Abstract
Objective:
High-resolution ultrasonography (HRUS) has been used recently to characterize median and ulnar nerves but is seldom used to characterize the lower extremity nerves. The reference standard for normal the lower extremity nerves has not been established. Thus, this study measured the cross-sectional areas (CSAs) of the sciatic nerve of 200 healthy male or female volunteers, aged 18–80 using HRUS. These data provide basic clinical data for the use of high-resolution ultrasound for the future diagnosis, treatment, and prognostic evaluation of peripheral neuropathies.
Methods:
Two hundred healthy volunteers with 400 lower extremities were studied with HRUS. According to their age, the subjects were assigned to young group (18-30 years, n = 75), middle group. (31-60 years, n = 70), and old group(61-80 year, n = 55). Age, sex, height, weight were recorded and CSAs of sciatic nerve were obtained at every predetermined sites.
Results:
The mean CSAs of sciatic nerves at GS and MGPF were 0.527 ± 0.028 cm2 and 0.444 ± 0.026 cm2 respectively. Pearson's correlation analysis showed that the mean CSAs were correlated with height and weight. There was no difference in mean CSAs among the three groups (P > 0.05). Women had smaller CSAs of the normal Sciatic nerves than men in two measuring sites (GS, MGPF) (P < 0.05).
Conclusion:
Peripheral nerve ultrasonography is a reliable and reproducible diagnostic method in the hands of experienced examiners. Normal values for the sciatic nerve nerves are provided by our study. Thus, reference values of Sciatic nerve CSA of the lower extremity can facilitate the analysis of abnormal nerve conditions.
Keywords: Cross-sectional area, high-resolution ultrasonography, nerve lesion, peripheral nerve, sciatic nerve, the lower extremity peripheral nerve
INTRODUCTION
High-resolution ultrasonography (HRUS) is an emerging new tool in the investigation of peripheral nerves. We set out to assess the utility of HRUS performed at lower extremity nerves in normal people. Clinicians frequently encounter compressive neuropathies of the lower extremity. The long course of the sciatic nerve leaves it vulnerable to nerve injury from a variety of causes. The clinical history and physical examination, along with electrodiagnostic testing and imaging studies, lead to the correct diagnosis. The imaging characteristics of the compression neuropathies can include acute and chronic changes in the nerves and the muscles they innervate.[1] Peripheral nerves are affected by a number of disease processes such as trauma, infection, inflammation, benign and malignant tumors, as well as entrapment neuropathies. With its high resolution, clinicians experienced in ultrasound can detect and characterize these pathologies in a cost-effective manner.[2] In 1988, Fornage presented the first report on peripheral nerves using sonography.[3] Continued technologic improvements, including the availability of high-frequency transducers, have led to an increase in the use of sonography in imaging of peripheral nerves. The present study has demonstrated that ultrasonography not only clearly depicts the sciatic nerve but also provides accurate information on its involvement in recent or previous traumas.[4] Normal peripheral nerves have a typical sonographic appearance, exhibiting multiple longitudinal hypoechoic bands, which represent fascicular bundles.[5] The lower extremity nerve is more frequently involved in entrapment syndromes than the ulnar and median nerves. However, the causes of most entrapment neuropathies in the lower extremity may be divided into two major categories as follows: (a) mechanical causes, which occur at fibrous or fibro-osseous tunnels and (b) dynamic causes related to nerve injury during specific limb positioning.[4] Common entrapment neuropathies in the knee, leg, ankle, and foot include those of the common peroneal nerve, deep peroneal nerve, superficial peroneal nerve, tibial nerve and its branches, and sural nerve.[5] HRUS of the peripheral nervous system allows nerves to be readily visualized and to assess their morphology. Ultrasonography has brought pathophysiological insights and substantially added to diagnostic accuracy and treatment decisions among mononeuropathies.[6] Entrapment neuropathies of the knee, leg, ankle, and foot are often under diagnosed, as the results of clinical examination and electrophysiologic evaluation are not always reliable.[7] However, the reference standard for normal sciatic nerves has not been established. Thus, the present study sought to obtain HRUS images of sciatic nerve nerves and sought to assess possible relationships between the cross-sectional areas (CSAs) and the volunteers' height and weight.
SUBJECTS AND METHODS
Two hundred healthy volunteers free of medical history of peripheral neuropathy were recruited in our ultrasonography laboratory between March and October 2015, including hospital staff and patients. They were of Han nationality, with a mean age of 52 years (range 18-80years), a mean height of 165 cm (range152-182 cm), and a mean body mass of 59 kg (range 44-80 kg). Of the 200 subjects, 104 were males and 96 were females. According to their age, the subjects were assigned to young group (18-30 years, n = 75), middle group.(31-60 years, n = 70), and old group(61-80 year, n = 55). Age, sex, height, weight were recorded and CSAs of sciatic nerve were obtained at every predetermined sites. The clinical features of the included 200 normal volunteers are shown in Table 1. All participants provided written informed consent. The experimental protocol was approved by the Institutional Human Study Committee of the Affiliated Hospital of Guizhou Medical University, China.
Table 1.
Clinical features of healthy volunteers
| Item | Young group (18-30-year-old) | Middle group (31-60-year-old) | Old group (61-80-year-old) |
|---|---|---|---|
| n | 75 | 70 | 55 |
| Sex (male/female) (n) | 39/36 | 32/38 | 23/22 |
| Height (cm)+ | 167.0±8.6 | 168.4±6.0 | 162.7±7.4 |
| Weight (kg)+ | 58.1±8.7 | 62.2±7.5 | 57.2±7.1 |
| Left/right (n) | 10/65 | 8/62 | 5/50 |
+Data are expressed as the mean±SD. SD: Standard deviation
Ultrasound measurements
A HRUS system (Philip iu22 made in USA) with a linear array transducer of 18 MHz was used. Depth, gain, and dynamic range were adjusted appropriately for optimal differentiation between nerves and other soft tissue structures. The ultrasound images were obtained by experienced operators, the transducer was placed perpendicular to the nerves on the skin. Pressure of the transducer on the skin was kept to a minimum to minimize deformation of underlying structures. The scans were made throughout the lower extremity, The cross-sectional areas of the sciatic nerves of two measuring sites were collected, one was located at the lower edge of the gluteus maximus in the posterior midline of the thigh (the gluteal sulcus) GS, the second was located at the midpoint between the gluteal sulcus and the popliteal fossa (MGPF). The CSA was measured by tracing the nerve just inside its hyperechoic rim, and three measurements were obtained with the probe repositioned. The average value was used for each level.[8,9]
Statistical analysis
Data were presented as mean ± standard deviation (SD) and compared among the groups. Statistical analysis was performed using the independent sample t-test. with the SPSS statistical software for Windows version 11.5 (SPSS, Inc., Chicago, Ill., USA). P < 0.05 was considered statistically significant. To correlate the CSA of nerves with other parameters, Pearson's correlation analysis was used.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by the local ethics committee of the institute. Informed written consent was obtained from all patients prior to their enrollment in this study.
RESULTS
The clinical characteristics of the subjects are presented in [Table 1]. According to their age, the subjects were assigned to young group (18-30 years, n = 75), middle group. (31-60 years, n = 70), and old group (61-80 year, n = 55). The normal sciatic nerve presents as a tubular echogenic structure with parallel linear internal echoes in the longitudinal section, and as reticular pattern echoic structure in cross-section, with the perineurium producing bright boundary echoes. Figure 1a shows transverse sonogram of the sciatic nerve at bottom of gluteus maximus in healthy people. Figure 1b shows transverse sonogram of the sciatic nerve at the midpoint between the gluteal sulcus (GS) and the popliteal fossa (MGPF) throughout the lower extremity.
Figure 1.
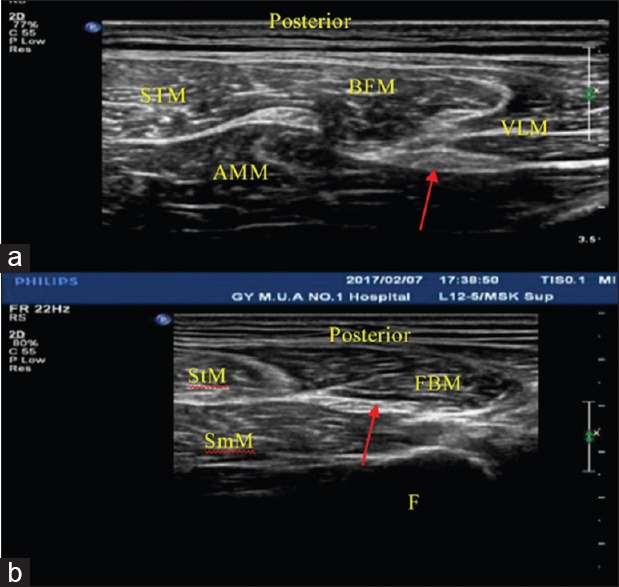
(a) At the gluteal sulcus. The red arrow indicates the sciatic nerve. BFM: Biceps femoris muscle, STM: Semitendinosus muscle, AMM: Adductor magnus muscle, VLM: Vastus lateralis muscle, (b) at the midpoint between the gluteal sulcus and the popliteal fossa. The red arrow indicates the sciatic nerve. FBM: Long head of biceps femoris muscle, STM: Semitendinosus muscle, SMM: Semimembranosus muscle, F: Femur
The following mean sciatic nerve CSAs at GS and MGPF were obtained respectively: 0.527 ± 0.028 cm2 and 0.444 ± 0.026 cm2 [Table 2]. There were no differences in the CSAs of the sciatic nerve at these two positions, either in the bilateral lower limbs or in the different age groups. However, the nerve in the male group was larger than the female group. [Table 3]. The mean CSAs of sciatic nerve were strongly correlated with height and weight (coefficient 0.34 and 0.39, P < 0.05).
Table 2.
CSAs of the normal sciatic nerves in different sites (x̄±S, cm2)
| MSs | x̄±S | The normal range |
|---|---|---|
| GS | 0.527±0.028 | 0.47-0.59 |
| MGPF | 0.444±0.026 | 0.39-0.50 |
GS: The gluteal sulcus, MGPF: The Midpoint between GS and the popliteal fossa, MSs: Measuring sites
Table 3.
Comparison of the CSAs of sciatic nerve of different ages , sexes, left and right sides in two measuring sites (x̄±S, cm2)
| Item | G S(, cm2) | P | MGPF(, cm2) | P |
|---|---|---|---|---|
| Age | ||||
| Young (n=75) | 0.495±0.021 | 0.426±0.013 | ||
| Middle (n=70) | 0.524±0.024+ | 0.14 | 0.450±0.022+ | 0.10 |
| Old (n=55) | 0.531±0.026+ | 0.09 | 0.461±0.038+ | 0.07 |
| Gender | ||||
| Male (n=104) | 0.539±0.029 | 0.487±0.023 | ||
| Female (n=96) | 0.443±0.027* | 0.01 | 0.421±0.023* | 0.04 |
| Side | ||||
| Right | 0.526±0.026 | 0.443±0.024 | ||
| Left | 0.527±0.033# | 0.28 | 0.446±0.029# | 0.20 |
Data are expressed as the mean±SD. Statistical analysis was performed using the independent sample t-test and one-way analysis of variance. *P<0.05 versus male. +Compare with young group P>0.05, #Compare with right side P>0.05. MGPF: The Midpoint between GS and the popliteal fossa, SD: Standard deviation
DISCUSSION
Ultrasound opens a window to detect the anatomy of the sciatic nerve in human. In addition, the surrounding anatomical structures can also be depicted. The ultrasonographic examination is currently increasingly used in imaging peripheral nerves, serving to supplement the physical examination, electromyography, and magnetic resonance imaging.[10] Peripheral nerves are often exposed to mechanical stress leading to compression neuropathies.[11] Nerve compression syndromes of the lower extremity present a challenge in differential diagnosis. Compression of the common peroneal nerve occurs relatively frequently; compression of the sciatic nerve occurs infrequently.[12] With the newer high-frequency probes with different footprints which allow high-resolution imaging at relatively superficial location, HRUS can detect and evaluate traumatic, inflammatory, infective, neoplastic, and compressive pathologies of the peripheral nerves.[13] Movement of the limb helps to differentiate nerve from tendons, whereas color Doppler helps to differentiate nerves from vessels. The current approach for localizing and assessing the severity of traumatic peripheral nerve injuries involves clinical evaluation and electrodiagnostic studies. However, the ability of these approaches to determine the extent of nerve damage within the first 6 weeks after trauma is limited.[14] In routine practice, a 18 MHz linear array transducer can be employed to scan the entire sciatic nerve in both the transverse and longitudinal planes and display variation in the shape of the nerve from a flat oval to a circle. Clinicians frequently encounter compressive neuropathies of the lower extremity.[1] The sciatic nerve is the largest branch of the sacral plexus. The long course of the sciatic nerve leaves it vulnerable to nerve injury from a variety of causes.[15] The course of the sciatic nerve along the thigh can be reliably identified.[16] We can find high-resolution of ultrasonographic imaging enables detailed imaging of nerve morphology (including fascicles, epineurium, perineurium, and echotexture) and size, and of the surrounding structures, such as muscles, soft tissues, and vessels. In ultrasonographic images, healthy sciatic nerves appear as cable-like structures that consist of hypoechoic fascicles and hyperechoic surrounding epineurium. On a transverse scan, they have an approximately round shape and a typical honeycomb appearance, with small dark areas (the fascicles) on a hyperechoic background (the perinerium). On a longitudinal scan, they appear as parallel hypoechoic (fascicles) and hyperecoic (perineurium and epineurium) lines. Such imaging can provide accurate evaluation of small nerves, such as the digital nerve. At each site, the CSA of the sciatic nerves was obtained by circumferentially tracing just inside the hyperechonic rim of the nerve, and care was made to ensure the transdurer was perpendicular to the nerve so the smallest and most accurate CSA was obtained. The images were obtained with the participant in the prone position throughout testing. The CSA value was measured three times. The following mean sciatic nerves CSAs ± SD were obtained: 0.527 ± 0.028 cm2 (GS) and 0.444 ± 0.026 cm2 (MGPF), respectively. At each site, the CSA of the sciatic nerve was obtained by tracing just inside the hyperechoic rim of the nerve. Sciatic nerve compression or injury may occur at any point along the anatomic course of the nerve.[17] This study aimed to obtain normal values for the nerve CSA of the lower extremity. The area of the nerve was consistent throughout its entire length. Nonetheless, the diameter rather than CSA was commonly used by others to evaluate nerve size.[18] The sciatic nerve was visible throughout the lower extremity and could be easily identified and measured at two sites. Clinical, laboratory, and electrodiagnostic studies are the main parameters used in the diagnosis of polyneuropathy. The CSAs of the sciatic nerve at GS was larger than those at the MGPF. There was no statistical difference in mean CSAs of the normal sciatic nerves among the three groups (P > 0.05). Our study has shown male CSAs were larger than the female population, indicating that women have a smaller sciatic nerve CSA. The precise reason for such discrepancy is largely unknown. Much of this variability may be due to differences in measurement techniques, along with differences between the populations studied. This sex-specific information could help define normal and abnormal states. The information on side-to-side variation and sex-specific differences will be particularly helpful for the diagnosis of peripheral neuropathies. There was no statistical difference in mean CSAs of the normal sciatic nerves among the three groups (P > 0.05). This study provides normative data on sciatic nerve sonoanatomy at the lower extremity. Also there were no difference in nerve size between the right and left sciatic nerves, it was consistent with our previous study of the upper limbs.[8,9,19] The sciatic nerves in the present study had quite different configurations, suggesting that calculations using a continuous boundary trace of the nerves should provide the most accurate CSAs. Reconstructing ultrasonographic planes with this high-resolution digitized anatomy not only enables an overview but also shows detailed views of the architecture of internal sciatic nerve.[20] Normal peripheral nerves have a typical sonographic appearance, exhibiting multiple longitudinal hypoechoic bands, which represent fascicular bundles. These are separated by discontinuous bands of increased echogenicity, corresponding to the surrounding epineurium.[21] Ultrasound measurements of the CSA variability have been recently introduced to quantify pathological changes in peripheral nerves (PN).[22] Reference nerve sizes are needed at evaluation sites to determine nerve enlargement.[23] The CSA is a measurement used to evaluate the lower extremity. It may be speculated that the CSA is a more stable and reliable measurement index than the diameter.[24] These results highlight that ultrasonography may be a complementary tool in differentiating polyneuropathies.[25] Similarly to entrapment neuropathies of lower extremities, the ultrasound constitutes a valuable supplementation of diagnostic examinations performed in patients with suspicions of nerve entrapment syndromes of the lower limb. This probably resulted from the lack of proper diagnostic tools (including high-frequency ultrasound transducers) as well as the lack of sufficient knowledge in this area.[26] High-resolution ultrasound is a powerful tool in the assessment of peripheral nerve disease. Nerve ultrasound is an evolving new discipline.[27] The results obtained indicate that high-frequency ultrasonography is a valuable method in qualifying patients for various types of treatment of peripheral neuropathies resulting from trauma.[28] The sciatic nerve along the thigh can be reliably identified and imaged with high-resolution ultrasound.[18] Of all the variables measured, linear regression analysis showed a significant correlation of the CSAs of sciatic nerves with weight and height in healthy adults. This finding implied that weight should be considered to have a major impact on the evaluation of the measured area of the sciatic nerve in normal patients. There are positive correlations between the sciatic nerve CSA and height (r = 0.34, P < 0.05) and positive correlations between the sciatic nerve CSA and weight (r = 0.39, P < 0.05). This finding implies that height and body mass has a major impact on the sciatic nerve CSA. However, further research is needed to investigate this relationship because little evidence shows a correlation between the size of other nerves and body mass. This variability may be due to differences in measurement technique along with differences in studied populations. Our study has shown that HRUS is a noninvasive, readily applicable imaging modality, capable of depicting real-time static and dynamic morphological information of the sciatic nerves and their surrounding tissues.
CONCLUSION
The normal sciatic nerve presents as a tubular echogenic structure with parallel linear internal echoes in the longitudinal section, and as reticular pattern echoic structure in cross-section, with the perineurium producing bright boundary echoes in two measuring's. Ultrasonography is capable of depicting these nerves morphological information, with respect to exact location, course, and extent.[29] Our experience seems to favor the conclusion that the examination of nerves in the extremities is a promising new application of high-resolution ultrasound. We feel the reference values obtained in this study are essential for facilitating the analysis of abnormal nerve conditions such as entrapment, hereditary neuropathies, acquired neuropathies, trauma, and nerve tumors result in an increase in nerve CSA. The reference values obtained in this study will facilitate the analysis abnormal nerve conditions, and the information on side-to-side variation and sex-specific differences should be particularly helpful. We conclude that the course of the sciatic nerve along the thigh can be reliably identified and imaged with HRUS.
Financial support and sponsorship
The study was supported by the Natural Science fund of Science and Technology Agency of Guizhou Province and Affiliated Hospital of Guizhou Medical University. No.[2015]7407.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We thank the staff from the Ultrasound Room of the Department of Neurology, the Affiliated Hospital of Guizhou Medical University for their help.
REFERENCES
- 1.Beltran LS, Bencardino J, Ghazikhanian V, Beltran J. Entrapment neuropathies III: Lower limb. Semin Musculoskelet Radiol. 2010;14:501–11. doi: 10.1055/s-0030-1268070. [DOI] [PubMed] [Google Scholar]
- 2.Lawande AD, Warrier SS, Joshi MS. Role of ultrasound in evaluation of peripheral nerves. Indian J Radiol Imaging. 2014;24:254–8. doi: 10.4103/0971-3026.137037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fornage BD. Peripheral nerves of the extremities: Imaging with US. Radiology. 1988;167:179–82. doi: 10.1148/radiology.167.1.3279453. [DOI] [PubMed] [Google Scholar]
- 4.Lazzari G. Ultrasonographic evaluation of the sciatic nerve and thigh trauma. Radiol Med. 1993;86:573–8. [PubMed] [Google Scholar]
- 5.Padua L, Pazzaglia C, Caliandro P, Granata G, Foschini M, Briani C, et al. Carpal tunnel syndrome: Ultrasound, neurophysiology, clinical and patient-oriented assessment. Clin Neurophysiol. 2008;119:2064–9. doi: 10.1016/j.clinph.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Goedee HS, Brekelmans GJ, van Asseldonk JT, Beekman R, Mess WH, Visser LH, et al. High resolution sonography in the evaluation of the peripheral nervous system in polyneuropathy – A review of the literature. Eur J Neurol. 2013;20:1342–51. doi: 10.1111/ene.12182. [DOI] [PubMed] [Google Scholar]
- 7.Donovan A, Rosenberg ZS, Cavalcanti CF. MR imaging of entrapment neuropathies of the lower extremity. Part 2. The knee, leg, ankle, and foot. Radiographics. 2010;30:1001–19. doi: 10.1148/rg.304095188. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Wang CL, Wu S, et al. The feasibility of using high-resolution ultrasonography to assess ulnar nerve in patients with diabetes. J Ultrason. 2017;17:160–6. doi: 10.15557/JoU.2017.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J, Wu S, Ren J. Ultrasonographic measurement of median nerve cross-sectional area reference values in a healthy Han population from Guiyang, China. Neural Regen Res. 2011;6:1883–7. [Google Scholar]
- 10.Kowalska B, Sudoł-Szopińska I. Normal and sonographic anatomy of selected peripheral nerves. Part III: Peripheral nerves of the lower limb. J Ultrason. 2012;12:148–63. doi: 10.15557/JoU.2012.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Otani Y, Yermakov LM, Dupree JL, Susuki K. Chronic peripheral nerve compression disrupts paranodal axoglial junctions. Muscle Nerve. 2017;55:544–54. doi: 10.1002/mus.25273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stuart JD, Morgan RF, Persing JA. Nerve compression syndromes of the lower extremity. Am Fam Physician. 1989;40:101–12. [PubMed] [Google Scholar]
- 13.Stacy MR, Dearth CL. Multimodality Imaging Approaches for Evaluating Traumatic Extremity Injuries: Implications for Military Medicine. Adv Wound Care (New Rochelle) 2017;6:241–51. doi: 10.1089/wound.2016.0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cartwright MS, Chloros GD, Walker FO, Wiesler ER, Campbell WW. Diagnostic ultrasound for nerve transection. Muscle Nerve. 2007;35:796–9. doi: 10.1002/mus.20761. [DOI] [PubMed] [Google Scholar]
- 15.Yuen EC, So YT. Sciatic neuropathy. Neurol Clin. 1999;17:617–31. doi: 10.1016/s0733-8619(05)70155-9. viii. [DOI] [PubMed] [Google Scholar]
- 16.Schon LC, Baxter DE. Neuropathies of the foot and ankle in athletes. Clin Sports Med. 1990;9:489–509. [PubMed] [Google Scholar]
- 17.Kincaid BR, Barrett SL. Use of high-resolution ultrasound in evaluation of the forefoot to differentiate forefoot nerve entrapments. J Am Podiatr Med Assoc. 2005;95:429–32. doi: 10.7547/0950429. [DOI] [PubMed] [Google Scholar]
- 18.Grechenig W, Clement HG, Peicha G, Klein A, Weiglein A. Ultrasound anatomy of the sciatic nerve of the thigh. Biomed Tech (Berl) 2000;45:298–303. doi: 10.1515/bmte.2000.45.11.298. [DOI] [PubMed] [Google Scholar]
- 19.Chen J, Wu S, Ren J. Ultrasonographic reference values for assessing normal radial nerve ultrasonography in the normal population. Neural Regen Res. 2014;18:44–9. doi: 10.4103/1673-5374.143433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moayeri N, van Geffen GJ, Bruhn J, Chan VW, Groen GJ. Correlation among ultrasound, cross-sectional anatomy, and histology of the sciatic nerve: A review. Reg Anesth Pain Med. 2010;35:442–9. doi: 10.1097/AAP.0b013e3181ef4cab. [DOI] [PubMed] [Google Scholar]
- 21.Tagliafico A, Pugliese F, Bianchi S, Bodner G, Padua L, Rubino M, et al. High-resolution sonography of the palmar cutaneous branch of the median nerve. AJR Am J Roentgenol. 2008;191:107–14. doi: 10.2214/AJR.07.3383. [DOI] [PubMed] [Google Scholar]
- 22.Kerasnoudis A, Pitarokoili K, Behrendt V, Gold R, Yoon MS. Cross sectional area reference values for sonography of peripheral nerves and brachial plexus. Clin Neurophysiol. 2013;124:1881–8. doi: 10.1016/j.clinph.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Sugimoto T, Ochi K, Matsumoto M. Ultrasonographic diagnosis of demyelinating neuropathy. Rinsho Shinkeigaku. 2013;23:1208–11. doi: 10.5692/clinicalneurol.53.1208. [DOI] [PubMed] [Google Scholar]
- 24.Chiou HJ, Chou YH, Chiou SY, Liu JB, Chang CY. Peripheral nerve lesions: Role of high-resolution US. Radiographics. 2003;23:e15. doi: 10.1148/rg.e15. [DOI] [PubMed] [Google Scholar]
- 25.Scheidl E, Böhm J, Simó M, Bereznai B, Bereczki D, Arányi Z, et al. Different patterns of nerve enlargement in polyneuropathy subtypes as detected by ultrasonography. Ultrasound Med Biol. 2014;40:1138–45. doi: 10.1016/j.ultrasmedbio.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 26.Kowalska B, Sudoł-Szopińska I. Ultrasound assessment of selected peripheral nerves pathologies. Part II: Entrapment neuropathies of the lower limb. J Ultrason. 2012;12:463–71. doi: 10.15557/JoU.2012.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hobson-Webb LD, Padua L, Martinoli C. Ultrasonography in the diagnosis of peripheral nerve disease. Expert Opin Med Diagn. 2012;6:457–71. doi: 10.1517/17530059.2012.692904. [DOI] [PubMed] [Google Scholar]
- 28.Kowalska B. Assessment of the utility of ultrasonography with high-frequency transducers in the diagnosis of posttraumatic neuropathies. J Ultrason. 2015;15:15–28. doi: 10.15557/JoU.2015.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang W, Zhong W, Yang M, Shi J, Guowei L, Ma Q, et al. Evaluation of the clinical efficacy of multiple lower-extremity nerve decompression in diabetic peripheral neuropathy. Br J Neurosurg. 2013;27:795–9. doi: 10.3109/02688697.2013.798854. [DOI] [PubMed] [Google Scholar]


