Abstract
Human body motion can generate a biological electric field and a current, creating a voltage gradient of −10 to −90 mV across cell membranes. In turn, this gradient triggers cells to transmit signals that alter cell proliferation and differentiation. Several cell types, counting osteoblasts, neurons and cardiomyocytes, are relatively sensitive to electrical signal stimulation. Employment of electrical signals in modulating cell proliferation and differentiation inspires us to use the electroactive polymers to achieve electrical stimulation for repairing impaired tissues. Electroactive polymers have found numerous applications in biomedicine due to their capability in effectively delivering electrical signals to the seeded cells, such as biosensing, tissue regeneration, drug delivery, and biomedical implants. Here we will summarize the electrical characteristics of electroactive polymers, which enables them to electrically influence cellular function and behavior, including conducting polymers, piezoelectric polymers, and polyelectrolyte gels. We will also discuss the biological response to these electroactive polymers under electrical stimulation. In particular, we focus this review on their applications in regenerating different tissues, including bone, nerve, heart muscle, cartilage and skin. Additionally, we discuss the challenges in tissue regeneration applications of electroactive polymers. We conclude that electroactive polymers have a great potential as regenerative biomaterials, due to their ability to stimulate desirable outcomes in various electrically responsive cells.
Keywords: Electroactive Polymers, Conducting Polymers, Piezoelectric Polymers, Polyelectrolyte Gels, Tissue Regeneration
1. Introduction
The biological electric field that the human body generates plays a pivotal role in wound healing due to the steady, direct current and electric field, which drives cells to migrate to the point of injury [1]. Moreover, a voltage gradient, called “action potential”, of −10 to −90 mV can trigger different cell types to change proliferation and differentiation by signaling across cell membranes [1,2]. The potential for harnessing the electric fields in cells to enhance growth and differentiation in biological systems has gained the attention of researchers. As is known, regeneration of damaged tissue begins with the growth and proliferation of cells [1,3]. Thus, to stimulate and enhance this regenerative process and thereby promote rapid healing of the damaged tissue, electroactive biomaterials are often considered for use as a tissue regeneration scaffold.
Electroactive materials provide a direct method for various forms of electrical stimulation to reach cells [3]. The electroactive materials include inorganic electroactive materials, metals and organic electroactive polymers. Recently, it is shown that specific amounts of electrical stimulation via electroactive materials could enhance the regeneration of cardiac [4], nerve [5,6] and bone through directing cell adhesion [7], growth, migration, apoptosis [8] and differentiation [9]. Thus, electroactive materials have the potential to evolve tissue healing and engineering treatments (e.g., bone [10,11] and nerve [12] regeneration). Particularly, electroactive polymers (EAPs) have received increasing attention (Figures 1 and 2) because the human body contains many electro-sensitive tissues such as bone, skin, nerve, heart and vessels [13]. In particularly, they have seen varied and extensive employment in tissue engineering (Table 1) because there are some advantages, such as the possibility of being constructed into varying shapes with attractive morphological features and a large selection of physical and chemical properties. To date, there are many reviews about the medical application of conducting polymers [5,14–16] and the tissue engineering application of piezoelectric materials [17,18]. However, there are few reviews for the application of electroactive polymers, including conducting polymers (CPs), piezoelectric polymers and polyelectrolyte gels, in tissue regeneration. This report will attempt to fill this gap.
Figure 1.
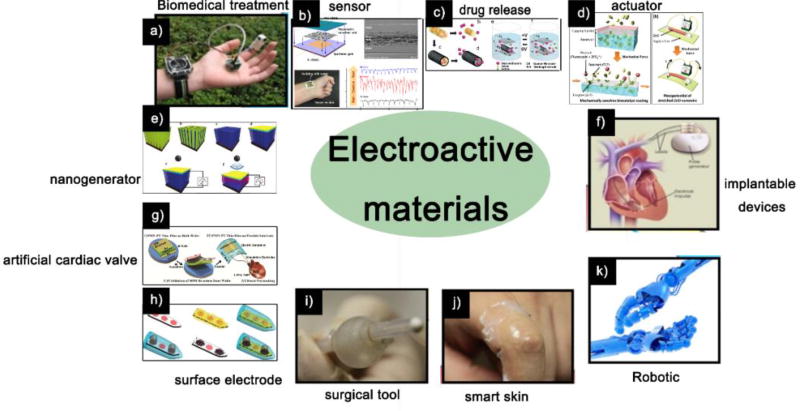
The applications of electroactive materials. a) Piezoelectric materials used for biomedical treatment application; b) Piezoelectric sensor; c) Drug release from the PEDOT nanotubes was achieved through the contraction or expansion of the PEDOT nanotubes under external electrical stimulation; d) ZnO nanowire actuator resulted in mechanical stretching, which activate the enzymes; e) Potential of PVDF piezoelectric nanogenerator generated by sonic wave; f) Using piezoelectric materials in implantable devices; g) Use of piezoelectric materials in artificial cardiac valves; h) Conducting polymers on neural microelectrodes; i) Piezoelectric materials as surgical tools; j) Piezoelectric materials as smart skins; k) Piezoelectric materials as Robotics.
Sources: (a, f, i, j, k) [19], Copyright 2013, All reproduced with permission from Elsevier Ltd; (b) [20], Copyright 2016, (e) [23], Copyright 2011, Both reproduced with permission from the American Chemical Society; (c) [21], Copyright 2006, (d) [22], Copyright 2012, (g) [24], Copyright 2014, (h) [25], Copyright 2012, All reproduced with permission from John Wiley and Sons.
Figure 2.
Tissues in the human body are mechanically and/or electrically sensitive to specific environment, indicating that the electroactive materials have potential applications in tissue regeneration.
Table 1.
Electroactive polymers in tissue regeneration application
| Tissue regeneration application |
Advantages | Limitations | Currently explored electroactive polymers |
|---|---|---|---|
| Bone tissue regeneration | Good biocompatibility and electroactivity, conducive to cell differentiation | Lack of biodegradability, hydrophobicity, needing external power source | |
| Neural regeneration | Good biocompatibility, conductivity, stability. high specific surface area, Easy processing, conducive to cell differentiation | Decreased electrical contact at interface | |
| Myocardial regeneration | Being electroactive, biocompatible, porous, fibrous, conducive to cell differentiation | Hydrophobicity, not biodegradable, needing external power source | |
| Cartilage regeneration | Being biocompatible, flexible, electroactive, conducive to cell differentiation | Poor biodegradability, needing external power source |
Abbreviations: Polypyrrole (PPy); Polypyrrole/Poly-Dl-Lactic Acid (PPy/PDLLA); Polycaprolactone-Polypyrrole (PCL-PPy); chitosan/polypyrrole (CS/PPy); Polyaniline (PANi); Poly(3,4-ethylenedioxythiophene) (PEDOT); Polyvinylidene Fluoride (PVDF); Polyhydroxybutyrate (PHB); polyurethane (PU)
2. Electroactive polymers
Under a stimulus, EAPs convert one form of energy into a more desirable electrical state, thus affording tremendous promise in emerging technologies for responsive prosthetics [69–71]. A typical characteristic property of EAPs is that they will undergo a large amount of deformation while under the pressure or being stretched. When electric charges are on the top of a polymer, a redistribution of charges within the polymer is observed; this is dependent on the polymer’s ability of being responsively mobile [69]. The observed response of the polymer to an electric field is divided into two distinct categories. One is dielectric properties (dielectric constants and dielectric relaxation). Another is conductive properties (conductivity and dielectric strength) [70]. EAPs produce additional novel electrical properties, such as ferroelectric, photoconductive, piezoelectric, triboelectric, or pyroelectric characteristics [71]. Over the last ten years, EAP materials have garnered increasing attention as they are developed for different prospects in biomedicine such as tissue engineering scaffolds, drug delivery, biosensors, artificial muscles, actuators, power generators and various medical instruments and auxiliaries (Figure 1) [72–74]. So, the interest in EAPs is increased because these “smart materials” have the ability to be responsive under varied external stimuli [75]. EAPs are unique in the biomedical field because they can convert different types of signals, such as mechanical, thermal, and magnetic, into electrical ones. This provides the opportunity to use EAPs in scaffolds for the stimulation of cell growth in tissue regeneration [8, 76].
EAP history starts in 1880 with the discovery of electromechanical coupling effects from the experiment where the rubber fixed at one end changed from charged to discharged [77]. Sacerdote [78] then conducted the same experiment and revealed the relationship between strain and electric field. In 1925 a piezoelectric polymer was identified [78]. Despite a lack of further work being explored, EAPs have become known for their reaction to electrical stimulation and the promise of practical and convenient applications. EAPs are readily categorized into two groups (electronic and ionic, Table 2) on the basis of the differing activation principles [79]. Electronic EAPs function by using the electrostatic forces of two electrodes to cause actuation to contract a polymer; this includes materials such as piezoelectric, electrostrictive, and ferroelectric. Ionic EAPs, on the other hand, function by displacing the ions contained in the polymer to cause actuation [80]. Examples of the ionic EAPs include polyelectrolyte gels, conducting polymers, and polymer-metal composites [72,79].
Table 2.
The advantages and disadvantages of two types of EAP
| EAP type | Advantages | Disadvantages |
|---|---|---|
| Electronic | Can operate in air with no major constraints, generate deformation displacement under a DC voltage; Provide a greater mechanical energy density | Activation; requires high voltages |
| Ionic | Mostly induce bending displacement; Require low voltages | Maintain wetness; Do not hold strain under DC voltage |
The most common applications of EAPs are in actuators and sensors. In recent years, the tissue regeneration applications of some EAPs have been increasingly developed. For example, piezoelectric polymers function by delivering electrical stimulus with limited control, despite not having an external power supply [81]. CPs are biocompatible, with high conductivity/weight ratio and good optical properties. They are also capable of controlling electrical stimulation precisely. However, they need an externally powered electric field [5]. Additionally, conductive polymers allow fine-tuning of chemical, electrical, and physical properties to fit the needs of the biological moieties in which they are employed [3]. In this review, CPs, including Polypyrrole (PPy), Polyaniline (PANI), and Poly(3,4-ethylenedioxythiophene) (PEDOT), piezoelectric polymers, including Polyvinylidene fluoride (PVDF), Polyhydroxybutyrate (PHB), and Poly(L-lactic acid) (PLLA), as well as polyelectrolyte gels, will be discussed regarding their properties and applications in tissue regeneration.
2.1. Conducting polymers
The interest in the potential biomedical capabilities of CPs has driven researchers to develop novel techniques in order to harness CPs capabilities. CPs are unique in that they offer easy synthesis methods and exhibit desirable elecrical and optical properties that closely resemble those of metals and semiconductors [82–84]. Initially, polyacetylene was observed to be conductive after being oxidized by iodine vapor, intriguing the interest of scientists [85,86]. However, polyacetylene is unstable in air and difficutlt to synthesize [87,88]. Now PPy, PANi, PTh and, PEDOT are the most extesnively studied CPs [88,89] (Figure 3).
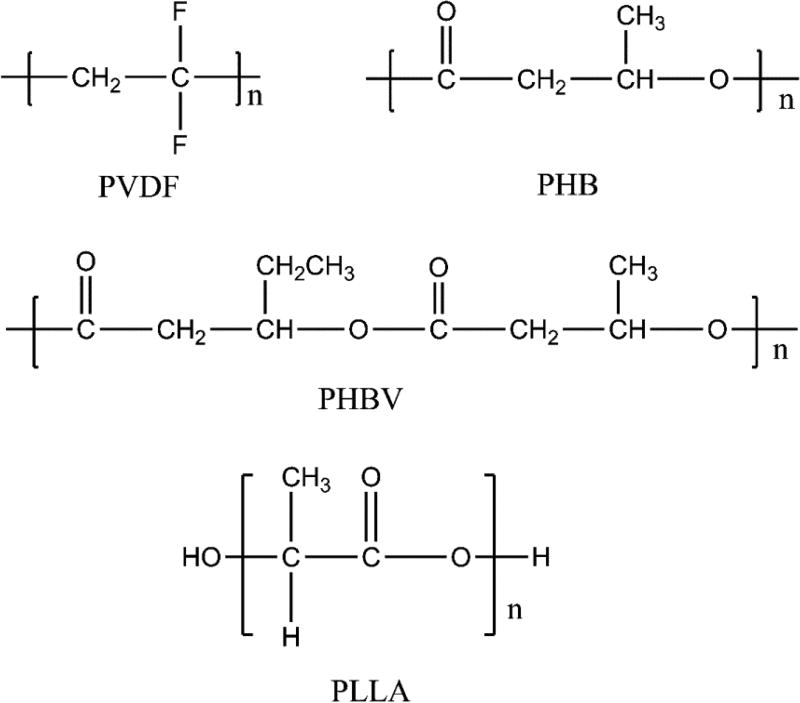
Figure 3.
Structures of some representative biomedical CPs.
CP synthesis can be done chemically or electrochemically. Chemical methods allow for customization of possible synthesis routes and scales, providing many variant CPs [90]. Despite the benefits of chemical synthesis, electrochemical synthesis provides an easier method. Therefore, it is the common procedure for making CPs [91]. Ease of synthesis and the ability to simultaneously dope and encase molecules are advantages to synthesizing CPs via electrochemical methods. However, in the electrochemical methods, challenge in covalent modification after synthesis of CPs and difficulty in removing films from electrodes are disadvantageous. Another limiting factor of electrochemical synthesis is its inability to function outside of a system where monomers are oxidized after applying potential, forming reactive radical ion intermediates allowing for polymerization. Despite the complexity of chemical synthesis, wider varieties of covalent modifications to the CP backbone and postsynthesis colvalent modifications are made possible with this method [92]. The chemical polymerization methods produce powders or exceptionally thick CP films; conversely electrochemical synthesis produces very thin CP films (20 nm), marking a major difference between the two. Several unique CPs are only synthesized while using chemcial methods but more known CPs (e.g. PPy, PANi, PTh (Polythiophene), PEDOT) are developed by utilizing chemical and electrochemical methods [14].
2.1.1. Polypyrrole
PPy is easy to be synthesized and modified on the surface and has a high electrical conductivity, so it is the most studied conjugated polymer [93]. PPy exhibits a high stability in environmental settings and stimuli-responsive properties. It helps cell attachment and proliferation, suggesting its possible use as a “smart” biomaterial [94]. Primarily, PPy is biocompatible [95,96], and chemically stable and conductive in physiological conditions [97]. PPy has easy and variable synthesis processes, allowing for large quantity synthesis at room temperature with multiple solvents [98,99]. PPy has the ability to be made usable in biomedicine by combining it with bioactive molecules [100,101]. Unfortunately, PPy is challenging to manipulate further after synthesis [102]. It also has a non-thermoplastic structure [99] after synthesis that is found to be insoluble, mechanically rigid, and brittle [102]. PPy today is used in fuel cells [103], corrosion protection [104], biosensors [105], drug delivery systems [106], biomaterials for neural tissue engineering [106], neural probes [48], and nerve guidance channels [107].
Despite the attractive processing properties of PPy, long term toxicity studies have yet to be evaluated in vivo. Additionally, the insoluble, rigid, and brittle composition of PPy limits post synthesis processing capabilities. This makes unmodified PPy a poor choice for many biomedical applications. In order to apply PPy as a biomaterial, further in vivo testing is needed due to its insoluble and rigid nature.
2.1.2 Polyaniline
Polyaniline (PANi) or aniline black, much like PPy, has been heavily studied [108]. PANi has three forms of existence, i.e., pernigraniline base (fully oxidized), emeraldine base (half-oxidized) and leucoemeraldine base (completely reduced) [108]. Additionally, PANi emeraldine provides the highest level of conductivity and stability [108]. PANi is cheap, environmentally stable, and easy to synthesize. It also has the capability to alternate between conductive and resistive states electrically [109]. Unfortunately, PANi lacks plasticity, is non-biodegradable and shows low cellular compatibility. Furthermore, once implanted chronic inflammation has been documented [86,110]. PANi is currently being tested for applications in biomedicine such as biosensors [111,112], neural prostheses, drug delivery and release system [113,114], and tissue engineering [60,115].
2.1.3. Polythiophene (PTh) derivative
PEDOT, a PTh derivative, is the third very interesting conjugated polymer [52]. PEDOT is synthesized by the polymerization of the bicyclic monomer 3,4-ethylenedioxythiophene (EDOT). Compared to PTh, PEDOT has improved properties in lowered band gap and its reduction and oxidation potential [116]. These properties provide PEDOT with a valuable electrical, chemical and environmental stability. When compared to PPy, PEDOT is more thermally established and conductive [117]. Investigations on PEDOTs capabilities and characteristics are fairly young when compared to PPy and PANI studies. Despite the fairly recent development of these investigations, PEDOT’s biocompatibility is already well established. Today PEDOT is used in biosensing and tissue engineering [84], neural electrodes [51,118] and heart muscle patches [89].
2.1.4. Conducting polymer composites
CP’s most attractive qualities are good stability, high electrical conductivity, and the capability to dispense biomolecules. CP’s electrical, chemical, physical, and biocompatibility properties can also be modified to aid the applications. Despite the positive qualities, CPs is limited in biomedical applications because it is difficult to handle and brittle, while larger dopants can make these conditions worse. In order to bypass these technical challenges, CPs are blended or composited together with another polymer, which allows exploitation of positive aspects from both materials. By using CP composites, higher solubility and improved mechanical properties are obtainable for employment in biomedical applications while avoiding significant compromises to other properties, such as conductivity [14,119].
Effectively, researchers have managed to develop CP composites by combining CPs with other polymers for desired properties. For example, researchers have paired PPy and PANi as conducting fillers with natural polymers in an attempt to increase the processing ability of CPs, which can also improve the conductivity of insulating polymers [120,121]. CP composites have better mechanical properties resulting from doping with large molecules. These alternative processes aren’t a perfect fix; the use of insulating molecules can produce electron conjugation interference within the CPs.
A lot of studies have been done on the in vitro capabilities of the CP composite materials. While this is important, there is a lack of animal studies, which must be done in order to move to human clinical trials in the future. CPs show promise in fulfilling a role for use as medical implant materials, specifically for neural stimulation and sensing. These materials also show promise in regenerating tissues that need electrical conductivity to assist with cell growth.
2.2. Piezoelectric polymers
Piezoelectric polymeric materials have no energy or power supply requirements to promote transient surface charge. Inspired from the piezoelectric character of the bone [122], the idea of using piezoelectric materials to correct bone defects emerged in the 1970s [123] because they have been shown to actively stimulate tissue by electrically and mechanically solicited responses. Piezoelectric materials have been studied by in vivo tests, and they have been shown to accelerate bone regeneration. Of the varying types of piezoelectric materials, piezoelectric polymers have shown to exhibit simple processing, flexibility and physical properties, making it an option for various applications. PLLA, PHB, PVDF and their co-polymers are the EAPs with the largest piezo, pyro, and ferroelectricity responses (Figure 4) [7,9]. To date, the most common piezoelectric polymers are PVDF, collagen, PHB and their composites.
Figure 4.
Representative structures of some biomedical piezoelectric polymers.
2.2.1. Polyvinylidene fluoride
Piezoelectric properties are displayed by a small number of synthetic polymers, such as PVDF, PLA and PHB [81]. PVDF and its copolymers showed the highest piezoelectric constant among this group. Thus, they are the best applicable polymer of the piezoelectric response materials for specific applications [124]. PVDF exhibits impressive polymorphism. It has at least 4 crystalline phases, including α, β, γ and δ [125]. β-phase PVDF is considered the most appropriate for electrical response applications because it bears the best piezoelectric coefficients [126].
PVDF has significant electroactive properties for a biocompatible material [7]. The piezoelectric effect exhibits an electrical potential brought on by the induction of mechanical stress. The positive or negative surface charge carried by PVDF films is known to influence hydrophobicity of the specimens, developing a diversity in absorbed extracellular matrix protein conformation. This eventually leads to the control of stem cell adhesion and induced osteogenic differentiation. Studies have shown that osteoblast attachment and growth is heavily dependent on the surface charge [127], indicating that PVDF has the ability to be used as an osteogenic material when the surface charge of the substrates is needed to increase functionality [128].
2.2.2. Polyhydroxyalkanoates
Polyhydroxyalkanoates (PHAs), a unique biodegradable polymer, is a natural byproduct of bacteria as a carbon/energy complex [129,130]. This group of biopolymers, whether naturally occurring or engineered polyesters, exhibits a wide range of physical properties [129]. PHAs generally exhibit multi-lamellar structures that have aggregated into lamellar crystals that have varying orientations when in plane, or other bulky forms like films [130]. When PHA chains crystalize following melting of bulk materials, spherulites generally form; these crystals form in twisted radial lamellar structures. The polymers formed in this way exhibit piezoelectric and optical activity. These polymers are insoluble in water. They are also inert, non-toxic and highly stable in air. PHAs are usually broken up into two groups. Short chain PHAs have 3–5 carbon atoms in monomeric units whereas alternately medium chain length PHAs have a 6–18 carbon atoms in monomeric units. Poly(3-hydroxybutyrate) (P3HB) as well as poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (P(3HB-co-3HV)) are the most abundant PHAs [23,131].
2.2.3. Poly (L-Lactic acid)
Poly (lactic acid) (PLA) is a frequently used biomaterial. Primary characteristics include low density and processing power, as well as corrosion resistance, elastomeric behavior, and multi-purpose construction [72]. Due to its bio-resorption, low toxicity, degradation, biocompatibility and useful mechanical performance, its presence in the medical field continues to grow. PLA can exist as linear polyester stereoisomer forms, including (poly(L-lactic acid) (PLLA), poly(D-lactic acid) (PDLA) and poly(D,L-lactic acid) (PDLLA) [72,132]. PLLA has optical activity characteristics and PDLA exists as its optical isomer. Piezoelectric effects of drawn PLLA and PDLA have been studied in the past using rods and films [133,134]. It was shown that shear piezoelectric constant d14 increased with the draw ratio, obtaining values of approximately 10 pC/N. This happens because there is a noticeable increase of PLA polymers chain alignment caused by stretching [135,136]. PLLA is the most common form of PLA and is mainly used in biomedical applications [137]. PLLA shows promise as a biomaterial for applications in bone regeneration and growth as well as neural recovery because it can display the electro-response induced by stress.
2.2.4. Piezoelectric polymer composites
Despite the positive qualities of PVDF and other piezoelectric materials, their ability for external stimulation control is restricted. Piezoelectric polymers are blended or composited together with another material, which allows the resultant composite materials to integrate beneficial properties from both materials. By using piezoelectric polymer composites, higher stability and improved piezoelectricity can be achieved, enabling their biomedical applications. In general, piezoelectric polymer composites can be formed by blending piezoelectric polymers with other piezoelectric materials. PVDF and piezoelectric ceramics are mostly used to form piezoelectric polymer composites [138,139]. Thus, piezoelectric polymer composites exhibit the advantages of both piezoelectric polymers and ceramics, such as good flexibility, processability and biocompatibility as well as high piezoelectric constant. Moreover, compared to either piezoelectric ceramics or polymers alone, the piezoelectric polymer composites have improved mechanical stability [140], further favoring potential tissue regeneration applications.
2.3. Polyelectrolyte gel
Polyelectrolyte gel is a kind of polymer with ionized groups. It can be ionized in water or polar solvents to produce charges along the polymer chain [67,141,142]. Polyelectrolytes differ from neutral polymers due to the presence of ionic groups which are covalently attached [141]. Polyelectrolytes, like the well-known bio-polyelectrolyte DNA that has charged groups, carry both charged or chargeable groups. A polyelectrolyte multilayer can be made with a layer by layer (LbL) technique [67]. Polyelectrolyte gels share properties with both their macromolecules and electrolytes. Polyelectrolyte gels can induce charge movement or volume change in response to temperature, pH value, and environmental factors such as electric field or magnetic field changes. Thus, they can convert external stimuli into electrical and mechanical signals. They can be employed in biology due to their biocompatibility and extensive charge density [67]. A diverse set of polyelectrolytes can be used for biological tissues, such as polysaccharides, proteins, heparin, chitosan, and hyaluronic acid. Particularly, drug delivery and tissue engineering can be facilitated with polysaccharide polyelectrolytes. Heparin, chitosan, and hyaluronic acid are biomedical polyelectrolytes that bear sulfate, amino, and carboxylate groups, respectively [142].
3. The biological response to electric stimulation
3.1. Protein behavior under electric stimulation
In many cases of biotechnological applications and biological phenomena, protein absorption is naturally occurring [143]. It is generally accepted that proteins take on a prominent role in guiding biological signaling and functions [144]. The first step of integration for surrounding cells and tissues with biomaterials is initiated by protein absorption [117]. Understanding cell-substrate interactions is critical when judging the biological reaction caused by implanted biomaterials. From the biological view point of the cells, proteins first coat implanted materials from blood and interstitial fluids before the cell adhesion occurs [145]. Protein adhesion generally mediates the cell adhesion, cell growth, and differentiation. However, interfacial protein absorption is found to be nonspecific, which can damage the usefulness of the biomaterial [117]. Therefore, biocompatibility and consequently the success of implants depend on the interfacial interactions between proteins and the synthetic materials [144].
A better understanding of the process starts with the knowledge about the polarization effect on the adsorption of proteins at the early stages of material implantation. This first stage is decisive because it will mediate the cells’ attachment and subsequent tissue growth. Indeed, the nature, quantity, density, and conformation of adsorbed proteins all depend markedly on the biomaterial surface characteristics. In fact, the behavior of protein absorption is dependent on internal properties (e.g., protein, surface and solvent) and external stimuli (e.g., electric field, thermal, light, and magnetic field) [143]. Over the years, many studies have been done on protein absorption, concentrating on internal factors to control protein behavior on the surface [146,147]. However, when discerning which way is more favorable to control protein behavior, external factors prove to be both more convenient and flexible. In this case, it is possible to apply external stimuli to the system without modifying the properties of the component materials. With this method the external stimuli can be tuned to the desired direction and/or strength in order to optimize the system for various specific applications. Additionally the use of external stimuli can assist with interfacial property control [148], allowing for protein behavior control to happen indirectly at the interfaces. When considering external factors, a possible point of control of the spatial homogeneity is offered with the application of an electrical field; this method provides the same possible control for the growth and direction of protein monolayers [143]. Zhou et al elucidated that absorption of lysozymes was increased when surfaces were negatively charged, conversely, it was dampened by positive electric fields [143]. Primarily, proteins migrate and orient under an electrical potential gradient due to the overall net charge and permanent dipole. Electrical stimulation has the ability to electrically drive changes in the polymer’s redox state in order to actively and precisely control protein interactions.
3.2. The cellular response to electric stimulation
Cellular response to electrical stimulation on a biochemical and physical level is extremely complicated and is still primarily a mystery. The cell membrane is the primary environmental contact and the surface of the membrane carries negatively charged chemical groups such as carboxylates and phosphates. Concentrations of soluble ions inside the variant ion pumps, transporters, and channels are the primary sources of regulation for cell activity. These various sources generally regulate membrane proteins that are sensitive to externally induced electrical stimuli, among other stimuli [149]. Certain regulatory membrane proteins as well as enzymes are considered voltage sensing proteins. These molecules sense and also use external electric fields as the mechanism of regulation. Once a cell is attached to a surface, it uses these ion channels and receptors built into the membrane to evaluate the environment. The cell then evaluates the chemical and physical signals being presented by the environment before integrins are used as a way to associate with proteins that link to the cytoskeleton intracellularly. This develops focal adhesions by binding the cluster integrin receptor and ligands of the extracellular matrix (ECM) [145].
Some studies [53,117] investigated electrical control with PEDOT substrates on extracellular matrix proteins, including cellular material interaction moderators like fibronectin (Fn). The goal of these investigations was to arrive at a possible electric control that works through changing cellular redox properties to assist in applications for culturing cells in vitro (e.g., to enhance cell proliferation). While electrical control is being applied, it can induce the change of the conformational states of the ECM protein, which causes shifts in cellular responses like migration or adhesion. Fn on oxidized PEDOT has possible applications in a specific conformation that exhibits motifs for cellular binding (i.e., RGD domain), which can assist in cell attachment and proliferation (Figure 5). Heparin doped oxidized PEDOT has mediated commanded release of fibroblast growth factors anchored to the surface to upregulate stem cell differentiation recently. This shows a correlation between electrochemical switches and accurate sequential mediation of stem cell states.
Figure 5.
Possible mechanism of cell interactions with reduced and oxidized PEDOT. When the cells grew on the reduced films, fibronectin was extended to present RGD sites for controlling cell adhesion. When the cells grew on the oxidized films, fibronectin became more compacted to hide RGD sites, preventing cell adhesion. [117], Copyright 2012, reproduced with permission from the American Chemical Society.
Our research group has found that PPy nano arrays on a titanium surface could be switched by redox potential [150,151]. We further studied a potential switchable PPy nanotubes doped with taurocholic acid (TCA). Such nanotubes showed reversible surface wettability, which further led to possible adsorption preference switching between three model proteins with different isoelectric points. The protein adsorption control further directed the adhesion and spreading of MC3T3-E1 osteoblasts (Figure 6A) [152]. Additionally, our group showed that β-Naphthalene sulphonic acid (NSA) doped PPy nanocone arrays controlled protein adsorption and bacterial adhesion as the surface potential and wettability were regulated by applying a redox potential [153]. Such control enabled the controlled cell adhesion to or detachment from the surface (Figure 6) [154]. The potential-switchable surface chemistry may provide clues for designing biointerfaces in biomaterials, and offer guidance for studying the dynamic cell behaviors. It would also shed light into intelligent drug delivery and release systems as well as controlling biological activities on the biomedical implants.
Figure 6.
Cell fate on different nanostructured conducting polymer PPy substrates. (A) Immunofluorescence stained bone forming cells after the cells were cultured on TCA-doped PPy nanotubes in their original state (a), potential-off states (b, d and f) and potential-on states (c, e and g); (B) Schematic and SEM image of cells on an oxidized and reduced PPy nanoarrays. The cells on oxidized PPy nanoarrays presented more and longer filopodia. They began to detach from the nanoarrays when the nanoarrays were reduced. Sources: (A) [152], Copyright 2014, reproduced with permission from the John Wiley and Sons; (B) [154], Copyright 2016, reproduced with permission from the John Wiley and Sons.
It has been reported that endogenous electric fields are 40 to 500 mV/mm in living tissue [155]. Transportation of macromolecules and ionic species connected with such fields is important in treating neurological injuries and wound healing [156,157]. Intra- and extra-cellular ionic concentrations present a variation that results in a potential across the membrane of 10 to 90 mV in various cell types. Such shifts of potential have been indicative of a change in cell proliferation and differentiation [158]; When the excitable cells have been excited after being in a resting state, they show rapid spreading in response to an action potential [159].
Application of electrical stimuli is possible through either the medium or substrate [160]. Current application through CPs like PPy and PANI provide indirect routes for electrical stimulation [161]. CPs have shown a positive trend for cell proliferation and extension when they are used as a substrate even without the addition of electrical current, when compared to samples that did not have a CP based substrate [162,163]. Fn adsorption increased heavily when a 10 μA current was applied onto the surface directly, this was observed especially when Fn had high concentrations at early exposure stages. PC-12 cells seeded on the films of this nature had up to 50% longer neurite growth when compared to films that went unstimulated [164]. Schmidt et al [165] applied an electrical field at 100 mV/mm to a PPy-coated electrospun scaffold. Then they found the lengths and number of neurite-bearing cells significantly increased. This demonstrated that there is a tissue regeneration application potential with electrical stimulation through electroactive polymers [166].
4. Electroactive polymers for tissue regeneration applications
Non-electroactive polymers, such as polydimethylsiloxane (PDMS) [167–169] and poly (lactic-co-glycolic acid) (PLGA) [170,171] have been widely used in tissue engineering. Although they bear good biocompatibility and can be easily processed, they are not “smart” in that they do not respond to electrical stimuli. However, EAPs, while still having biocompatibility and easy processibility, can respond to electrical/mechanical stimuli, enabling them to influence the fate of cells or tissues electrically or mechanically. This unique feature allows them to serve as a unique “smart” candidate for developing the scaffolds capable of directing cell fate and promoting tissue regeneration.
4.1. Conducting polymers
CPs as a novel organic material originally began production in the mid-1970s [172]. CPs continue to attract a lot of attention as do their composites for two reasons. One is due to their interesting electrical and optical characteristics that are akin to inorganic semiconductors and metals. Another is because CPs also have classic polymer characteristics, like easy synthesis and processing flexibility [173]. The fact that electrical stimuli of CPs generate tissue response makes CPs increasingly interesting for a large diversity of biomedical applications including biosensors, modified neural electrodes, tissue engineering[174] as well as drug delivery [173,175,176]. In tissue engineering areas, the primarily used CPs include PPy [93], PANI [177], PEDOT [117].
4.1.1. Polypyrrole
PPy is generally the most studied CP for applications in biomedicine because of its good conductivity, environmental stability, facile preparation, redox and excellent biocompatibility [178,179]. A number of studies have shown PPy is compatible with various cells, including nerve cells, endothelial cells, myocardial cells, osteoblasts and mesenchymal stem cells (MSCs) [29,43]. With the development of biomedical material technology, PPy has been shown increasingly promising in repairs and regeneration of damaged tissue.
Neural cells communicate via electric pulse as their membrane has voltage gated ion channels. These channels are triggered by variations occurring in electrical potential near them. An extensive amount of research has been done on the electric stimulation of neuron growth as it mimics neuron-neuron communication. Examination of PPy, both as neural probes [12] and as scaffolds, was implemented for nerve conduit since axon was elongated through electric stimulation. For example, Yow et al [180] fabricated a collagen-based 3D fibrous scaffold containing PPy and showed that human MSCs (hMSCs) grown on PPy fibers expressed characteristic markers denoting a neural lineage. They also demonstrated that cellular function could be influenced by external electric field utilization and that when stimulation was extended over a long period, it could be detrimental to the culture system [181].
Currently it is believed that Schwann cell (SC) migration precedes and enhances axonal repair in the peripheral nervous system [47]. Schmidt et al [43] have explored the SC behavior on PPy by utilizing electrical stimulation and the effect of SC behavior on the neural regeneration. Their study revealed average displacement of the SCs increased with the electrical stimulation, which had an overall net anodic migration effect. Additionally, protein adsorption had indirect effects after oxidation of the films due to electrical stimuli causing an extensive effect on the speed of migration but less so on the directionality. SCs would directly migrate from the proximal to the distal end of the injury induced by electrical stimulation through a conduit. The increase in SC migration to the distal end would result in a higher degree of neural regeneration and possibly an increase in functional recovery. Huang et al [47] provided evidence that the conductive PPy/chitosan films heightened the viability, adhesion as well as spreading of SCs with or without electrical stimuli (Figure 7). Interestingly, the electrical stimuli applied through the PPy/chitosan composites caused an extreme increase in the production of nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) in comparison with cells that were not exposed to electrical stimuli [182]. These interesting results proved PPy to be invaluable in nerve repair.
Figure 7.
SEM images of SCs cultured on PPy/chitosan films. Twenty hours later, SCs were spindle shaped (A) and in the division stage on PPy/chitosan membranes (B). SCs are shown from the conductive films without ES (M−ES) group (C) and conductive films with ES (M+ES) group: (D) 100 mV/mm, (E) 600 mV/mm, and (F) 1000 mV/mm. [47], Copyright 2009, reproduced with permission from the John Wiley and Sons.
Cardiovascular diseases are the primary cause for death compared to other illnesses [183]. Recently, attention has been paid to generating cardiac patches [58] and heart valves as “spare parts”, and developing functional myocardial stents for cardiac repair [184]. Electrical conductivity of the PPy scaffolds has the possibility to be a critical part of cardiac and neural tissue engineering. Electrical stimulation producing a reaction is a pivotal characteristic of neuron and myocyte differentiation and function [27,28,58] (Figure 8). Recently, cardiac differentiation has been promoted by using porous and fibrous scaffolds or 2D substrates incorporated with PPy or PANi [59–62,185].
Figure 8.
Cardiac differentiation on day 14 from embryonic stem cells. (a–b) Typical images of unstimulated (a) and stimulated EBs (b). Red: areas where cardiac/ventricular differentiation occurred. Blue: nuclear DNA. It should be noted that red areas are corresponding to spontaneously contractile regions. [58], Copyright 2011, reproduced with permission from the Elsevier.
Cardiac cells need to develop the right intracellular networks and matrix architectures, enabling the transmission of electrical impulses at a normal speed in the correct direction [186]. Cardiac muscle contraction, which forces the natural mechanical stretch of the heart, is triggered by waves of electrical impulses. Due to this fact, any hindrance in the electrical signal conducting pathway develops into cardiovascular disease [187]. It is at this point that CP scaffold based methods have the chance to be applied to cardiac tissue regeneration.
For instance, PPy and heparin composites were found to be a positive substrate for endothelial cell proliferation [188] and vascularization was also promoted by PPy-hyaluronic acid composites [189]. In addition, the blood Rhesus factor, an antigen present on the surface of red blood cells, could be detected with erythrocytes doped PPy because Rhesus factor antigens were on the surface of cells. ELISA test confirmed that undoped PPy bound less antibodies than the erythrocytes doped PPy [25].
Bone is sensitive to electrical stimulation because of its piezoelectricity. Therefore, many experiments have been executed to modulate osteogenesis through implants transmitting electrical stimulation to cells. CPs are suitable materials as implants due to their responsivity of electrical signal. Thus, PPy also holds promise in bone tissue regeneration. In recent years, our team has constructed various biomolecules doped PPy nanostructures [190] and verified their biocompatibility suitable for bone repair. We have electrochemically constructed different kinds of PPy nanostructures doped with chondroitin sulphate (CS) [191], polydopamine (PDA) [192], taurine (Tau) [193] or citric acid [194]. We found that the surface wettability was reversibly switched under the redox potential [195,196] and that biomolecule doped PPy improved osteogenic differentiation. These works are important in driving the development of nanostructured PPy application in bone tissue regeneration.
4.1.2. Polyaniline
PPy has negative mechanical properties and poor processibility, limiting its possible applications. This disadvantage of PPy makes PANi a plausible alternative and a highly studied CP because PANi is cheap while having better processibility and good environmental stability [114,197]. Hence, many of PANi characteristics are known, including many structural forms, low cost production, good processibility, environmental stability, and the capacity to transport charges via the “doping/dedpoing” process [198]. Despite the possible benefits, PPy has been more studied than PANi for tissue engineering applications [173,175,199]. PANi was later proven to be biocompatible in vitro and in vivo [200]. Bidez et al. found that PANi and its variants could support the adhesion and proliferation of H9c2 cardiac myoblasts [201,202]. PANi and gelatin blends could be electrospun to form nanofibers for favoring H9c2 cell adhesion and growth [181]. These studies indicated the potential application of electroactive PANi in cardiac and nerve tissue regeneration.
The nervous system is a close network of neurons, cells that are excitable by electric signaling that transmit signals at an accelerated pace. Earlier many methods were proposed to repair and regenerate damage to the central and peripheral nervous systems, using several different non-conductive scaffolds [203]. With the development of CPs, scaffolds based on CPs have been exploited in the neural regeneration since neuronal function can be triggered by electrical stimuli. Several theories have evolved to explain how electrical stimulation positively regulates neurite growth and nerve regeneration [204,205]. CPs like PPy and PANi have provided a new way to accommodate and increase growth and regeneration of nerve tissue without growth factors, when constructed into scaffolds and stimulated by electrical signals [5, 206].
4.1.3. Poly(3,4-ethylenedioxythiophene)
PEDOT has a lot of potential as a regenerative material because successful application of PEDOT has led to the development of a neurotransmitter delivery system made of electronic ion pumps in animal models [207]. PEDOT is also used for synthesizing organic electrochemical transistors that can be applied to biosensing [208]. Richardson et al [209] studied relationships between neural cells and PEDOT in order to construct biomaterials that possessed an electric conductivity and could form functional interface with electrically active tissues (e.g. heart and skeletal muscle or the nervous system). They also developed microelectrodes and CP-live cell electrodes by using PEDOT to coat neural cells [5].
Implantable electrodes with PEDOT can be employed to induce nerve impulses electrically or record neuron signals [210]. For example, Green et al [211] prepared platinum electrodes coated with laminin peptides doped PEDOT. They then investigated the bioactivity of the peptides and the vitality and proliferation of PC12 cells on the electrodes. The results provide evidence that dopants of large peptides generate softer PEDOT films. Outgrowth of longer neurites was shown on the PEDOT films doped with laminin peptides than on those without being doped. Peramo et al [212] studied a process for in situ chemical polymerization of PEDOT in acellular muscle tissue constructs. These experiments showed in situ polymerization happened all through the tissue; this altered the substrate from a non-antigenic substrate to a substrate with extensive acellular properties, a pivotal alteration in order to test nerve repair and artificial prosthesis.
When neural cells were first seeded onto electrodes, direct electrochemical polymerization of PEDOT formed CP matrixes around adhered cells [25,213]. It was found that there is an intimate relationship between PEDOT surfaces and the neuronal membrane, causing the exhibition of an unusual polymer-electrode interface when the surface was coated by fragile filopodia and neurites [213]. Yu et al [214] have constructed CPs that have several interesting properties when they’re designed to mimic cell membranes, displaying elevated nonspecific enzyme/cell binding resistance as well as targeted cell recognition used for electrical communications persisting an extensive length of time. They determined that these membrane mimics were able to promote neural cellular behavior by incorporating biochemical and electrical stimuli. Neurite extension was highly impacted and increased on the CPs (Figure 9). Additionally, Schwan cells have the ability to secrete proteins due to electrical stimulation. These biomimetic PEDOTs display elevated electrical/ionic conductivities, making electrical communication possible and efficient at the cell-material interfaces. This method has far reaching opportunities for employment. CPs of this nature can be used to target ligand-receptor interactions as well as cell-electronic interfaces. Polymers such as these are attractive and necessary for in vivo testing of bioeletronic devices.
Figure 9.
The design and synthesis of conducting polymers to mimic cell membranes. (a) General idea of the biomimetic design so that interactions between cells and ECM can be mimicked. (b–d) Morphologies of differentiated PC12 cells that were cultured in NGF-supplemented medium for five days on different substrates, including (b) a PEDOT film, (c) a biomimetic PEDOT film, and (d) the biomimetic PEDOT film under electrical stimulation. The insets are magnified images. Scale bars=200 µm. [214], Copyright 2014, reproduced with permission from the Nature Publishing Group.
4.1.4. Conducting polymer composites
Allowing CPs to form composites with other materials is an alternative strategy for developing novel CP materials for tissue regeneration applications. For example, fiber materials coated with PPy doped dodecylbenzenesulfonate (DBS) were studied to mimic the ECM, providing a 3D microenvironment for cardiac progenitor cells (CPCs). Cell types with increasing sensitivity to these scaffolds were presented [215]. Petrov et al [216] have fabricated novel nanocomposite cryogels made of electrically conductive 2-hydroxyethylcellulose/polyaniline (HEC/PANi). It showed positive response to cell growth and survival. Moreover, cell morphology was altered by electrical stimulation and cell arrangement was found to be parallel to the applied electrical field. PPy functionalized graphene (PPy-G) was originally synthesized by a simple but effective mall milling method [217]. PPy-G based aligned nanofibers were then designed to provide electrical stimulation and guide the growth of retinal ganglion cells (RGCs). Once electrical stimulation was applied to RGCs, their viability, neurite elongation, and antiaging properties showed significant enhancement. This result suggests applications for optic nerve regeneration with the same method is possible. Guo et al [218] have synthesized the CP composites made of polylactide and adjustable components of the aniline oligomer. The MC3T3-E1 cells and BMSCs cultured on the resultant CP composites exhibited cytocompatibility and a significant increase of cellular propagation [15]. Osteogenic differentiation of BMSCs was promoted by the CP composites. If such composites elicit the desired cellular response, they might be useful for modifying neural, cardiac and bone tissue implants.
4.2. Piezoelectric polymers
Piezoelectric materials, capable of generating electrical charges when exposed to a mechanical pressure, are the materials of choice for all the applications in which electro-mechanical transduction is needed. They represent one of the most valuable biomedical materials. Many studies confirmed that mechanical forces play a role in increasing osteogenic differentiation and morphogenetic maintenance [219]. Osteoclasts, osteoblasts, osteocytes and MSCs are sensitive to force stimulation in vivo, and have the unique capability to respond to it, because they are exposed to a dynamic and balanced mechanical environment. This environment, including tension, shear, stress, fluid strain and flow-potential, is the mechanical environment in nature. Due to the complexity of the bone, it is unlikely that the specific effects will ever be separated, but it is understood that multiple factors have the capability to independently regulate cell response and drive remodeling events within bone [220].
Bone bears piezoelectricity as a result of its constituent piezoelectric collagen fibers. Both dielectric and piezoelectric properties of bone are dependent on the frequency, direction, and intensity of the stimuli, as well as the humidity of bone. Piezoelectric coefficients of human bone have been recently measured up to 0.7 pC/N [221]. The recognition of the piezoelectric character of the bone inspired the idea emerging in the 1970s of using piezoelectric materials to correct bone defects. The suitability of a few piezoelectric materials (ceramics of barium titanate (BTO), PVDF, collagen, PLLA, and their composites) for bone substitution has been studied by in vivo tests. These tests have been shown to accelerate bone regeneration [222–225].
4.2.1. Polyvinylidene fluoride
PVDF and its copolymers are piezoelectric materials most suitable for biomedical application due to their highest piezoelectric properties [226]. The biocompatibility of PVDF has been investigated [227]. Studies have indicated that poled PVDF films controlled MSCs adhesion and activated osteogenic differentiation [7] due to the variations of the surface charges. Therefore, PVDF may find applications where surface charge may influence the interaction between material’s interface and tissue [228].
Piezoelectric nerve guidance channels made of PVDF have been tested in vivo on mice with transected sciatic nerve [229]. After four and twelve weeks of implantation, poled and unpoled PVDF channels were compared, and nerve regeneration was evaluated. In every tested animal, when comparing nerve regeneration, larger amounts of myelinated axons were found in piezoelectric channels than nerves that were regenerated under non-piezoelectric channels. These results were achieved without any external stimulus, only exploiting the positive effect of the presence of a piezoelectric material. Young et al [230] designed membranes of microporous PVDF immobilized with the lysine component in order to assist nerve tissue regeneration. PC-12 cells cultured on these membranes exhibited good cell attachment and propagation, supporting the possible applications for designing strategies that promote nerve tissue regrowth and regeneration. It was found that neurite extension was greatly increased on the piezoelectric materials such as PVDF [203]. PVDF nerve conduits with piezoelectricity were constructed and then the effect on a sciatic mouse nerve model was assessed [229]. It was found that piezoelectric nerve conduits increased peripheral nerve regeneration, allowing for further investigations into how electrical activity could manipulate nerve regeneration. Fine et al [231] fabricated nerve guidance channel by use of a vinylidenefluoride–trifluoroethylene copolymer and found that the resultant piezoelectric copolymer tubes greatly increased the nerve regeneration. Increased neurite elongation was identified due to the presence of surface and transient charges [230,232]. The advantages related to using piezoelectric materials for neural tissue regeneration are not only dependent on the enhanced neural cell function and tissue regeneration on such materials, but they are also related, in some cases, to a decreased glial cell adhesion and proliferation. Figure 10 showed the typical neurite tracing for a cell grown on the stimulated piezoelectric PVDF (S-PZ) [55].
Figure 10.
Spinal cord neurons immunostained with a mouse anti-MAP2 antibody on stimulated piezoelectric PVDF after 5 days in vitro. Scale bar =15 µm. [55], Copyright 2012, reproduced with permission from the Springer.
Piezoelectric materials are well suited for employment in bone tissue engineering because bone has piezoelectric properties [233–235]. Varying morphologies or surface charges of PVDF materials could cause unusual behavior of bone cells [236]. Unlike characteristics exhibited by muscle cells, MC3T3-E1 cells presented better adhesion and propagation on positively poled piezoelectric PVDF membranes [38,237]. Different mechanical methods were used in varying conditions to test electrical response of cell types on the piezoelectric materials. Mechanical stimulus in this case is used as a mimic for the natural environment needed for cell development, as bone is generally constructed under these conditions. Mechanical stimulus can be varied to achieve higher cell proliferation rates [38]. Differentiation can also be established under multiple conditions in poled β-PVDF samples unlike under static conditions [238]. Non-poled β-PVDF samples did not exhibit this response due to a differing surface charge; this effect along with the addition of topography can enhance osteoblast cell differentiation and growth. These studies all indicated that poled β-PVDF samples improved cell behaviors. Our team recently has prepared a PVDF film on the Ti substrate (PVDF-Ti) as an electroactive implant to mimic the electrical microenvironment of bone [239]. Our cell assays showed that polarized PVDF-Ti induced the osteogenesis behavior of MSCs by altering its surface charge. Hence, PVDF can be used as a scaffold for bone regeneration.
Myocardium is an electroactive tissue. Because of the unusual characteristics of piezoelectric scaffolds, PVDF is a desirable material for cardiovascular tissue engineering. Lee et al [240] studied the vitality and function of cardiomyocytes (CM) taken from mouse embryonic stem cells (mES-CM) and endothelial cells (mES-EC) on the piezoelectric PVDF scaffolds. They have found that mES-CM and mES-EC both exhibited high viability and good adhesion on PVDF (Figure 11). Furthermore, mES-CM and mES-EC showed cardiac markers when cultured on the piezoelectric PVDF scaffolds. The piezoelectric scaffolds deliver the electrical stimuli needed for specific cell lines, facilitating new tissue engineering applications. Hence, there is a need for bioreactors that mimic various stimuli occurring in human body, so as to promote tissue regeneration using EAPs [18,241].
Figure 11.
Characterization of mES-CM and mES-EC cells grown on PVDF scaffolds for 6 days. (A) mES-CM stained by a live/dead assay, Scale bar=50 µm. (B) Strong cTnT-eGFP expression, Scale bar=20 µm. (C) Protein expression of in mES-CM grown in 2D substrates or on PVDF scaffolds. (D) mES-EC stained by a live/dead assay, Scale bar=50 µm. (E) Uptake of LDL (red) by mES-EC grown on PVDF scaffolds, Scale bar=50 µm. (F) Protein expression in mES-EC. Arrows represent principle fiber axis. [240], Copyright 2016, reproduced with permission from the John Wiley and Sons.
4.2.2. Polyhydroxybutyrate
PHAs are a kind of polymer produced by bacterial metabolism. Such polyesters produced by bacterial fermentation is one material with possible biomedical uses due to their natural, renewable, biodegradable, and biocompatible thermoplastic properties. These compounds can promote cell adhesion and proliferation; piezoelectric polymers PHB and poly (β-hydroxy butyric acid ester and β-hydroxy valeric acid) copolymers (PHBV) belong to this family. Their piezoelectric properties have allowed them to play a large role in health care and disease treatment.
To further develop possible repair techniques for peripheral nerve faults, synthetic neural conduits have been designed. PHAs are altered in various aspects to maintain proper neural prosthesis. For neural regeneration to be successful, the inner and outer microenvironments of the prosthesis must be in communication. Therefore, polymers that are porous are a viable choice for neural regeneration application and fibrous polymer tubes are a possible choice to improve regeneration because SCs can use them to align and trigger axonal elongation [242].
As another example, Bian et al [243] designed neuronal conduits out of PHB using various techniques. They found that PHB conduits did not perform as well as autologous nerve grafts in rate or amount of regeneration, but they did provide positive axonal regeneration with minimal inflammatory infiltration. When Fn and alginate were covered on the PHB fibers, it was found that such fibers could repair the spinal cord injury [244]. Similar success was found by other groups as well [245].
Studies of PHBV as a possible tissue engineering substrate are now increasing quickly. Rivard et al [246] showed that PHBV supported fibroblast growth rates comparable to those seen when collagen sponges were used for over one month. PHBV materials were stable throughout the culturing period even after four weeks of culturing. Conversely, collagen foams were found to contract. This result suggests that PHBV is a better choice than collagen as a polymeric substrate used for cell culturing. These piezoelectric polymer substrates are considered as transducers that can convert the mechanical energy of bone deformation into electrical stimuli and eventually control bone growth.
4.2.3. Poly(L-lactic acid)
PLLA is a slow crystallizing, semi-crystalline polymer. It has the ability to degrade into L-lactic acid, a substance that is non-toxic to humans. PLLA has been tested for 3D cell culture and dental transplantation for years, because of its biocompatibility, non-toxic degradation products and easy processability [247]. PLLA is now employed in various biomedical applications as scaffolds, sutures, and drug carriers. PLLA has recently been employed as a tissue engineering material. For instance, Chang et al [248] showed that PLLA/chitosan electro-spun composite membrane minimized undesirable effects from fibroblasts and guided periodontal tissue regeneration. PLLA/PCL/hydroxyapatite nanofibrous scaffolds were also found to be a biocomposite for supporting the proliferation, differentiation and mineralization of osteoblasts, favoring their use in bone tissue regeneration [249].
Previous study showed that ECM-mimicking nanofibrous PLLA scaffolds facilitated adhesion, elongation and propagation of cardiovascular progenitor cells (CPCs) [250]. In vivo experiments showed that the scaffolds were in favor of cardiac tissue regeneration from CPCs. Park et al fabricated human fibroblast-derived matrix (hFDM) coated PLGA/PLLA fiber scaffolds [251]. They demonstrated that the hFDM-coated microfiber scaffolds could support the attachment and migration of human umbilical cord blood-derived mesenchymal stem cells (UCB-MSCs), and promoted the chondrogenic differentiation in vitro. Ma et al [252] successfully synthesized the electroactive polylactide (PLA)/aniline trimer (AT) shape memory polymers networks (SMP) with well-developed biodegradablity. It is evident that electroactive ESMP has a high level of biocompatibility and significantly increases the proliferation of C2C12 cells. SMPs are biocompatible and electroactive. They have intense mechanical characteristics and adjustable degradability. Hence, when electrically active, they are promising bone tissue engineering candidates.
4.2.4. Piezoelectric polymer composites
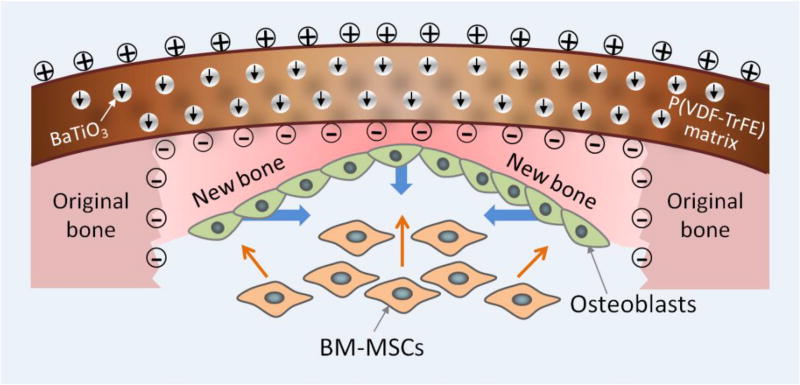
In order to advance osseointegration, osteogenesis and ossification in animal models, composites based on piezoelectric polymers have been employed. For instance, Deng et al [253] fabricated a nanocomposite membrane with polydopamine@BaTiO3 nanoparticles (Dopa@BTO NPs) in P(VDF-PTrFE) to mimic the endogenous electric potential for bone defect repair. It was found that these membranes sustain the electric microenvironment, improving osteogenic differentiation of BMSCs and even rapid bone regeneration. They also presented the conceptual proof on the use of piezoelectric polymer-based composite membranes for bone defect repair (Figure 12). This study revealed that piezoelectricity is a critical parameter for designing biomaterials in tissue engineering.
Figure 12.
Illustration of biomimetic electric microenvironment created by BTO NP/P(VDF-TrFE) composite membranes encouraging bone defect repair. Electrical dipoles of BTO NPs are reoriented in the direction of poling electric field after corona poling treatment, and consequently induced charges on the membrane. When the composite membranes are implanted like native periosteum covering the bone defect, endogenous BMSCs can be recruited by galvanotaxis and induced to differentiate into osteoblasts. Consequently, the electric microenvironment in the membrane resulted in swift bone regeneration and mature bone formation. The short black arrows denote the direction of electrical dipole in BTO NPs. The blue thick arrows denote the direction of new bone growth. The orange thin arrows denote the recruitment and osteogenic differentiation of BMSCs. [253], Copyright 2016, reproduced with permission from the American Chemical Society.
4.3. Polyelectrolyte gel
Polyelectrolyte gel can absorb enormous amounts of water and swell. Polyelectrolyte gel is unusual in their reaction to environmental stimuli. For example, the induction of chemo-mechanical contraction electrically produces a biological response, unlike classic hydrogels that show no alteration in equilibrium swelling when the surrounding environment changes [254–256]. Additionally, polyelectrolytes like polysaccharides and charged filamentous proteins are found in biological tissues [257]. Because of these characteristics, polyelectrolyte gels show promise in employment as model systems for biological tissues, allowing them to serve as biomaterials that replace tissue damage [258]. The capacity of polyelectrolyte gels to be used in regenerative medicine should not be overlooked because they bear desired functions such as supporting cell adhesion, tunable chemistry, structural integrity, biodegradability, and biocompatibility [259,260]. For example, cartilage-like structures in rabbits were formed due to the negative charge of the alginate gels after implantation for 4–6 months [261]. Further, negatively charged alginate microgels within hyaluronic acid hydrogels were found to promote cartilage regeneration [67,262].
5. Challenges in electroactive polymers for tissue regeneration
Electrostimulation, also commonly referred to as electro-therapy, is inspired by the fact that the torpedo fish produced a series of electric shocks to reduce and control the painful area of the body [263]. In fact, some cells such as nerve cells, myocardial cells and osteocytes, are sensitive to electrical stimulations. With the possibility to achieve an indirect or direct stimulation inside cells, electroactive materials are therefore extremely exciting and open a wide spectrum of applications in the tissue regeneration. For examples, conductive and piezoelectric EAPs were found to promote cardiac [264] and neural [265] regeneration compared to their non-electroactive counterparts.
However, the introduction of electroactive materials in biomedical research is often followed by a huge debate about their biological adaptation with living matter. The complexity of regenerating tissue (including bone, nerve, cardiac and cartilage) necessitates vigilant attention to structure, physicochemical property, biological performance, vascularization, neurotization, infection probability, and external stimulation competently matching human biology. Nevertheless, it is difficult to maintain all of these requirements for tissue regeneration simultaneously since they are often conflicting factors. Therefore, the major challenges of electroactive materials for tissue regeneration applications include the following aspects: 1) Improving biocompatibility, histocompatibility and antibacterial property of EAP; 2) Achieving rapid regeneration induced by EAP under stimuli; 3) Adaptation of the electrical/mechanical stimuli to tissue environments at a valid range; 4) Triggering multi-tissues regeneration by EAPs under stimuli.
When EAP based scaffolds are implanted in vivo for tissue regeneration purpose, they should be biocompatible, histocompatible and antibacterial. While much attention has been paid to the biocompatibility and histocompatibility in the past, the antibacterial property of EAPs has received less attention. We believe more work should be done to modify the EAPs to increase their antibacterial capability. Recently it was found that piezoelectric material exhibited an antibacterial property by producing reactive oxygen species (ROS) after polarization [266]. It was also found that doping CPs with antibacterial polypeptides could improve their antibiotic property and biocompatibility [267]. These successes suggest that EAPs can be made biocompatible, histocompatible and antibacterial by their own electroactive property or modification with bioactive (e.g. antibacterial) agents.
Rapid regeneration is important for the ease of pain. Currently, studies on the electroactive materials mainly focus on improving the differentiation of cells and verifying the biocompatibility of electroactive materials with tissues. However, some more long-term in vivo experiments need to be studied. In particular, some attempts should be implemented about modifying the electroactive materials for rapid tissue regeneration.
Bone, nerve, cardiac and cartilage cells and tissues are sensitive to electrical/mechanical stimulation. Electroactive materials can transmit the signals to these cells or tissues and improve cell function or tissue regeneration since the substrates provide the stimulatory cues for cells. However, the adaptation of electroactive materials to cells/tissues is challenging. The valid range of electrical stimulation to cells is now uncertain. Some more efforts should be made to determine the electrical stimulation range on the cells/tissues. Fundamental studies should be done to find how cells or tissues respond to different electrical/mechanical signals in vitro and in vivo.
Finally, it is challenging to incorporate a signal on electroactive materials to simultaneously trigger the regeneration of multiple tissues such as bone and blood vessels. To overcome such challenge, the scaffolds made of individual or multiple EAPs should be developed and implanted into the defects to exam their capability in inducing or promoting the multi-tissue regeneration in vivo under a single or multiple stimuli. Experiments in vivo with large animal models are necessary for further developing and improving electroactive scaffolds mimicking living tissue.
6. Conclusions and outlook
The development of EAPs is far from perfect, which leaves some issues to be studied. Electricity is well known to be present in living tissues, caused by resting potentials, action potentials and stress generated potentials. Extensive work has been conducted to elucidate whether mimicking these biologically active electric fields can increase the rate of growth and repair. These efforts of producing medically acceptable treatments have paid off in the form of approved treatments and clinical trials. EAP implants could generate charge and potential in vivo by body movement and physiological stress, which encourages nerve repair, bone formation and wound healing. They could also promote cellular adhesion, differentiation and migration under the mechanical or electrical stimulation. Hence, EAP scaffolds have the potential to be applied for the next level of tissue regeneration. However, most pure EAPs scaffolds are not proper for tissue regeneration because they need external power source or additional surface electrodes for conducting electrical stimulation. Therefore, their modifications such as composite formation are needed. Chemists should seek more modification methods to improve the adaptability of EAPs to tissue regeneration applications. Combining the advantage of different EAPs, for example piezoelectric and conducting, in physiological environments, by forming a composite of piezoelectric and conductive polymers may find further potential application in tissue regeneration. In addition, the use of EAPs as biomimetic membranes, ion channels and skin dressings are also worth further studying by biologists due to their capability of stimuli responsivity.
Acknowledgments
The authors gratefully acknowledge the financial support of the National High Technology Research and Development Program of China (863 Program) (2015AA033502), the National Natural Science Foundation of China (51372087, 51541201, 51673168), the National Key Research and Development Program of China (2016YFA0100900), Science and Technology Planning Project of Guangdong Province, China (2014A010105048), the Natural Science Foundation of Guangdong Province (2015A030313493, 2016A030308014), State Key Laboratory for Mechanical Behavior of Materials, China (20141607), and Zhejiang Provincial Natural Science Foundation of China (LZ16E030001). YZ and CBM would also like to thank the financial support from National Institutes of Health (CA200504, CA195607, and EB021339), Department of Defense Office of Congressionally Directed Medical Research Programs (W81XWH-15-1-0180), Oklahoma Center for Adult Stem Cell Research (434003), and Oklahoma Center for the Advancement of Science and Technology (HR14-160).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jiang T, Carbone EJ, Lo KWH, Laurencin CT. Electrospinning of polymer nanofibers for tissue regeneration. Prog Polym Sci. 2015;46:1–24. [Google Scholar]
- 2.Balint R, Cassidy NJ, Cartmell SH. Conductive polymers: towards a smart biomaterial for tissue engineering. Acta Biomater. 2014;10:2341–53. doi: 10.1016/j.actbio.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Ohki T, Yamato M, Ota M, Takagi R, Kondo M, Kanai N, Okano T, Yamamoto M. Application of regenerative medical technology using tissue-engineered cell sheets for endoscopic submucosal dissection of esophageal neoplasms. Digest Endosc. 2015;27:182–8. doi: 10.1111/den.12354. [DOI] [PubMed] [Google Scholar]
- 4.Mooney E, Mackle JN, Blond DJ, O'Cearbhaill E, Shaw G, Blau WJ, Barry FP, Barron V, Murphy JM. The electrical stimulation of carbon nanotubes to provide a cardiomimetic cue to MSCs. Biomaterials. 2012;33:6132–9. doi: 10.1016/j.biomaterials.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 5.Ghasemi-Mobarakeh L, Prabhakaran MP, Morshed M, Nasr-Esfahani MH, Baharvand H, Kiani S, Al-Deyab SS, Ramakrishna S. Application of conductive polymers, scaffolds and electrical stimulation for nerve tissue engineering. J Tissue Eng Regener Med. 2011;5:e17–35. doi: 10.1002/term.383. [DOI] [PubMed] [Google Scholar]
- 6.Wu Y, Wang L, Guo B, Shao Y, Ma PX. Electroactive biodegradable polyurethane significantly enhanced Schwann cells myelin gene expression and neurotrophin secretion for peripheral nerve tissue engineering. Biomaterials. 2016;87:18–31. doi: 10.1016/j.biomaterials.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Parssinen J, Hammaren H, Rahikainen R, Sencadas V, Ribeiro C, Vanhatupa S, Miettinen S, Lanceros-Mendez S, Hytonen VP. Enhancement of adhesion and promotion of osteogenic differentiation of human adipose stem cells by poled electroactive poly(vinylidene fluoride) J Biomed Mater Res Part A. 2015;103:919–28. doi: 10.1002/jbm.a.35234. [DOI] [PubMed] [Google Scholar]
- 8.Costa R, Ribeiro C, Lopes AC, Martins P, Sencadas V, Soares R, Lanceros-Mendez S. Osteoblast, fibroblast and in vivo biological response to poly(vinylidene fluoride) based composite materials. J Mater Sci Mater Med. 2013;24:395–403. doi: 10.1007/s10856-012-4808-y. [DOI] [PubMed] [Google Scholar]
- 9.Meng S, Rouabhia M, Zhang Z. Electrical stimulation modulates osteoblast proliferation and bone protein production through heparin-bioactivated conductive scaffolds. Bioelectromagnetics. 2013;34:189–99. doi: 10.1002/bem.21766. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Q, Mochalin VN, Neitzel I, Knoke IY, Han J, Klug CA, Zhou JG, Lelkes PI, Gogotsi Y. Fluorescent PLLA-nanodiamond composites for bone tissue engineering. Biomaterials. 2011;32:87–94. doi: 10.1016/j.biomaterials.2010.08.090. [DOI] [PubMed] [Google Scholar]
- 11.Sultana N, Wang M. PHBV/PLLA-based composite scaffolds fabricated using an emulsion freezing/freeze-drying technique for bone tissue engineering: surface modification and in vitro biological evaluation. Biofabrication. 2012;4 doi: 10.1088/1758-5082/4/1/015003. 015003 |1–14. [DOI] [PubMed] [Google Scholar]
- 12.Fattahi P, Yang G, Kim G, Abidian MR. A review of organic and inorganic biomaterials for neural interfaces. Adv Mater. 2014;26:1846–85. doi: 10.1002/adma.201304496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim HN, Jiao A, Hwang NS, Kim MS, Kang do H, Kim DH, Suh KY. Nanotopography-guided tissue engineering and regenerative medicine. Adv Drug Deliv Rev. 2013;65:536–58. doi: 10.1016/j.addr.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaur G, Adhikari R, Cass P, Bown M, Gunatillake P. Electrically conductive polymers and composites for biomedical applications. RSC Adv. 2015;5:37553–67. [Google Scholar]
- 15.Yang M, Liang Y, Gui Q, Chen J, Liu Y. Electroactive biocompatible materials for nerve cell stimulation. Mater Res Express. 2015;2 042001 |1–14. [Google Scholar]
- 16.Gelmi A, Zhang J, Cieslar-Pobuda A, Ljunngren MK, Los MJ, Rafat M, Jager EWH. Electroactive polymer scaffolds for cardiac tissue engineering. Proc of SPIE. 2015;9430 94301T1 |1–7. [Google Scholar]
- 17.Rajabi AH, Jaffe M, Arinzeh TL. Piezoelectric materials for tissue regeneration: A review. Acta Biomater. 2015;24:12–23. doi: 10.1016/j.actbio.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Ribeiro C, Correia DM, Ribeiro S, Sencadas V, Botelho G, Lanceros-Méndez S. Piezoelectric poly(vinylidene fluoride) microstructure and poling state in active tissue engineering. Eng Life Sci. 2015;15:351–6. [Google Scholar]
- 19.Wu W, Pan C, Zhang Y, Wen X, Wang ZL. Piezotronics and piezo-phototronics – From single nanodevices to array of devices and then to integrated functional system. Nano Today. 2013;8:619–42. [Google Scholar]
- 20.Park SH, Lee HB, Yeon SM, Park J, Lee NK. Flexible and Stretchable Piezoelectric Sensor with Thickness-Tunable Configuration of Electrospun Nanofiber Mat and Elastomeric Substrates. ACS Appl Mater Interfaces. 2016;8:24773–81. doi: 10.1021/acsami.6b07833. [DOI] [PubMed] [Google Scholar]
- 21.Abidian MR, Kim DH, Martin DC. Conducting-Polymer Nanotubes for Controlled Drug Release. Adv Mater. 2006;18:405–9. doi: 10.1002/adma.200501726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ariga K, Mori T, Hill JP. Mechanical control of nanomaterials and nanosystems. Adv Mater. 2012;24:158–76. doi: 10.1002/adma.201102617. [DOI] [PubMed] [Google Scholar]
- 23.Cha S, Kim SM, Kim H, Ku J, Sohn JI, Park YJ, Song BG, Jung MH, Lee EK, Choi BL, Park JJ, Wang ZL, Kim JM, Kim K. Porous PVDF as effective sonic wave driven nanogenerators. Nano Lett. 2011;11:5142–7. doi: 10.1021/nl202208n. [DOI] [PubMed] [Google Scholar]
- 24.Hwang GT, Park H, Lee JH, Oh S, Park KI, Byun M, Park H, Ahn G, Jeong CK, No K, Kwon H, Lee SG, Joung B, Lee KJ. Self-powered cardiac pacemaker enabled by flexible single crystalline PMN-PT piezoelectric energy harvester. Adv Mater. 2014;26:4880–7. doi: 10.1002/adma.201400562. [DOI] [PubMed] [Google Scholar]
- 25.Abidian MR, Martin DC. Multifunctional Nanobiomaterials for Neural Interfaces. Adv Funct Mater. 2009;19:573–85. [Google Scholar]
- 26.Hu WW, Hsu YT, Cheng YC, Li C, Ruaan RC, Chien CC, Chung CA, Tsao CW. Electrical stimulation to promote osteogenesis using conductive polypyrrole films. Mater Sci Eng C. 2014;37:28–36. doi: 10.1016/j.msec.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 27.Quigley AF, Razal JM, Kita M, Jalili R, Gelmi A, Penington A, Ovalle-Robles R, Baughman RH, Clark GM, Wallace GG, Kapsa RM. Electrical stimulation of myoblast proliferation and differentiation on aligned nanostructured conductive polymer platforms. Adv Healthc Mater. 2012;1:801–8. doi: 10.1002/adhm.201200102. [DOI] [PubMed] [Google Scholar]
- 28.Gilmore KJ, Kita M, Han Y, Gelmi A, Higgins MJ, Moulton SE, Clark GM, Kapsa R, Wallace GG. Skeletal muscle cell proliferation and differentiation on polypyrrole substrates doped with extracellular matrix components. Biomaterials. 2009;30:5292–304. doi: 10.1016/j.biomaterials.2009.06.059. [DOI] [PubMed] [Google Scholar]
- 29.Castano H, O'Rear EA, McFetridge PS, Sikavitsas VI. Polypyrrole thin films formed by admicellar polymerization support the osteogenic differentiation of mesenchymal stem cells. Macromol Biosci. 2004;4:785–94. doi: 10.1002/mabi.200300123. [DOI] [PubMed] [Google Scholar]
- 30.Alvarez-Mejia L, Morales J, Cruz GJ, Olayo M-G, Olayo R, Díaz-Ruíz A, Ríos C, Mondragón-Lozano R, Sánchez-Torres S, Morales-Guadarrama A. Functional recovery in spinal cord injured rats using polypyrrole/iodine implants and treadmill training. J Mater Sci Mater Med. 2015;26 doi: 10.1007/s10856-015-5541-0. 209 |1–11. [DOI] [PubMed] [Google Scholar]
- 31.Xu H, Holzwarth JM, Yan Y, Xu P, Zheng H, Yin Y, Li S, Ma PX. Conductive PPY/PDLLA conduit for peripheral nerve regeneration. Biomaterials. 2014;35:225–35. doi: 10.1016/j.biomaterials.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hardy JG, Villancio-Wolter MK, Sukhavasi RC, Mouser DJ, Aguilar D, Geissler SA, Kaplan DL, Schmidt CE. Electrical Stimulation of Human Mesenchymal Stem Cells on Conductive Nanofibers Enhances their Differentiation toward Osteogenic Outcomes. Macromol Rapid Commun. 2015;36:1884–90. doi: 10.1002/marc.201500233. [DOI] [PubMed] [Google Scholar]
- 33.Sajesh K, Jayakumar R, Nair SV, Chennazhi K. Biocompatible conducting chitosan/polypyrrole–alginate composite scaffold for bone tissue engineering. Int J Biol Macromol. 2013;62:465–71. doi: 10.1016/j.ijbiomac.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 34.Jun I, Jeong S, Shin H. The stimulation of myoblast differentiation by electrically conductive sub-micron fibers. Biomaterials. 2009;30:2038–47. doi: 10.1016/j.biomaterials.2008.12.063. [DOI] [PubMed] [Google Scholar]
- 35.Cao J, Man Y, Li L. Electrical stimuli improve osteogenic differentiation mediated by aniline pentamer and PLGA nanocomposites. Biomed Rep. 2013;1:428–32. doi: 10.3892/br.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shahini A, Yazdimamaghani M, Walker KJ, Eastman MA, Hatami-Marbini H, Smith BJ, Ricci JL, Madihally SV, Vashaee D, Tayebi L. 3D conductive nanocomposite scaffold for bone tissue engineering. Int J Nanomed. 2014;9:167–81. doi: 10.2147/IJN.S54668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ribeiro C, Parssinen J, Sencadas V, Correia V, Miettinen S, Hytonen VP, Lanceros-Mendez S. Dynamic piezoelectric stimulation enhances osteogenic differentiation of human adipose stem cells. J Biomed Mater Res Part A. 2015;103:2172–5. doi: 10.1002/jbm.a.35368. [DOI] [PubMed] [Google Scholar]
- 38.Ribeiro C, Moreira S, Correia V, Sencadas V, Rocha JG, Gama FM, Gómez Ribelles JL, Lanceros-Méndez S. Enhanced proliferation of pre-osteoblastic cells by dynamic piezoelectric stimulation. RSC Adv. 2012;2:11504–9. [Google Scholar]
- 39.Hayati AN, Rezaie H, Hosseinalipour S. Preparation of poly (3-hydroxybutyrate)/nano-hydroxyapatite composite scaffolds for bone tissue engineering. Mater Lett. 2011;65:736–9. [Google Scholar]
- 40.Ramier J, Bouderlique T, Stoilova O, Manolova N, Rashkov I, Langlois V, Renard E, Albanese P, Grande D. Biocomposite scaffolds based on electrospun poly (3-hydroxybutyrate) nanofibers and electrosprayed hydroxyapatite nanoparticles for bone tissue engineering applications. Mater Sci Eng C. 2014;38:161–9. doi: 10.1016/j.msec.2014.01.046. [DOI] [PubMed] [Google Scholar]
- 41.Coimbra P, Ferreira P, De Sousa H, Batista P, Rodrigues M, Correia I, Gil MH. Preparation and chemical and biological characterization of a pectin/chitosan polyelectrolyte complex scaffold for possible bone tissue engineering applications. Int J Biol Macromol. 2011;48:112–8. doi: 10.1016/j.ijbiomac.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen HT, Sapp S, Wei C, Chow JK, Nguyen A, Coursen J, Luebben S, Chang E, Ross R, Schmidt CE. Electric field stimulation through a biodegradable polypyrrole-co-polycaprolactone substrate enhances neural cell growth. J Biomed Mater Res Part A. 2014;102:2554–64. doi: 10.1002/jbm.a.34925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Forciniti L, Ybarra J, 3rd, Zaman MH, Schmidt CE. Schwann cell response on polypyrrole substrates upon electrical stimulation. Acta Biomater. 2014;10:2423–33. doi: 10.1016/j.actbio.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 44.Yue Z, Moulton SE, Cook M, O'Leary S, Wallace GG. Controlled delivery for neuro-bionic devices. Adv Drug Deliv Rev. 2013;65:559–69. doi: 10.1016/j.addr.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 45.Thompson BC, Moulton SE, Richardson RT, Wallace GG. Effect of the dopant anion in polypyrrole on nerve growth and release of a neurotrophic protein. Biomaterials. 2011;32:3822–31. doi: 10.1016/j.biomaterials.2011.01.053. [DOI] [PubMed] [Google Scholar]
- 46.Liu X, Yue Z, Higgins MJ, Wallace GG. Conducting polymers with immobilised fibrillar collagen for enhanced neural interfacing. Biomaterials. 2011;32:7309–17. doi: 10.1016/j.biomaterials.2011.06.047. [DOI] [PubMed] [Google Scholar]
- 47.Huang J, Hu X, Lu L, Ye Z, Zhang Q, Luo Z. Electrical regulation of Schwann cells using conductive polypyrrole/chitosan polymers. J Biomed Mater Res Part A. 2009;93:164–74. doi: 10.1002/jbm.a.32511. [DOI] [PubMed] [Google Scholar]
- 48.Richardson RT, Wise AK, Thompson BC, Flynn BO, Atkinson PJ, Fretwell NJ, Fallon JB, Wallace GG, Shepherd RK, Clark GM, O'Leary SJ. Polypyrrole-coated electrodes for the delivery of charge and neurotrophins to cochlear neurons. Biomaterials. 2009;30:2614–24. doi: 10.1016/j.biomaterials.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang J, Qiu K, Sun B, Fang J, Zhang K, Hany E-H, Al-Deyab SS, Mo XM. The aligned core–sheath nanofibers with electrical conductivity for neural tissue engineering. J Mater Chem B. 2014;2:7945–54. doi: 10.1039/c4tb01185f. [DOI] [PubMed] [Google Scholar]
- 50.Wang L, Huang Q, Wang JY. Nanostructured Polyaniline Coating on ITO Glass Promotes the Neurite Outgrowth of PC 12 Cells by Electrical Stimulation. Langmuir. 2015;31:12315–22. doi: 10.1021/acs.langmuir.5b00992. [DOI] [PubMed] [Google Scholar]
- 51.Collazos-Castro JE, Polo JL, Hernandez-Labrado GR, Padial-Canete V, Garcia-Rama C. Bioelectrochemical control of neural cell development on conducting polymers. Biomaterials. 2010;31:9244–55. doi: 10.1016/j.biomaterials.2010.08.057. [DOI] [PubMed] [Google Scholar]
- 52.Ludwig KA, Uram JD, Yang J, Martin DC, Kipke DR. Chronic neural recordings using silicon microelectrode arrays electrochemically deposited with a poly(3,4-ethylenedioxythiophene) (PEDOT) film. J Neural Eng. 2006;3:59–70. doi: 10.1088/1741-2560/3/1/007. [DOI] [PubMed] [Google Scholar]
- 53.Pires F, Ferreira Q, Rodrigues CA, Morgado J, Ferreira FC. Neural stem cell differentiation by electrical stimulation using a cross-linked PEDOT substrate: Expanding the use of biocompatible conjugated conductive polymers for neural tissue engineering. Biochim Biophys Acta. 2015;1850:1158–68. doi: 10.1016/j.bbagen.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 54.Sebaa M, Nguyen TY, Dhillon S, Garcia S, Liu H. The effects of poly (3, 4-ethylenedioxythiophene) coating on magnesium degradation and cytocompatibility with human embryonic stem cells for potential neural applications. J Biomed Mater Res Part A. 2015;103:25–37. doi: 10.1002/jbm.a.35142. [DOI] [PubMed] [Google Scholar]
- 55.Royo-Gascon N, Wininger M, Scheinbeim JI, Firestein BL, Craelius W. Piezoelectric substrates promote neurite growth in rat spinal cord neurons. Ann Biomed Eng. 2013;41:112–22. doi: 10.1007/s10439-012-0628-y. [DOI] [PubMed] [Google Scholar]
- 56.Khorasani M, Mirmohammadi S, Irani S. Polyhydroxybutyrate (PHB) scaffolds as a model for nerve tissue engineering application: fabrication and in vitro assay. Int J Polym Mater. 2011;60:562–75. [Google Scholar]
- 57.Chang H, Wang Z, Luo H, Xu M, Ren X, Zheng G, Wu BJ, Zhang XH, Lu XY, Chen F. Poly (3-hydroxybutyrate-co-3-hydroxyhexanoate)-based scaffolds for tissue engineering. Braz J Med Biol Res. 2014;47:533–9. doi: 10.1590/1414-431X20143930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bidez PR, Kresh JY, Wei Y, Lelkes PI. Enhanced Cardiac Differentiation of Mouse Embryonic Stem Cells by Electrical Stimulation. In: Artmann GM, Minger S, Hescheler J, editors. Stem Cell Engineering: Principles and Applications. Berlin: Springer-Verlag; 2011. pp. 119–41. [Google Scholar]
- 59.Kai D, Prabhakaran MP, Jin G, Ramakrishna S. Polypyrrole-contained electrospun conductive nanofibrous membranes for cardiac tissue engineering. J Biomed Mater Res Part A. 2011;99:376–85. doi: 10.1002/jbm.a.33200. [DOI] [PubMed] [Google Scholar]
- 60.Borriello A, Guarino V, Schiavo L, Alvarez-Perez MA, Ambrosio L. Optimizing PANi doped electroactive substrates as patches for the regeneration of cardiac muscle. J Mater Sci Mater Med. 2011;22:1053–62. doi: 10.1007/s10856-011-4259-x. [DOI] [PubMed] [Google Scholar]
- 61.Cui H, Liu Y, Cheng Y, Zhang Z, Zhang P, Chen X, Wei Y. In vitro study of electroactive tetraaniline-containing thermosensitive hydrogels for cardiac tissue engineering. Biomacromolecules. 2014;15:1115–23. doi: 10.1021/bm4018963. [DOI] [PubMed] [Google Scholar]
- 62.Qazi TH, Rai R, Dippold D, Roether JE, Schubert DW, Rosellini E, Barbani N, Boccaccini AR. Development and characterization of novel electrically conductive PANI–PGS composites for cardiac tissue engineering applications. Acta Biomater. 2014;10:2434–45. doi: 10.1016/j.actbio.2014.02.023. [DOI] [PubMed] [Google Scholar]
- 63.Martins AM, Eng G, Caridade SG, Mano JoF, Reis RL, Vunjak-Novakovic G. Electrically conductive chitosan/carbon scaffolds for cardiac tissue engineering. Biomacromolecules. 2014;15:635–43. doi: 10.1021/bm401679q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Karagkiozaki V, Karagiannidis PG, Gioti M, Kavatzikidou P, Georgiou D, Georgaraki E, Logothetidis S. Bioelectronics meets nanomedicine for cardiovascular implants: PEDOT-based nanocoatings for tissue regeneration. Biochim Biophys Acta. 2013;1830:4294–304. doi: 10.1016/j.bbagen.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 65.Alarifi S, Aphale A, Patra P. Piezoelectric Materials Based Scaffolds Fabrication for Cardiamyocyets Cell Growth. UB Scholar Works. 2015:1. [Google Scholar]
- 66.Baheiraei N, Yeganeh H, Ai J, Gharibi R, Azami M, Faghihi F. Synthesis, characterization and antioxidant activity of a novel electroactive and biodegradable polyurethane for cardiac tissue engineering application. Mater Sci Eng C. 2014;44:24–37. doi: 10.1016/j.msec.2014.07.061. [DOI] [PubMed] [Google Scholar]
- 67.Kwon HJ, Yasuda K, Gong JP, Ohmiya Y. Polyelectrolyte hydrogels for replacement and regeneration of biological tissues. Macromol Res. 2014;22:227–35. [Google Scholar]
- 68.Zhao W, Jin X, Cong Y, Liu Y, Fu J. Degradable natural polymer hydrogels for articular cartilage tissue engineering. J Chem Technol Biotechnol. 2013;88:327–39. [Google Scholar]
- 69.Biggs J, Danielmeier K, Hitzbleck J, Krause J, Kridl T, Nowak S, Orselli E, Quan X, Schapeler D, Sutherland W, Wagner J. Electroactive polymers: developments of and perspectives for dielectric elastomers. Angew Chem Int Ed. 2013;52:9409–21. doi: 10.1002/anie.201301918. [DOI] [PubMed] [Google Scholar]
- 70.Kundu T, Guyomar D, Cottinet PJ, Lallart M. Energy harvesting in electroactive materials: a comparison between ferroelectrics and electrostrictive polymers. Proc of SPIE. 2011;7984:79841L–6L. [Google Scholar]
- 71.Givaja G, Amo-Ochoa P, Gomez-Garcia CJ, Zamora F. Electrical conductive coordination polymers. Chem Soc Rev. 2012;41:115–47. doi: 10.1039/c1cs15092h. [DOI] [PubMed] [Google Scholar]
- 72.Masmoudi S, El Mahi A, El Guerjouma R. Mechanical behaviour and health monitoring by acoustic emission of sandwich composite integrated by piezoelectric implant. Composites Part B. 2014;67:76–83. [Google Scholar]
- 73.Bar-Cohen Y, Kim KJ, Choi HR, Madden JD. Electroactive polymer materials. Smart Mater Struct. 2007;16:1–2. [Google Scholar]
- 74.Finkenstadt V, Willett J. Preparation and characterization of electroactive biopolymers. Macromol Symp. 2005;227:367–72. [Google Scholar]
- 75.Shankar R, Ghosh TK, Spontak RJ. Mechanical and actuation behavior of electroactive nanostructured polymers. Sens Actuators A. 2009;151:46–52. [Google Scholar]
- 76.Qazi TH, Rai R, Boccaccini AR. Tissue engineering of electrically responsive tissues using polyaniline based polymers: A review. Biomaterials. 2014;35:9068–86. doi: 10.1016/j.biomaterials.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 77.Bar-Cohen Y. Current and future developments in artificial muscles using electroactive polymers. Expert Rev Med Devices. 2005;2:731–40. doi: 10.1586/17434440.2.6.731. [DOI] [PubMed] [Google Scholar]
- 78.Bar-Cohen Y. Biologically inspired robots as artificial inspectors. Proc of SPIE. 2002;4702:41–8. [Google Scholar]
- 79.Bar-Cohen Y. Biomimetics using electroactive polymers (EAP) as artificial muscles-: A review. J Adv Mater. 2006;38:3–9. [Google Scholar]
- 80.Shankar R, Ghosh TK, Spontak RJ. Dielectric elastomers as next-generation polymeric actuators. Soft Matter. 2007;3:1116–29. doi: 10.1039/b705737g. [DOI] [PubMed] [Google Scholar]
- 81.Ramadan KS, Sameoto D, Evoy S. A review of piezoelectric polymers as functional materials for electromechanical transducers. Smart Mater Struct. 2014;23 033001 |1–26. [Google Scholar]
- 82.Xia L, Wei Z, Wan M. Conducting polymer nanostructures and their application in biosensors. J Colloid Interface Sci. 2010;341:1–11. doi: 10.1016/j.jcis.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 83.Wallace G, Spinks G. Conducting polymers ? bridging the bionic interface. Soft Matter. 2007;3:665–71. doi: 10.1039/b618204f. [DOI] [PubMed] [Google Scholar]
- 84.Nambiar S, Yeow JT. Conductive polymer-based sensors for biomedical applications. Biosens Bioelectron. 2011;26:1825–32. doi: 10.1016/j.bios.2010.09.046. [DOI] [PubMed] [Google Scholar]
- 85.Dubey N, Leclerc M. Conducting polymers: Efficient thermoelectric materials. J Polym Sci Part B Polym Phys. 2011;49:467–75. [Google Scholar]
- 86.Ates M. A review study of (bio)sensor systems based on conducting polymers. Mater Sci Eng C. 2013;33:1853–9. doi: 10.1016/j.msec.2013.01.035. [DOI] [PubMed] [Google Scholar]
- 87.Lee R, Wanekaya AK. Conducting Polymer Nanowires and Their Biomedical Applications. In: Kumar CSSR, editor. Nanotechnologies for the Life Sciences. Vol. 10. Weinheim; Wiley-VCH: 2011. pp. 455–71. [Google Scholar]
- 88.MacDiarmid AG. “Synthetic metals”: A novel role for organic polymers (Nobel lecture) Angew Chem Int Ed. 2001;40:2581–90. doi: 10.1002/1521-3773(20010716)40:14<2581::AID-ANIE2581>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 89.Otero TF, Martinez JG, Arias-Pardilla J. Biomimetic electrochemistry from conducting polymers. A review. Electrochim Acta. 2012;84:112–28. [Google Scholar]
- 90.Luo S-C, Zhu B, Nakao A, Nakatomi R, Yu H-h. Functionalized Conducting Polymer Nano-Networks from Controlled Oxidation Polymerization toward Cell Engineering. Adv Eng Mater. 2011;13:B423–B7. [Google Scholar]
- 91.Ge D, Wang J, Wang Z, Wang S. Electrochemical synthesis of polypyrrole nanowires on composite electrode. Synth Met. 2002;132:93–5. [Google Scholar]
- 92.Long Y-Z, Li M-M, Gu C, Wan M, Duvail J-L, Liu Z, Fan ZY. Recent advances in synthesis, physical properties and applications of conducting polymer nanotubes and nanofibers. Prog Polym Sci. 2011;36:1415–42. [Google Scholar]
- 93.Ateh DD, Navsaria HA, Vadgama P. Polypyrrole-based conducting polymers and interactions with biological tissues. J R Soc Interface. 2006;3:741–52. doi: 10.1098/rsif.2006.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Serra Moreno J, Panero S, Materazzi S, Martinelli A, Sabbieti MG, Agas D, Materazzi G. Polypyrrole-polysaccharide thin films characteristics: electrosynthesis and biological properties. J Biomed Mater Res Part A. 2009;88:832–40. doi: 10.1002/jbm.a.32230. [DOI] [PubMed] [Google Scholar]
- 95.Ramanaviciene A, Kausaite A, Tautkus S, Ramanavicius A. Biocompatibility of polypyrrole particles: an in-vivo study in mice. J Pharm Pharmacol. 2007;59:311–5. doi: 10.1211/jpp.59.2.0017. [DOI] [PubMed] [Google Scholar]
- 96.Gomez N, Lee JY, Nickels JD, Schmidt CE. Micropatterned Polypyrrole: A Combination of Electrical and Topographical Characteristics for the Stimulation of Cells. Adv Funct Mater. 2007;17:1645–53. doi: 10.1002/adfm.200600669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nickels JD, Schmidt CE. Surface modification of the conducting polymer, polypyrrole, via affinity peptide. J Biomed Mater Res Part A. 2013;101:1464–71. doi: 10.1002/jbm.a.34435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hong J-Y, Jeon SO, Jang J, Song K, Kim SH. A facile route for the preparation of organic bistable memory devices based on size-controlled conducting polypyrrole nanoparticles. Org Electron. 2013;14:979–83. [Google Scholar]
- 99.Han Y, Zhang Q, Han F, Li C, Sun J, Lu Y. Fabrication of conducting polypyrrole film with microlens arrays by combination of breath figures and replica molding methods. Polymer. 2012;53:2599–603. [Google Scholar]
- 100.Leprince L, Dogimont A, Magnin D, Demoustier-Champagne S. Dexamethasone electrically controlled release from polypyrrole-coated nanostructured electrodes. J Mater Sci Mater Med. 2010;21:925–30. doi: 10.1007/s10856-010-4008-6. [DOI] [PubMed] [Google Scholar]
- 101.Meng S, Zhang Z, Rouabhia M. Accelerated osteoblast mineralization on a conductive substrate by multiple electrical stimulation. J Bone Miner Metab. 2011;29:535–44. doi: 10.1007/s00774-010-0257-1. [DOI] [PubMed] [Google Scholar]
- 102.Kopecká J, Kopecký D, Vrňata M, Fitl P, Stejskal J, Trchová M, Bober P, Morávková Z, Prokeš J, Sapurina I. Polypyrrole nanotubes: mechanism of formation. RSC Adv. 2014;4:1551–8. [Google Scholar]
- 103.Wei L, Sevilla M, Fuertes AB, Mokaya R, Yushin G. Polypyrrole-Derived Activated Carbons for High-Performance Electrical Double-Layer Capacitors with Ionic Liquid Electrolyte. Adv Funct Mater. 2012;22:827–34. [Google Scholar]
- 104.Mert BD, Solmaz R, Kardaş G, Yazıcı B. Copper/polypyrrole multilayer coating for 7075 aluminum alloy protection. Prog Org Coat. 2011;72:748–54. [Google Scholar]
- 105.Ramanavicius A, Oztekin Y, Ramanaviciene A. Electrochemical formation of polypyrrole-based layer for immunosensor design. Sens Actuators B. 2014;197:237–43. [Google Scholar]
- 106.Jiang S, Sun Y, Cui X, Huang X, He Y, Ji S, Shi W, Ge DT. Enhanced drug loading capacity of polypyrrole nanowire network for controlled drug release. Synth Met. 2013;163:19–23. [Google Scholar]
- 107.Cui X, Wiler J, Dzaman M, Altschuler RA, Martin DC. In vivo studies of polypyrrole/peptide coated neural probes. Biomaterials. 2003;24:777–87. doi: 10.1016/s0142-9612(02)00415-5. [DOI] [PubMed] [Google Scholar]
- 108.Li D, Huang J, Kaner RB. Polyaniline nanofibers: a unique polymer nanostructure for versatile applications. Acc Chem Res. 2008;42:135–45. doi: 10.1021/ar800080n. [DOI] [PubMed] [Google Scholar]
- 109.Ayad M, El-Hefnawy G, Zaghlol S. Facile synthesis of polyaniline nanoparticles; its adsorption behavior. Chem Eng J. 2013;217:460–5. [Google Scholar]
- 110.Bhadra S, Khastgir D, Singha NK, Lee JH. Progress in preparation, processing and applications of polyaniline. Prog Polym Sci. 2009;34:783–810. [Google Scholar]
- 111.Anu Prathap MU, Chaurasia AK, Sawant SN, Apte SK. Polyaniline-based highly sensitive microbial biosensor for selective detection of lindane. Anal Chem. 2012;84:6672–8. doi: 10.1021/ac301077d. [DOI] [PubMed] [Google Scholar]
- 112.Malhotra BD, Chaubey A, Singh SP. Prospects of conducting polymers in biosensors. Anal Chim Acta. 2006;578:59–74. doi: 10.1016/j.aca.2006.04.055. [DOI] [PubMed] [Google Scholar]
- 113.Svirskis D, Travas-Sejdic J, Rodgers A, Garg S. Electrochemically controlled drug delivery based on intrinsically conducting polymers. J Controlled Release. 2010;146:6–15. doi: 10.1016/j.jconrel.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 114.Xia H, Tao X. In situ crystals as templates to fabricate rectangular shaped hollow polyaniline tubes and their application in drug release. J Mater Chem. 2011;21:2463–65. [Google Scholar]
- 115.Liu S, Wang J, Zhang D, Zhang P, Ou J, Liu B, Yang SR. Investigation on cell biocompatible behaviors of polyaniline film fabricated via electroless surface polymerization. Appl Surf Sci. 2010;256:3427–31. [Google Scholar]
- 116.Temmer R, Maziz A, Plesse C, Aabloo A, Vidal F, Tamm T. In search of better electroactive polymer actuator materials: PPy versus PEDOT versus PEDOT–PPy composites. Smart Mater Struct. 2013;22 104006 |1–16. [Google Scholar]
- 117.Higgins MJ, Molino PJ, Yue Z, Wallace GG. Organic Conducting Polymer–Protein Interactions. Chem Mater. 2012;24:828–39. [Google Scholar]
- 118.Chang J, Dommer M, Chang C, Lin L. Piezoelectric nanofibers for energy scavenging applications. Nano Energy. 2012;1:356–71. [Google Scholar]
- 119.Romero IS, Bradshaw NP, Larson JD, Severt SY, Roberts SJ, Schiller ML, Leger JM, Murphy AR. Biocompatible Electromechanical Actuators Composed of Silk-Conducting Polymer Composites. Adv Funct Mater. 2014;24:3866–73. [Google Scholar]
- 120.Saïdi S, Bouzitoun M, Mannaî A, Gmati F, Derouiche H, Mohamed AB. Effect of PANI rate percentage on morphology, structure and charge transport mechanism in PANI–PVDF composites above percolation threshold. J Phys D. 2013;46 355101 |1–11. [Google Scholar]
- 121.Merlini C, Barra GMO, Medeiros Araujo T, Pegoretti A. Electrically pressure sensitive poly(vinylidene fluoride)/polypyrrole electrospun mats. RSC Adv. 2014;4:15749–58. [Google Scholar]
- 122.Fukada E, Yasuda I. On the piezoelectric effect of bone. J Phys Soc Jpn. 1957;12:1158–62. [Google Scholar]
- 123.Marino AA, Becker RO, Soderholm SC. Origin of the piezoelectric effect in bone. Calcif Tissue Res. 1971;8:177–80. doi: 10.1007/BF02010135. [DOI] [PubMed] [Google Scholar]
- 124.Persano L, Dagdeviren C, Maruccio C, De Lorenzis L, Pisignano D. Cooperativity in the enhanced piezoelectric response of polymer nanowires. Adv Mater. 2014;26:7574–80. doi: 10.1002/adma.201403169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Martins P, Lopes AC, Lanceros-Mendez S. Electroactive phases of poly(vinylidene fluoride): Determination, processing and applications. Prog Polym Sci. 2014;39:683–706. [Google Scholar]
- 126.Damaraju SM, Wu S, Jaffe M, Arinzeh TL. Structural changes in PVDF fibers due to electrospinning and its effect on biological function. Biomed Mater. 2013;8 doi: 10.1088/1748-6041/8/4/045007. 045007 |1–11. [DOI] [PubMed] [Google Scholar]
- 127.Low YK, Zou X, Fang YM, Wang JL, Lin WS, Boey FY, Ng KW. beta-Phase poly(vinylidene fluoride) films encouraged more homogeneous cell distribution and more significant deposition of fibronectin towards the cell-material interface compared to alpha-phase poly(vinylidene fluoride) films. Mater Sci Eng C. 2014;34:345–53. doi: 10.1016/j.msec.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 128.Low YKA, Meenubharathi N, Niphadkar ND, Boey FYC, Ng KW. α- and β-Poly(Vinylidene Fluoride) Evoke Different Cellular Behaviours. J Biomater Sci Polym Ed. 2011;22:1651–67. doi: 10.1163/092050610X519471. [DOI] [PubMed] [Google Scholar]
- 129.Petrasovits LA, Zhao L, McQualter RB, Snell KD, Somleva MN, Patterson NA, Nielsen LK, Brumbley SM. Enhanced polyhydroxybutyrate production in transgenic sugarcane. Plant Biotechnol J. 2012;10:569–78. doi: 10.1111/j.1467-7652.2012.00686.x. [DOI] [PubMed] [Google Scholar]
- 130.Hazer DB, Kılıçay E, Hazer B. Poly(3-hydroxyalkanoate)s: Diversification and biomedical applications. Mater Sci Eng C. 2012;32:637–47. [Google Scholar]
- 131.Laycock B, Halley P, Pratt S, Werker A, Lant P. The chemomechanical properties of microbial polyhydroxyalkanoates. Prog Polym Sci. 2014;39:397–443. [Google Scholar]
- 132.Inuzuka Y, Onishi K, Kinoshita S, Nakashima Y, Nagata T, Yamane H, Nakai T, Kataoka T, Ito S, Tajitsu Y. Fundamental Study of Application of Piezoelectric Chiral Polymer to Actuator. Jpn J Appl Phys. 2012;51 09LD15 |1–4. [Google Scholar]
- 133.Yoshida M, Onogi T, Onishi K, Inagaki T, Tajitsu Y. High piezoelectric performance of poly(lactic acid) film manufactured by solid-state extrusion. Jpn J Appl Phys. 2014;53 09PC02 |1–6. [Google Scholar]
- 134.Smyth M, Poursorkhabi V, Mohanty AK, Gregori S, Misra M. Electrospinning highly oriented and crystalline poly(lactic acid) fiber mats. J Mater Sci. 2014;49:2430–41. [Google Scholar]
- 135.Barroca NB, Daniel-da-Silva AL, Gomes PS, Fernandes MHR, Lanceros-Méndez S, Sharma P, Gruverman A, Fernandes MHV, M Vilarinho P. Suitability of PLLA as Piezoelectric Substrates for Tissue Engineering Evidenced by Microscopy Techniques. Microsc Microanal. 2012;18:63–4. [Google Scholar]
- 136.Barroca N, Vilarinho PM, Fernandes MHV, Sharma P, Gruverman A. Stability of electrically induced-polarization in poly (L-lactic) acid for bone regeneration. Appl Phys Lett. 2012;101:023701–4. [Google Scholar]
- 137.Lee SJ, Arun AP, Kim KJ. Piezoelectric properties of electrospun poly(l-lactic acid) nanofiber web. Mater Lett. 2015;148:58–62. [Google Scholar]
- 138.Liu ZH, Pan CT, Lin LW, Lai HW. Piezoelectric properties of PVDF/MWCNT nanofiber using near-field electrospinning. Sens Actuators, A. 2013;193:13–24. [Google Scholar]
- 139.Park KI, Xu S, Liu Y, Hwang GT, Kang SJ, Wang ZL, Lee KJ. Piezoelectric BaTiO(3) thin film nanogenerator on plastic substrates. Nano Lett. 2010;10:4939–43. doi: 10.1021/nl102959k. [DOI] [PubMed] [Google Scholar]
- 140.Wang M, Fang DW, Wang NN, Jiang S, Nie J, Yu Q, Ma GP. Preparation of PVDF/PVP core–shell nanofibers mats via homogeneous electrospinning. Polymer. 2014;55:2188–96. [Google Scholar]
- 141.Yan S, Zhang K, Liu Z, Zhang X, Gan L, Cao B, Chen XS, Cui L, Yin JB. Fabrication of poly(l-glutamic acid)/chitosan polyelectrolyte complex porous scaffolds for tissue engineering. J Mater Chem B. 2013;1:1541–51. doi: 10.1039/c2tb00440b. [DOI] [PubMed] [Google Scholar]
- 142.Ma G, Wang Z, Chen J, Yin R, Chen B, Nie J. Freeze-dried chitosan–sodium hyaluronate polyelectrolyte complex fibers as tissue engineering scaffolds. New J Chem. 2014;38:1211–17. [Google Scholar]
- 143.Xie Y, Liao C, Zhou J. Effects of external electric fields on lysozyme adsorption by molecular dynamics simulations. Biophys Chem. 2013;179:26–34. doi: 10.1016/j.bpc.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 144.Wei Q, Becherer T, Angioletti-Uberti S, Dzubiella J, Wischke C, Neffe AT, Lendlein A, Ballauff M, Haag R. Protein interactions with polymer coatings and biomaterials. Angew Chem Int Ed. 2014;53:8004–31. doi: 10.1002/anie.201400546. [DOI] [PubMed] [Google Scholar]
- 145.Song W, Mano JF. Interactions between cells or proteins and surfaces exhibiting extreme wettabilities. Soft Matter. 2013;9:2985–99. [Google Scholar]
- 146.Zhang Z, Liang Y, Liang P, Li C, Fang S. Protein adsorption materials based on conducting polymers: polypyrrole modified with ω-(N-pyrrolyl)-octylthiol. Polym Int. 2011;60:703–10. [Google Scholar]
- 147.Wang X, Liu G, Zhang G. Effect of surface wettability on ion-specific protein adsorption. Langmuir. 2012;28:14642–53. doi: 10.1021/la303001j. [DOI] [PubMed] [Google Scholar]
- 148.Wang LP, Wang W, Di L, Lu YN, Wang JY. Protein adsorption under electrical stimulation of neural probe coated with polyaniline. Colloids Surf B. 2010;80:72–8. doi: 10.1016/j.colsurfb.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 149.Cakmak AS, Cakmak S, White JD, Raja WK, Kim K, Yigit S, Kaplan DL, Gumusderelioglu M. Synergistic effect of exogeneous and endogeneous electrostimulation on osteogenic differentiation of human mesenchymal stem cells seeded on silk scaffolds. J Orthop Res. 2016;34:581–90. doi: 10.1002/jor.23059. [DOI] [PubMed] [Google Scholar]
- 150.Liao JW, Zhang Y, Tan GX, Ning CY. Nanostructured PPy coating on titanium fabricated via template-free electrochemical polymerization in PBS. Surf Coat Technol. 2013;228:S41–S3. [Google Scholar]
- 151.Liao JW, Huang SS, Ning CY, Tan GX, Pan HB, Zhang Y. Potential-induced reversible switching in the tubular structure of conducting polypyrrole nanotube arrays. RSC Adv. 2013;3:14946–9. [Google Scholar]
- 152.Liao JW, Zhu Y, Zhou ZN, Chen JQ, Tan GX, Ning CY, Mao CB. Reversibly Controlling Preferential Protein Adsorption on Bone Implants by Using an Applied Weak Potential as a Switch. Angew Chem Int Ed. 2014;53:13068–72. doi: 10.1002/anie.201406349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhou ZN, Li WP, He TR, Yu P, Tan GX, Ning CY. Controllable Protein Adsorption and Bacterial Adhesion on Polypyrrole Nanocone Arrays. J Mater Sci Technol. 2016;32:950–5. [Google Scholar]
- 154.Li YY, Wei Y, Liao JW, Hao YW, Ning CY, Jiang L, Wang ST. Surface Wettability Switched Cell Adhesion and Detachment on Conducting Polymer Nanoarray. Adv Mater Interfaces. 2016;3 1600598 |1–4. [Google Scholar]
- 155.Thompson DM, Koppes AN, Hardy JG, Schmidt CE. Electrical stimuli in the central nervous system microenvironment. Annu Rev Biomed Eng. 2014;16:397–430. doi: 10.1146/annurev-bioeng-121813-120655. [DOI] [PubMed] [Google Scholar]
- 156.Nuccitelli R. A role for endogenous electric fields in wound healing. Curr Top Dev Biol. 2003;58:1–26. doi: 10.1016/s0070-2153(03)58001-2. [DOI] [PubMed] [Google Scholar]
- 157.McCaig CD, Rajnicek AM, Song B, Zhao M. Has electrical growth cone guidance found its potential? Trends Neurosci. 2002;25:354–9. doi: 10.1016/s0166-2236(02)02174-4. [DOI] [PubMed] [Google Scholar]
- 158.Sundelacruz S, Levin M, Kaplan DL. Role of membrane potential in the regulation of cell proliferation and differentiation. Stem Cell Rev Rep. 2009;5:231–46. doi: 10.1007/s12015-009-9080-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.McCormick DA. Membrane potential and action potential. San Diego: Academic Press; 2004. p. 359. [Google Scholar]
- 160.Stoppel WL, Kaplan DL, Black LD., 3rd Electrical and mechanical stimulation of cardiac cells and tissue constructs. Adv Drug Deliv Rev. 2016;96:135–55. doi: 10.1016/j.addr.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sarvari R, Massoumi B, Jaymand M, Beygi-Khosrowshahi Y, Abdollahi M. Novel three-dimensional, conducting, biocompatible, porous, and elastic polyaniline-based scaffolds for regenerative therapies. RSC Adv. 2016;6:19437–51. [Google Scholar]
- 162.Prabhakaran MP, Ghasemi-Mobarakeh L, Jin G, Ramakrishna S. Electrospun conducting polymer nanofibers and electrical stimulation of nerve stem cells. J Biosci Bioeng. 2011;112:501–7. doi: 10.1016/j.jbiosc.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 163.Jeong SI, Jun ID, Choi MJ, Nho YC, Lee YM, Shin H. Development of Electroactive and Elastic Nanofibers that contain Polyaniline and Poly (Leong SI, Jun caprolactone) for the Control of Cell Adhesion. Macromol Biosci. 2008;8:627–37. doi: 10.1002/mabi.200800005. [DOI] [PubMed] [Google Scholar]
- 164.Kotwal A, Schmidt CE. Electrical stimulation alters protein adsorption and nerve cell interactions with electrically conducting biomaterials. Biomaterials. 2001;22:1055–64. doi: 10.1016/s0142-9612(00)00344-6. [DOI] [PubMed] [Google Scholar]
- 165.Lee JY, Bashur CA, Goldstein AS, Schmidt CE. Polypyrrole-coated electrospun PLGA nanofibers for neural tissue applications. Biomaterials. 2009;30:4325–35. doi: 10.1016/j.biomaterials.2009.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Motamedi AS, Mirzadeh H, Hajiesmaeilbaigi F, Bagheri-Khoulenjani S, Shokrgozar MA. Piezoelectric electrospun nanocomposite comprising Au NPs/PVDF for nerve tissue engineering. J Biomed Mater Res Part A. 2017;105:1984–93. doi: 10.1002/jbm.a.36050. [DOI] [PubMed] [Google Scholar]
- 167.Yu H, Lui YS, Xiong SJ, Leong SW, Wen F, Nurkahfianto H, Rana S, Leong DT, Ng KW, Tan LP. Insights into the role of focal adhesion modulation in myogenic differentiation of human mesenchymal stem cells. Stem Cells Dev. 2012;22:136–147. doi: 10.1089/scd.2012.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yu HY, Tay CY, Pal M, Leong SW, Li HQ, Li H, Wen F, Ng KW, Tan LP. A Bio-inspired Platform to Modulate Myogenic Differentiation of Human Mesenchymal Stem Cells Through Focal Adhesion Regulation. Adv Healthc Mater. 2013;2:442–9. doi: 10.1002/adhm.201200142. [DOI] [PubMed] [Google Scholar]
- 169.Li HQ, Wen F, Wong YS, Boey FYC, Venkatraman S, Leong DT, Ng KW, Ng GKL, Tan LP. Direct laser machining-induced topographic pattern promotes up-regulation of myogenic markers in human mesenchymal stem cells. Acta Biomater. 2012;8:531–9. doi: 10.1016/j.actbio.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 170.Tay CY, Pal M, Yu HY, Leong WS, Tan NS, Ng KW, Venkatraman S, Boey FYC, Leong DT, Tan LP. Bio-inspired Micropatterned Platform to Steer Stem Cell Differentiation. Small. 2011;7:1416–21. doi: 10.1002/smll.201002298. [DOI] [PubMed] [Google Scholar]
- 171.Tay CY, Yu HY, Pal M, Leong WS, Tan NS, Ng KW, Leong DT, Tan LP. Micropatterned matrix directs differentiation of human mesenchymal stem cells towards myocardial lineage. Exp Cell Res. 2010;316:1159–68. doi: 10.1016/j.yexcr.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 172.Guo B, Glavas L, Albertsson A-C. Biodegradable and electrically conducting polymers for biomedical applications. Prog Polym Sci. 2013;38:1263–86. [Google Scholar]
- 173.Guimard NK, Gomez N, Schmidt CE. Conducting polymers in biomedical engineering. Prog Polym Sci. 2007;32:876–921. [Google Scholar]
- 174.Cui Z, Yang B, Li R-K. Application of Biomaterials in Cardiac Repair and Regeneration. Engineering. 2016;2:141–8. [Google Scholar]
- 175.Schultze JW, Karabulut H. Application potential of conducting polymers. Electrochim Acta. 2005;50:1739–45. [Google Scholar]
- 176.Green RA, Lovell NH, Wallace GG, Poole-Warren LA. Conducting polymers for neural interfaces: challenges in developing an effective long-term implant. Biomaterials. 2008;29:3393–9. doi: 10.1016/j.biomaterials.2008.04.047. [DOI] [PubMed] [Google Scholar]
- 177.Tahir ZM, Alocilja EC, Grooms DL. Polyaniline synthesis and its biosensor application. Biosens Bioelectron. 2005;20:1690–5. doi: 10.1016/j.bios.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 178.Otero TF, Martinez JG. Structural Electrochemistry: Conductivities and Ionic Content from Rising Reduced Polypyrrole Films. Adv Funct Mater. 2014;24:1259–64. [Google Scholar]
- 179.Zha Z, Deng Z, Li Y, Li C, Wang J, Wang S, Qu EZ, Dai ZF. Biocompatible polypyrrole nanoparticles as a novel organic photoacoustic contrast agent for deep tissue imaging. Nanoscale. 2013;5:4462–7. doi: 10.1039/c3nr00627a. [DOI] [PubMed] [Google Scholar]
- 180.Yow S-Z, Lim TH, Yim EK, Lim CT, Leong KW. A 3D electroactive polypyrrole-collagen fibrous scaffold for tissue engineering. Polymers. 2011;3:527–44. [Google Scholar]
- 181.Guo B, Sun Y, Finne-Wistrand A, Mustafa K, Albertsson AC. Electroactive porous tubular scaffolds with degradability and non-cytotoxicity for neural tissue regeneration. Acta Biomater. 2012;8:144–53. doi: 10.1016/j.actbio.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 182.Qi FY, Wang YQ, Ma T, Zhu S, Zeng W, Hu XY, Liu ZY, Huang JH, Luo ZJ. Electrical regulation of olfactory ensheathing cells using conductive polypyrrole/chitosan polymers. Biomaterials. 2013;34:1799–809. doi: 10.1016/j.biomaterials.2012.11.042. [DOI] [PubMed] [Google Scholar]
- 183.O'Neill HS, Gallagher LB, O'Sullivan J, Whyte W, Curley C, Dolan E, Hameed A, O'Dwyer J, Payne C, O'Reilly D, Ruiz-Hernandez E, Roche ET, O'Brien FJ, Cryan SA, Kelly H, Murphy B, Duffy GP. Biomaterial-Enhanced Cell and Drug Delivery: Lessons Learned in the Cardiac Field and Future Perspectives. Adv Mater. 2016;28:5648–61. doi: 10.1002/adma.201505349. [DOI] [PubMed] [Google Scholar]
- 184.Parsa H, Ronaldson K, Vunjak-Novakovic G. Bioengineering methods for myocardial regeneration. Adv Drug Deliv Rev. 2016;96:195–202. doi: 10.1016/j.addr.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Baheiraei N, Yeganeh H, Ai J, Gharibi R, Ebrahimi-Barough S, Azami M, Vahdat S, Baharvand H. Preparation of a porous conductive scaffold from aniline pentamer-modified polyurethane/PCL blend for cardiac tissue engineering. J Biomed Mater Res Part A. 2015;103:3179–87. doi: 10.1002/jbm.a.35447. [DOI] [PubMed] [Google Scholar]
- 186.Zimmermann T, Bordeanu N, Strub E. Properties of nanofibrillated cellulose from different raw materials and its reinforcement potential. Carbohydr Polym. 2010;79:1086–93. [Google Scholar]
- 187.Zimmermann W-H, Didié M, Wasmeier GH, Nixdorff U, Hess A, Melnychenko I, Boy O, Neuhuber WL, Weyand M, Eschenhagen T. Cardiac grafting of engineered heart tissue in syngenic rats. Circulation. 2002;106:I-151–I-7. [PubMed] [Google Scholar]
- 188.Shi W, Ge D, Wang J, Jiang Z, Ren L, Zhang Q. Heparin-Controlled Growth of Polypyrrole Nanowires. Macromol Rapid Commun. 2006;27:926–30. [Google Scholar]
- 189.Collier CP, Mattersteig G, Wong EW, Luo Y, Beverly K, Sampaio J, Raymo FM, Stoddart JF, Heath JR. A [2] catenane-based solid state electronically reconfigurable switch. Science. 2000;289:1172–5. doi: 10.1126/science.289.5482.1172. [DOI] [PubMed] [Google Scholar]
- 190.Liao JW, Wu SL, Yin ZY, Huang SS, Ning CY, Tan GX, Chu PK. Surface-dependent self-assembly of conducting polypyrrole nanotube arrays in template-free electrochemical polymerization. ACS Appl Mater Interfaces. 2014;6:10946–51. doi: 10.1021/am5017478. [DOI] [PubMed] [Google Scholar]
- 191.Zhou ZN, Zhu WJ, Liao JW, Huang SS, Chen JQ, He TR, Tan GX, Ning CY. Chondroitin sulphate-guided construction of polypyrrole nanoarchitectures. Mater Sci Eng C. 2015;48:172–8. doi: 10.1016/j.msec.2014.11.070. [DOI] [PubMed] [Google Scholar]
- 192.Wang ZG, Zhou L, Yu P, Liu Y, Chen JQ, Liao JW, Li WP, Chen W, Zhou WH, Yi X, Ouyang Polydopamine-Assisted Electrochemical Fabrication of Polypyrrole Nanofibers on Bone Implants to Improve Bioactivity. Macromol Mater Eng. 2016;301:1288–94. [Google Scholar]
- 193.Liao JW, Pan HB, Ning CY, Tan GX, Zhou ZN, Chen JQ, Huang SS. Taurine-induced fabrication of nano-architectured conducting polypyrrole on biomedical titanium. Macromol Rapid Commun. 2014;35:574–8. doi: 10.1002/marc.201300843. [DOI] [PubMed] [Google Scholar]
- 194.Liao JW, Zhu Y, Yin ZY, Tan GX, Ning CY, Mao CB. Tuning nano-architectures and improving bioactivity of conducting polypyrrole coating on bone implants by incorporating bone-borne small molecules. J Mater Chem B. 2014;2014:7872–6. doi: 10.1039/c4tb01053a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Liao JW, Ning CY, Tan GX, Ni GX, Pan HB. Conducting Polypyrrole Nanotube Arrays as an Implant Surface: Fabricated on Biomedical Titanium with Fine-Tunability by Means of Template-Free Electrochemical Polymerization. Chem Plus Chem. 2014;79:524–30. doi: 10.1002/cplu.201300385. [DOI] [PubMed] [Google Scholar]
- 196.Liao JW, Ning CY, Yin ZY, Tan GX, Huang SS, Zhou ZN, Chen JQ, Pan HB. Nanostructured conducting polymers as intelligent implant surface: fabricated on biomedical titanium with a potential-induced reversible switch in wettability. Chem phys chem. 2013;14:3891–4. doi: 10.1002/cphc.201300746. [DOI] [PubMed] [Google Scholar]
- 197.Xia Y, Lu X, Zhu H. Natural silk fibroin/polyaniline (core/shell) coaxial fiber: Fabrication and application for cell proliferation. Compos Sci Technol. 2013;77:37–41. [Google Scholar]
- 198.Wu J, Tang Q, Li Q, Lin J. Self-assembly growth of oriented polyaniline arrays: A morphology and structure study. Polymer. 2008;49:5262–7. [Google Scholar]
- 199.Bendrea AD, Cianga L, Cianga I. Review paper: progress in the field of conducting polymers for tissue engineering applications. J Biomater Appl. 2011;26:3–84. doi: 10.1177/0885328211402704. [DOI] [PubMed] [Google Scholar]
- 200.Mattioli-Belmonte M, Giavaresi G, Biagini G, Virgili L, Giacomini M, Fini M, Giantomassi F, Natali D, Torricelli P, Giardino R. Tailoring biomaterial compatibility: in vivo tissue response versus in vitro cell behavior. Int J Artif Organs. 2003;26:1077–85. doi: 10.1177/039139880302601205. [DOI] [PubMed] [Google Scholar]
- 201.Guterman E, Cheng S, Palouian K, Bidez P, Lelkes P, Wei Y. Peptide-modified electroactive polymers for tissue engineering applications. Abstr Pap Am Chem Soc. 2002;224:U433–3. [Google Scholar]
- 202.Bidez PR, Li S, MacDiarmid AG, Venancio EC, Wei Y, Lelkes PI. Polyaniline, an electroactive polymer, supports adhesion and proliferation of cardiac myoblasts. J Biomater Sci Polym Ed. 2006;17:199–212. doi: 10.1163/156856206774879180. [DOI] [PubMed] [Google Scholar]
- 203.Schmidt CE, Leach JB. Neural tissue engineering: strategies for repair and regeneration. Annu Rev Biomed Eng. 2003;5:293–347. doi: 10.1146/annurev.bioeng.5.011303.120731. [DOI] [PubMed] [Google Scholar]
- 204.Patel N, Poo M-M. Orientation of neurite growth by extracellular electric fields. J Neurosci. 1982;2:483–96. doi: 10.1523/JNEUROSCI.02-04-00483.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sisken B, Kanje M, Lundborg G, Herbst E, Kurtz W. Stimulation of rat sciatic nerve regeneration with pulsed electromagnetic fields. Brain Res. 1989;485:309–16. doi: 10.1016/0006-8993(89)90575-1. [DOI] [PubMed] [Google Scholar]
- 206.Li GN, Hoffman-Kim D. Tissue-engineered platforms of axon guidance. Tissue Eng Part B. 2008;14:33–51. doi: 10.1089/teb.2007.0181. [DOI] [PubMed] [Google Scholar]
- 207.Simon DT, Kurup S, Larsson KC, Hori R, Tybrandt K, Goiny M, Jager EWH, Berggren M, Canlon B, Richter-Dahlfors A. Organic electronics for precise delivery of neurotransmitters to modulate mammalian sensory function. Nat Mater. 2009;8:742–6. doi: 10.1038/nmat2494. [DOI] [PubMed] [Google Scholar]
- 208.Mabeck JT, Malliaras GG. Chemical and biological sensors based on organic thin-film transistors. Anal Bioanal Chem. 2006;384:343–53. doi: 10.1007/s00216-005-3390-2. [DOI] [PubMed] [Google Scholar]
- 209.Richardson-Burns SM, Hendricks JL, Martin DC. Electrochemical polymerization of conducting polymers in living neural tissue. J Neural Eng. 2007;4:L6–L13. doi: 10.1088/1741-2560/4/2/L02. [DOI] [PubMed] [Google Scholar]
- 210.Asplund M, Thaning E, Lundberg J, Sandberg-Nordqvist A, Kostyszyn B, von Holst H. Toxicity evaluation of PEDOT/biomolecular composites intended for neural communication electrodes. Biomed Mater. 2009;4 doi: 10.1088/1748-6041/4/4/045009. 045009 |1–12. [DOI] [PubMed] [Google Scholar]
- 211.Green RA, Lovell NH, Poole-Warren LA. Cell attachment functionality of bioactive conducting polymers for neural interfaces. Biomaterials. 2009;30:3637–44. doi: 10.1016/j.biomaterials.2009.03.043. [DOI] [PubMed] [Google Scholar]
- 212.Peramo A, Urbanchek MG, Spanninga SA, Povlich LK, Cederna P, Martin DC. In situ polymerization of a conductive polymer in acellular muscle tissue constructs. Tissue Eng Part A. 2008;14:423–32. doi: 10.1089/tea.2007.0123. [DOI] [PubMed] [Google Scholar]
- 213.Richardson-Burns SM, Hendricks JL, Foster B, Povlich LK, Kim D-H, Martin DC. Polymerization of the conducting polymer poly (3, 4-ethylenedioxythiophene)(PEDOT) around living neural cells. Biomaterials. 2007;28:1539–52. doi: 10.1016/j.biomaterials.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zhu B, Luo S-C, Zhao H, Lin H-A, Sekine J, Nakao A, Chen C, Yamashita Y, Yu HH. Large enhancement in neurite outgrowth on a cell membrane-mimicking conducting polymer. Nat Commun. 2014;5 doi: 10.1038/ncomms5523. 4523 |1–9. [DOI] [PubMed] [Google Scholar]
- 215.Bar-Cohen Y, Gelmi A, Zhang J, Cieslar-Pobuda A, Ljunngren MK, Los MJ, Rafat M, Jager EWH. Electroactive 3D materials for cardiac tissue engineering. Proc of SPIE. 2015;9430 94301T |1–7. [Google Scholar]
- 216.Petrov P, Mokreva P, Kostov I, Uzunova V, Tzoneva R. Novel electrically conducting 2-hydroxyethylcellulose/polyaniline nanocomposite cryogels: Synthesis and application in tissue engineering. Carbohydr Polym. 2016;140:349–55. doi: 10.1016/j.carbpol.2015.12.069. [DOI] [PubMed] [Google Scholar]
- 217.Yan L, Zhao B, Liu X, Li X, Zeng C, Shi H, Xu XX, Lin T, Dai LM, Liu Y. Aligned Nanofibers from Polypyrrole/Graphene as Electrodes for Regeneration of Optic Nerve via Electrical Stimulation. ACS Appl Mater Interfaces. 2016;8:6834–40. doi: 10.1021/acsami.5b12843. [DOI] [PubMed] [Google Scholar]
- 218.Li L, Yu M, Ma PX, Guo B. Electroactive degradable copolymers enhancing osteogenic differentiation from bone marrow derived mesenchymal stem cells. J Mater Chem B. 2016;4:471–81. doi: 10.1039/c5tb01899d. [DOI] [PubMed] [Google Scholar]
- 219.Sittichockechaiwut A, Scutt AM, Ryan AJ, Bonewald LF, Reilly GC. Use of rapidly mineralising osteoblasts and short periods of mechanical loading to accelerate matrix maturation in 3D scaffolds. Bone. 2009;44:822–9. doi: 10.1016/j.bone.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 220.Fernandez-Yague MA, Abbah SA, McNamara L, Zeugolis DI, Pandit A, Biggs MJ. Biomimetic approaches in bone tissue engineering: Integrating biological and physicomechanical strategies. Adv Drug Deliv Rev. 2015;84:1–29. doi: 10.1016/j.addr.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 221.Cui H, Wang Y, Cui L, Zhang P, Wang X, Wei Y, Chen XS. In vitro studies on regulation of osteogenic activities by electrical stimulus on biodegradable electroactive polyelectrolyte multilayers. Biomacromolecules. 2014;15:3146–57. doi: 10.1021/bm5007695. [DOI] [PubMed] [Google Scholar]
- 222.Xin YC, Jiang J, Huo KF, Hu T, Chu PK. Bioactive SrTiO3 Nanotube Arrays: Strontium Delivery Platform on Ti-Based Osteoporotic Bone Implants. ACS Nano. 2009;3:3228–34. doi: 10.1021/nn9007675. [DOI] [PubMed] [Google Scholar]
- 223.Bagchi A, Meka SRK, Rao BN, Chatterjee K. Perovskite ceramic nanoparticles in polymer composites for augmenting bone tissue regeneration. Nanotech. 2014;25 doi: 10.1088/0957-4484/25/48/485101. 485101 |1–13. [DOI] [PubMed] [Google Scholar]
- 224.Ferreira AM, Gentile P, Chiono V, Ciardelli G. Collagen for bone tissue regeneration. Acta Biomater. 2012;8:3191–200. doi: 10.1016/j.actbio.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 225.Schofer MD, Tunnermann L, Kaiser H, Roessler PP, Theisen C, Heverhagen JT, Hering J, Voelker M, Agarwal S, Efe T, Fuchs-Winkelmann S, Paletta JRJ. Functionalisation of PLLA nanofiber scaffolds using a possible cooperative effect between collagen type I and BMP-2: impact on colonization and bone formation in vivo. J Mater Sci Mater Med. 2012;23:2227–33. doi: 10.1007/s10856-012-4697-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Dias JC, Correia DC, Lopes AC, Ribeiro S, Ribeiro C, Sencadas V, Botelho G, Esperança JMSS, Laza JM, Vilas JL, León LM, Lanceros-Méndez S. Development of poly(vinylidene fluoride)/ionic liquid electrospun fibers for tissue engineering applications. J Mater Sci. 2016;51:4442–50. [Google Scholar]
- 227.Correia DM, Ribeiro C, Sencadas V, Vikingsson L, Oliver Gasch M, Gómez Ribelles JL, Botelho G, Lanceros-Méndez S. Strategies for the development of three dimensional scaffolds from piezoelectric poly(vinylidene fluoride) Mater Des. 2016;92:674–81. [Google Scholar]
- 228.Schneider GB, English A, Abraham M, Zaharias R, Stanford C, Keller J. The effect of hydrogel charge density on cell attachment. Biomaterials. 2004;25:3023–8. doi: 10.1016/j.biomaterials.2003.09.084. [DOI] [PubMed] [Google Scholar]
- 229.Aebischer P, Valentini RF, Dario P, Domenici C, Galletti PM. Piezoelectric guidance channels enhance regeneration in the mouse sciatic nerve after axotomy. Brain Res. 1987;436:165–8. doi: 10.1016/0006-8993(87)91570-8. [DOI] [PubMed] [Google Scholar]
- 230.Young T-H, Lu J-N, Lin D-J, Chang C-L, Chang H-H, Cheng L-P. Immobilization of l-lysine on dense and porous poly (vinylidene fluoride) surfaces for neuron culture. Desalination. 2008;234:134–43. [Google Scholar]
- 231.Fine EG, Valentini RF, Bellamkonda R, Aebischer P. Improved nerve regeneration through piezoelectric vinylidenefluoride-trifluoroethylene copolymer guidance channels. Biomaterials. 1991;12:775–80. doi: 10.1016/0142-9612(91)90029-a. [DOI] [PubMed] [Google Scholar]
- 232.Schmidt CE, Shastri VR, Vacanti JP, Langer R. Stimulation of neurite outgrowth using an electrically conducting polymer. Proc Natl Acad Sci USA. 1997;94:8948–53. doi: 10.1073/pnas.94.17.8948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kubo T. Piezoelectricity of bone and electrical callus. J Orthop Sci. 2012;17:105–6. doi: 10.1007/s00776-012-0219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Ma PX. Biomimetic materials for tissue engineering. Adv Drug Deliv Rev. 2008;60:184–98. doi: 10.1016/j.addr.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ficat JJ, Thiechart M, Ficat P, Lacabanne C, Micheron F, Bab I. Piezoelectric induction of bone formation: Ultrastructural observations. Ferroelectrics. 1984;60:313–6. [Google Scholar]
- 236.Nunes-Pereira J, Ribeiro S, Ribeiro C, Gombek CJ, Gama FM, Gomes AC, Patterson DA, Lanceros-Méndez S. Poly(vinylidene fluoride) and copolymers as porous membranes for tissue engineering applications. Polym Test. 2015;44:234–41. [Google Scholar]
- 237.Ribeiro C, Panadero J, Sencadas V, Lanceros-Méndez S, Tamaño M, Moratal D, Salmerón-Sánchez M, Ribelles JLG. Fibronectin adsorption and cell response on electroactive poly (vinylidene fluoride) films. Biomed Mater. 2012;7 doi: 10.1088/1748-6041/7/3/035004. 035004 |1–10. [DOI] [PubMed] [Google Scholar]
- 238.Ribeiro C, Correia V, Martins P, Gama FM, Lanceros-Mendez S. Proving the suitability of magnetoelectric stimuli for tissue engineering applications. Colloids Surf B. 2016;140:430–6. doi: 10.1016/j.colsurfb.2015.12.055. [DOI] [PubMed] [Google Scholar]
- 239.Zhou ZN, Li WP, He TR, Qian L, Tan GX, Ning CY. Polarization of an electroactive functional film on titanium for inducing osteogenic differentiation. Sci Rep. 2016;6 doi: 10.1038/srep35512. 35512 |1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Hitscherich P, Wu S, Gordan R, Xie LH, Arinzeh T, Lee EJ. The effect of PVDF-TrFE scaffolds on stem cell derived cardiovascular cells. Biotechnol Bioeng. 2016;113:1577–85. doi: 10.1002/bit.25918. [DOI] [PubMed] [Google Scholar]
- 241.Correia DM, Gonçalves R, Ribeiro C, Sencadas V, Botelho G, Ribelles JLG, Lanceros-Méndez S. Electrosprayed poly(vinylidene fluoride) microparticles for tissue engineering applications. RSC Adv. 2014;4:33013–21. [Google Scholar]
- 242.Mosahebi A, Fuller P, Wiberg M, Terenghi G. Effect of allogeneic Schwann cell transplantation on peripheral nerve regeneration. Exp Neurol. 2002;173:213–23. doi: 10.1006/exnr.2001.7846. [DOI] [PubMed] [Google Scholar]
- 243.Bian Y-Z, Wang Y, Aibaidoula G, Chen G-Q, Wu Q. Evaluation of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) conduits for peripheral nerve regeneration. Biomaterials. 2009;30:217–25. doi: 10.1016/j.biomaterials.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 244.Novikov LN, Novikova LN, Mosahebi A, Wiberg M, Terenghi G, Kellerth J-O. A novel biodegradable implant for neuronal rescue and regeneration after spinal cord injury. Biomaterials. 2002;23:3369–76. doi: 10.1016/s0142-9612(02)00037-6. [DOI] [PubMed] [Google Scholar]
- 245.Yucel D, Kose GT, Hasirci V. Tissue engineered, guided nerve tube consisting of aligned neural stem cells and astrocytes. Biomacromolecules. 2010;11:3584–91. doi: 10.1021/bm1010323. [DOI] [PubMed] [Google Scholar]
- 246.Rivard C, Chaput C, Rhalmi S, Selmani A. Bio-absorbable synthetic polyesters and tissue regeneration. A study of three-dimensional proliferation of ovine chondrocytes and osteoblasts. Ann Chir. 1996;50:651–8. [PubMed] [Google Scholar]
- 247.Narayanan G, Vernekar VN, Kuyinu EL, Laurencin CT. Poly (lactic acid)-based biomaterials for orthopaedic regenerative engineering. Adv Drug Deliv Rev. 2016;107:247–76. doi: 10.1016/j.addr.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Chen S, Hao Y, Cui W, Chang J, Zhou Y. Biodegradable electrospun PLLA/chitosan membrane as guided tissue regeneration membrane for treating periodontitis. J Mater Sci. 2013;48:6567–77. [Google Scholar]
- 249.Qi H, Ye Z, Ren H, Chen N, Zeng Q, Wu X, Lu TL. Bioactivity assessment of PLLA/PCL/HAP electrospun nanofibrous scaffolds for bone tissue engineering. Life Sci. 2016;148:139–44. doi: 10.1016/j.lfs.2016.02.040. [DOI] [PubMed] [Google Scholar]
- 250.Liu Q, Tian S, Zhao C, Chen X, Lei I, Wang Z, Ma PX. Porous nanofibrous poly(L-lactic acid) scaffolds supporting cardiovascular progenitor cells for cardiac tissue engineering. Acta Biomater. 2015;26:105–14. doi: 10.1016/j.actbio.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Kim IG, Ko J, Lee HR, Do SH, Park K. Mesenchymal cells condensation-inducible mesh scaffolds for cartilage tissue engineering. Biomaterials. 2016;85:18–29. doi: 10.1016/j.biomaterials.2016.01.048. [DOI] [PubMed] [Google Scholar]
- 252.Xie M, Wang L, Ge J, Guo B, Ma PX. Strong electroactive biodegradable shape memory polymer networks based on star-shaped polylactide and aniline trimer for bone tissue engineering. ACS Appl Mater Interfaces. 2015;7:6772–81. doi: 10.1021/acsami.5b00191. [DOI] [PubMed] [Google Scholar]
- 253.Zhang XH, Zhang CG, Lin YH, Hu PH, Shen Y, Wang K, Meng S, Chai Y, Dai XH, Liu X, Liu Y, Mo XJ, Cao C, Li S, Deng XL, Chen LL. Nanocomposite Membranes Enhance Bone Regeneration Through Restoring Physiological Electric Microenvironment. ACS Nano. 2016;10:7279–86. doi: 10.1021/acsnano.6b02247. [DOI] [PubMed] [Google Scholar]
- 254.Tanaka T, Nishio I, Sun S-T, Ueno-Nishio S. Collapse of gels in an electric field. Science. 1982;218:467–9. doi: 10.1126/science.218.4571.467. [DOI] [PubMed] [Google Scholar]
- 255.Osada Y, Okuzaki H, Hori H. A polymer gel with electrically driven motility. Nature. 1992;355:242–4. [Google Scholar]
- 256.Yoshida R. Self-Oscillating Gels Driven by the Belousov–Zhabotinsky Reaction as Novel Smart Materials. Adv Mater. 2010;22:3463–83. doi: 10.1002/adma.200904075. [DOI] [PubMed] [Google Scholar]
- 257.Tang JX, Janmey PA. The polyelectrolyte nature of F-actin and the mechanism of actin bundle formation. J Biol Chem. 1996;271:8556–63. doi: 10.1074/jbc.271.15.8556. [DOI] [PubMed] [Google Scholar]
- 258.Kwon HJ, Gong JP. Negatively charged polyelectrolyte gels as bio-tissue model system and for biomedical application. Curr Opin Colloid Interface Sci. 2006;11:345–50. [Google Scholar]
- 259.Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337–51. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 260.Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA. Hydrogels in regenerative medicine. Adv Mater. 2009;21:3307–29. doi: 10.1002/adma.200802106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Fragonas E, Valente M, Pozzi-Mucelli M, Toffanin R, Rizzo R, Silvestri F, Vittur F. Articular cartilage repair in rabbits by using suspensions of allogenic chondrocytes in alginate. Biomaterials. 2000;21:795–801. doi: 10.1016/s0142-9612(99)00241-0. [DOI] [PubMed] [Google Scholar]
- 262.Bian L, Zhai DY, Tous E, Rai R, Mauck RL, Burdick JA. Enhanced MSC chondrogenesis following delivery of TGF-β3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials. 2011;32:6425–34. doi: 10.1016/j.biomaterials.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Meng SY, Rouabhia M, Zhang Z. Electrical Stimulation in Tissue Regeneration. In: Gargiulo GD, McEwan A, editors. Applied Biomedical Engineering. Rijeka Croatia: InTech; 2011. pp. 37–62. [Google Scholar]
- 264.Spearman BS, Hodge AJ, Porter JL, Hardy JG, Davis ZD, Xu T, Zhang X, Schmidt CE, Hamilton MC, Lipke EA. Conductive interpenetrating networks of polypyrrole and polycaprolactone encourage electrophysiological development of cardiac cells. Acta Biomater. 2015;28:109–20. doi: 10.1016/j.actbio.2015.09.025. [DOI] [PubMed] [Google Scholar]
- 265.Lee YS, Collins G, Arinzeh TL. Neurite extension of primary neurons on electrospun piezoelectric scaffolds. Acta Biomater. 2011;7:3877–86. doi: 10.1016/j.actbio.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 266.Tan GX, Wang SY, Zhu Y, Zhou L, Yu P, Wang XL, He TR, Chen JQ, Mao CB, Ning CY. Surface-Selective Preferential Production of Reactive Oxygen Species on Piezoelectric Ceramics for Bacterial Killing. ACS Appl Mater Interfaces. 2016;8:24306–9. doi: 10.1021/acsami.6b07440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Hackett AJ, Malmström J, Travas-Sejdic J. Functionalization of conducting polymers for biointerface applications. Prog Polym Sci. 2017;70:18–33. [Google Scholar]