Abstract
Background
The ethical implications of the utilization of kidneys with solid renal masses (SRMs) in transplantation are the subject of lively debate in the transplantation community and beyond. One of such implications is that as the life expectancy of renal transplant patients improve, the prevalence of SRMs in donors is likely to increase. We report a case of an oncocytoma in a renal allograft complicating a deceased-donor kidney transplant.
Case presentation
A 60-year-old woman received and underwent deceased-donor renal transplantation for end-stage renal disease after a waiting-list period of 11 years. Kidney Doppler ultrasound (DUS) of the deceased donor was negative for any nodular lesion. The finding of the DUS, done on postoperative day 1, to assess the patency of the graft, was suspicious for an acute arterial thrombosis but did not reveal any focal irregularities. An ensuing computed tomography (CT) scan did not show any arterial complications but serendipitously revealed a 2.4-cm lesion on the upper pole of the renal allograft, which was not detected during the back-table or ultrasonography monitoring. Histology of the biopsied lesion was consistent with oncocytoma. However, because the eosinophilic variant of chromophobe renal cell carcinoma may morphologically resemble renal oncocytoma, immunohistochemical staining was performed. The results were negative, ruling out chromophobe RCC. After discussing the therapeutic options and potential related outcomes with the patient, we found no reason for resection of the lesion or an allograft nephrectomy, given the low risk of malignant transformation in an oncocytoma. Active surveillance of the benign tumor was done with ultrasonography, every 2 months, for the first year and, then, with magnetic resonance imaging, every year. The patient received mycophenolate-mofetil, tacrolimus, and prednisone throughout the 5-year follow-up period, and the regimen for immunosuppression was not changed despite the presence of the renal mass. After 60 months, we report that none of the radiological findings have shown any morphological changes of the lesion, and the patient is well.
Conclusion
To the best of our knowledge, we report the first case of an oncocytoma in a renal allograft complicating a deceased-donor kidney transplant, which was successfully managed by active surveillance.
Keywords: Kidney transplantation, Renal transplantation, Solid renal mass, Oncocytoma
Background
The ethical implications of the utilization of kidneys that have been found to have solid renal masses (SRMs) in transplantation are the subject of lively debate in the transplantation community and beyond. One of such implications is that as the life expectancy of renal transplant patients improve, the prevalence of SRMs in donors is likely to increase [1, 2]. A recent review of the literature on SRMs in transplant allograft kidneys proposed a detailed management algorithm for the tumors in allograft kidneys that mirrors the clinical decision-making in the non-transplant population [3]. We report a case of an oncocytoma in a renal allograft complicating a deceased-donor kidney transplant in a 60-year-old woman.
Case presentation
The deceased donor was a 67-year-old man with a kidney Doppler ultrasound (DUS) that was negative for any nodular lesion. As part of the routine postoperative follow-up management, the recipient underwent DUS to assess the patency of the graft on postoperative day 1. The DUS finding was suspicious for an acute arterial thrombosis but did not reveal any focal irregularities. Consequently, a computed tomography (CT) scan was urgently obtained but it did not show any arterial complications. However, it serendipitously revealed a 2.4-cm lesion on the upper pole of the renal allograft which was not detected during the back-table or ultrasonography monitoring. A biopsy of the lesion was performed, and its histology revealed an epithelial proliferation of large cells with finely granular cytoplasm and medium round nucleus vesicular acidophilus, arranged tubules, and alveoli and cords immersed in a connective tissue stroma. This picture was consistent with oncocytoma. However, because the eosinophilic variant of chromophobe renal cell carcinoma (RCC) may morphologically resemble renal oncocytoma, immunohistochemical staining was performed using Ki-67 antibodies and RCC antigens. The results were negative, ruling out chromophobe RCC. The therapeutic options and potential related outcomes were clearly discussed with the patient. Given the low risk of malignant transformation in an oncocytoma [4], we found no reason for resection of the lesion or an allograft nephrectomy. Consequently, we opted for active surveillance of the benign tumor with ultrasonography, every 2 months, for the first year and, then, with magnetic resonance imaging (MRI), every year (Fig. 1). The patient received mycophenolate-mofetil, tacrolimus, and prednisone throughout the 5-year follow-up period and the regimen for immunosuppression was not changed despite the presence of the renal mass. After 60 months of active surveillance, we report that radiological studies have shown no growth, regression, or any other interim morphological changes to the lesion, and the patient is alive and well (Fig. 2).
Fig. 1.
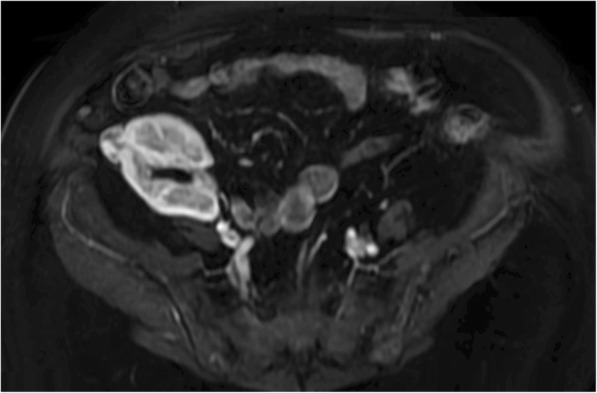
Magnetic resonance imaging (MRI) was used for the follow-up evaluation of renal mass because of its multi-planar capabilities, as well as its ability to demonstrate of enhancement and to provide soft-tissue contrast. A MRI scan at 60 months after transplantation showed a well-circumscribed oncocytoma with expansive net margins at the cortical surrounding of the kidney (white arrows). It has not infiltrative margins with extension in peri-renal fat or renal sinus, in the medulla of the kidney or in the major renal venous vessels
Fig. 2.
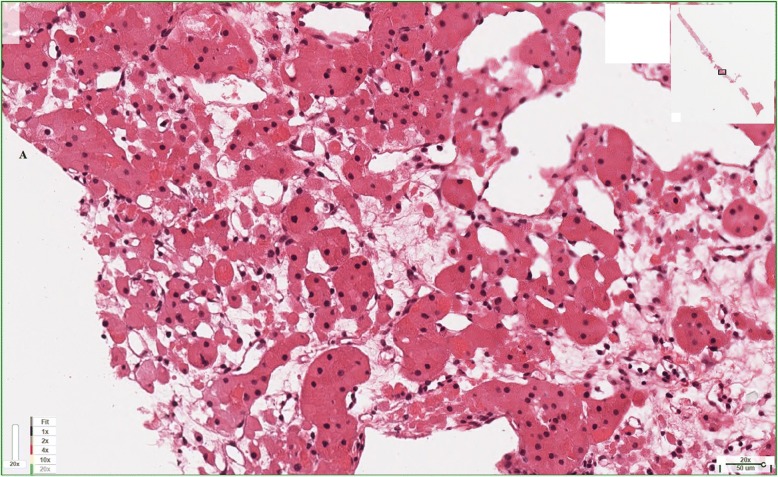
Histological examination showed small islands and microcysts of large, mostly round, eosinophilic cells (oncocytes) within hypocellular loose connective tissue, consistent with oncocytoma. Renal cell carcinoma has been ruled out
Discussion
Renal oncocytomas are considered benign kidney tumors [5] with an incidence of 3–7% among all solid renal neoplasms [6]. They are often diagnosed incidentally as SRMs and have traditionally been managed by nephrectomy as it is often suspected to be chromophobe RCC [6]. Treatment results are excellent, with only one case of metastatic tissue proven as oncocytoma reported in the literature [7, 8]. Thus, making a correct preoperative diagnosis of renal oncocytoma is vital as it can allow for the prevention of unnecessary and aggressive surgical procedures in both transplant and non-transplant patients. A recent study in the UK on the surgical management of renal oncocytomas showed a 0.4% 60-day mortality rate and a 20% in-hospital complication rate, including bleeding, pneumothorax, chest infection, splenectomy, wound infection, ileus, and deep vein thrombosis/pulmonary embolus [9]. Furthermore, an accurate preoperative diagnosis could be of benefit when an SRM is detected during living kidney donor evaluation or is found in kidneys from deceased donors. However, cohort studies are needed to better understand the effect of the active surveillance approach in the management of asymptomatic renal oncocytoma in the transplant population. This could, potentially, help alleviate some of the burden of an increasing demand for kidneys for transplantation as kidneys that were once abandoned could be put into good use in the right patients.
There are case reports of coexistent RCC associated with or even within oncocytomas, and we are not aware of any reports indicating that immunosuppressive regimen facilitates oncocytoma malignant transformation [4, 10]. Nevertheless, we recommend that clinicians remain mindful that the growth patterns of renal oncocytoma and those of RCC can be similar. Transparency and clinical clarity are two cornerstones in this act of sharing experiences with the medical community at large. To the best of our knowledge, this is the first case report of oncocytoma managed by active surveillance in a transplant allograft kidney. Whether this scarcity in the literature reflects the rarity of the clinical phenomenon or a reluctance to report it when detected is not for our institute to speculate [3]. We would suggest to consider this clinical entity as a non-surgical oncologic disease, believing that although the risk of malignant transformation of oncocytoma is low in the non-transplant population, awareness of the risk and the use of appropriate protocols for informed consent and surgical decision-making is paramount for continued success in this delicate field of medicine.
Conclusion
We report a case of an oncocytoma in a renal allograft complicating a deceased-donor kidney transplant in a 60-year-old woman, managed by active surveillance.
Funding
We hereby certify that all the authors whose names are listed immediately below certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Abbreviations
- CT
Computed tomography
- DUS
Doppler ultrasound
- MRI
Magnetic resonance imaging
- RCC
Renal cell carcinoma
- SRM
Solid renal mass
Authors’ contributions
DP took part in the concept/design and in drafting article. CAN contributed in drafting and revising the article. SLP, RL, and FdF did the critical revision of the article. SG provided critical revision and the approval of the article. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable. In details, our manuscript does not report on or involve the use of any animal or human data or tissue, and not contain any individual persons’ data, so this section is not applicable to our submission.
Consent for publication
Not applicable. In details, our manuscript does not contain any individual persons’ data, so this section is not applicable to our submission.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Duilio Pagano, Email: dpagano@ismett.edu.
Fabrizio di Francesco, Email: fdifrancesco@ismett.edu.
Liotta Rosa, Email: rliotta@ismett.edu.
Chibueze A. Nwaiwu, Email: can46@pitt.edu
Sergio Li Petri, Email: slipetri@ismett.edu.
Salvatore Gruttadauria, Phone: +39 091 219 24 75, Email: sgruttadauria@ismett.edu.
References
- 1.Flechner SM, Campbell SC. The use of kidneys with small renal tumors for transplantation: who is taking the risk? Am J Transplant. 2012;12(1):48–54. doi: 10.1111/j.1600-6143.2011.03794.x. [DOI] [PubMed] [Google Scholar]
- 2.Tillou X, Guleryuz K, Doerfler A, et al. Nephron sparing surgery for de novo kidney graft tumor: results from a multicenter national study. Am J Transplant. 2014;14:2120–2125. doi: 10.1111/ajt.12788. [DOI] [PubMed] [Google Scholar]
- 3.Griffith JJ, Amin KA, Waingankar N, Lerner SM, Delaney V, Ames SA, Badani K, et al. Solid renal masses in transplanted allograft kidneys: a closer look at the epidemiology and management. Am J Transplant. 2017;17(11):2775–2781. doi: 10.1111/ajt.14366. [DOI] [PubMed] [Google Scholar]
- 4.Van der Kwast T, Perez-Ordoñez B. Renal oncocytoma, yet another tumour that does not fit in the dualistic benign/malignant paradigm? J Clin Pathol. 2007;60(6):585–586. doi: 10.1136/jcp.2006.044438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yap FY, Hwang DH, Cen SY, Varghese BA, Desai B, Quinn BD, Gupta MN, Rajarubendra N, Desai MM, Aron M, Liang G, Aron M, Gill IS, Duddalwar VA. Quantitative contour analysis as an image-based discriminator between benign and malignant renal tumors. Urology. 2018. doi:10.1016/j.urology.2017.12.018. [Epub ahead of print]. [DOI] [PubMed]
- 6.Chao DH, Zisman A, Pantuck AJ, Freedland SJ, Said JW, Belldegrun AS. Changing concepts in the management of renal oncocytoma. Urology. 2002;59:635–642. doi: 10.1016/S0090-4295(01)01630-2. [DOI] [PubMed] [Google Scholar]
- 7.Perez-Ordonez B, Hamed G, Campbell S, Erlandson RA, Russo P, Gaudin PB, Reuter VE. Renal oncocytoma: a clinicopathologic study of 70 cases. Am J Clin Path. 1997;21(8):871–883. doi: 10.1097/00000478-199708000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Kawaguchi S, Fernandes KA, Finelli A, Robinette M, Fleshner N, Jewett MA. Most renal oncocytomas appear to grow: observations of tumor kinetics with active surveillance. J Urol. 2011;186:1218–1222. doi: 10.1016/j.juro.2011.05.080. [DOI] [PubMed] [Google Scholar]
- 9.Neves JB, Withington J, Fowler S, Patki P, Barod R, Mumtaz F, O'Brien T, Aitchison M, Bex A, Tran MGB. Contemporary surgical management of renal oncocytoma: a nation's outcome. British Association of Urological Surgeons (BAUS). BJU Int. 2018; 10.1111/bju.14159. [DOI] [PubMed]
- 10.Fontaine A, Thuret R, Garrigues V, Taourel P. Post-transplant renal tumors: a report of three cases. J Radiol. 2010;91(4):491–494. doi: 10.1016/S0221-0363(10)70064-0. [DOI] [PubMed] [Google Scholar]