Summary
The influenza A virus genome consists of eight segmented, single-stranded, negative-sense RNAs. Each viral RNA (vRNA) segment forms a ribonucleoprotein complex (RNP) together with nucleoproteins and a polymerase complex, which is a fundamental unit for transcription and replication of the viral genome. Although the exact structure of the intact RNP remains poorly understood, recent electron microscopic studies have revealed certain structural characteristics of the RNP. This review focuses on the findings of these various electron microscopic analyses of RNPs extracted from virions and RNPs inside virions. Based on the morphological and structural observations, we present the architecture of RNPs within a virion and discuss the genome packaging mechanism by which the vRNA segments are incorporated into virions.
Keywords: influenza virus, ribonucleoprotein complex, electron microscopy
Introduction
Influenza A, B, and C viruses are members of the Orthomyxoviridae, which is a family of enveloped viruses with segmented, single-stranded, negative-sense RNA genomes (1). They are classified by antigenic differences in their nucleoprotein (NP) and matrix protein (M1), which are present within the virions. Influenza A viruses are further classified into 16 hemagglutinin (HA) subtypes (H1–H16) and 9 neuraminidase (NA) subtypes (N1–N9) on the basis of the antigenicities of their HA and NA (2). All subtypes can be found in their natural reservoir of wild aquatic birds, but they can also infect mammalian species, such as humans, pigs, and horses. Influenza A viruses cause annual epidemics in humans and occasional pandemics that spread on a global scale with severe consequences for human health. Influenza B viruses naturally infect humans, and occasionally seals, and cause more limited epidemics than Influenza A viruses in humans every few years (3). Influenza C viruses infect humans and pigs (4). Seroepidemiological studies suggest that influenza C virus has been globally distributed, although it is clinically benign in humans (5).
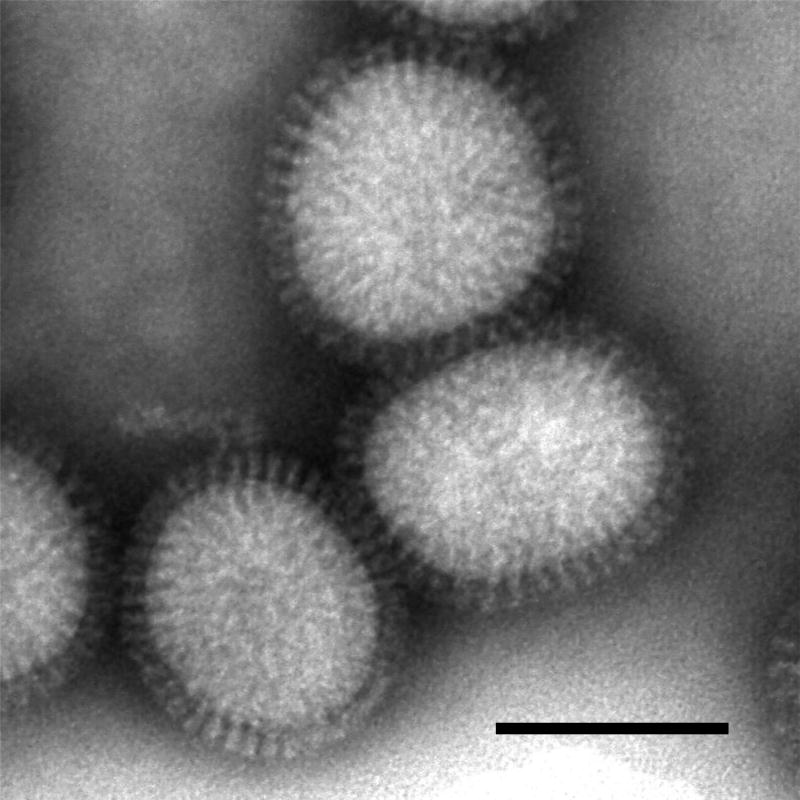
Influenza A virions possess a lipid envelope that is acquired from the apical plasma membrane of infected cells during the budding process. The virions released from infected cells are generally spherical, ranging from approximately 80 to 120 nm in diameter (Figure 1). On the other hand, budding virions on the surface of infected cells present as mostly elongated particles and occasionally filamentous particles of uniform diameter. These virions are covered with projections called spikes. A large number of two glycoproteins, HA and NA, and small amounts of an ion channel protein (M2) are inserted into the envelope. The two glycoproteins form the spikes on the viral surface. The HA spikes are rod-shaped, while the NA spikes are mushroom-shaped with a box-shaped head that is connected to the lipid membrane by a stalk. M1, a peripheral membrane protein, is one of the most abundant viral proteins in the virion. It binds to the lipid envelope and is thought to form a layer beneath it to maintain the spherical or filamentous structure of the virion. The viral genome is enclosed in a shell mainly composed of a layer of M1 protein, HA and NA spikes, and the lipid envelope.
Figure 1.
Purified influenza virions visualized by negative staining electron microscopy. The spherical virions, approximately 120 nm in diameter, are covered with spikes composed of HA and NA. Bar; 100 nm.
The genomes of influenza A and B viruses consist of eight single-stranded negative-sense RNA segments, while that of influenza C virus consists of seven RNA segments. Each viral RNA (vRNA) segment forms a ribonucleoprotein (RNP) complex which creates a twisted rod-like structure that is folded back and coiled on itself (6–10). The RNPs, but not the genomic RNA alone, are transcriptionally active. In the RNP complexes, the vRNA is associated with NP and a heterotrimeric RNA-dependent RNA polymerase complex that is composed of basic polymerase protein 1 (PB1), basic polymerase protein 2 (PB2), and acidic polymerase protein (PA). PB1 forms the core structure of the heterotrimeric RNA polymerase complex. The N-terminal region of PB1 binds to the C-terminal region of PA, and C-terminal region of PB1 binds to the N-terminal region of PB2 (11,12).
Unlike most negative-sense RNA viruses, transcription and replication of the influenza virus genome occurs in the nucleus of infected cells. After synthesis of the genomic RNAs and viral proteins, RNPs are synthesized in nucleus and exported to the cytoplasm mediated by two viral proteins, M1 (13,14) and nuclear export protein (NEP/NS2) (15–17), through a cellular chromosome region maintenance 1 (Crm1) protein-dependent pathway (18–23). The RNPs are intracellularly transported to the budding site (i.e., the lipid rafts on the apical plasma membrane of polarized cells (24,25), while the transmembrane HA, NA, and M2 proteins are conveyed to the cell surface by the standard exocytic pathway. The RNPs are presumed to interact with the M1 proteins and/or the cytoplasmic tails of HA, NA, and M2 at the plasma membrane, to be packaged into virus particles (26,27). Finally, all of the viral components assemble into progeny virions, leading to budding from the apical plasma membrane by membrane fission.
The RNP is the fundamental unit for vRNA synthesis and thus plays important roles in the virus life cycle. Although a detailed structure of the intact RNP is still lacking, recent electron microscopic analyses have revealed important structural characteristics of the RNPs. Here, we will use the findings of these various electron microscopic analyses to describe the architecture of RNPs within the virions as well as the structure of RNPs extracted from virions. We also discuss a mechanism by which the vRNA segments are packaged into virions from a morphological viewpoint.
The structure of purified RNPs from virions
The eight vRNA segments of the influenza A virus genome range in size from 890 to 2341 bases. All eight segments contain conserved sequences at the 3’ and 5’ ends that are partially complimentary to each other (28–30). The complementary terminus is the core promoter, which is essential for the regulation of vRNA synthesis; it forms a base-pairing necessary for the formation of a corkscrew structure (31,32). The heterotrimeric RNA polymerase is associated with the complimentary terminus at the 3’ and 5’ ends of the vRNA (33). Each vRNA segment is separately encapsidated by multiple copies of NP molecules forming a twisted rod-like structure. Each NP molecule is associated with 20–25 nucleotides of vRNA (6,8,34) through its phosphate-sugar backbone (35). The vRNA in the form of RNP is RNase-sensitive (35,36) and can be replaced by negatively charged polyvinylsulfate (37), suggesting that the vRNA has its nucleotide bases exposed on the outside of the RNP and that there are charge interactions between the NP molecules and the phosphate backbone of the vRNA. This is consistent with recent crystal structure data on NP that show that the possible RNA-binding site is composed of many basic residues and is located at the exterior of the NP oligomers (38,39). NP has two sites for NP-NP interaction and has an intrinsic ability to form RNP-like structures even in the absence of viral RNA (10), indicating that NP is the major determinant of the rod-like structure of the RNPs and that the vRNA is wrapped around the NP scaffold. Immunoelectron microscopy of isolated RNPs demonstrates that one copy or a small number of heterotrimeric RNA polymerases is located at, or very close to, an end of each rod-like RNP (40). Taken together, the RNA polymerase is likely located at an end of the rod-like RNP, where it is associated with the complimentary terminus of the vRNA.
The NP monomer, as observed by negative staining electron microscopy, forms small rods with a length of approximately 6.2 nm and a width of 3.5 nm (10). The negatively stained RNPs isolated from virions show twisted rod-like structures and each RNP seems to consist of a single strand of NP monomers that is folded back on itself to give a loop at one end and is coiled on itself to form a double-stranded arrangement except at the loop (6, 8, 37). Given that the RNA polymerase complex is associated with the complementary terminus of vRNA, which exists as double-stranded RNA (33), the polymerase complex would be located at the opposite side of the single-strand loop in the RNP, although the polymerase complex is not directly detectable by negative staining electron microscopy. The diameter of the single strand seen in the loop at the end of RNPs is approximately 7.5 nm (37), which is close to the size of the NP monomer (10). RNPs have major grooves at a periodicity of approximately 15 nm and minor grooves at a periodicity of approximately 7.5 nm in their mid-section (6,41), supporting the idea that the RNP is a single strand of NP co-polymer folded back and coiled upon itself (Figure 2). However, the number of NP monomers per turn in the helix is still uncertain. It can be addressed by three-dimensional structural analysis of the intact RNPs by electron tomography, but such an analysis has not yet been done.
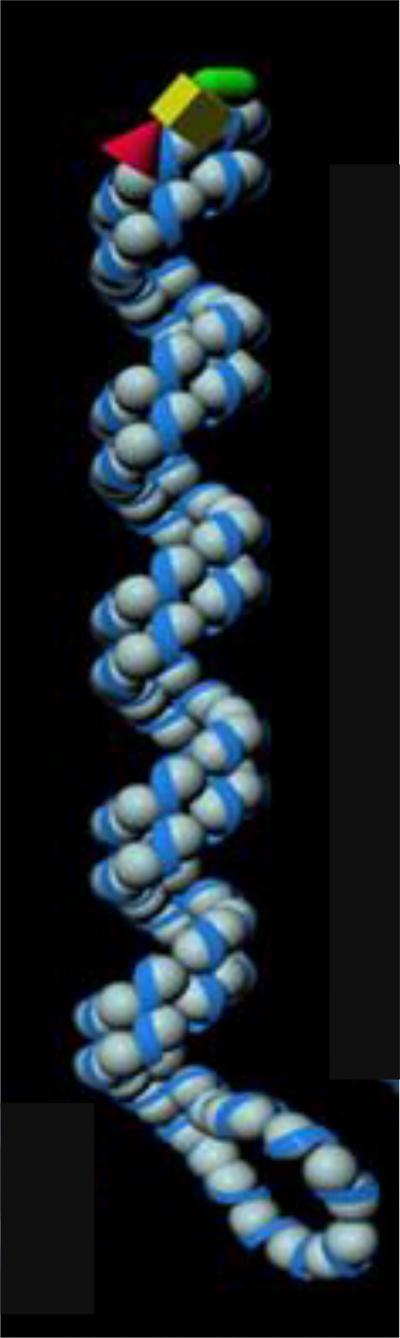
Figure 2.
Schematic diagram of an extracted RNP. The RNP consists of a strand of NP monomers folded back on itself creating a loop at the end, and coiled on itself, forming a double-stranded helical body except at the loop. The NP monomers and vRNA are depicted as gray spheres and a blue ribbon, respectively The polymerase complex is depicted by a yellow box, a red cone, and a green rod.
RNPs extracted from virions by ether or detergent treatment are separated into different size classes by velocity sedimentation in glycerol gradients. The isolated RNPs that possess twisted rod-like structures are about 13 nm in diameter but vary in length from approximately 30 to 110 nm (6,37).. They are broadly classified into three size classes, in which the large RNPs have a peak length of 90 to 110 nm, the intermediate, 60 to 90 nm, and the small, 30 to 50 nm (6). The length of the RNP correlates with the length of each vRNA segment (6), suggesting that the large RNPs would contain the large vRNA segments such as PB2, PB1 and PA, the intermediate RNPs would contain intermediate vRNA segments such as HA, NP, and NA, and the small RNPs would contain the small vRNA segments such as M and NS.
Although the overall morphology of the isolated RNP has been well characterized at low resolution by negative staining electron microscopy as described above, the flexibility and heterogeneity of the RNP structures have prevented high-resolution structural analyses by electron microscopy and image processing based on averaging many identical structures. To obtain samples with sufficient structural rigidity for structural analysis, recombinant RNPs are artificially generated from NP, the three polymerase subunits, and the short vRNA-like genome (34,42–45). Negative staining electron microscopy of the recombinant RNPs shows circular or elliptic structures, depending on the length of the model vRNA-like genome. Each NP monomer is associated with approximately 24 nucleotides in the recombinant RNPs (34), which is consistent with previous reports of NP in authentic RNPs (6,8). A recombinant RNP with a vRNA-like genome of 248 nucleotides, which gives homogeneous structure and yields a good average image, shows a circular shape and contains nine NP monomers, two of which are associated with the RNA polymerase complex (42). Each NP monomer shows a curvature structure and consists of two domains, an upper head domain and a centered body (42,44). The NP monomers are tightly connected to each other at the bottom, which may represent the presence of the model vRNA bound to the NP monomers (42).
The heterotrimeric RNA polymerase complex in the recombinant RNPs shows a very compact structure without apparent boundaries among the PB1, PB2 and PA subunits (43). The positions of PB1, PB2, and PA in the heterotrimeric complex is determined from RNP-monoclonal antibody complexes or tagged RNPs, which reveal that the N-terminal region of PB2 and the C-terminal region of PA are located opposite the polymerase-NP interaction side and that the C-terminal region of PB1 is situated on the polymerase-NP interaction side (43,44). In the three-dimensional RNP model, the polymerase complex is connected to the mid-section of two NP monomers through PB1 and PB2 (42,44), which is consistent with biochemical data indicating that NP interacts with both PB1 and PB2 (46–48). On the other hand, a three-dimensional model reconstituted from a soluble heterotrimeric RNA polymerase complex shows a more open conformation, although there are similarities in the general structural features between the soluble form and the RNP-bound form (45). The conformational difference is more evident in an area implicated in interactions between NP monomers in recombinant RNPs (42), and a substantial displacement of PB2 appears to take place as a result of forming the RNPs, suggesting that the interaction of the polymerase complex with NP or RNA will trigger conformational changes to activate different functions (42). Further structural studies are needed to understand the conformational changes of the polymerase complex during formation of the RNP complex as well as during transcription and replication of the vRNA segments.
The structure of RNPs inside virions
The conformation of RNPs inside virions had been the source of considerable controversy. When examined by negative staining, a continuous strand 7–8 nm in diameter is arranged in the form of a helix within disrupted virions (Figure 3a) (49–54). The helices vary considerably from virion to virion with respect to the number of turns and overall diameter (49,50,52,55). Because the continuous strand had been the only structure seen within the virions by negative staining, it was thought to represent the natural organization of the viral RNP. Thus, it was proposed that the viral RNP exists as a single continuous helix within the virions and that the continuous helix would be fragmented into multiple RNPs during the purification process (51,52). However, it is difficult to morphologically reconcile the single continuous helix with the twisted rod-like structures seen when RNPs are extracted from virions. In addition, there is no evidence that the continuous helices are actually composed of NP molecules. Later, Ruigrok et al (1989) revealed, by negative staining electron microscopy, that a wide variety of the continuous helices within virions consist of the same units that have a dimension of 4 by 4 nm (56). They also demonstrated that isolated M1 protein formed regular strands on liposomes when it was reassociated with lipids in vitro. Because influenza virus has two major internal proteins, NP and M1, and the NP monomer has dimensions of 6.2 by 3.5 nm (10), they concluded that the 4 × 4 nm unit represents an M1 molecule. Thus, the continuous helices of 7–8 nm in diameter seen in the negatively stained disrupted virions are probably pairs of M1 strands, representing a layer of M1 proteins underneath the lipid envelope (56). It is possible that similar continuous helices observed in purified virions by cryoelectron microscopy also represent the layer of M1 molecules (57,58).
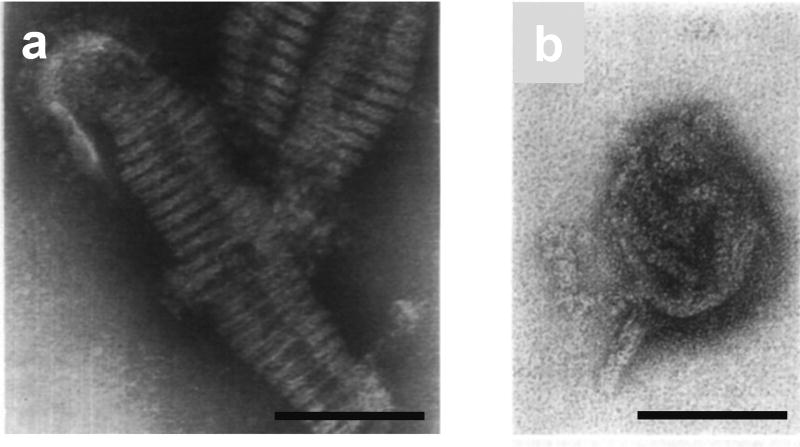
Figure 3.
Internal structure of purified virions observed by negative staining electron microscopy. (a) A continuous helix approximately 8 nm in diameter is observed in a disrupted virion. (b) Irregularly compressed rod-like structures approximately 12 nm in diameter. Two rod-like structures can be seen protruding from the virion. These electron micrographs have been reproduced with permission from those published in Schulze (1972) (54). Bars; 100 nm.
One explanation for the difficulty in elucidating the internal structure of influenza virions by negative staining electron microscopy may originate with poor penetration of stain solutions. To observe the RNPs inside virions by negative staining electron microscopy, purified virions were briefly treated with trypsin to partially remove glycoproteins on the envelope (54). The trypsin-treated virions were infectious and had HA activity but were devoid of neuraminidase activity. Irregularly compressed rod-like structures with diameters similar to those of mid-sectioned RNPs extracted from virions were observed inside these trypsin-treated virions (Figure 3b) (54). Further, twisted rod-like structures, which were morphologically identical to purified RNPs, “spilled” from some of the trypsin-treated virions (Figure 3b) (54). Such twisted rod-like structures also protruded from virions disrupted by freeze-drying and reacted with anti-NP monoclonal antibodies (59), suggesting that the RNPs exist within the virions as fragmented rod-like structures. Thin-section electron microscopy of purified virions also supports this notion. Thin-section electron microscopy of purified virions showed some compressed rod-like structures of 10–13 nm in diameter inside the virions (54), which were morphologically similar to both the purified RNPs and the RNPs seen in trypsin-treated virions by negative staining electron microscopy (54). The good agreement between images obtained by negative staining of purified virions and those obtained by thin sectioning of pelleted virions suggests that the RNPs are present as separate rod-like structures within intact virions.
Arrangement of RNPs inside a virion
It remains unclear how the fragmented RNPs are organized within the virions. Once this issue is resolved, we will finally be able to elucidate the genome packaging mechanism by which vRNA segments are incorporated into each virion. In early proposals, it was predicted that the vRNA segments were bound to a single backbone for cooperative packaging of the segmented vRNAs (37,50–52). However, because this idea is based on the electron microscopic observations of the single continuous helix seen in negatively stained disrupted virions (Figure 3a), which has since been shown to represent the layer of M1 molecules (56), it is an unlikely scenario. Information about the organization of the fragmented rod-like RNPs within the virions is limited. Thus, many questions remain concerning the packaging mechanisms of fragmented RNPs: how many RNPs are incorporated into each virion? How are the RNPs arranged within each virion? Do the RNPs interaction with each other within virions? Are there any specific mechanisms to recruit a full component of eight RNPs into a virion? To answer these questions, the organization of the RNPs within the virions must be revealed in detail.
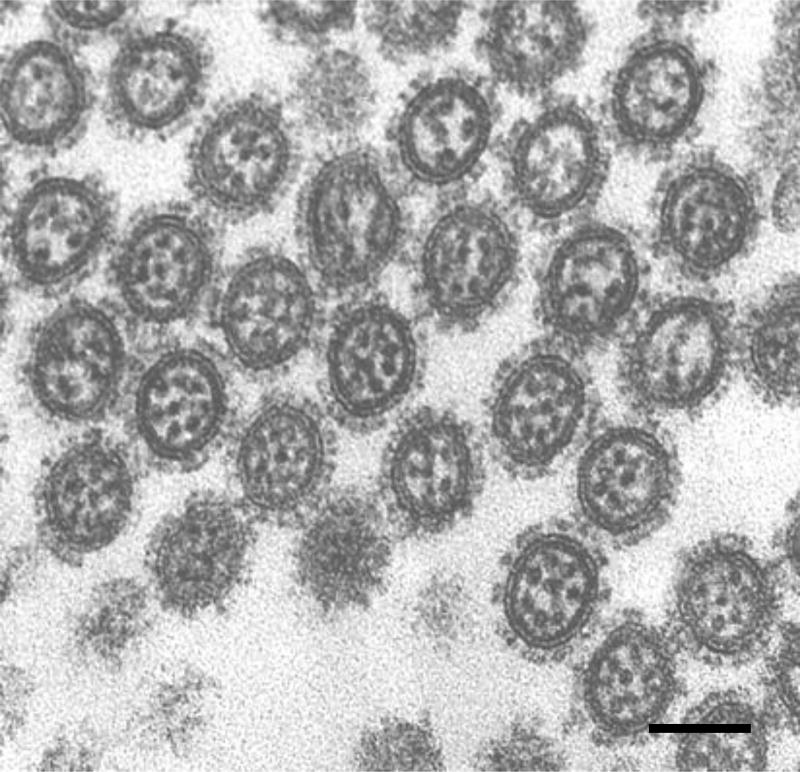
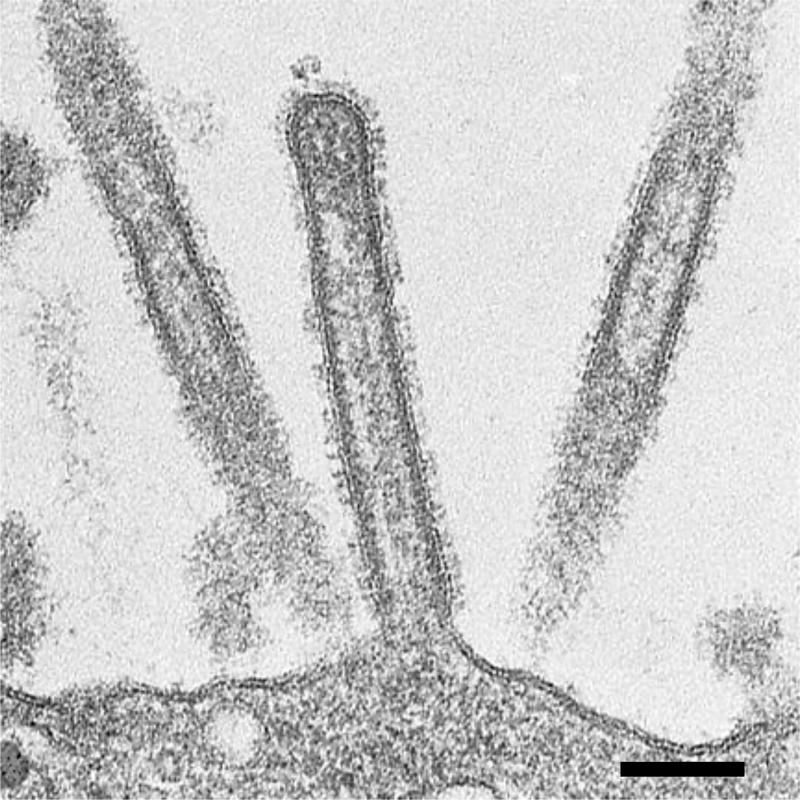
We have demonstrated by thin-section electron microscopy that elongated virions budding from the plasma membrane of infected cells contain distinct arrangement of RNPs inside (59). When the elongated budding virions were sectioned longitudinally, nearly all of them contained rod-like RNPs that were always suspended from the interior of the viral envelope at the distal end of the elongated virions and were oriented perpendicular to the budding tip. They were about 12 nm in width and up to 130 nm in length, consistent with the size of purified RNPs (6, 37). RNPs were similarly observed not only in budded virions but also virions in the course of budding (59), suggesting that the incorporation of fragmented RNPs is coordinated with bud growth. In transversely sectioned budding virions, electron-dense dots of about 12 nm in diameter, confirmed as RNPs by immunoelectron microscopy with anti-nucleoprotein monoclonal antibodies, were observed (59). These dots represent-cross sections of the rod-like RNPs and were apparent inside each budding virion. Interestingly, we found that many of the transversely sectioned budding virions contained a regular arrangement of eight RNPs, in which a central RNP was surrounded by seven others [(7+1) configuration] (Figure 4). No more than eight RNPs were observed in a virion in our study. Notably, serial transverse sections of whole budding virions revealed that all budding virions contained the maximum of eight RNPs, but that they differed in length. Because the length of the RNPs correlates with the length of each vRNA segment (6), these results suggest that each virion contains a highly organized set of eight RNPs composed of different kinds of vRNA segments. The (7+1) configuration of the eight RNPs was also observed in huge filamentous virions as well as different viruses isolated from humans, pigs, and birds (59). Some transversely sectioned filamentous virions showed the typical configuration of eight RNPs, although most were empty. In the longitudinal sections, the RNPs were confined to the distal end of each filamentous virion and the remainder of the filamentous virion was empty (Figure 5), which is consistent with the apparent lack of internal components in most of transversely sectioned filamentous virions (59). Thus, it is likely that huge filamentous virions also incorporate a set of eight RNPs. These results suggest that all budding virions, independent of the virion shape, incorporate an organized set of eight fragmented RNPs that are associated with the inner envelope at the distal end of the virion.
Figure 4.
Internal structure of budding virions revealed by thin-section electron microscopy. Transversely sectioned budding virions show a well-organized arrangement of eight RNPs within the virions. Some virions contain less than eight RNPs because the RNPs differ in length. Thus, the number of RNPs seen in the virions varies depending on where the budding virions are transversely sectioned. Bar; 100 nm.
Figure 5.
Longitudinal section of filamentous virions budding from the cell surface. The rod-like RNPs are associated with the envelope at the distal end of the budding virion. Note that the RNPs are present only at the distal end of the virion. Bar; 100 nm.
In contrast, isolated virions released into environmental solution and purified by ultracentrifugation showed a somewhat different organization of the RNPs within the virions. In an earlier report, the internal structures of purified virions, which were uniformly spherical at approximately 100 nm in diameter, were examined by thin-section electron microscopy (54). Thin-sections of virion pellets showed a wide variety of organization patterns of compressed rod-like RNPs in most of the purified virions, although some virions exhibited the (7+1) configuration of the RNPs. In theory, when the rod-like RNPs are perpendicular to the electron beam, rod-like structures are observed in the virions. On the other hand, when the rod-like RNPs are parallel to the electron beam, round dots are observed in the virions. Because the purified virions are randomly oriented with respect to the electron beam in the pellets, it is natural that only some particles show the (7+1) configuration of eight RNPs. In addition, because some RNPs (from approximately 30 to 120 nm in length) are larger than typical spherical virions (approximately 80 to 120 nm in diameter, including surface spikes), it is possible that some RNPs are compressed and the configurations they had at the time of budding are partially destroyed within the spherical virions. Therefore, it is conceivable that the spherical virions after purification show disordered arrangements of RNPs as well as the regular arrangement of eight RNPs (54), while the elongated budding virions on the cell surface show the (7+1) configuration within the virions when they are transversely sectioned (59). Taken together, it appears that the eight fragmented RNPs hold the well-organized configuration when the virions are budding, but this configuration becomes partially distorted or completely destroyed within the spherical virions upon detachment from the cell surface.
Recently, Harris et al (2006) and Nayak et al (2009) resolved detailed internal structures of purified virions by cryoelectron tomography (58,61). Although they also found the (7+1) configuration of eight RNPs within the purified virions, the eight RNPs were not that well aligned in most virions and the (7+1) configuration was observed at only a low frequency. Such disordered arrangement of the eight RNPs, as well as the limited observation of the (7+1) configuration, suggests that the purified virions were randomly oriented to the electron beam and that some RNPs were compressed in spherical virions, as described above. Nayal et al (2009) also noted that, in their experiment, the purified virions were substantially pleomorphic, differing in size and shape, and that most of the virions contained fewer than eight RNPs (61). They concluded that the incorporation of a complete set of eight RNPs may not occur in each virion and that the arrangement of fragmented RNPs within virions may not be uniform from virion to virion. These discrepancies between budding virions and purified virions could arise from the difference in virion morphology that accompanies sample processing for virion purification. Budding virions on the plasma membrane of infected cells show relatively regular structure, such as elongated or huge filamentous particles with uniform diameter, and are not pleomorphic (59,60,62). On the other hand, purified virions show not only typical spherical structures but also pleomorphic structures of different sizes which have never been reported on thin-section or scanning electron microscopy of budding virions at the plasma membrane. Because morphological changes readily occur in released virions, even when they are kept for a few days at 4°C during the purification process (54), these pleomorphic particles seen only in purified samples could be artifacts introduced during purification. These pleomorphic particles cannot, therefore, reflect the native structure of the virions as the arrangement of RNPs in these particles would be altered.
A possible mechanism of genome packaging
Two models have been proposed thus far to explain the mechanism by which vRNA segments are packaged into virions: the random packaging (63,64) and selective packaging models (65,66). The former model assumes a common packaging signal in all vRNA segments, which differentiates between vRNAs and cellular RNAs but not among vRNAs, enabling any number and combination of vRNAs to be incorporated randomly into virions. The selective packaging model predicts the presence of specific packaging signals for each vRNA segment, which differentiate not only between vRNAs and cellular RNAs but also between individual vRNAs, leading to the incorporation of a complete set of eight vRNA segments into virions. Conclusive evidence in support of either model is lacking, and controversy over the mechanism of genome packaging ensues. Recently, we (67–72) and others (73–80) by virtue of reverse genetics, revealed that all of the eight vRNA segments possess segment-specific packaging signals for efficient incorporation into virions. These packaging signals include bipartite sequences at the 5’ and 3’ ends of the vRNA, which house not only conserved promoter sequences but also coding and segment-specific noncoding regions adjacent to the promoter region. These findings, together with electron microscopic observations of the influenza virion interior, support the selective packaging model and refute the random packaging model.
The (7+1) configuration of eight RNPs observed in virions likely requires specific interactions among the vRNAs (RNPs) to stably maintain the configuration. Reverse genetics studies showed that mutations or deletions in a packaging signal of a vRNA segment reduced incorporation of the other vRNA segments into virions, suggesting that incorporation of the eight vRNA segments are unlikely to be independent but, rather coordinated events involving inter-segment interactions (70,76,77,79,80). However, it remains uncertain whether there are specific interactions among the eight RNPs in virions. The physical contacts among the eight RNPs observed in tomograms of transversely sectioned budding virions provides potential morphological evidence supporting the presence of interactions among RNPs (54). Because the vRNA is wrapped around the NP scaffold (35–37), such contacts may represent vRNA-vRNA interactions through the packaging signals in the respective vRNA segments that are necessary for efficient incorporation into virions (67–80). Although speculative, the interactions among RNPs though the packaging signals may contribute to recruitment of a complete set of eight RNPs as well as the (7+1) configuration. Elucidation of the fine structure of RNPs within virions, as well as the three-dimensional position of the packaging signals on RNPs, could provide the missing details of the packaging mechanism of the influenza virus genome.
A schematic diagram of RNPs in a virion
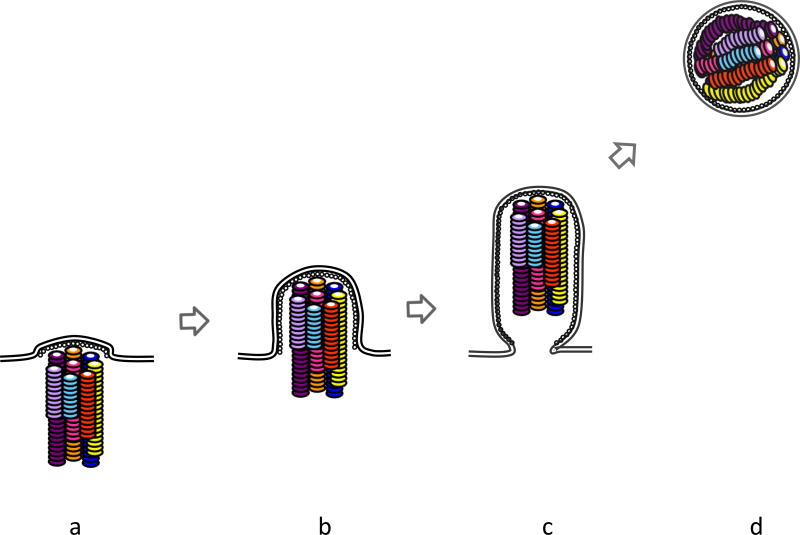
Finally, based on previous electron microscopic analyses, a schematic diagram of RNPs within virions during the budding process is shown in Figure 6. A lipid bilayer of the envelope is depicted as a double line. HA, NA, and M2 embedded in the envelope are omitted in this schematic representation in the interest of clarity. The M1 protein is depicted as white dots underneath the lipid envelope. Each RNP is illustrated as a twisted rod-like structure in different colors (6,8,37). The eight RNPs, which differ in length, are arranged in a specific pattern, whereby seven peripheral RNPs are shown to surround a core RNP (59). However, the respective RNPs in the (7+1) configuration are portrayed arbitrarily due to the lack of information on their alignment. Elucidation of the alignment of eight RNPs will help us understand the mechanism(s) in which a complete set of eight vRNAs is selected. Incorporation of the eight RNPs coordinates with bud growth (Figure 6a–c) (59). The well-organized eight RNPs are enclosed within an elongated budding virion (Figure 6c). They are associated with the inner envelope at the distal end of the virion and oriented perpendicular to the budding tip of the virion (Figure 6c) (59), suggesting that there might be “molecular glue” between the RNP and the inner envelope at the distal end. Although the heterotrimeric polymerase complex should be associated with each RNP at either end of the rod-like structure (40), it is uncertain at which end of the RNP it is located. Although NEP/NS2 likely associates with RNPs via M1 (81), its location in virions remains unsolved. Because the heterotrimeric polymerase complex or NEP/NS2 on RNPs may function as the molecular glue for RNP incorporation into virions, it is important to reveal their localization within virions. Once the elongated virion is released into the environmental solution, it morphs into a spherical shape (59,60). Because some RNPs are apparently longer than the diameter of typical spherical virions excluding the spikes (6, 37), it is possible that these RNPs are compressed in the spherical virions and that the (7+1) configuration of eight RNPs is physically distorted (Figure 6d) (54).
Figure 6.
Schematic diagram of RNPs within a budding virion. (a–c) Incorporation of the eight RNPs is coordinated with bud growth. In an elongated budding virion, eight RNPs are arranged into the (7+1) configuration. (d) In a spherical virion released from the plasma membrane, the RNPs are compressed and the arrangement of eight RNPs is disordered.
Acknowledgments
We thank Susan Watson for editing the manuscript. Our original research was supported by Grants-in-Aid for Specially Promoted Research and for Scientific Research, by a Contract Research Fund for the Program of Founding Research Centers for Emerging and Reemerging Infectious Diseases, by Exploratory Research for Advanced Technology (Japan), by the Special Coordination Funds for Promoting Science and Technology from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, and by National Institute of Allergy and Infectious Diseases Public Health Service research grants. T.N. was supported by a Grant-in-Aid for Young Scientists from the Japan Society for the Promotion of Science, a Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science, and Technology, and by the Takeda Science Foundation.
Abbreviations
- vRNA
viral RNA
- RNP
ribonucleoprotein complex
- NP
nucleoprotein
- M1
matrix protein
- HA
hemagglutinin
- NA
neuraminidase
- M2
ion channel protein
- PB1
basic polymerase protein 1
- PB2
basic polymerase protein 2
- PA
acidic polymerase protein
- NEP/NS2
nuclear export protein
- Crm1
cellular chromosome region maintenance 1
References
- 1.Palese P. Orthomyxoviridae. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE, editors. Fields virology. 5. Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 1647–1689. [Google Scholar]
- 2.Fouchier RA, Munster V, Wallensten A, et al. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. J Virol. 2005;79:2814–2822. doi: 10.1128/JVI.79.5.2814-2822.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Osterhaus AD, Rimmelzwaan GF, Martina BE, et al. Influenza B virus in seals. Science. 2000;288:1051–1053. doi: 10.1126/science.288.5468.1051. [DOI] [PubMed] [Google Scholar]
- 4.Guo YJ, Jin FG, Wang P, et al. Isolation of influenz C virus from pigs and experimental infection of pigs with influenza C virus. J Gen Virol. 1983;64:177–182. doi: 10.1099/0022-1317-64-1-177. [DOI] [PubMed] [Google Scholar]
- 5.Taylor RM. Studies on survival of influenza virus between epidemics and antigenic variants of the virus. Am J Public Health Nations Health. 1949;39:171–178. doi: 10.2105/ajph.39.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Compans RW, Content J, Duesberg PH. Structure of the ribonucleoprotein of influenza virus. J Virol. 1972;10:795–800. doi: 10.1128/jvi.10.4.795-800.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heggeness MH, Smith PR, Ulmanen I, et al. Studies on the helical nucleocapsid of influenza virus. Virology. 1982;118:466–470. doi: 10.1016/0042-6822(82)90367-1. [DOI] [PubMed] [Google Scholar]
- 8.Jennings PA, Finch JT, Winter G, et al. Does the higher order structure of the influenza virus ribonucleoprotein guide sequence rearrangements in influenza viral RNA? Cell. 1983;34:619–627. doi: 10.1016/0092-8674(83)90394-x. [DOI] [PubMed] [Google Scholar]
- 9.Oxford JS, Hockley DJ. Orthomyxoviridae. In: Nermut MV, Steven AC, editors. Animal Virus Structure. Elsevier; New York: 1987. pp. 213–232. [Google Scholar]
- 10.Ruigrok RW, Baudin F. Structure of influenza virus ribonucleoprotein particles. II. Purified RNA-free influenza virus ribonucleoprotein forms structures that are indistinguishable from the intact influenza virus ribonucleoprotein particles. J Gen Virol. 1995;76:1009–1014. doi: 10.1099/0022-1317-76-4-1009. [DOI] [PubMed] [Google Scholar]
- 11.He X, Zhou J, Bartlam M, et al. Crystal structure of the polymerase PA(C)-PB1(N) complex from an avian influenza H5N1 virus. Nature. 2008;454:1123–1126. doi: 10.1038/nature07120. [DOI] [PubMed] [Google Scholar]
- 12.Obayashi E, Yoshida H, Kawai F, et al. The structural basis for an essential subunit interaction in influenza virus RNA polymerase. Nature. 2008;454:1127–1131. doi: 10.1038/nature07225. [DOI] [PubMed] [Google Scholar]
- 13.Bui M, Whittaker G, Helenius A. Effect of M1 protein and low pH on nuclear transport of influenza virus ribonucleoproteins. J Virol. 1996;70:8391–8401. doi: 10.1128/jvi.70.12.8391-8401.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bui M, Wills EG, Helenius A, et al. Role of the influenza virus M1 protein in nuclear export of viral ribonucleoproteins. J Virol. 2000;74:1781–1786. doi: 10.1128/jvi.74.4.1781-1786.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baudin F, Petit I, Weissenhorn W, et al. In vitro dissection of the membrane and RNP binding activities of influenza virus M1 protein. Virology. 2001;281:102–108. doi: 10.1006/viro.2000.0804. [DOI] [PubMed] [Google Scholar]
- 16.O'Neill RE, Talon J, Palese P. The influenza virus NEP (NS2 protein) mediates the nuclear export of viral ribonucleoproteins. EMBO J. 1998;17:288–296. doi: 10.1093/emboj/17.1.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye Z, Liu T, Offringa DP, et al. Association of influenza virus matrix protein with ribonucleoproteins. J Virol. 1999;73:7467–7473. doi: 10.1128/jvi.73.9.7467-7473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akarsu H, Burmeister WP, Petosa C, et al. Crystal structure of the M1 protein-binding domain of the influenza A virus nuclear export protein (NEP/NS2) EMBO J. 2003;22:4646–4655. doi: 10.1093/emboj/cdg449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elton D, Simpson-Holley M, Archer K, et al. Interaction of the influenza virus nucleoprotein with the cellular CRM1-mediated nuclear export pathway. J Virol. 2001;75:408–419. doi: 10.1128/JVI.75.1.408-419.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwatsuki-Horimoto K, Horimoto T, Fujii Y, et al. Generation of influenza A virus NS2 (NEP) mutants with an altered nuclear export signal sequence. J Virol. 2004;78:10149–10155. doi: 10.1128/JVI.78.18.10149-10155.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma K, Roy AM, Whittaker GR. Nuclear export of influenza virus ribonucleoproteins: identification of an export intermediate at the nuclear periphery. Virology. 2001;282:215–220. doi: 10.1006/viro.2001.0833. [DOI] [PubMed] [Google Scholar]
- 22.Neumann G, Hughes MT, Kawaoka Y. Influenza A virus NS2 protein mediates vRNP nuclear export through NES-independent interaction with hCRM1. EMBO J. 2000;19:6751–6758. doi: 10.1093/emboj/19.24.6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watanabe K, Takizawa N, Katoh M, et al. Inhibition of nuclear export of ribonucleoprotein complexes of influenza virus by leptomycin B. Virus Res. 2001;77:31–42. doi: 10.1016/s0168-1702(01)00263-5. [DOI] [PubMed] [Google Scholar]
- 24.Scheiffele P, Rietveld A, Wilk T, et al. Influenza viruses select ordered lipid domains during budding from the plasma membrane. J Biol Chem. 1999;274:2038–2044. doi: 10.1074/jbc.274.4.2038. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, Pekosz A, Lamb RA. Influenza virus assembly and lipid raft microdomains: a role for the cytoplasmic tails of the spike glycoproteins. J Virol. 2000;74:4634–4644. doi: 10.1128/jvi.74.10.4634-4644.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J, Leser GP, Pekosz A, et al. The cytoplasmic tails of the influenza virus spike glycoproteins are required for normal genome packaging. Virology. 2000;269:325–334. doi: 10.1006/viro.2000.0228. [DOI] [PubMed] [Google Scholar]
- 27.McCown MF, Pekosz A. The influenza A virus M2 cytoplasmic tail is required for infectious virus production and efficient genome packaging. J Virol. 2005;79:3595–3605. doi: 10.1128/JVI.79.6.3595-3605.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skehel JJ, Hay AJ. Nucleotide sequences at the 5' termini of influenza virus RNAs and their transcripts. Nucleic Acids Res. 1978;5:1207–1219. doi: 10.1093/nar/5.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robertson JS. 5' and 3' terminal nucleotide sequences of the RNA genome segments of influenza virus. Nucleic Acids Res. 1979;6:3745–3757. doi: 10.1093/nar/6.12.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desselberger U, Racaniello VR, Zazra JJ, et al. The 3' and 5'-terminal sequences of influenza A, B and C virus RNA segments are highly conserved and show partial inverted complementarity. Gene. 1980;8:315–328. doi: 10.1016/0378-1119(80)90007-4. [DOI] [PubMed] [Google Scholar]
- 31.Fodor E, Pritlove DC, Brownlee GG. Characterization of the RNA-fork model of virion RNA in the initiation of transcription in influenza A virus. J Virol. 1995;69:4012–4019. doi: 10.1128/jvi.69.7.4012-4019.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flick R, Neumann G, Hoffmann E, et al. Promoter elements in the influenza vRNA terminal structure. RNA. 1996;2:1046–1057. [PMC free article] [PubMed] [Google Scholar]
- 33.Klumpp K, Ruigrok RW, Baudin F. Roles of the influenza virus polymerase and nucleoprotein in forming a functional RNP structure. EMBO J. 1997;16:1248–1257. doi: 10.1093/emboj/16.6.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ortega J, Martín-Benito J, Zürcher T, et al. Ultrastructural and functional analyses of recombinant influenza virus ribonucleoproteins suggest dimerization of nucleoprotein during virus amplification. J Virol. 2000;74:156–163. doi: 10.1128/jvi.74.1.156-163.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baudin F, Bach C, Cusack S, et al. Structure of influenza virus RNP. I. Influenza virus nucleoprotein melts secondary structure in panhandle RNA and exposes the bases to the solvent. EMBO J. 1994;13:3158–3165. doi: 10.1002/j.1460-2075.1994.tb06614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duesberg PH. Distinct subunits of the ribonucleoprotein of influenza virus. J Mol Biol. 1969;42:485–499. doi: 10.1016/0022-2836(69)90237-x. [DOI] [PubMed] [Google Scholar]
- 37.Pons MW, Schulze IT, Hirst GK, et al. Isolation and characterization of the ribonucleoprotein of influenza virus. Virology. 1969;39:250–259. doi: 10.1016/0042-6822(69)90045-2. [DOI] [PubMed] [Google Scholar]
- 38.Ye Q, Krug RM, Tao YJ. The mechanism by which influenza A virus nucleoprotein forms oligomers and binds RNA. Nature. 2006;444:1078–1082. doi: 10.1038/nature05379. [DOI] [PubMed] [Google Scholar]
- 39.Ng AK, Zhang H, Tan K, et al. Structure of the influenza virus A H5N1 nucleoprotein: implications for RNA binding, oligomerization, and vaccine design. FASEB J. 2008;22:3638–3647. doi: 10.1096/fj.08-112110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murti KG, Webster RG, Jones IM. Localization of RNA polymerases on influenza viral ribonucleoproteins by immunogold labeling. Virology. 1988;164:562–566. doi: 10.1016/0042-6822(88)90574-0. [DOI] [PubMed] [Google Scholar]
- 41.Schulze IT, Pons MW, Hirst GK. In: The Biology of Large RNA Viruses. Barry RD, Mahy BWJ, editors. Academic Press; New York: 1970. pp. 324–346. [Google Scholar]
- 42.Martín-Benito J, Area E, Ortega J, et al. Three-dimensional reconstruction of a recombinant influenza virus ribonucleoprotein particle. EMBO Rep. 2001;2:313–317. doi: 10.1093/embo-reports/kve063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Area E, Martín-Benito J, Gastaminza P, et al. 3D structure of the influenza virus polymerase complex: localization of subunit domains. Proc Natl Acad Sci USA. 2004;101:308–313. doi: 10.1073/pnas.0307127101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coloma R, Valpuesta JM, Arranz R, et al. The structure of a biologically active influenza virus ribonucleoprotein complex. PLoS Pathog. 2009;5:e1000491. doi: 10.1371/journal.ppat.1000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Torreira E, Schoehn G, Fernández Y, et al. Three-dimensional model for the isolated recombinant influenza virus polymerase heterotrimer. Nucleic Acids Res. 2007;35:3773–3783. doi: 10.1093/nar/gkm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Biswas SK, Boutz PL, Nayak DP. Mutational analysis of the conserved motifs of influenza A virus polymerase basic protein 1. J Virol. 1994;68:1819–1826. doi: 10.1128/jvi.68.3.1819-1826.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Medcalf L, Poole E, Elton D, et al. Temperature-sensitive lesions in two influenza A viruses defective for replicative transcription disrupt RNA binding by the nucleoprotein. J Virol. 1999;73:7349–7356. doi: 10.1128/jvi.73.9.7349-7356.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Poole E, Elton D, Medcalf L, et al. Functional domains of the influenza A virus PB2 protein: identification of NP- and PB1-binding sites. Virology. 2004;321:120–133. doi: 10.1016/j.virol.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 49.Hoyle L, Horne RW, Waterson AP. The structure and composition of the myxoviruses. II. Components released from the influenza virus particle by ether. Virology. 1961;13:448–459. doi: 10.1016/0042-6822(61)90276-8. [DOI] [PubMed] [Google Scholar]
- 50.Apostolov K, Flewett TH. Internal structure of influenza virus. Virology. 1965;26:506–508. doi: 10.1016/0042-6822(65)90014-0. [DOI] [PubMed] [Google Scholar]
- 51.Almeida JD, Brand CM. A morphological study of the internal component of influenza virus. J Gen Virol. 1975;27:313–318. doi: 10.1099/0022-1317-27-3-313. [DOI] [PubMed] [Google Scholar]
- 52.Murti KG, Bean WJ, Jr, Webster RG. Helical ribonucleoproteins of influenza virus: an electron microscopic analysis. Virology. 1980;104:224–229. doi: 10.1016/0042-6822(80)90380-3. [DOI] [PubMed] [Google Scholar]
- 53.Murti KG, Brown PS, Bean WJ, Jr, et al. Composition of the helical internal components of influenza virus as revealed by immunogold labeling/electron microscopy. Virology. 1992;186:294–299. doi: 10.1016/0042-6822(92)90084-3. [DOI] [PubMed] [Google Scholar]
- 54.Schulze IT. The structure of influenza virus. II. A model based on the morphology and composition of subviral particles. Virology. 1972;47:181–196. doi: 10.1016/0042-6822(72)90251-6. [DOI] [PubMed] [Google Scholar]
- 55.Almeida JD, Waterson AP. In: The Biology of Large RNA Viruses. Barry RD, Mahy BWJ, editors. Academic Press; New York: 1970. pp. 27–51. [Google Scholar]
- 56.Ruigrok RW, Calder LJ, Wharton SA. Electron microscopy of the influenza virus submembranal structure. Virology. 1989;173:311–316. doi: 10.1016/0042-6822(89)90248-1. [DOI] [PubMed] [Google Scholar]
- 57.Booy FP, Ruigrok RW, van Bruggen EF. Electron microscopy of influenza virus. A comparison of negatively stained and ice-embedded particles. J Mol Biol. 1985;184:667–676. doi: 10.1016/0022-2836(85)90312-2. [DOI] [PubMed] [Google Scholar]
- 58.Harris A, Cardone G, Winkler DC. Influenza virus pleiomorphy characterized by cryoelectron tomography. Proc Natl Acad Sci USA. 2006;103:19123–19127. doi: 10.1073/pnas.0607614103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Noda T, Sagara H, Yen A, et al. Architecture of ribonucleoprotein complexes in influenza A virus particles. Nature. 2006;439:490–492. doi: 10.1038/nature04378. [DOI] [PubMed] [Google Scholar]
- 60.Compans RW, Dimmock NJ. An electron microscopic study of single-cycle infection of chick embryo fibroblasts by influenza virus. Virology. 1969;39:499–515. doi: 10.1016/0042-6822(69)90098-1. [DOI] [PubMed] [Google Scholar]
- 61.Nayak DP, Balogun RA, Yamada H, et al. Influenza virus morphogenesis and budding. Virus Res. 2009;143:147–161. doi: 10.1016/j.virusres.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Herrero-Uribe L, Mann GF, Zuckerman AJ, et al. Replication of influenza A and B viruses in human diploid cells. J Gen Virol. 1983;64:471–475. doi: 10.1099/0022-1317-64-2-471. [DOI] [PubMed] [Google Scholar]
- 63.Enami M, Sharma G, Benham C, et al. An influenza virus containing nine different RNA segments. Virology. 1991;185:291–298. doi: 10.1016/0042-6822(91)90776-8. [DOI] [PubMed] [Google Scholar]
- 64.Bancroft CT, Parslow TG. Evidence for segment-nonspecific packaging of the influenza a virus genome. J Virol. 2002;76:7133–7139. doi: 10.1128/JVI.76.14.7133-7139.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Duhaut SD, McCauley JW. Defective RNAs inhibit the assembly of influenza virus genome segments in a segment- specific manner. Virology. 1996;216:326–337. doi: 10.1006/viro.1996.0068. [DOI] [PubMed] [Google Scholar]
- 66.Odagiri T, Tashiro M. Segment-specific noncoding sequences of the influenza virus genome RNA are involved in the specific competition between defective interfering RNA and its progenitor RNA segment at the virion assembly step. J Virol. 1997;71:2138–2145. doi: 10.1128/jvi.71.3.2138-2145.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fujii Y, Goto H, Watanabe T, et al. Selective incorporation of influenza virus RNA segments into virions. Proc Natl Acad Sci USA. 2003;100:2002–2007. doi: 10.1073/pnas.0437772100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Watanabe T, Watanabe S, Noda T, et al. Exploitation of nucleic acid packaging signals to generate a novel influenza virus-based vector stably expressing two foreign genes. J Virol. 2003;77:10575–10583. doi: 10.1128/JVI.77.19.10575-10583.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fujii K, Fujii Y, Noda T, et al. Importance of both the coding and the segment-specific noncoding regions of the influenza A virus NS segment for its efficient incorporation into virions. J Virol. 2005;79:3766–3774. doi: 10.1128/JVI.79.6.3766-3774.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Muramoto Y, Takada A, Fujii K, et al. Hierarchy among viral RNA (vRNA) segments in their role in vRNA incorporation into influenza A virions. J Virol. 2006;80:2318–2325. doi: 10.1128/JVI.80.5.2318-2325.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ozawa M, Maeda J, Iwatsuki-Horimoto K, et al. Nucleotide sequence requirements at the 5' end of the influenza A virus M RNA segment for efficient virus replication. J Virol. 2009;83:3384–3388. doi: 10.1128/JVI.02513-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fujii K, Ozawa M, Iwatsuki-Horimoto K, et al. Incorporation of influenza A virus genome segments does not absolutely require wild-type sequences. J Gen Virol. 2009;90:1734–1740. doi: 10.1099/vir.0.010355-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liang Y, Hong Y, Parslow TG. cis-Acting packaging signals in the influenza virus PB1, PB2, and PA genomic RNA segments. J Virol. 2005;79:10348–10355. doi: 10.1128/JVI.79.16.10348-10355.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.de Wit E, Spronken MI, Rimmelzwaan GF, et al. Evidence for specific packaging of the influenza A virus genome from conditionally defective virus particles lacking a polymerase gene. Vaccine. 2006;24:6647–6650. doi: 10.1016/j.vaccine.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 75.Gog JR, Afonso Edos S, Dalton RM, et al. Codon conservation in the influenza A virus genome defines RNA packaging signals. Nucleic Acids Res. 2007;35:1897–1907. doi: 10.1093/nar/gkm087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marsh GA, Hatami R, Palese P. Specific residues of the influenza A virus hemagglutinin viral RNA are important for efficient packaging into budding virions. J Virol. 2007;81:9727–9736. doi: 10.1128/JVI.01144-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marsh GA, Rabadán R, Levine AJ, et al. Highly conserved regions of influenza a virus polymerase gene segments are critical for efficient viral RNA packaging. J Virol. 2008;82:2295–2304. doi: 10.1128/JVI.02267-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liang Y, Huang T, Ly H, et al. Mutational analyses of packaging signals in influenza virus PA, PB1, and PB2 genomic RNA segments. J Virol. 2008;82:229–236. doi: 10.1128/JVI.01541-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hutchinson EC, Curran MD, Read EK, et al. Mutational analysis of cis-acting RNA signals in segment 7 of influenza A virus. J Virol. 2008;82:11869–11879. doi: 10.1128/JVI.01634-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hutchinson EC, Wise HM, Kudryavtseva K, et al. Characterisation of influenza A viruses with mutations in segment 5 packaging signals. Vaccine. 2009;27:6270–6275. doi: 10.1016/j.vaccine.2009.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yasuda J, Nakada S, Kato A, et al. Molecular assembly of influenza virus: association of the NS2 protein with virion matrix. Virology. 1993;196:249–255. doi: 10.1006/viro.1993.1473. [DOI] [PubMed] [Google Scholar]