Abstract
Group living can help individuals defend against predators and acquire nutrition. However, conflicts between group members can arise (food sharing, mating, etc), requiring individuals to know the social status of each member to promote survival. In our recent paper, we sought to understand how the brain represents the social status of monkeys living in the same colony. Primates learn the social status of their peers through experience, including observation and direct interactions, just like they learn the rewarding or aversive nature of stimuli that predict different types of reinforcement. Group members may thereby be viewed as differing in value. We found in the amygdala, a brain area specialized for emotion, a neural representation of social hierarchy embedded in the same neuronal ensemble engaged in the assignment of motivational significance to previously neutral stimuli. Interestingly, we found 2 subpopulations of amygdala neurons encoding the social status of individuals in an opposite manner. In response to a stimulus, one population encodes similarly appetitive nonsocial images and dominant monkeys as well as aversive nonsocial stimuli and submissive monkeys. The other population encodes the opposite pattern later in time. This mechanism could reflect the emotional ambiguity we face in social situations as each interaction is potentially positive (eg, food access, protection, promotion) or negative (eg, aggression, bullying).
Keywords: Social neuroscience, primate, reward, neurophysiology, amygdala
Comment on: Munuera J, Rigotti M, Salzman CD. Shared neural coding for social hierarchy and reward value in primate amygdala. Nat Neurosci.2018;21:415–423. doi:10.1038/s41593-018-0082-8. Epub February 19, 2018. PubMed PMID: 29459764.
Primates are social animals, living in groups to find mates, forage for food, and protect themselves from external threats, among other functions. Within a group of primates, conflicts also arise during activities that include food sharing and mating. In many primate species, the resolution of these conflicts is related to the social status of individuals,1,2 with the most dominant individual imposing her or his will. How and where the brain creates and maintains neural representations of social status remained poorly understood. Our recent paper3 established that the amygdala represents social hierarchy of a rhesus macaque colony. Moreover, the same neural ensembles that encodes social hierarchy also encodes the learned value of nonsocial stimuli.
We developed a behavioral paradigm for evaluating monkeys’ knowledge of the social status of other members of their group. Viewer monkeys performed 2 types of blocks of a trace conditioning task. In one block type, monkeys fixated fractal images (conditioned stimulus [CS]) for 400 ms followed, before reward delivery, by a free viewing epoch of 1 second. Three different fractal images were associated with 3 different rewards (large, medium, or no juice delivery). In the second block type, CS consisted of pictures of monkey faces belonging to the viewer monkey’s group. In these blocks, all completed trials resulted in delivery of a medium reward. We computed a social index based on the trial completion rate per social image, the viewing times of the entire image at the end of the free viewing epoch as well as the proportion of time spent looking specifically at the monkey’s eyes, and the type of error related to a failure to maintain fixation at image presentation. Importantly, the computed social index was correlated with the social hierarchy determined by independent observers, allowing us to conclude that viewer monkeys were indeed actively assessing the social status of the monkeys in the pictures presented.
While viewer monkeys were performing this task, we investigated the neurophysiological mechanisms that represent social hierarchy and characterized the relationship between neural representations of social hierarchy and of the reward value of nonsocial stimuli. We performed single-cell recordings simultaneously in the amygdala and the orbitofrontal and anterior cingulate cortices, 3 interconnected brain regions and known to encode reward value and social stimuli.4–7 We found that amygdala represents both the reward value of nonsocial images and the social status of viewed monkeys. In contrast, both prefrontal areas lacked strong neural representation of the social hierarchy while encoding the value of nonsocial images similar to the amygdala. Furthermore, the same population of neurons in the amygdala can be used to decode the reward value of CS and the social status of group members. These results suggest a common neural ensemble for processing motivational significance of social and nonsocial stimuli. The acquisition of neural representations of social hierarchy therefore arise in the same amygdala neural circuits that link representations of nonsocial sensory stimuli with innately rewarding or aversive reinforcement. That mechanism emphasizes the idea that organisms acquire social status representation of individuals through experience as it happens to be by observation or direct interaction. Thereby, social status assessment processes could be achieved through reinforcement learning,8–10 a mechanism mainly known to be involved in the learning of the value (positive/appetitive vs negative/aversive) of nonsocial stimuli.
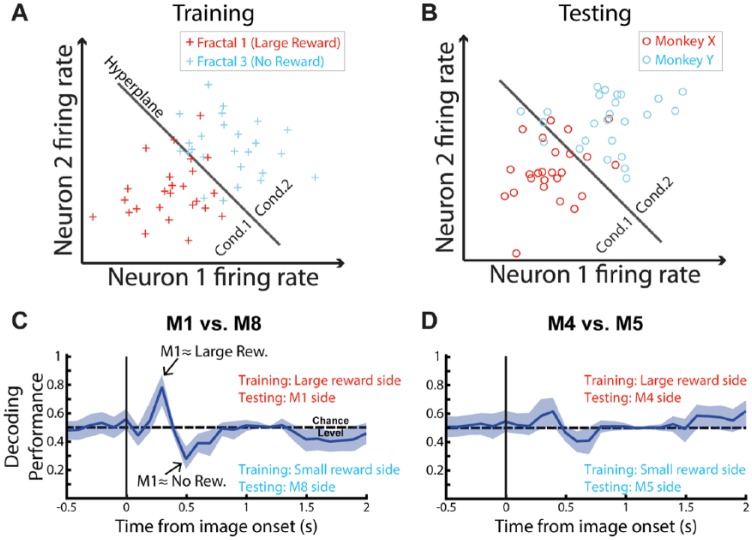
Our main approach was based on a linear decoder that was trained on nonsocial fractal images with opposite reward value (large vs no reward, Figure 1A) and then tested on social images with different pairs of viewed monkeys (Figure 1B), eg, most dominant (M1) vs most submissive monkeys (M8) (Figure 1C) in the hierarchy or M4 vs M5 (Figure 1D). Using a set of stimuli different from the ones used to train the decoder allowed us to investigate any common neural signal between 2 different sets of stimuli (ie, fractal and social images). Overall, our main results show that the larger the social distance between 2 viewed monkeys, the better the decoding performance (see Figure 1C vs Figure 1D). Interestingly, from 100 to 400 ms after image onset, the more dominant monkey in any pair comparison was classified by the decoder as more comparable with the appetitive fractal image (large reward) (and inversely for the more submissive one). Nevertheless, from 400 to 700 ms after image onset (equivalent to the first 300 ms of the 1 second of free viewing where viewer monkeys were free to look at the pictures) we found the opposite pattern; ie, the dominant monkeys were classified as aversive fractal image (no reward), whereas the more submissive ones were classified as the appetitive fractal image.
Figure 1.
Decoding method to investigate the shared neural mechanisms between social and nonsocial stimuli in amygdala (adapted from Figure 3 of our original publication).3 (A) We trained the linear decoder on fractal images (ie, finding the best hyperplane position maximizing the separation between the 2 different types of fractal images). (B) We then tested it on different pairs of monkey faces. (C) Decoding performance plotted as a function of time when pair of monkeys used for testing were the most dominant (M1) vs the most submissive (M8) monkey (ie, the pair with the largest social distance). We set the decoder in a way that if any common signal was detected, the decoding performance would be positive (above chance level) or negative (below chance level) for the most dominant and submissive monkey of the tested pair. (D) Same analysis using a pair of monkeys with a similar social status (M4 vs M5) in the group hierarchy. Shading area represents the 95% confidence intervals (bootstrap, 1000 iterations of the decoder). Analysis time bins were set at 300 ms with 100-ms increments across the trial. During decoding analysis, each bin training and testing were independent.
This flip in the neural representation of social hierarchy may come from 2 populations of neurons with an opposite pattern of selectivity at a dedicated time epoch: when the viewer monkeys had to fixate the social images at the image onset, dominant agents are encoded like a positive item and inversely for the submissive ones. When the viewer monkeys were free to look at the social image, we found the opposite pattern. The main evidence for 2 different subpopulations is the lack of selectivity changes of the neurons across the 2 time epochs used to perform our analyses during nonsocial and social images presentation. In other words, a neuron preferring large reward fractal images in the first epoch also preferred this type of fractal images in the second epoch. The same logic applied for social images. This lack of selectivity change was true whether we considered only the amygdala neurons with a significant modulation (P < .05) for fractal or social images (r = .49, P < 1e−06, n = 94 neurons for fractal images and r = .79, P < 1e−15, n = 70 for social images) or all the cells irrespectively of their selectivity (r = .47, P < 1e−10 for fractal images and r = .75, P < 1e−33 for social images; n = 180). These 2 different neuronal populations encoding the agent’s social status in an opposite manner encompassed the ambiguity of our social interaction. It is indeed often a mixture of repulsion and attraction, especially in the very hierarchical primate world.1 A dominant subject can indeed be positive by providing protection or promotion. They can also be negative by firing you or bullying you. However, a submissive agent is in general not so dangerous (we can relax in his presence) and can be eventually positive as it is easier to steal his food or to ask him to do a task we do not want to do.
A potential neural network used to create these inverted social hierarchy representations could involve the amygdala which receives information about face identity from the temporal cortex.11 The amygdala could also receive information about the nature (positive or negative) of present and past outcomes associated with each face from the ventral tegmental area (dopaminergic reward system) and the lateral habenula (“negative reward” system activated by the absence of reward or by punishments),12 2 areas involved in reinforcement learning. As a social agent can be both positive and negative for survival, the reward and negative-reward system should be involved alternatively for any agent and thus create a different population of neurons. Yet, the current context in which the face is viewed (eg, free to look or not) could change the meaning of the social stimulus and its associated value. One interpretation could be that looking at a very dominant monkey is primarily interesting and positive because it allows the viewer to easily gather some information about an important member of the group. Then, gazing for too long can trigger a defensive mechanism signaling that this type of individual is also potentially harmful as prolonged direct gaze (specially on the eyes) can be viewed as a threat in primates.
Despite the decoder classifying dominant monkey images as something unpleasant in the second time epoch of neural analysis (400 to 700 ms after image onset), the viewer monkey completed more trials when a dominant monkey was on the screen and looked more at their entire pictures (but avoided eye region) at the end of the free viewing epoch13 (see Supplementary Figure 2A and B of our original publication3), ie, more than 1 seconds after image onset. This may represent a kind of rubbernecking effect. A typical example of this effect is when people slow down and look at a car crash they drive past. Thus, rubbernecking, a mechanism impaired in schizophrenia14 and after amygdala lesions,15 is defined as staring compulsively at something emotionally relevant even if it could be potentially unpleasant.
Our results may have implications for improving management and educational practice. Indeed, managers and educators certainly want to be considered as a positive item but being represented as something too negative can decrease the productivity and learning rate of their employees or students. Social and nonsocial aversive stimuli generate an amygdala fear responses which can alter amygdala neurons and increase stress or anxiety16–18 when the aversive events are repetitively experienced during an extensive period. Among many other potential issues, this could result in behavioral inhibition, such as freezing,19 that will affect efficiency and creativity. Conversely, being represented as a positive stimulus should improve the confidence of the peers and allows for better information intake. A recent study suggests this mechanism is applicable to advertisements because primates, including rhesus macaques, show preference for brand logos associated with peers of different sex or social status over control logos without sexual or social association.20
Our results also provide evidence that not all social behavior uses a dedicated neural network to process social information as postulated by some extreme versions of the social brain hypothesis.21 In psychiatric disorders such as autism spectrum disorder (ASD) where social functioning is impaired, data increasingly suggest that the amygdala plays a significant role.22,23 Other recent data also suggest that the autistic deficits go beyond the impairment of social skills and can affect nonsocial function.24 Our results could provide additional evidence to implement targeted strategies in clinical ASD studies and to improve and promote the efficiency of Applied Behavior Analysis therapy for patients with ASD,25 a therapy that primarily used nonsocial reward to reinforce suitable social and nonsocial behavior.
In summary, peers are extremely important for one individual because they can promote his survival by protecting or promoting him or giving him access to food. They can also negatively affect his daily life by stealing his food or his ideas. These different outcomes will strongly depend on the social status of the peers, but all social agents carried intrinsically a potential positive and negative value. Being able to represent the social hierarchy of a group is thus crucial. Our new data reveal that the amygdala, an area involved in value coding of nonsocial stimuli, also signals the hierarchical rank of peers within a social group in the same neuronal ensembles. This neural mechanism changes over time, reflecting the emotional ambiguity of social interactions. This likely mediates appropriate social and emotional behavior, 2 types of behavior that are closely related in many social settings.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: The author was supported by the Fyssen Foundation.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: JM wrote the manuscript.
ORCID iD: Jérôme Munuera  https://orcid.org/0000-0001-9532-8735
https://orcid.org/0000-0001-9532-8735
References
- 1. Thierry B. Covariation of conflict management patterns across macaque species.In: Aureli F, de Waal FBM. eds. Natural Conflict Resolution. Berkeley, CA: University of California Press; 2000:106–128. [Google Scholar]
- 2. Cowlishaw G, Dunbar R. Dominance rank and mating success in male primates. Anim Behav. 1991;41:1045–1056. [Google Scholar]
- 3. Munuera J, Rigotti M, Salzman CD. Shared neural coding for social hierarchy and reward value in primate amygdala. Nat Neurosci. 2018;21:415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saez A, Rigotti M, Ostojic S, Fusi S, Salzman CD. Abstract context representations in primate amygdala and prefrontal cortex. Neuron. 2015;87:869–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leonard CM, Rolls ET, Wilson FA, Baylis GC. Neurons in the amygdala of the monkey with responses selective for faces. Behav Brain Res. 1985;15:159–176. [DOI] [PubMed] [Google Scholar]
- 6. Azzi JC, Sirigu A, Duhamel JR. Modulation of value representation by social context in the primate orbitofrontal cortex. Proc Natl Acad Sci U S A. 2012;109:2126–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chang SW, Gariepy JF, Platt ML. Neuronal reference frames for social decisions in primate frontal cortex. Nat Neurosci. 2013;16:243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sutton RS, Barto AG. Reinforcement Learning. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- 9. Joiner J, Piva M, Turrin C, Chang SWC. Social learning through prediction error in the brain. npj Sci Learn. 2017;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dunne S, O’Doherty JP. Insights from the application of computational neuroimaging to social neuroscience. Curr Opin Neurobiol. 2013;23:387–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tsao DY, Freiwald WA, Tootell RBH, Livingstone MS. A cortical region consisting entirely of face-selective cells. Science. 2006;311:670–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bromberg-Martin ES, Matsumoto M, Nakahara H, Hikosaka O. Multiple timescales of memory in lateral habenula and dopamine neurons. Neuron 2010;67:499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deaner RO, Khera AV, Platt ML. Monkeys pay per view: adaptive valuation of social images by rhesus macaques. Curr Biol. 2005;15:543–548. [DOI] [PubMed] [Google Scholar]
- 14. Eich TS, Smith EE. Schizophrenia and emotional rubbernecking. Cogn Affect Behav Neurosci. 2014;14:202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Anderson AK, Phelps EA. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411:305–309. [DOI] [PubMed] [Google Scholar]
- 16. Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. [DOI] [PubMed] [Google Scholar]
- 17. Patel D, Anilkumar S, Chattarji S, Buwalda B. Repeated social stress leads to contrasting patterns of structural plasticity in the amygdala and hippocampus. Behav Brain Res. 2018;347:314–324. [DOI] [PubMed] [Google Scholar]
- 18. Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annu Rev Physiol. 2005;67:259–284. [DOI] [PubMed] [Google Scholar]
- 19. Roelofs K. Freeze for action: neurobiological mechanisms in animal and human freezing. Philos Trans R Soc B Biol Sci. 2017;372:20160206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Acikalin MY, Watson KK, Fitzsimons GJ, Platt ML. Rhesus macaques form preferences for brand logos through sex and social status based advertising. PLoS ONE. 2018;13:e0194055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dunbar RIM. The social brain hypothesis. Evol Anthropol. 1998;6:178–190. [Google Scholar]
- 22. Gaigg SB. The interplay between emotion and cognition in autism spectrum disorder: implications for developmental theory. Front Integr Neurosci. 2012;6:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bachevalier J, Loveland KA. The orbitofrontal-amygdala circuit and self-regulation of social-emotional behavior in autism. Neurosci Biobehav Rev. 2006;30:97–117. [DOI] [PubMed] [Google Scholar]
- 24. Solomon M, Smith AC, Frank MJ, Ly S, Carter CS. Probabilistic reinforcement learning in adults with autism spectrum disorders. Autism Res. 2011;4:109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Foxx RM. Applied behavior analysis treatment of autism: the state of the art. Child Adolesc Psychiatr Clin N Am. 2008;17:821–834. [DOI] [PubMed] [Google Scholar]