (Fig. 5D and E). The heterogeneity in growth trends through serial transfers of cocultures with E. coli ΔNtrC is in stark contrast to the stability of cocultures with WT E. coli, which we have serially transferred over 100 times with no extinction events (J. B. McKinlay, unpublished data). The nitrogen starvation response is thus important for the long-term survival of the mutualism.
FIG 5.
Deletion of ntrC in E. coli results in variable coculture growth trends across serial transfers. Shown are net changes in cell densities (A) and replicate growth curves (B to E) of cocultures pairing the E. coli (Ec) ΔNtrC strain with R. palustris (Rp) Nx across serial transfers. Cocultures were initially inoculated (transfer zero) at a 1:1 starting species ratio based on CFU per milliliter from R. palustris and E. coli monocultures. A 1% inoculum was used for each serial transfer. Transfers were performed every 10 days. Error bars indicate standard deviations (n = 4).
Increased NH4+ cross-feeding levels can compensate for the absence of a nitrogen starvation response.
The NtrC regulon is critical during periods of nitrogen starvation, activating a wide variety of genes that are important for scavenging diverse nitrogen sources (20). We hypothesized that higher R. palustris NH4+ cross-feeding levels could mitigate the poor growth of E. coli ΔNtrC in coculture by making the nitrogen starvation response less important for survival. Previously, we engineered an R. palustris Nx strain that excretes 3-fold more NH4+ by deleting R. palustris NH4+ transporters encoded by amtB1 and amtB2 (NxΔAmtB) (10). N2-fixing bacteria use AmtB to reacquire NH4+ that leaks outside the cell, and ΔAmtB mutants thus accumulate NH4+ in the supernatant (10, 12, 21). In agreement with our hypothesis, cocultures with R. palustris NxΔAmtB exhibited similar growth trends regardless of the E. coli strain used (Fig. 4B and D). As R. palustris NxΔAmtB excretes more NH4+ than does R. palustris Nx, it was shown previously to result in higher levels of WT E. coli growth and subsequent fermentation rates in coculture, ultimately leading to the accumulation of consumable organic acids (see Fig. S4B in the supplemental material) and acidification of the medium, inhibiting R. palustris growth (10). Cocultures pairing R. palustris NxΔAmtB with either WT E. coli or the ΔNtrC strain exhibited growth (Fig. 4B and D) and fermentation profile trends (Fig. S4B) similar to those observed previously (10). The similar trends of R. palustris NxΔAmtB cocultures with either E. coli strain indicate that high-level R. palustris NH4+ excretion can eliminate the trends observed when the E. coli nitrogen starvation response is compromised due to a ΔNtrC mutation.
One possibility for why high NH4+ cross-feeding levels eliminate the need for E. coli ntrC is that the free NH4+ levels might be sufficiently high enough to prevent the activation of the E. coli NtrC regulon. However, comparative RNA-seq and proteomic analyses revealed that the same E. coli genes within the NtrC regulon that were highly upregulated in cocultures pairing WT E. coli with R. palustris Nx were also upregulated in cocultures with R. palustris NxΔAmtB (Tables 1 and 2). Thus, even though the E. coli nitrogen starvation response is activated when E. coli is cocultured with R. palustris NxΔAmtB, this response is likely dispensable if there is a sufficiently high level of NH4+ cross-feeding.
E. coli NtrC is required for adequate AmtB expression to access cross-fed NH4+ in coculture.
While a high level of R. palustris NH4+ excretion can compensate for an improper E. coli nitrogen starvation response, less NH4+ excretion could potentially exaggerate problems emerging from the absence of NtrC. We previously constructed an R. palustris ΔAmtB strain that excreted one-third of the NH4+ produced by R. palustris Nx in monoculture and that could not coexist in coculture with an E. coli ΔAmtB strain (12). The reason for this lack of coexistence was due to R. palustris ΔAmtB outcompeting E. coli ΔAmtB for the lower level of transiently available NH4+, thus limiting E. coli growth and thereby the reciprocal supply of fermentation products to R. palustris (12). The expression of E. coli amtB is thus important in cocultures in order to maintain coexistence. Indeed, RNA-seq and proteomic analyses revealed that E. coli AmtB transcript and protein levels were upregulated in all cocultures pairing WT E. coli with any of the three R. palustris strains (Nx, NxΔAmtB, and ΔAmtB) (Tables 1 and 2). We thus wondered whether E. coli ΔNtrC would coexist with the low-NH4+-excreting R. palustris ΔAmtB strain in coculture, as E. coli amtB expression is transcriptionally activated by NtrC. Consistent with our previous findings, R. palustris ΔAmtB supported a relatively large WT E. coli population in coculture (Fig. 4D) (12). When cocultured with WT E. coli, R. palustris ΔAmtB responds to NH4+ loss to E. coli by upregulating nitrogenase activity, since it has a wild-type copy of NifA (12). As a result, R. palustris ΔAmtB cross-feeds enough NH4+ to stimulate a high WT E. coli frequency and the subsequent accumulation of consumable organic acids, similar to cocultures with R. palustris NxΔAmtB (Fig. 4D; see also Fig. S4B in the supplemental material) (12). In contrast, when we paired E. coli ΔNtrC with R. palustris ΔAmtB, little to no coculture growth was observed (Fig. 4C), similar to previous observations of cocultures pairing E. coli ΔAmtB with R. palustris ΔAmtB (12). Cocultures inoculated with single colonies of each species in this pairing grew to low cell densities (Fig. S4A), and cocultures inoculated from these cocultures resulted in little to no growth, even after prolonged incubation (Fig. 4C).
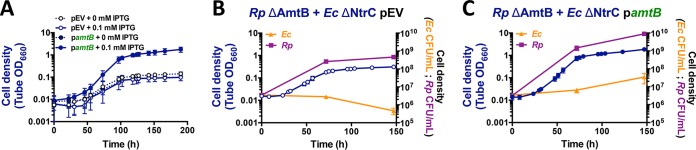
As AmtB is under the control of NtrC (20), we hypothesized that cocultures pairing E. coli ΔNtrC with R. palustris ΔAmtB would result in insufficient E. coli amtB expression, leading to a decreased ability of E. coli to capture NH4+, which R. palustris will reacquire if given the chance (12). We thus predicted that increasing expression of amtB in the E. coli ΔNtrC strain would result in increased net growth of both species, as E. coli ΔNtrC would be more competitive for essential NH4+ and be able to grow and produce more organic acids for R. palustris ΔAmtB. To test this prediction, we obtained a plasmid harboring an isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible copy of amtB (pamtB) for use in E. coli ΔNtrC cells. AmtB is typically tightly regulated and expressed only when NH4+ concentrations are below 20 μM, as cells acquire sufficient NH4+ through the passive diffusion of NH3 across the membrane at higher concentrations (22). Additionally, excessive NH4+ uptake through AmtB transporters that exceeds the rate of assimilation can result in a futile cycle, as excess NH3 inevitably diffuses outside the cell (19). We therefore first tested the effect of pamtB on WT E. coli monocultures with 15 mM NH4Cl. Induction with 1 mM IPTG prevented growth, whereas 0.1 mM IPTG permitted growth albeit at a decreased growth rate (Fig. S5). We thus decided to use 0.1 mM IPTG to induce amtB expression in all cocultures described below. In cocultures pairing E. coli ΔNtrC/pamtB with R. palustris ΔAmtB, more growth was observed than in cocultures with E. coli ΔNtrC harboring an empty vector (pEV) (Fig. 6A). In cocultures with E. coli ΔNtrC/pEV, the R. palustris ΔAmtB cell density increased, whereas the E. coli cell density did not (Fig. 6B). R. palustris growth was likely due to growth-independent cross-feeding of fermentation products from E. coli maintenance metabolism, a phenomenon that we described previously (11). In contrast, cell densities of both species increased in cocultures pairing R. palustris ΔAmtB with E. coli ΔNtrC/pamtB (Fig. 6C), in agreement with our hypothesis that poor E. coli amtB expression contributed to the lack of growth in this coculture pairing. While E. coli amtB expression in this coculture pairing was sufficient to restore the growth of both species, there could be other genes within the NtrC regulon that contribute to E. coli growth in coculture. For example, the E. coli NtrC-regulated serine/threonine kinase YeaG has been shown to play a role in survival during nitrogen starvation by promoting metabolic heterogeneity (23). Indeed, both E. coli YeaG and its associated protein of unknown function YeaH are highly upregulated in coculture (Table 1). Thus, while we cannot rule out that other genes within the E. coli NtrC regulon are not important for coculture growth, the necessity of NtrC for the upregulation of amtB is clearly important.
FIG 6.
Ectopic expression of amtB in the E. coli ΔNtrC strain permits mutualistic growth with the R. palustris ΔAmtB strain. Shown are growth curves (A to C) and cell densities for each species (B and C) from cocultures pairing the R. palustris (Rp) ΔAmtB strain with the E. coli (Ec) ΔNtrC strain harboring a plasmid encoding an IPTG-inducible copy of amtB (pamtB) or an empty vector (pEV). To maintain plasmids, all cocultures were supplemented with 5 μg/ml chloramphenicol, which is otherwise lethal to E. coli but not to R. palustris (see Fig. S6 in the supplemental material). Cocultures were inoculated with a single colony of each species (A) or at a 1:1 starting species ratio based on equivalent CFU per milliliter from starter R. palustris and E. coli monocultures (B and C). A total of 0.1 mM IPTG was added to the cocultures at the initial time point. Error bars indicate standard deviations (n = 3).
DISCUSSION
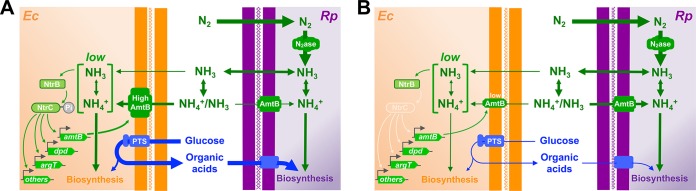
In this study, we found that reciprocal nutrient cross-feeding between E. coli and R. palustris resulted in significant changes in gene expression in both species compared to monocultures. For E. coli, our results indicate a model wherein the low level of NH4+ cross-feeding from R. palustris induces an E. coli nitrogen starvation response mediated by NtrBC (Fig. 7A). As part of this response, E. coli increases the expression level of the NH4+ transporter AmtB, giving E. coli an advantage in acquiring NH4+ before it is recaptured by the R. palustris population (Fig. 7A). Without NtrC, E. coli expresses less AmtB and thus is less competitive against R. palustris for acquiring excreted NH4+ (Fig. 7B). This decreased ability of E. coli to acquire NH4+ leads to a lower E. coli growth rate and a lower level of organic acid excretion, thereby starving R. palustris for carbon (Fig. 7B) and leading to variable population outcomes (Fig. 5). Thus, the alteration of E. coli physiology to a nitrogen-starved state is important for the coexistence of the two species under the conditions tested here.
FIG 7.
Summary of how an E. coli nitrogen starvation response impacts cross-feeding. The arrow thickness indicates relative flux. (A) Low NH4+ excretion levels by R. palustris (Rp) limit the ability of E. coli (Ec) to obtain NH4+ by diffusion across the membrane as NH3. Low NH4+ availability is sensed by E. coli through the sensor kinase NtrB, which phosphorylates the response regulator NtrC (see reference 19 for details on how nitrogen availability is sensed and transmitted). NtrC upregulates the expression of many genes involved in scavenging nitrogen, including the gene for the high-affinity NH4+ transporter AmtB. Higher AmtB levels allow E. coli to acquire the small amounts of NH4+ excreted by R. palustris, supporting E. coli growth and the mutualistic excretion of organic acids, which R. palustris uses as a carbon source. (B) Without NtrC, E coli AmtB levels remain low, and R. palustris has a competitive advantage in reacquiring excreted NH4+. Starved for nitrogen, E. coli growth and organic acid cross-feeding slow, thereby threatening the stability of the mutualism. PTS, phosphotransferase system. (Adapted from reference 12.)
Mutualistic nutrient cross-feeding has also been shown to change the lifestyles of interacting partners in other systems. In natural communities, nutrient cross-feeding can alter gene expression patterns to adapt each species to a syntrophic lifestyle (24–27). In some cases, the lifestyles exhibited within a mutualism might not even be possible during growth in isolation. For example, in synthetic communities that pair the sulfate reducer Desulfovibrio vulgaris with the methanogen Methanococcus maripaludis, the methanogen consumes H2, which maintains low partial pressures that permit the sulfate reducer to adopt a fermentative lifestyle that would otherwise be thermodynamically infeasible (5). Similarly, in an experimental Geobacter coculture, direct electron transfer from Geobacter metallireducens to Geobacter sulfurreducens makes ethanol fermentation by G. metallireducens thermodynamically possible (7).
Similar to our mutualistic system, the mutualism between D. vulgaris and M. maripaludis represents a facultative mutualism, at least in the short term prior to the evolutionary erosion of independent lifestyles (28). For mutualistic relationships to persist between partners that are conditionally capable of a free-living lifestyle, the relationship must exhibit resilience, or the ability to recover its function after a disturbance (29). One important resilience factor is the activation of regulatory networks that allow microbes to quickly respond to environmental perturbations. Whereas flexible gene expression is useful for an individual microbe's survival, excessive flexibility can sometimes lead to community collapse between mutualists in a fluctuating environment (30, 31). In the coculture of D. vulgaris and M. maripaludis, alternating between coculture and monoculture conditions, which require different metabolic lifestyles, resulted in community collapse (30, 31). Surprisingly, community collapse could be avoided by mutations that disrupted the D. vulgaris regulatory response needed to adapt cells for optimal growth rates in monoculture (30). A disruption of this regulatory response resulted in a heterogeneous D. vulgaris population, ensuring that a subpopulation would be primed for immediate mutualistic growth upon transitions between growth conditions (31). In our system, the E. coli nitrogen starvation regulatory network was activated by coculturing with R. palustris and was important for coculture stability. It is currently unclear if transitioning E. coli between monoculture and coculture conditions would result in a similar community collapse or whether the NtrC-regulated network would adjust rapidly to meet the demands of each condition.
Nutrient starvation and other stress responses are widely conserved in diverse microbes and are primarily regarded as being necessary for an individual's survival in nutrient-limited environments (32–35). Many microbial communities are composed of primarily slow-growing or even nongrowing subpopulations (36–38). However, the lack of microbial growth in these communities does not imply a cessation of cross-feeding, as bacteria often carry out growth-independent maintenance processes at low rates (39), and such activities can be coupled to cross-feeding (11). Our findings suggest that nutrient starvation and perhaps other stress responses can help stabilize microbial cross-feeding interactions, especially at low nutrient cross-feeding levels. The extent to which specific starvation or stress responses are active in diverse mutualistic relationships remains unclear yet likely depends on the environmental context. Together, our results highlight the important role that alternate physiological states, including stress responses, can play in establishing and maintaining mutualistic cross-feeding.
MATERIALS AND METHODS
Strains and growth conditions.
Strains, plasmids, and primers are listed in Table 3. All R. palustris strains contained ΔuppE and ΔhupS mutations to facilitate accurate CFU measurements by preventing cell aggregation (40) and to prevent H2 uptake, respectively. E. coli was cultivated on Luria-Bertani (LB) agar, and R. palustris was cultured on defined photosynthetic medium (PM) agar (41) with 10 mM succinate. (NH4)2SO4 was omitted from PM agar for determining R. palustris CFU. Monocultures and cocultures were grown in 10 ml of defined M9-derived coculture medium (MDC) (10) in 27-ml anaerobic test tubes under 100% N2, as described previously (10). For harvesting RNA and protein, 100-ml cultures were grown in 260-ml serum vials. In both cases, MDC was supplemented with a cation solution (1% [vol/vol]; 100 mM MgSO4 and 10 mM CaCl2) and glucose (25 mM), unless indicated otherwise. R. palustris monocultures were further supplemented with 15 mM sodium bicarbonate, 7.8 mM sodium acetate, 8.7 mM disodium succinate, 1.5 mM sodium lactate, 0.3 mM sodium formate, and 6.7 mM ethanol. E. coli monocultures were further supplemented with 2.5 mM NH4Cl. Kanamycin was added to a final concentration of 30 μg/ml for E. coli where appropriate. Chloramphenicol was added to a final concentration of 5 μg/ml for both R. palustris and E. coli where appropriate. All cultures were grown at 30°C, lying horizontally under a 60-W incandescent bulb with shaking at 150 rpm. Starter cocultures were inoculated with 200 μl MDC containing a suspension of a single colony of each species. Test cocultures and serial transfers were inoculated by using a 1% dilution from starter cocultures. For experiments requiring a starting species ratio of 1:1, E. coli and R. palustris starter monocultures were grown to equivalent cell densities and inoculated at equal volumes.
TABLE 3.
Strains, plasmids, and primers
| Strain, plasmid, or primer | Descriptiona or sequence (5′–3′) | Reference or purpose |
|---|---|---|
| Strains | ||
| R. palustris | ||
| CGA009 | Wild-type strain; spontaneous Cmr derivative of CGA001 | 45 |
| CGA4005 | CGA009 ΔhupS ΔuppE nifA*; Nx | 10 |
| CGA4021 | CGA4005 ΔamtB1 ΔamtB2; NxΔAmtB | 10 |
| CGA4026 | CGA009 ΔhupS ΔuppE ΔamtB1 ΔamtB2; ΔAmtB | 12 |
| E. coli | ||
| MG1655 | Wild-type K-12 strain; WT | 53 |
| K-12 JW1483 | Keio collection; ΔddpX::Km | 54 |
| K-12 JW5240 | Keio collection; ΔddpA::Km | 54 |
| K-12 JW0997 | Keio collection; ΔrutA::Km | 54 |
| K-12 JW2307 | Keio collection; ΔargT::Km | 54 |
| K-12 JW5510 | Keio collection; ΔpatA::Km | 54 |
| K-12 JW0838 | Keio collection; ΔpotF::Km | 54 |
| K-12 JW3840 | Keio collection; ΔntrC::Km | 54 |
| K-12/pCA24N(pASKA) | ASKA collection; pCA24N | 52 |
| MG1655/pCA24N −GFP | ASKA collection; pCA24N with GFP removed using NotI digestion | This study |
| K-12 JW0441-AM/pASKAamtB | ASKA collection; pCA24N-N-His-amtB (GFP minus) | 52 |
| MG1655ΔDdpX | MG1655 ΔddpX::Km; ΔDdpX | This study |
| MG1655ΔDdpA | MG1655 ΔddpA::Km; ΔDdpA | This study |
| MG1655ΔRutA | MG1655 ΔrutA::Km; ΔRutA | This study |
| MG1655ΔArgT | MG1655 ΔargT::Km; ΔArgT | This study |
| MG1655ΔPatA | MG1655 ΔpatA::Km; ΔPatA | This study |
| MG1655ΔPotF | MG1655 ΔpotF::Km; ΔPotF | This study |
| MG1655ΔNtrC | MG1655 ΔntrC::Km; ΔNtrC | This study |
| MG1655/pEV | MG1655/pCA24N; WT pEV | This study |
| MG1655ΔNtrC/pEC | MG1655 ΔntrC::Km/pCA24N; ΔNtrC/pEV | This study |
| MG1655/pamtB | MG1655/pCA24N-N-His-amtB+; WT/pamtB | This study |
| MG1655ΔNtrC/pamtB | MG1655 ΔntrC::Km/pCA24N-N-His-amt+; ΔNtrC/pamtB | This study |
| Plasmids | ||
| pCA24N | Cmr; ASKA collection empty vector with IPTG-inducible promoter | 52 |
| pCA24N-amtB+ | Cmr; ASKA collection vector with IPTG-inducible promoter in front of N-terminal His-tagged amtB gene | 52 |
| Primers | ||
| ALM47 | CGGAAAGCGCAGCAATTTTTGT | ddpX upstream (E. coli) |
| ALM48 | GAGCAATGTGGGACGAAACG | ddpX downstream (E. coli) |
| ALM45 | ATATCCCCTGGCACACAGC | ddpA upstream (E. coli) |
| ALM46 | CCAGCAGCGTTGGCGTAAAATA | ddpX downstream (E. coli) |
| ALM51 | CCGCTTTGCAAACAAGCC | rutA upstream (E. coli) |
| ALM52 | ATCAGCGCACTTTGCTGC | rutA downstream (E. coli) |
| ALM49 | GCAAACACACAACACAATACACAAC | argT upstream (E. coli) |
| ALM50 | CCATCAGGTACAGCTTCCCA | argT downstream (E. coli) |
| ALM53 | TGAAAGCGTGCTGTTAACGC | patA upstream (E. coli) |
| ALM54 | ATCCCGATTTTCGCGATCG | patA downstream (E. coli) |
| ALM55 | CTGGCCGGGAGAAAGTTCT | potF upstream (E. coli) |
| ALM56 | TTACGGGTTTTCGCCTGC | potF downstream (E. coli) |
| MO 7 | CAATCTTTACACACAAGCTGTGAATC | ntrC upstream (E. coli) |
| MO 8 | CCTGCCTATCAGGAAATAAAGG | ntrC downstream (E. coli) |
| pCA24N.for | GATAACAATTTCACACAGAATTCATTAAAGAG | pCA24N upstream of cloned gene |
| pCA24N.rev | CCCATTAACATCACCATCTAATTCAAC | pCA24N downstream of cloned gene |
Underlining indicates strain designations used in this study. GFP, green fluorescent protein.
Generation of E. coli mutants.
P1 transduction (42) was used to introduce deletions from Keio collection strains into MG1655. The genotype of kanamycin-resistant colonies was confirmed by PCR and sequencing.
Analytical procedures.
The cell density was assayed by determining the optical density at 660 nm (OD660) using a Genesys 20 visible spectrophotometer (Thermo-Fisher, Waltham, MA, USA). Growth curve readings were taken in culture tubes without sampling (i.e., tube OD660). Specific growth rates were determined by using readings between OD660 values of 0.1 and 1.0, where there is a linear correlation between the cell density and OD660. E. coli percentages of the total population in coculture, as determined by CFU counts, are also constant between these OD values (10). Final OD660 measurements were taken in cuvettes, and samples were diluted into the linear range as necessary. Cell densities measured by the OD660 are correlated with measurements of CFU per milliliter, throughout both the exponential growth and stationary phases. Levels of glucose, organic acids, formate, and ethanol were quantified by using a Shimadzu high-performance liquid chromatograph, as described previously (43).
Sample collection for transcriptomics and proteomics.
Monocultures and cocultures were grown in 100-ml volumes to late exponential phase and chilled in an ice water bath. A 1-ml sample was collected for protein quantification by using a Pierce bicinchoninic acid (BCA) protein assay kit according to the manufacturer's protocol. A 5-ml sample was removed for RNA extraction, and 90 ml was used for proteomic analysis. All samples were centrifuged at 4°C, supernatants were discarded, and cell pellets were frozen in liquid N2 and stored at −80°C.
RNA-seq.
Total RNA was isolated from cell pellets by using the RNeasy kit (Qiagen, Valencia, CA, USA) according to the manufacturer's protocol. To calculate baseline expression levels, RNA sequencing reads resulting from monocultures were mapped to their corresponding reference genomes (E. coli strain K-12 substrain MG1655 [44] [NCBI RefSeq accession number NC_000913.3] and R. palustris CGA0009 [45] [NCBI RefSeq accession number NC_005296.1]) by using the Tuxedo protocol for RNA expression analysis (46) (workflow deposited at https://github.com/MURI2/Task3/tree/master/RNA-Seq). Specifically, split reads were aligned to the reference genome with Tophat2 (v.2.1.0) (47) and Bowtie2 (v.2.1.0) (48). Following mapping, transcripts were assembled with Cufflinks (v.2.2.0) (49), and differential expression was identified with the Cufflinks tool Cuffdiff (v.2.2.0). To ensure that cross-mapping of homologous sequencing reads would not complicate expression analysis of data from the coculture experiments, monoculture reads were additionally mapped to the opposing genome, as described above. As all potential cross-mapping was confined to residual rRNA reads, these regions were excluded from the analysis, and the coculture RNA-seq reads were analyzed by mapping the sequenced reads to both reference genomes, with no further correction.
Preparation of protein samples for mass spectrometry (MS).
Cell pellets were resuspended in 1 ml total protein buffer (20 mM HEPES-NaOH [pH 7.4], 150 mM NaCl, 2 mM EDTA, 0.2 mM dithiothreitol, 1:100 dilution of phenylmethylsulfonyl fluoride, 1:100 dilution of protease inhibitors cocktail IV) and sonicated at 20% intensity (7 s on and 7 s off) for 5 min in an ice bath. A 1/10 volume of 20% SDS was then added. Samples were vortexed, boiled for 5 min, and immediately placed on ice. Debris was cleared by centrifugation for 30 s at 10,000 × g at 4°C, and the supernatant was collected. The protein contents of different lysates were analyzed by Coomassie staining following SDS-PAGE, and sample aliquots containing 200 μg protein were subjected to chloroform-methanol protein extraction, as described previously (50).
Analysis by LC-MS/MS.
Mass spectrometry was performed at the Mass Spectrometry and Proteomics Research Laboratory (MSPRL), FAS Division of Science, Harvard University. Samples were individually labeled with tandem mass tag (TMT) 10-plex reagents, according to the manufacturer's protocol (Thermo-Fisher Scientific), and mixed. The mixed sample was dried in a SpeedVac and rediluted with buffer A (0.1% formic acid in water) for injection for high-performance liquid chromatography (HPLC) runs. The sample was submitted for a single liquid chromatography-tandem mass spectrometry (LC-MS/MS) experiment, which was performed on an LTQ Orbitrap Elite instrument (Thermo-Fisher Scientific) equipped with a Waters (Milford, MA) NanoAcquity HPLC pump. Peptides were separated onto a 100-μm-inner-diameter microcapillary trapping column packed first with approximately 5 cm of C18 Reprosil resin (5 μm, 100 Å; Dr. Maisch GmbH, Germany) followed by a ∼20-cm analytical column of Reprosil resin (1.8 μm, 200 Å; Dr. Maisch GmbH, Germany). Separation was achieved by applying a gradient from 5 to 27% acetonitrile in 0.1% formic acid over 90 min at 200 nl min−1. Electrospray ionization was enabled by applying a voltage of 1.8 kV using a homemade electrode junction at the end of the microcapillary column and sprayed from fused silica pico tips (New Objective, MA). The LTQ Orbitrap Elite instrument was operated in data-dependent mode for the mass spectrometry methods. The mass spectrometry survey scan was performed with the Orbitrap instrument in the range of 395 to 1,800 m/z at a resolution of 6 × 104, followed by the selection of the 20 most intense ions (TOP20) for collision-induced dissociation (CID)–second MS (MS2) fragmentation in the ion trap using a precursor isolation width window of 2 m/z, an automatic gain control (AGC) setting of 10,000, and maximum ion accumulation at 200 ms. Singly charged ion species were not subjected to CID fragmentation. The normalized collision energy was set to 35 V and an activation time of 10 ms. Ions in a 10-ppm m/z window around ions selected for MS2 were excluded from further selection for fragmentation for 60 s. The same TOP20 ions were subjected to a higher-energy collisional dissociation (HCD) MS2 event in the Orbitrap part of the instrument. The fragment ion isolation width was set to 0.7 m/z, the AGC was set to 50,000, the maximum ion time was 200 ms, the normalized collision energy was set to 27 V, and an activation time of 1 ms was used for each HCD MS2 scan.
Mass spectrometry data analysis.
Raw data were submitted for analysis in MaxQuant 1.5.6.5 (13). Assignment of MS/MS spectra was performed by searching the data against a protein sequence database including all entries from the E. coli MG1655 proteome (51), the R. palustris CGA009 proteome (45), and other known contaminants, such as human keratins and common laboratory contaminants. MaxQuant searches were performed by using a 20-ppm precursor ion tolerance with a requirement that each peptide had N termini consistent with trypsin protease cleavage, allowing up to two missed cleavage sites. The 10-plex TMTs on peptide amino termini and lysine residues were set as static modifications, while methionine oxidation and deamidation of asparagine and glutamine residues were set as variable modifications. MS2 spectra were assigned a false discovery rate (FDR) of 1% at the protein level by a target-decoy database search. Per-peptide reporter ion intensities were exported from MaxQuant (evidence.txt). Only peptides with a parent ion fraction of ≥0.5 were used for subsequent analysis (6,063 of 9,987 peptides). Intensities were calculated as the sum of peptide intensities. Ratios between conditions were computed at the peptide level, and the protein ratio was computed as the mean of peptide ratios. All ratios were normalized by dividing by the median value for proteins from the same species. The ratio significance for coculture conditions at an FDR of 1% was computed by determining the ratio, r, at which 99% of genes have a ratio less than r when comparing biological-replicate monocultures.
Expression of E. coli amtB in coculture.
The ASKA collection (52) plasmid harboring an IPTG-inducible copy of amtB (pCA24N amtB) was purified from strain JW0441-AM and introduced by electroporation into WT E. coli and the E. coli ΔNtrC mutant. Cocultures were inoculated either with single colonies of each species or at a 1:1 starting species ratio, as indicated in the figure legends. IPTG and 5 μg/ml chloramphenicol were added to cocultures to induce E. coli amtB expression in cocultures and maintain the plasmid, respectively.
Accession number(s).
RNA-seq reads are available at the NCBI Sequence Read Archive under BioProject accession number PRJNA449071 (https://www.ncbi.nlm.nih.gov/sra). Raw proteomics data are available at the Chorus database (https://chorusproject.org/) under file 194480.
Supplementary Material
ACKNOWLEDGMENTS
We thank B. A. Budnik and R. A. Robins (Harvard MSPRL) for assistance with mass spectrometry. We thank P. L. Foster for providing the Keio and ASKA E. coli collections.
This work was supported in part by the U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research, under award number DE-SC0008131 to J.B.M.; by U.S. Army Research Office grant W911NF-14-1-0411 to M.L., D.A.D., and J.B.M.; by National Institutes of Health national service award F32GM123703 to M.G.B.; and by the Indiana University College of Arts and Sciences.
We declare no conflict of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.00404-18.
REFERENCES
- 1.Seth EC, Taga ME. 2014. Nutrient cross-feeding in the microbial world. Front Microbiol 5:350. doi: 10.3389/fmicb.2014.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hammer ND, Cassat JE, Noto MJ, Lojek LJ, Chadha AD, Schmitz JE, Creech CB, Skaar EP. 2014. Inter- and intraspecies metabolite exchange promotes virulence of antibiotic-resistant Staphylococcus aureus. Cell Host Microbe 16:531–537. doi: 10.1016/j.chom.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramsey MM, Rumbaugh KP, Whiteley M. 2011. Metabolite cross-feeding enhances virulence in a model polymicrobial infection. PLoS Pathog 7:e1002012. doi: 10.1371/journal.ppat.1002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iannotti EL, Kafkewit D, Wolin MJ, Bryant MP. 1973. Glucose fermentation products of Ruminococcus albus grown in continuous culture with Vibrio succinogenes: changes caused by interspecies transfer of H2. J Bacteriol 114:1231–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stolyar S, Van Dien S, Hillesland KL, Pinel N, Lie TJ, Leigh JA, Stahl DA. 2007. Metabolic modeling of a mutualistic microbial community. Mol Syst Biol 3:92. doi: 10.1038/msb4100131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker CB, Redding-Johanson AM, Baidoo EE, Rajeev L, He Z, Hendrickson EL, Joachimiak MP, Stolyar S, Arkin AP, Leigh JA, Zhou J, Keasling JD, Mukhopadhyay A, Stahl DA. 2012. Functional responses of methanogenic archaea to syntrophic growth. ISME J 6:2045–2055. doi: 10.1038/ismej.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Summers ZM, Fogarty HE, Leang C, Franks AE, Malvankar NS, Lovley DR. 2010. Direct exchange of electrons within aggregates of an evolved syntrophic coculture of anaerobic bacteria. Science 330:1413–1415. doi: 10.1126/science.1196526. [DOI] [PubMed] [Google Scholar]
- 8.Widder S, Allen RJ, Pfeiffer T, Curtis TP, Wiuf C, Sloan WT, Cordero OX, Brown SP, Momeni B, Shou W, Kettle H, Flint HJ, Haas AF, Laroche B, Kreft J. 2016. Challenges in microbial ecology: building predictive understanding of community function and dynamics. ISME J 10:2557–2568. doi: 10.1038/ismej.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindemann SR, Bernstein HC, Song H-S, Fredrickson JK, Fields MW, Shou W, Johnson DR, Beliaev AS. 2016. Engineering microbial consortia for controllable outputs. ISME J 10:2077–2084. doi: 10.1038/ismej.2016.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LaSarre B, McCully AL, Lennon JT, McKinlay JB. 2017. Microbial mutualism dynamics governed by dose-dependent toxicity of cross-fed nutrients. ISME J 11:337–348. doi: 10.1038/ismej.2016.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCully AL, LaSarre B, McKinlay JB. 2017. Growth-independent cross-feeding modifies boundaries for coexistence in a bacterial mutualism. Environ Microbiol 19:3538–3550. doi: 10.1111/1462-2920.13847. [DOI] [PubMed] [Google Scholar]
- 12.McCully AL, LaSarre B, McKinlay JB. 2017. Recipient-biased competition for an intracellularly generated cross-fed nutrient is required for coexistence of microbial mutualists. mBio 8:e01620-17. doi: 10.1128/mBio.01620-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cox J, Mann M. 2008. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol 26:1367–1372. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- 14.Jozefczuk S, Klie S, Catchpole G, Szymanski J, Cuadros-Inostroza A, Steinhauser D, Selbig J, Willmitzer L. 2010. Metabolomic and transcriptomic stress response of Escherichia coli. Mol Syst Biol 6:364. doi: 10.1038/msb.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lessard IAD, Pratt SD, McCaffertyl DG, Bussiere DE, Hutchins C, Wanner BL, Katz L, Walsh CT. 1998. Homologs of the vancomycin resistance d-Ala-d-Ala dipeptidase VanX in Streptomyces toyocaensis, Escherichia coli and Synechocystis: attributes of catalytic efficiency, stereoselectivity and regulation with implications for function. Chem Biol 5:489–504. doi: 10.1016/S1074-5521(98)90005-9. [DOI] [PubMed] [Google Scholar]
- 16.Kim KS, Pelton JG, Inwood WB, Andersen U, Kustu S, Wemmer DE. 2010. The Rut pathway for pyrimidine degradation: novel chemistry and toxicity problems. J Bacteriol 192:4089–4102. doi: 10.1128/JB.00201-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caldara M, Charlier D, Cunin R. 2006. The arginine regulon of Escherichia coli: whole-system transcriptome analysis discovers new genes and provides an integrated view of arginine regulation. Microbiology 152:3343–3354. doi: 10.1099/mic.0.29088-0. [DOI] [PubMed] [Google Scholar]
- 18.Kashiwagi K, Pistocchi R, Shibuya S, Sugiyama S, Morikawa K, Igarashi K. 1996. Spermidine-preferential uptake system in Escherichia coli. J Biol Chem 271:12205–12208. doi: 10.1074/jbc.271.21.12205. [DOI] [PubMed] [Google Scholar]
- 19.van Heeswijk WC, Westerhoff HV, Boogerd FC. 2013. Nitrogen assimilation in Escherichia coli: putting molecular data into a systems perspective. Microbiol Mol Biol Rev 77:628–695. doi: 10.1128/MMBR.00025-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zimmer DP, Soupene E, Lee HL, Wendisch VF, Khodursky AB, Peter BJ, Bender RA, Kustu S. 2000. Nitrogen regulatory protein C-controlled genes of Escherichia coli: scavenging as a defense against nitrogen limitation. Proc Natl Acad Sci U S A 97:14674–14679. doi: 10.1073/pnas.97.26.14674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barney BM, Eberhart LJ, Ohlert JM, Knutson CM, Plunkett MH. 2015. Gene deletions resulting in increased nitrogen release by Azotobacter vinelandii: application of a novel nitrogen biosensor. Appl Environ Microbiol 81:4316–4328. doi: 10.1128/AEM.00554-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim M, Zhang Z, Okano H, Yan D, Groisman A, Hwa T. 2012. Need-based activation of ammonium uptake in Escherichia coli. Mol Syst Biol 8:616. doi: 10.1038/msb.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Figueira R, Brown DR, Ferreira D, Eldridge MJG, Burchell L, Pan Z, Helaine S, Wigneshweraraj S. 2015. Adaptation to sustained nitrogen starvation by Escherichia coli requires the eukaryote-like serine/threonine kinase YeaG. Sci Rep 5:17524. doi: 10.1038/srep17524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenthal AZ, Matson EG, Eldar A, Leadbetter JR. 2011. RNA-seq reveals cooperative metabolic interactions between two termite-gut spirochete species in co-culture. ISME J 5:1133–1142. doi: 10.1038/ismej.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Filkins LM, Graber JA, Olson DG, Dolben EL, Lynd LR, Bhuju S, Toole AO, O'Toole GA. 2015. Coculture of Staphylococcus aureus with Pseudomonas aeruginosa drives S. aureus towards fermentative metabolism and reduced viability in a cystic fibrosis model. J Bacteriol 197:2252–2264. doi: 10.1128/JB.00059-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Men Y, Feil H, VerBerkmoes NC, Shah MB, Johnson DR, Lee PKH, West KA, Zinder SH, Andersen GL, Alvarez-Cohen L. 2012. Sustainable syntrophic growth of Dehalococcoides ethenogenes strain 195 with Desulfovibrio vulgaris Hildenborough and Methanobacterium congolense: global transcriptomic and proteomic analyses. ISME J 6:410–421. doi: 10.1038/ismej.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giannone RJ, Huber H, Karpinets T, Heimerl T, Küper U, Rachel R, Keller M, Hettich RL, Podar M. 2011. Proteomic characterization of cellular and molecular processes that enable the Nanoarchaeum equitans-Ignicoccus hospitalis relationship. PLoS One 6:e22942. doi: 10.1371/journal.pone.0022942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hillesland KL, Lim S, Flowers JJ, Turkarslan S, Pinel N, Zane GM, Elliott N, Qin Y, Wu L, Baliga NS, Zhou J, Wall JD, Stahl DA. 2014. Erosion of functional independence early in the evolution of a microbial mutualism. Proc Natl Acad Sci U S A 111:14822–14827. doi: 10.1073/pnas.1407986111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song HS, Renslow RS, Fredrickson JK, Lindemann SR. 2015. Integrating ecological and engineering concepts of resilience in microbial communities. Front Microbiol 6:1298. doi: 10.3389/fmicb.2015.01298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turkarslan S, Raman AV, Thompson AW, Arens CE, Gillespie MA, von Netzer F, Hillesland KL, Stolyar S, López García de Lomana A, Reiss DJ, Gorman-Lewis D, Zane GM, Ranish JA, Wall JD, Stahl DA, Baliga NS. 2017. Mechanism for microbial population collapse in a fluctuating resource environment. Mol Syst Biol 13:919. doi: 10.15252/msb.20167058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson AW, Turkarslan S, Arens CE, López García de Lomana A, Raman AV, Stahl DA, Baliga NS. 2017. Robustness of a model microbial community emerges from population structure among single cells of a clonal population. Environ Microbiol 19:3059–3069. doi: 10.1111/1462-2920.13764. [DOI] [PubMed] [Google Scholar]
- 32.Kjelleberg S, Albertson N, Flardh K, Holmquist L, Jouper-Jaan A, Marouga R, Ostling J, Svenblad B, Weichart D. 1993. How do non-differentiating bacteria adapt to starvation? Antonie Van Leeuwenhoek 63:333–341. doi: 10.1007/BF00871228. [DOI] [PubMed] [Google Scholar]
- 33.Shimizu K. 2013. Regulation systems of bacteria such as Escherichia coli in response to nutrient limitation and environmental stresses. Metabolites 4:1–35. doi: 10.3390/metabo4010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schimel J, Balser TC, Wallenstein M. 2007. Microbial stress-response physiology and its implications for ecosystem function. Ecology 88:1386–1394. doi: 10.1890/06-0219. [DOI] [PubMed] [Google Scholar]
- 35.Roszak DB, Colwell RR. 1987. Survival strategies of bacteria in the natural environment. Microbiol Rev 51:365–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jørgensen BB, Marshall IPG. 2016. Slow microbial life in the seabed. Annu Rev Mar Sci 8:311–332. doi: 10.1146/annurev-marine-010814-015535. [DOI] [PubMed] [Google Scholar]
- 37.Bergkessel M, Basta DW, Newman DK. 2016. The physiology of growth arrest: uniting molecular and environmental microbiology. Nat Rev Microbiol 14:549–562. doi: 10.1038/nrmicro.2016.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lennon JT, Jones SE. 2011. Microbial seed banks: the ecological and evolutionary implications of dormancy. Nat Rev Microbiol 9:119–130. doi: 10.1038/nrmicro2504. [DOI] [PubMed] [Google Scholar]
- 39.Wanner U, Egli T. 1990. Dynamics of microbial growth and cell composition in batch culture. FEMS Microbiol Rev 6:19–43. doi: 10.1111/j.1574-6968.1990.tb04084.x. [DOI] [PubMed] [Google Scholar]
- 40.Fritts RK, LaSarre B, Stoner AM, Posto AL, McKinlay JB. 2017. A Rhizobiales-specific unipolar polysaccharide adhesin contributes to Rhodopseudomonas palustris biofilm formation across diverse photoheterotrophic conditions. Appl Environ Microbiol 83:e03035-16. doi: 10.1128/AEM.03035-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim M-K, Harwood CS. 1991. Regulation of benzoate-CoA ligase in Rhodopseudomonas palustris. FEMS Microbiol Lett 83:199–203. doi: 10.1111/j.1574-6968.1991.tb04440.x-i1. [DOI] [Google Scholar]
- 42.Thomason LC, Costantino N, Court DL. 2007. E. coli genome manipulation by P1 transduction. Curr Protoc Mol Biol Chapter 1:Unit 1.17. doi: 10.1002/0471142727.mb0117s79. [DOI] [PubMed] [Google Scholar]
- 43.McKinlay JB, Zeikus JG, Vieille C. 2005. Insights into Actinobacillus succinogenes fermentative metabolism in a chemically defined growth medium. Appl Environ Microbiol 71:6651–6656. doi: 10.1128/AEM.71.11.6651-6656.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hayashi K, Morooka N, Yamamoto Y, Fujita K, Isono K, Choi S, Ohtsubo E, Baba T, Wanner BL, Mori H, Horiuchi T. 2006. Highly accurate genome sequences of Escherichia coli K-12 strains MG1655 and W3110. Mol Syst Biol 2:2006.0007. doi: 10.1038/msb4100049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Larimer FW, Chain P, Hauser L, Lamerdin J, Malfatti S, Do L, Land ML, Pelletier DA, Beatty JT, Lang AS, Tabita FR, Gibson JL, Hanson TE, Bobst C, Torres JLTY, Peres C, Harrison FH, Gibson J, Harwood CS. 2004. Complete genome sequence of the metabolically versatile photosynthetic bacterium Rhodopseudomonas palustris. Nat Biotechnol 22:55–61. doi: 10.1038/nbt923. [DOI] [PubMed] [Google Scholar]
- 46.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. 2012. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc 7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. 2013. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol 14:R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Langmead B, Salzberg SL. 2012. Fast gapped-read alignment with Bowtie 2. Nat Methods 9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L. 2010. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 28:511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wallace EWJ, Kear-Scott JL, Pilipenko EV, Schwartz MH, Laskowski PR, Rojek AE, Katanski CD, Riback JA, Dion MF, Franks AM, Airoldi EM, Pan T, Budnik BA, Drummond DA. 2015. Reversible, specific, active aggregates of endogenous proteins assemble upon heat stress. Cell 162:1286–1298. doi: 10.1016/j.cell.2015.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.UniProt Consortium. 2017. UniProt: the universal protein knowledgebase. Nucleic Acids Res 45:D158–D169. doi: 10.1093/nar/gkw1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kitagawa M, Ara T, Arifuzzaman M, Ioka-Nakamichi T, Inamoto E, Toyonaga H, Mori H. 2005. Complete set of ORF clones of Escherichia coli ASKA library (a complete set of E. coli K-12 ORF archive): unique resources for biological research. DNA Res 12:291–299. doi: 10.1093/dnares/dsi012. [DOI] [PubMed] [Google Scholar]
- 53.Blattner F, Plunkett GI, Bloch C, Perna N, Burland V, Riley M, Collado-Vides J, Glasner J, Rode C, Mayhew G, Gregor J, Davis N, Kirkpatrick H, Goeden M, Rose D, Mau B, Shao Y. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453–1462. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 54.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:2006.0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.