Abstract
Objective:
The objective of the study was to perform quantitative failure and fault analysis to the diagnostic ultrasound (US) scanners in a radiology department after the implementation of the predictive maintenance (PdM) method; to study the reduction trend of machine failure; to understand machine operating parameters affecting the failure; to further optimize the method to maximize the machine clinically service time.
Materials and Methods:
The PdM method has been implemented to the 5 US machines since 2013. Log books were used to record machine failures and their root causes together with the time spent on repair, all of which were retrieved, categorized, and analyzed for the period between 2013 and 2016.
Results:
There were a total of 108 cases of failure occurred in these 5 US machines during the 4-year study period. The average number of failure per month for all these machines was 2.4. Failure analysis showed that there were 33 cases (30.5%) due to software, 44 cases (40.7%) due to hardware, and 31 cases (28.7%) due to US probe. There was a statistically significant negative correlation between the time spent on regular quality assurance (QA) by hospital physicists with the time spent on faulty parts replacement over the study period (P = 0.007). However, there was no statistically significant correlation between regular QA time and total yearly breakdown case (P = 0.12), although there has been a decreasing trend observed in the yearly total breakdown.
Conclusion:
There has been a significant improvement on the machine failure of US machines attributed to the concerted effort of sonographers and physicists in our department to practice the PdM method, in that system component repair time has been reduced, and a decreasing trend in the number of system breakdown has been observed.
Keywords: Diagnostic ultrasound machines, failure analysis, preventive and predictable maintenance
INTRODUCTION
Medical ultrasound (US) systems play an important role in the clinical diagnosis of patient care. A performance maintenance program is mandatoryto keep the system reliable, safe, and available for use when it is needed for patient service.[1,2] The program can prolong the system useful life and also minimize the repair cost to its ownership.[3] There are indeed many studies reporting the importance to have a maintenance management program to medical systems.[4,5,6]
For US systems in a radiology department, the department has the options either from the in-house technical repair service or from vendor technical service. However, most vendors conduct their preventive maintenance procedures according to their factory recommendations, and sometimes, the content of such procedures may not be clinically relevant.
The concept of predictive maintenance (PdM) to the US scanners in our department, performed as a teamwork by sonographers and physicists, has been implemented since 2013. PdM is the application of performance monitoring technologies with statistical process control to monitor equipment performance for the purpose of early detection and of elimination of equipment defects that could lead to unplanned downtime or unnecessary expenditures.[7]
Logbook and direct verbal communication, as the method of reporting in our PdM program, have been used to record events that sonographers may consider relevant to the machine performance. Routine quality assurance (QA) to each scanner, performed by hospital physicists with US phantoms, can ensure the machine performance consistency in clinical application. Performance parameters are analyzed as a time series so that any degrading machine performance can be predicted and then corrected after clinical hours of the machine. The whole system will then be tested with a series QA procedure in addition to electrical safety test before the system is released back to clinical service.
In the current study, failure analysis of the 5 US machines in our department after 4 years of PdM implementation has been studied with statistical trend analysis to optimize the clinical application of the systems.
MATERIALS AND METHODS
The structure of PdM has included reporting events of operational difficulties or problems of regular QA by physicists and of repair maintenance by vendor. Sonographers, who are with the daily use of these US machines, reported problems or faults through the use of logbooks or through immediate communication with the vendor engineer, who will check the machine to identify possible faulty components. If it is of no urgency for immediate action, the corrective action is performed after clinical hours. Physicists will perform acceptance tests of the system when the machine is back to its normal functionality.
Definition of failure
Machine failures have been defined as the inability to carry out its normal functionality. Failure analysis generally included consultation at three levels of personnel. First, sonographers provided detailed information how the problems were observed and whether there were any warning signs about the failure. Second, vendor engineers would diagnose the faulty. Third, if component has to be replaced, vendor engineers would provide the underlying causes of the failure after the machine was disassembled and then repaired. Physicists would carry out functional tests of the system after replacement. All the failure and associated solution documentation written in the study period were retrieved, categorized, and then analyzed.
Failure category and failure duration
There were more than fifty types of failures recorded. We first categorized failures into different categories, namely software, hardware, and US probe. Software category included failures related to the system software such as software corruption or system reboot. Hardware failures were subcategorized into input, core, and output. Input hardware failures included all sensing equipment, such as barcode scanner, and all keyboard command keys. Core hardware failures included the computer central processing unit, disc storage, batteries, and cables. Output hardware failures included image display issues such as image artifacts as observed on monitor, image artifacts as observed on hardcopy, and all printing problems.
Failures specific to the US probe ware categorized into six different subcategories of physical, defects of the probe itself, black shadows arising from the probe, sheath/shell, general defects, and cable and signal loss.
Statistical analysis
Events identified for machine failure were categorized and presented as proportions per year during the study period. Time spent for regular QA and by engineers for their preventive maintenance and repair was calculated as total hours per year of the study. Time trend of total hours spent on these services with a number of machine failures per year was assessed using regression data analysis. Statistical analysis was performed using SAS (SAS, version 9.4, Carly, NC, USA). P ≤ 0.05 was considered statistically significant.
RESULTS
There was a total of 108 cases of failure occurred in the 5 US machines during the 4-year study period. The analysis of failure shows that there were 33 cases (30.6%) due to software, 44 cases (40.7%) due to hardware, and 31 cases (29.7%) due to US probe [Table 1]. Figure 1 shows the frequency of failure for different categories. There was a decrease in failures of all categories over the study period except for US probe that showed a surge in 2014 [Table 1]. The average number of reported failure per month among these machines was 2.25. There has been a decreasing trend of frequency of failure per year over the study period.
Table 1.
Failure categories for each year between 2013 and 2016
| Failure category | Failure frequency for all machines at each year of the study period | Total | Average per year | |||
|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2016 | |||
| Software | 9 | 14 | 7 | 3 | 33 | 8.25 |
| Hardware | ||||||
| Input | 6 | 4 | 2 | 2 | 14 | 3.5 |
| Core | 2 | 2 | 1 | 1 | 6 | 1.5 |
| Output | 7 | 9 | 5 | 3 | 24 | 6 |
| US probe | 8 | 13 | 6 | 4 | 31 | 7.75 |
| Total | 32 | 42 | 21 | 13 | 108 | 27 |
US: Ultrasound
Figure 1.
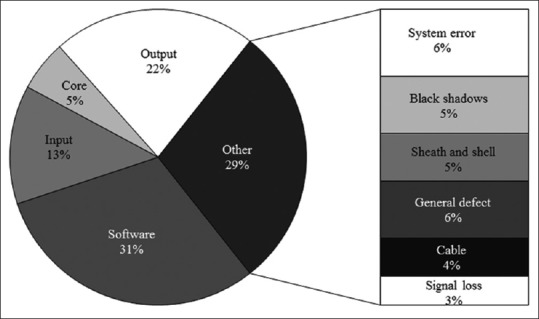
Proportion of failures by component for the whole study period 2013–2016
Figure 2 shows the failure category by brands of US machine during the study period. Among the different brands, the case of failure for brand A was the highest (27) and brand B and D machine over the study period was 19, which were the lowest among the 5 brands, the yearly average failure rate per machine was about 4.
Figure 2.
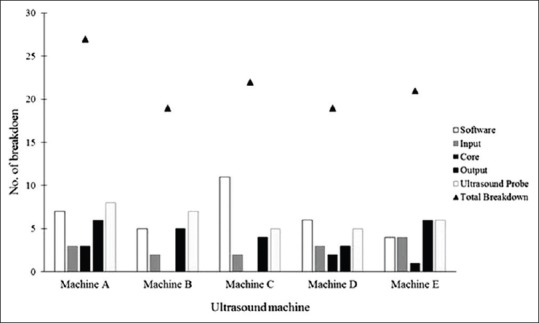
Failure category for each ultrasound machine during the study period
Table 2 tabulates the time spent by physicists for regular QA and time spent by vendor engineers for vendor's preventive and repair maintenance and time spent by engineers for parts replacement. There was a statistically significant negative correlation between regular QA time and repair time (correlation coefficient = 0.99, P = 0.007). However, there was no statistically significant negative correlation between regular QA time and total yearly breakdown case (correlation coefficient = −0.87, P = 0.12).
Table 2.
Regular quality assurance performed by hospital physicists and vendor preventive maintenance service and vendor repair time during the study period
| Different service | Average maintenance time per machine for the study period | Total | Average per year | |||
|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2016 | |||
| Regular QA (h) | 19 | 25 | 41 | 48 | 133 | 33.25 |
| Vendor PM services (h) | 10 | 10 | 10 | 10 | 40 | 10 |
| Repair time (h) | 64 | 49 | 21 | 14 | 148 | 37 |
QA: Quality assurance, PM: Preventive maintenance
DISCUSSION
There has been no uniform classification for causes of US machine failures.[8,9] We classified them by components of US machine as identified by sonographers and physicists. Based on this classification, the most common failure was the software program. This was mainly due to network connection with our departmental PACS system and infrequent computer virus alerts. The second most common failure was associated with the US probe. These two factors contributed equally to the events of failure [Table 1]. Apparently, there should be further reduction of machine failures through staff continuous education on US technology review and on informatics of system networking that we have not yet addressed.
The negative correlation between the QA time spent and the repair time spent by vendor engineers was observed as a statistical significance. This implies that the trend of machine performance has been regularly checked and any degradation in performance could be observed along the trend. When the degradation required a spare parts replacement, the replacement time was reduced because the problem related to the degradation has been noted for some time. In this case, replacement was performed after clinical hour. Therefore, the QA work could minimize the downtime during clinical hours of the machine.
CONCLUSION
Throughout the years, there has been a significant improvement on the maintenance services of US machines attributed to the concerted effort of sonographers and physicists. Enhancement of staff education to US technology advancement and network upgrade may help further reduce failure and should be one of the next goals of program enhancement.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.American Institute of Ultrasound in Medicine. AIUM practice guideline for the performance of sonohysterography. J Ultrasound Med. 2015;34:1–6. doi: 10.7863/ultra.34.8.15.13.0005. doi: 10.7863/ultra. 34.8.15.13.0005. [DOI] [PubMed] [Google Scholar]
- 2.American College of Radiology, Comp ACR–AAPM–SIIM Technical Standard for Electronic Practice of Medical Imaging. American College of Radiology, Comp. 2014:1–18. [Google Scholar]
- 3.World Health Organization. Medical Equipment Maintenance Programme Overview. World Health Organization. 2011:4–77. [Google Scholar]
- 4.Malkin R, Keane A. Evidence-based approach to the maintenance of laboratory and medical equipment in resource-poor settings. Med Biol Eng Comput. 2010;48:721–6. doi: 10.1007/s11517-010-0630-1. [DOI] [PubMed] [Google Scholar]
- 5.Mavalankar D, Raman P, Dwivedi H, Jain ML. Managing equipment for emergency obstetric care in rural hospitals. Int J Gynaecol Obstet. 2004;87:88–97. doi: 10.1016/j.ijgo.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Kutor JK, Agede P, Ali RH. Maintenance practice, causes of failure and risk assessment of diagnostic medical equipment. J Biomed Eng Med Device. 2017;2:123. [Google Scholar]
- 7.Sezdi M. Two different maintenance strategies in the hospital environment: Preventive maintenance for older technology devices and predictive maintenance for newer high-tech devices. J Healthc Eng. 2016:1–16. doi: 10.1155/2016/7267983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO. Medical Equipment Maintenance Programme Overview (WHO Medical Device Technical Series). Geneva, Switzerland: World Health Organization. 2012. [Last accessed on 2017 Jul 16]. Available from: http://www.apps.who.int/iris/bitstream/10665/44587/1/9789241501538_eng.pdf .
- 9.Med Sing Long. Probe Component of Common Failures and 6 Faults Solution. Cheap Medical Equipment. Med Sing Long. 2011. [Last accessed on 2017 Jul 17]. 30 November. Available from: http://www.medicalequipment-msl.com/htm/medical-equipment-news/Probe-component-of-common-failures-and-6-faults-solution.html .


