Abstract
In contrast with previous accounts, it is reported that a single, strongly hydrophilic Cu complex can control an electrochemically mediated atom transfer radical polymerization (eATRP) in oil-in-water miniemulsion in the presence of anionic surfactants, such as sodium dodecyl sulfate (SDS). The anionic surfactant interacted strongly with cationic copper complexes, enabling controlled polymerization by a combination of “interfacial” and “ion-pair” catalysis, whereby ion pairs transport the catalyst to the monomer droplets. The ion-pair system was assembled in situ by mixing commercially available reagents (NaBr, SDS, and traditional hydrophilic copper complexes). Polymer purification was very facile because after reaction >99% of the hydrophilic copper complexes spontaneously left the hydrophobic polymer particles.
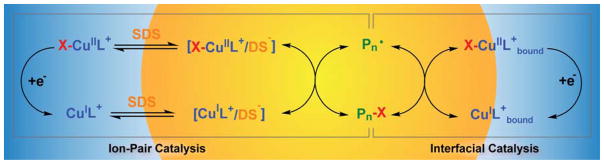
Graphical Abstract

Introduction
In an electrochemically mediated atom transfer radical polymerization (eATRP, Scheme 1) the active CuI catalyst is (re)generated by electrochemical reduction at a working electrode (WE).1 A successful ATRP in miniemulsion typically requires hydrophobic catalyst to be confined into organic phase.2–5 Thus, in a recently reported eATRP miniemulsion procedure, a dual catalytic system was utilized, composed of one hydrophobic and one hydrophilic catalyst.6 The presence of a second water-soluble catalyst was required to close the electrochemical circuit between the WE and the hydrophobic catalyst complex residing within monomer droplets. The WE was in contact with the aqueous phase, from which the water-soluble catalyst shuttled the electrochemical stimulus to the hydrophobic catalyst confined in the dispersed droplets.
Scheme 1.
Mechanism of eATRP
Unexpectedly, and in contrast with our previous results, we recently discovered that a single hydrophilic catalyst could control a miniemulsion eATRP, if the cationic copper complex is paired with an anionic surfactant. The catalyst/surfactant system then generates ion pairs that transport the catalyst into the hydrophobic monomer droplets, thus allowing a controlled polymerization.
Non-ionic surfactants are typically employed in miniemulsion ATRP.7,8 Until recently, anionic surfactants such as sodium dodecyl sulfate (SDS) were considered a poison for ATRP catalysts.9,10 Instead, in this work the interaction between SDS and CuII/L was exploited to concentrate the catalyst in the polymerization loci.
Different combinations of surfactant and hydrophilic Cu ligands were tested in the miniemulsion eATRP of n-butyl acrylate (BA), relevant structures of the reagents are provided in Scheme 2. The X-CuIIL+/SDS system was characterized by electrochemical and spectroscopic techniques in order to provide a detailed molecular description of the catalyst.
Scheme 2.
Structures of the Investigated Surfactants and Copper Ligands
Results
A typical list of reagents used in this miniemulsion eATRP in the presence of hydrophilic catalyst and anionic surfactant is shown in Table 1. Since the surfactant (DS− anion) can coordinate to CuIIL2+, displacing Br− from the deactivator Br-CuIIL2+,10 excess NaBr (0.1 M) was added to the reaction medium to avoid this undesired reaction. Under such conditions, Br-CuIIL+ was stable and was therefore considered the main CuII species present in the aqueous phase.11
Table 1.
Composition of Organic and Aqueous Phases in a Typical Miniemulsion Polymerizationa
| component | weight (g) | comments |
|---|---|---|
| organic phase | ||
| BA | 7.12 | 20 vol% (18 wt%) to total |
| Ethyl α-bromoisobutyrate (EBiB) | 0.039b | [BA]/[EBiB] = 280/1 |
| Hexadecane (HD) | 0.77 | 10.8 wt% to BA |
| aqueous phase | ||
| Water | 32 | Distilled water |
| SDSc | 0.44 | 6.2 wt% to BA |
| NaBr | 0.41 | [NaBr] = 0.1 M |
| CuIIBr2 | 8.9×10−3 | 1 mM with respect to Vtot |
| TPMAc | 0.023 | [CuIIBr2]/[L] = 1/2 |
Polymerization conditions: T = 65 °C; working electrode = Pt mesh with area ≈ 6 cm2; counter electrode = Pt mesh separated from reaction mixture by methylcellulose gel saturated with (C2H5)4NBF4; reference electrode = Ag/AgI/0.1 M (n-C4H9)4NI;
Initiator concentration, [EBiB], was varied to target different degrees of polymerization (DP);
Other employed surfactants and copper ligands are listed in Scheme 2; tris(2-pyridylmethyl)amine (TPMA), 1-(4-methoxy-3,5-dimethylpyridin-2-yl)-N-((4-methoxy-3,5-dimethylpyridin-2-yl)methyl)-N-(pyridin-2-ylme-thyl)methanamine (TPMA*2), tris[2-(dimethylamino)ethyl]amine (Me6TREN), N,N-bis(2-pyri-dylmethyl)-2-hydroxyethylamine (BPMEA), and bis[2-(4-methoxy-3,5-dimethyl)pyridyl-methyl]octadecylamine (BPMODA*).
Table 2 shows the results of eATRP of BA in miniemulsion with different surfactants and catalysts. In an eATRP, the active CuIL+ is (re)generated by electrochemical reduction of X-CuIIL (Scheme 1). The appropriate potential (Eapp) applied at the WE was chosen from the cyclic voltammetry (CV) of the catalyst in the polymerization mixture (Figure S1). Eapp ≈ Epc was chosen for each polymerization, in order to reduce X-CuIIL+ with similar rates (Epc = cathodic peak potential).
Table 2.
eATRP of BA in Miniemulsion with Different Surfactants and Catalysts, T = 65 °Ca
| entry | aq. phase ligand | org. phase ligand | surfactant | Eapp | t (h) | Qb(C) | conv (%) | k papp c (h−1) | Mn | Mn,th | Đ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | TPMA | - | Brij 98 | Epc − 0.03 V | 7.5 | 16.2 | 90 | 0.60 | 27800 | 3200 | 4.77 |
| 2 | TPMA | - | SDS | Epc − 0.03 V | 7.5 | 3.9 | 75 | 0.18 | 28400 | 27300 | 1.18 |
| 3 | TPMA | BPMODA* | SDS | Epc | 24 | 6.6 | 13 | 0.01 | 12400 | 5100 | 1.32 |
| 4d | BPMEA | BPMODA* | SDS | Epc | 24 | 5.8 | 61 | 0.04 | 31800 | 22700 | 1.25 |
| 5 | TPMA*2 | - | SDS | Epc − 0.03 V | 7.5 | 3.3 | 38 | 0.07 | 15900 | 14100 | 1.32 |
| 6 | Me6TREN | - | SDS | Epc − 0.03 V | 1.5 | 7.3 | 93 | 0.40 | 59500 | 33800 | 1.94 |
| 7 | Me6TREN | - | SDS | Epc + 0.12 V | 5 | 4.6 | 77 | 0.55 | 38700 | 27900 | 1.56 |
| 8d | BPMEA | - | SDS | Epc | 24 | 2.0 | 66 | 0.06 | 29100 | 24300 | 4.62 |
Effect of Surfactant
eATRP with a hydrophilic catalyst, such as Br-CuIITPMA+, was strongly affected by the nature of the surfactant (Table 2, entry 1 vs. 2). The presence of a non-ionic Brij-98 afforded an uncontrolled polymerization with very broad molecular weight dispersity, suggesting the presence of insufficient amount of deactivator in the polymerizing droplets (Figure S3). Conversely, the use of an anionic surfactant, SDS, provided good control (Figure 1). This indicated the presence of a specific interaction between Br-CuIIL+ and SDS that favored controlled polymerization within the dispersed monomer droplets, without requiring the presence of a dual hydrophilic/hydrophobic catalytic system.
Figure 1.
Miniemulsion eATRP of BA with different copper ligands (L = TPMA, TPMA*2, and Me6TREN; Table 2, entries 2, 5, 6). (A) Logarithmic kinetic plot and (B) molecular weight (MW) and Đ evolution vs. monomer conversion. Reaction conditions as in Table 1.
In fact, eATRP with the Br-CuIITPMA+/SDS system was much faster, and somewhat better controlled in terms of molecular weight and dispersity, than eATRP with the dual catalysts Br-CuIITPMA+/Br-CuII(BPMODA*)+ or Br-CuIIBPMEA+/Br-CuII(BPMODA*)+ (Table 2, entries 3–4).
Effect of Catalyst Hydrophilicity
With the single Br-CuIITPMA+ catalyst and SDS as surfactant, polymerization control increased with increasing hydrophilicity of the catalyst. Molecular-weight dispersity, Đ, sharply decreased, as shown in Table 3. Hydrophilicity was quantified from the distribution of the catalyst between water and BA, i.e. partition experiments in the absence of SDS. The catalyst Br-CuIITPMA+, completely distributed in the aqueous phase, provided very well defined poly(n-butyl acrylate), PBA (Figure 1). Unexpectedly, the super-active catalyst Br-CuII(TPMA*2)+ provided slower polymerization rate and poorer control, indicating that catalyst distribution and interfacial dynamics may play a more important role than the thermodynamics of RX activation.12
Table 3.
Partition of CuIIBr2/L Catalysts between Water and BAa
| L | [CuIIBr2/L]water/[CuIIBr2/L]tot
|
Ieffb | Đb | |
|---|---|---|---|---|
| 15 vol% BAc | 30 vol% BAd | |||
|
|
|
|||
| TPMAe,f | 1.00 | 1.00 | 0.96 | 1.18 |
| TPMA*2 | 1.00 | 0.99 | 0.89 | 1.32 |
| Me6TREN | 0.94 | 0.94 | 0.57 | 1.94 |
| BPMEAf | 0.54 | 0.73 | 0.84 | 4.62 |
[CuIIBr2/L]tot = 2.5 mM at room temperature in water. Ratios of [CuIIBr2/L]water/[CuIIBr2/L]tot were determined by calibration curve in water (Figures S4–S5);
Initiation efficiency (Ieff = Mn,th/Mn), from Table 2 (at Eapp = Epc − 0.03 V or Eapp = Epc).
13.5 wt% of BA;
27.8 wt% of BA.
No Cu complex was detected in the Vis-NIR spectrum of the organic phase.
From reference 6.
Overall, catalyst’s hydrophilicity or nature of the surfactant affected eATRP much more than the rate and amount of CuI generation. For example, control only partially improved by decreasing the rate of regeneration of CuIMe6TREN+ (Table 2, entry 6 vs. 7). CuII reduction rate was diminished by applying a 0.15 V more positive Eapp, which resulted in ~300 times lower CuI/CuII ratio on the surface of the working electrode.13 This drastic decrease in CuII reduction could not guarantee full control of eATRP, lowering Đ from 1.94 to 1.56. CuI generation rate was also varied in the case of X-CuIITPMA+, with even smaller effects on polymerization control, which was always good (Table S2). These results confirmed that the specific catalyst/surfactant interaction was the most important parameter affecting control.
The best performing system, Br-CuIITPMA+/SDS, was also used to produce very well defined PBA (Đ = 1.1) in a much simplified eATRP system (seATRP), i.e. a two-electrode system with a sacrificial Al anode under fixed current conditions (Figures S7–S8).14 This setup can be performed with a simpler instrumentation, a current generator instead of a potentiostat.15
Effect of Catalyst Loading
The efficiency of the Br-CuIITPMA+/SDS complex catalyst was tested over the range of 1200 to 300 ppm of Cu (Table 4, entries 1–3). Controlled eATRP was obtained in each case, with linear increase of MW with conversion (Figure S9). However, polymerization rate tended to slow down at high conversion with lower catalyst concentration. Higher catalyst loadings provided higher rates of polymerization and higher conversions with narrower Đ.
Table 4.
eATRP of BA in Miniemulsion at Different Copper Concentrations and Degree of Polymerizationa
| [M]/[EBiB]/[CuIIBr2] | Qb (C) | Conv (%) | kpapp c (h−1) | Mn | Mn,th | Đ | dv,finald (nm) | |
|---|---|---|---|---|---|---|---|---|
| 1 | 280/1/0.09 | 2.6 | 60 | 0.14 | 24600 | 21800 | 1.19 | 105±1 |
| 2 | 280/1/0.20 | 3.9 | 75 | 0.18 | 28400 | 27300 | 1.18 | 126±2 |
| 3 | 280/1/0.34 | 4.8 | 87 | 0.27 | 33700 | 31900 | 1.16 | 174±1 |
| 4 | 100/1/0.07 | 4.9 | 80 | 0.22 | 10700 | 10600 | 1.26 | 117±1 |
| 5 | 500/1/0.36 | 1.8 | 47 | 0.09 | 31600 | 30200 | 1.09 | 100±1 |
Conditions as in Table 1 unless otherwise stated; Eapp = Epc − 0.03 V, selected from CV response; reaction time = 7.5 h.
Charge passed, determined from the chronoamperometry recorded during electrolysis (e.g. Figure S9).
The slope of the ln([M]0/[M]) vs time plot.
Final average particle diameter, calculated from volume, determined by dynamic light scattering (DLS).
Targeted Degree of Polymerization (DP)
Polymerizations of BA were also performed at three different [M]0/[EBiB]0 ratios, targeting DP = 100, 280, and 500 (Table 4). Polymerizations were well controlled with linear first-order kinetics (Figure 2a), Mn matching the theoretical values, and low Đ (Figure 2b). Dispersity and polymerization rate decreased with increasing [M]0/[EBiB]0.13,16
Figure 2.
Miniemulsion eATRP of BA with different target DP (100, 280, and 500). (A) Kinetic plot and (B) MW and Đ vs. monomer conversion. Reaction conditions as in Table 1.
Chain-End Functionality
Chain-end functionality was completely retained, within the GPC detection limit, which was proven by chain extension of a PBA78-Br macroinitiator prepared by miniemulsion eATRP with Br-CuIITPMA+/SDS (DP = 78, Figure 3). PBA78-Br was seamlessly extended with tert-butyl acrylate (tBA) in situ, generating PBA78-b-P(tBA67-stat-BA18), where stat indicates the formation of a statistical copolymer with composition tBA/BA 67/18. PBA78-Br was also chain extended after purification of the macroinitiator and re-emulsification, generating PBA78-b-P(tBA)67 (experimental details in Table S3). In each case, linear first-order kinetics, predetermined MWs, and low dispersities were obtained (Figure S11–S12).
Figure 3.
Chain extension of PBA78-Br by simplified electrochemically mediated ATRP (se-ATRP) in miniemulsion (red = in situ chain extension, blue = chain extension after purification of the PBA-Br macroinitiator; Table S3). (A) MW and Đ vs. monomer conversion. (B–C) GPC traces. Reaction conditions as in Table 1, with [tBA]/[PBA78-Br]/[CuII] = 80/1/0.06, 20% v/v tBA.
By recording the total consumed charge (Q) during eATRP, it was determined that ≤1% of chains terminated by radical–radical reactions (see SI).
Discussion
The strongly hydrophilic Br-CuIITPMA+ catalyst complex efficiently controlled a miniemulsion eATRP inside the dispersed hydrophobic BA droplets stabilized with an anionic surfactant. This suggested that the catalyst could associate with the anionic surfactant present at the droplets’ surface, realizing an “interfacial catalysis” procedure similar in nature to heterogeneous catalysis. The interaction between surfactant and catalyst at the interface was studied by CV.
Interfacial Catalysis and Interaction between Br-CuIITPMA+ and SDS
CV was first applied to a model heterogeneous system, composed of catalyst and surfactant, but without monomer. In this medium, micelles with diameter ca. 2 nm were formed, as compared to larger miniemulsion droplets with diameter ca. 100 nm. The CV of Br-CuIITPMA+ transition metal complex in water drastically changed after addition of SDS (Figure 4). The lower current indicated that the diffusion coefficient (D) of Br-CuIITPMA+ diminished, because the catalyst was bound to the much larger micelles, which diffused slowly throughout the solution.17 A similar behavior was observed for other CuII complexes.18,19 Another feature of the CV in the presence of SDS is a −0.03 V shift of the half-wave potential (E1/2). This indicated that the CuII complex (deactivator) had ca. 3 times more affinity for the micellar environment than the CuI complex (activator, see Eq. S1). This is an important aspect of the disclosed procedure that can enhance deactivation and control in ATRP conducted within the distributed micelles.20 Indeed, the poor polymerization control obtained with Br-CuIIMe6TREN+ could be due to its weaker interaction with SDS, which favored activation over deactivation (Figure S17). Br-CuIIMe6TREN+ could also be partially reduced to Cu0, subtracting catalyst from the polymerization environment thus reducing its efficiency (Figure S2).
Figure 4.
CV of Br-CuIITPMA+ in (
 ) water + 0.1 M NaBr; (
) water + 0.1 M NaBr; (
 ) water + 0.1 NaBr + 0.028 M SDS; (
) water + 0.1 NaBr + 0.028 M SDS; (
 ) miniemulsion as described in Table 1, but without initiator EBiB. v = 0.1 V s−1, T = 65 °C.
) miniemulsion as described in Table 1, but without initiator EBiB. v = 0.1 V s−1, T = 65 °C.
The CV pattern of Br-CuIITPMA+ in miniemulsion was similar to that in the micellar environment. In the miniemulsion, much lower current was observed because of the bigger size, and thus smaller D, of droplets compared to micelles. In this case, E1/2 was influenced by the presence of both SDS and BA (Figure S16).
The fraction of Br-CuIITPMA+ bound to SDS micelles (fbound) was determined by comparing D of Br-CuIITPMA+ in pure water to its D in the presence of micelles, according to a literature procedure (Figure S20).21,22 A similar procedure was applied for the first time to estimate the fraction of catalyst bound to the droplets’ interface in a polymerization environment, i.e. in the SDS miniemulsion. The detailed procedure is described in the SI, while results are summarized in Table 5; fbound of Br-CuIITPMA+ was similar in micellar and in miniemulsion environments: 79% of the catalyst was bound to the interface, confirming the strong interaction between Br-CuIITPMA+ and SDS. Conversely, the less effective Br-CuIIMe6TREN+ and Br-CuIIBPMEA+ exhibited weak bonding to the droplets’ interface.
Table 5.
Fraction of Br-CuIIL+ Bound to SDS Interfaces in Micellar Systems or in Miniemulsions, T = 65 °C.
| catalyst | environment | fbound |
|---|---|---|
| Br-CuIITPMA+ | micellesa | 0.79 |
| Br-CuIITPMA+ | miniemulsionb | 0.95 |
| Br-CuIIMe6TREN+ | miniemulsionb | <0.50 |
| Br-CuIIBPMEA+ | miniemulsionb | 0.64 |
Water + 0.1 M NaBr + 10−3 M CuIIBr2/TPMA + 0.028 M SDS.
Cn-BuA/CNaBr/CCuIIBr2/CL= 280/20/0.2/0.4, 20% v/v BA in water, SDS = 28 mM = 4.6 wt % relative to BA, HD = 10.8 wt % relative to BA.
The strong interaction between catalyst and droplets did not destabilize the miniemulsion during polymerization; a roughly one-to-one copy of the original dispersion was obtained after each eATRP, as determined by dynamic light scattering (Table S2–S3).
In conclusion, a large fraction of Br-CuIITPMA+ was present at the droplets’ surface, from where it could activate and deactivate the growing chains by an interfacial catalysis procedure. However, such heterogeneous catalysts are typically inefficient deactivators which would lead to broad Đ.23,24 Therefore, we investigated if, in the presence of SDS, some of the catalyst could be transported inside the monomer droplets, acting as a traditional homogenous catalyst and supplementing the interfacial catalysis.
Ion pairing between Br-CuIITPMA+ and DS−
In the absence of surfactant, no CuII complexes were detected in the Vis-NIR spectrum of the organic phase (Figure S22), indicating that Br-CuIITPMA+ was completely distributed in the aqueous phase (Table 3). The same was observed in the presence of Brij-98. Conversely, partition experiment in the presence of SDS (Figure S22) showed that 0.4% of the catalyst was present in the organic phase (i.e. ca. 3 ppm of Cu, out of a total 700 ppm Cu). This indicated that Br–CuIITPMA+ and DS− formed hydrophobic ion pairs that transported some deactivator molecules into the organic phase, enhancing deactivation. Indeed, less than 10 ppm of deactivators were found to be sufficient for the controlled polymerization of acrylic monomers.25 The neutral Br–CuIITPMA+/DS− ion pair, containing the long SDS alkyl chain, was significantly more hydrophobic than the cationic Br-CuII TPMA+ complex. However, when Br–CuIITPMA+ and DS− are mixed in water, no precipitate was observed, because in polar media Br–CuIITPMA+ and DS− interact forming soluble aggregates.
ICP-MS of the obtained polymer, before any purification procedure, showed the presence of a small mount (<20 ppm) of copper, which can also improve the stability of the latex. 26,27 Polymers obtained with Br-CuIITPMA+/SDS were perfectly clear and transparent (Figure S15); in comparison, samples obtained with similar amounts of hydrophobic complexes (Figure S14) contained >400 ppm of residual Cu and were intensely colored.
Regarding the activator complex, the ion pair [CuIL+/DS−] or the neutral complex Br-CuIL could enter the organic phase at concentrations slightly higher than that of [Br-CuIIL+/DS−]. Indeed, we estimated a CuI/CuII ratio of ca. 4 in the monomer droplets (calculations for L = TPMA in the SI).
Overall Mechanism of Interfacial and Ion-Pair Catalysis
Scheme 3 presents the proposed mechanism of miniemulsion eATRP with X-CuIITPMA+/SDS catalytic system. Atom transfer was carried out both by catalyst bound to the droplets’ surface, “interfacial catalysis”, and by ion-pairs distributed in the monomer droplets, “ion-pair catalysis”. Electrochemical regeneration of the catalyst involved both a small amount of X-CuIIL+ dissolved in the aqueous phase and X-CuIIL+ bound to the droplets’ interface. The same catalyst could be reduced at the WE and then control polymerization in the monomer droplets, without the need of a dual (hydrophilic + hydrophobic) catalytic system.
Scheme 3.
Mechanism of Ion-Pair and Interfacial Catalysis in Miniemulsion eATRP
Table 6 presents a molecular description of the ATRP catalytic system in a single miniemulsion droplet, which can be considered an individual mini-bulk polymerization reactor. For a reaction volume of 40 mL, the number of droplets was determined as 7.6×1015, considering each droplet as a sphere with diameter 126 nm (measured by DLS). The amount of reagents in each droplet was computed by determining the number of particles (N) of each species and dividing it by the number of droplets. The amount of catalyst inside each droplet was derived from the average 14 ppm of residual copper as determined by ICP-MS. Finally, the catalyst at the interface was calculated as 95% of the total catalyst concentration (Table 5).
Table 6.
Molecular Description of the Br-CuIITPMA+/SDS System in a Single Miniemulsion Dropleta
| species | concentration (M)b | Nc | ratiod |
|---|---|---|---|
| SDSe | - | 9.2×104 | 1.5×103 |
| Hexadecane | - | 2.7×105 | 4.4×103 |
| Br-CuIITPMA+bound | - | 3.0×103 | 48 |
| [Br-CuIITPMA+/DS−] | 9.9×10−5 | 62 | 1 |
| BA | 7.0 | 4.4×106 | 7.1×105 |
| EBiB | 2.5×10−2 | 1.6×104 | 2.6×102 |
| Pn• f | 0/11.7×10−6 (1.3×10−9) | 0/1 (8.2×10−4) | 0/2.9×10−2 (2.3×10−5) |
Conditions as in Table 1.
Concentration of the species in the organic phase (V = 8 mL).
Average number of particles of each species in a single droplet.
N/Ncatalyst.
The surfactant available to stabilize the droplets was considered as CSDS – cmc (critical micellar concentration, see SI).
Two different values are reported, considering that during polymerization in most droplets the concentration of radical is either 0 or 1. In parentheses the average of the whole organic phase.
With 700 ppm of total copper present in the miniemulsion, each ion-pair catalyst was responsible for the activation/deactivation of 260 chain ends. Conversely, each catalyst bound to the interface activated and deactivated an average of 5 chains. On the surface, one catalyst molecule (Br-CuIITPMA+bound) was present every ~30 surfactant molecules. Interestingly, during polymerization only 1 in 1200 droplets was active (i.e. contained an active radical) at any given moment, which suggests that radical-radical termination events could be limited.28–30
The concentration of residual copper determined by ICP (average 14 ppm) was higher than the concentration determined from the partition experiments (3 ppm, Figure S22). The concentration of CuII can increase during polymerization due to some radical termination reactions. Moreover, excess activator CuIL+ can access the monomer droplets, resulting in overall higher catalyst loading in the organic phase during polymerization.
Conclusions
In contrast to our previous report, it is now described that miniemulsion eATRP can be carried out with an anionic surfactant and a single, strongly hydrophilic catalyst. The obtained PBA was well defined, with MWs matching theoretical values and low Đ. Chain extension experiments confirmed that chain-end functionality was very well retained. These results are in contrast with a previous paradigm that super-hydrophobic catalysts are required for ATRP in dispersed media.
In this work, the catalytic system was generated in situ by mixing commercially available reagents (NaBr, SDS, traditional copper catalysts). Only a few ppm of catalyst were present inside the monomer droplets. Polymer purification was simplified, because, after crashing the miniemulsion, >99% of the hydrophilic catalyst was present in the aqueous phase.
Controlled polymerization was favored by the strong interaction between copper complexes and an anionic surfactant, SDS. This interaction, once considered a poison for the ATRP catalyst, generated hydrophobic ion pairs [Br-CuIITPMA+/DS−] at the droplet surface that transported a fraction of the catalyst into the monomer droplets, enabling controlled polymerization via “ion-pair catalysis”. Control was further enhanced by catalyst bound to the droplets’ surface via “interfacial catalysis”.
The ideal Cu catalyst has the following characteristics: (i) high activity, (ii) ability to form ion pairs with SDS, and (iii) stronger affinity for the surfactant when present in its CuII oxidation state.
Supplementary Material
Acknowledgments
The support from the National Science Foundation (CHE 1400052) and the National Institutes of Health (R01DE020843) is acknowledged. The authors acknowledge Tom Ribelli and Sangwoo Park for the preparation of copper ligands, and Xiaoyu Gao for help in ICP-MS tests. P. Chmielarz acknowledges Kosciuszko Foundation Fellowship.
Footnotes
Supporting Information. Additional polymerization results and complete electrochemical characterization of the catalyst are supplied in the supporting information. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Chmielarz P, Fantin M, Park S, Isse AA, Gennaro A, Magenau AJD, Sobkowiak A, Matyjaszewski K. Electrochemically Mediated Atom Transfer Radical Polymerization (eATRP) Prog Polym Sci. doi: 10.1016/j.progpolymsci.2017.02.005. [DOI] [Google Scholar]
- 2.Qiu J, Charleux B, Matyjaszewski K. Controlled/Living Radical Polymerization in Aqueous Media: Homogeneous and Heterogeneous Systems. Prog Polym Sci. 2001;26:2083–2134. [Google Scholar]
- 3.Cunningham MF. Controlled/Living Radical Polymerization in Aqueous Dispersed Systems. Prog Polym Sci. 2008;33:365–398. [Google Scholar]
- 4.Zetterlund PB, Kagawa Y, Okubo M. Controlled/Living Radical Polymerization in Dispersed Systems. Chem Rev. 2008;108:3747–3794. doi: 10.1021/cr800242x. [DOI] [PubMed] [Google Scholar]
- 5.Elsen AM, Burdyńska J, Park S, Matyjaszewski K. Active Ligand for Low Ppm Miniemulsion Atom Transfer Radical Polymerization. Macromolecules. 2012;45:7356–7363. [Google Scholar]
- 6.Fantin M, Park S, Wang Y, Matyjaszewski K. Electrochemical Atom Transfer Radical Polymerization in Miniemulsion with a Dual Catalytic System. Macromolecules. 2016;49:8838–8847. doi: 10.1021/acs.macromol.6b02037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simms RW, Cunningham MF. High Molecular Weight Poly(Butyl Methacrylate) by Reverse Atom Transfer Radical Polymerization in Miniemulsion Initiated by a Redox System. Macromolecules. 2007;40:860–866. [Google Scholar]
- 8.Kitayama Y, Yorizane M, Kagawa Y, Minami H, Zetterlund PB, Okubo M. Preparation of Onion-Like Multilayered Particles Comprising Mainly Poly(Iso-Butyl Methacrylate)-Block-Polystyrene by Two-Step Aget ATRP. Polymer. 2009;50:3182–3187. [Google Scholar]
- 9.Gaynor SG, Qiu J, Matyjaszewski K. Controlled/“Living” Radical Polymerization Applied to Water-Borne Systems. Macromolecules. 1998;31:5951–5954. [Google Scholar]
- 10.Teo VL, Davis BJ, Tsarevsky NV, Zetterlund PB. Successful Miniemulsion ATRP Using an Anionic Surfactant: Minimization of Deactivator Loss by Addition of a Halide Salt. Macromolecules. 2014;47:6230–6237. [Google Scholar]
- 11.Fantin M, Isse AA, Gennaro A, Matyjaszewski K. Understanding the Fundamentals of Aqueous ATRP and Defining Conditions for Better Control. Macromolecules. 2015;48:6862–6875. [Google Scholar]
- 12.Kaur A, Ribelli TG, Schröder K, Matyjaszewski K, Pintauer T. Properties and ATRP Activity of Copper Complexes with Substituted Tris(2-Pyridylmethyl)Amine-Based Ligands. Inorg Chem. 2015;54:1474–1486. doi: 10.1021/ic502484s. [DOI] [PubMed] [Google Scholar]
- 13.Magenau AJD, Bortolamei N, Frick E, Park S, Gennaro A, Matyjaszewski K. Investigation of Electrochemically Mediated Atom Transfer Radical Polymerization. Macromolecules. 2013;46:4346–4353. [Google Scholar]
- 14.Park S, Chmielarz P, Gennaro A, Matyjaszewski K. Simplified Electrochemically Mediated Atom Transfer Radical Polymerization Using a Sacrificial Anode. Angew Chem Int Ed. 2015;54:2388–2392. doi: 10.1002/anie.201410598. [DOI] [PubMed] [Google Scholar]
- 15.Fantin M, Lorandi F, Isse AA, Gennaro A. Sustainable Electrochemically_Mediated Atom Transfer Radical Polymerization with Inexpensive Non_Platinum Electrodes. Macromol Rapid Commun. 2016;37:1318–1322. doi: 10.1002/marc.201600237. [DOI] [PubMed] [Google Scholar]
- 16.Chmielarz P, Sobkowiak A, Matyjaszewski K. A Simplified Electrochemically Mediated ATRP Synthesis of PEO-b-PMMA Copolymers. Polymer. 2015;77:266–271. [Google Scholar]
- 17.Harwell JH, Hoskins JC, Schechter RS, Wade WH. Pseudophase Separation Model for Surfactant Adsorption: Isomerically Pure Surfactants. Langmuir. 1985;1:251–262. [Google Scholar]
- 18.Sivagnanam U, Palaniandavar M. Electrochemical Behaviour of Certain Biomimetic Copper(II) Complexes in Aqueous and Aqueous Micellar Solutions. J Electroanal Chem. 1996;410:43–53. [Google Scholar]
- 19.Anitha N, Balamurugan R, Palaniandavar M. Spectral and Electrochemical Studies of Bis(Diimine)Copper(II) Complexes in Anionic, Cationic and Nonionic Micelles. J Colloid Interface Sci. 2011;362:243–252. doi: 10.1016/j.jcis.2011.05.075. [DOI] [PubMed] [Google Scholar]
- 20.Lanzalaco S, Fantin M, Scialdone O, Galia A, Isse AA, Gennaro A, Matyjaszewski K. Atom Transfer Radical Polymerization with Different Halides (F, Cl, Br, and I): Is the Process “Living” in the Presence of Fluorinated Initiators? Macromolecules. 2017;50:192–202. [Google Scholar]
- 21.Mandal AB, Nair BU, Ramaswamy D. Determination of the Critical Micelle Concentration of Surfactants and the Partition Coefficient of an Electrochemical Probe by Using Cyclic Voltammetry. Langmuir. 1988;4:736–739. [Google Scholar]
- 22.Mandal AB, Nair BU. Cyclic Voltammetric Technique for the Determination of the Critical Micelle Concentration of Surfactants, Self-Diffusion Coefficient of Micelles, and Partition Coefficient of an Electrochemical Probe. The Journal of Physical Chemistry. 1991;95:9008–9013. [Google Scholar]
- 23.Hong SC, Paik H-j, Matyjaszewski K. An Immobilized/Soluble Hybrid Catalyst System for Atom Transfer Radical Polymerization. Macromolecules. 2001;34:5099–5102. [Google Scholar]
- 24.Wei Y, Jia Y, Wang WJ, Li BG, Zhu S. Surfactant–Ligand Design for Ab Initio Emulsion Atom Transfer Radical Polymerization. Macromolecules. 2014;47:7701–7706. [Google Scholar]
- 25.Williams VA, Ribelli TG, Chmielarz P, Park S, Matyjaszewski K. A Silver Bullet: Elemental Silver as an Efficient Reducing Agent for Atom Transfer Radical Polymerization of Acrylates. J Am Chem Soc. 2015;137:1428–1431. doi: 10.1021/ja512519j. [DOI] [PubMed] [Google Scholar]
- 26.Wei Y, Liu P, Wang WJ, Li BG, Zhu S. Well-Controlled and Stable Emulsion ATRP of Mma with Low Surfactant Concentration Using Surfactant-Ligand Design as the Copper Capture Agent. Polymer Chemistry. 2015;6:2837–2843. [Google Scholar]
- 27.Wei Y, Zhang Q, Wang WJ, Li BG, Zhu S. Improvement on Stability of Polymeric Latexes Prepared by Emulsion ATRP through Copper Removal Using Electrolysis. Polymer. 2016;106:261–266. [Google Scholar]
- 28.Kagawa Y, Zetterlund PB, Minami H, Okubo M. Compartmentalization in Atom Transfer Radical Polymerization (ATRP) in Dispersed Systems. Macromol Theory Simul. 2006;15:608–613. [Google Scholar]
- 29.Zetterlund PB, Kagawa Y, Okubo M. Compartmentalization in Atom Transfer Radical Polymerization of Styrene in Dispersed Systems: Effects of Target Molecular Weight and Halide End Group. Macromolecules. 2009;42:2488–2496. [Google Scholar]
- 30.Thomson ME, Cunningham MF. Compartmentalization Effects on the Rate of Polymerization and the Degree of Control in ATRP Aqueous Dispersed Phase Polymerization. Macromolecules. 2010;43:2772–2779. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.