Abstract
Background:
Impaired left atrial (LA) reservoir deformation has been found to be associated with poor functional capacity and outcomes in severe chronic mitral regurgitation (MR). Among patients with primary MR (valve incompetence due to mitral valve pathology), we focus on Carpentier II classification (prolapse or flail mitral valve) and aim to investigate determinants for decreased LA reservoir deformation and its impact on LA remodeling in severe MR.
Methods:
Among 159 consecutive patients with severe chronic Carpentier II MR (left ventricular ejection fraction ≥ 60%), 55 underwent follow-up echocardiography, which was compared with their baseline study. We used the change of LA volume index as the rapidity of LA remodeling, LA eccentricity index as LA sphericity, and peak LA reservoir strain as well as reservoir strain rate (LASRR) derived from two-dimensional speckle-tracking echocardiography as LA reservoir function.
Results:
Older age, elongated left atrium, increased LA volume index, as well as reduced left ventricular global longitudinal strain and LA ejection fraction all linked to a poor baseline LASRR (all p < 0.001). A second echocardiography during a mean follow-up of 15.3 ± 8.3 months revealed an enlarged left atrium (increased interval change of LA volume index; p < 0.001). In multivariate analysis, only the difference between the baseline and follow-up LASRR values (Δ: LASRR; odds ratio (OR) 0.037, 95% confidence interval (CI) 0.003–0.496, p = 0.013) predicted accelerated LA remodeling. A poor baseline LASRR was significantly associated with its profound deterioration during the follow-up period (β: = -0.424, p = 0.002).
Conclusion:
In severe chronic Carpentier II MR, a reduced follow-up LASRR predicted future accelerated LA remodeling. Patients with a poor baseline LASRR are at a higher risk of its deterioration.
KEYWORDS: left atrium, mitral regurgitation, speckle-tracking echocardiography, strain rate
Introduction
Severe chronic mitral regurgitation (MR) is characterized by excessive volume load, leading to serial cardiac adaptations [1].Among which, left atrial (LA) dilatation arises early, maintaining a balanced LA pressure while prohibiting pulmonary congestion. However, the rapidity of this compensatory process per se forecasts dire outcomes. LA volume index (LAVi), signifying LA remodeling, has been well acknowledged as a prognostic indicator with respect to atrial fibrillation (AF), stroke, and congestive heart failure [2,3,4]. Similarly, in primary MR (characterized by pathology involving mitral leaflets, chordae tendineae, papillary muscle, and annulus), LAVi is a harbinger of increased mortality and more cardiac events, including subsequent occurrence of AF [2,5,6,7]. As a matter of fact, identifying patients with severe primary MR who are at risk of accelerated LA remodeling is pivotal in guiding patient-tailored therapeutic strategy to improve their survival and restore their quality of life.
By contrast, the association between severe primary MR and impaired LA deformation assessed by two-dimensional (2D) speckle-tracking echocardiography (STE) has been demonstrated [8,9]. Changes of LA deformation mechanics, reflecting LA ultrastructural change, may occur before changes in LA dimension [10]. LA deformation indexes have been utilized in the prediction of functional capacity, future cardiac events, as well as postoperative survival in severe primary MR, suggesting their value in clinical practice [11,12,13]. It is of interest whether accelerated LA remodeling and LA deformation deterioration are closely interwoven.
Using 2D STE, phasic LA deformation can be evaluated, including the reservoir, conduit, and contractile functions. Notably, the prognostic implication of a reduced LA reservoir function, represented by decreased LA reservoir strain (LAεR) and strain rate (LASRR), has been recognized, assuring its value and importance in chronic MR [12,13,14]. During ventricular systole, LA strain (ɛ) and strain rate (SR) reflect LA expansibility and stiffness. Our previous studies found that deformation in the reservoir phase is associated with functional capacity and prognosis in severe chronic primary MR, especially for Carpentier II classification (mitral valve prolapse or flail) [12,13]. However, determinants of reservoir deformation have not been elucidated in this group. Hence, the current study aims to explore the (1) determinants of a poor LA reservoir function, (2) determinants of accelerated LA remodeling, and (3) interconnection between LA remodeling and LA reservoir mechanics in patients with chronic severe Carpentier II MR.
Methods
Study population
Figure 1 shows the study flow diagram of this prospective observational cohort study. Patients with chronic severe primary MR undergoing echocardiography in the outpatient clinic were screened between December 2010 and August 2013. We did not enroll patients with: (1) left ventricular ejection fraction (LVEF) < 60%; (2) New York Heart Association (NYHA) functional classification III or IV; (3) ischemic MR; (4) infiltrative cardiomyopathy; (5) coexistent aortic valve disease and mitral stenosis ≥ mild degree; (6) prior open heart surgery; and (7) congenital heart disease. Eighteen of 177 patients with chronic severe Carpentier II MR were excluded because of an anticipated mitral valve surgery at the time of the index echocardiography or inadequate image acquisition.
Figure 1.

Study flow diagram–dpatients included and excluded from the study. EF = ejection fraction; LV = left ventricular; LVEF = left ventricular ejection fraction; MR = mitral regurgitation; MV = mitral valve; NYHA = New York Heart Association functional classification;
Severe MR was diagnosed using a multiparametric approach via transthoracic echocardiography, including evaluation of the vena contracta width, effective regurgitant orifice area, regurgitant volume, and presence of systolic pulmonary venous flow reversal aligned with the European Association of Echocardiography criteria [15]. Patients were followed up (interval ≤3 months), by their original cardiovascular specialists, in the clinic where symptoms and signs of heart failure were carefully evaluated. Follow-up echocardiography was arranged based on the discretion of the care physician. The functional class at enrollment and past history were obtained from the medical records. The study adhered to the Declaration of Helsinki and received approval from the Human Research and Ethics Committee of National Cheng Kung University Hospital (A-ER-102-322).
Echocardiography
Standard transthoracic echocardiography was performed (Vivid 7; GE-VingMed, Horten, Norway) using a 3.5-MHz multiphase array probe in individuals respiring quietly at a left lateral decubitus position. Chamber dimension and wall thickness were measured by the 2D-guided M-mode method, and LVEF was measured by the 2D biplane method of discs [16]. Left ventricular (LV) mass was obtained by the M-mode-derived linear measurements based on modeling of the left ventricle as a prolate ellipse and indexed by the body surface area. The Doppler sample volume was placed at the tips of the mitral leaflets to obtain the LV inflow blood flow velocity waveforms on the apical four-chamber view. Pulse wave tissue Doppler imaging was obtained from the septal and lateral annulus from the apical four-chamber view, from which average value of early diastolic annulus velocity (e’) was derived. LV apical four-chamber, two-chamber, and long-axis views for consecutive three cardiac cycles were acquired at a high frame rate (50–90 frames/s). The mean global LV longitudinal systolic strain (GLS) was measured from three apical views by automated function imaging software, as we had reported in detail previously, with very low intra- and interobserver variability [17]. The ultrasonic data were analyzed offline using dedicated software EchoPac PC 09 (GE-VingMed).
Sphericity, volumetric, and deformation analyses of left atrium
The sphericity of the left atrium was assessed by the LA eccentricity index, with a larger or a smaller value indicating an elongated or a spherical left atrium, respectively [18]. The LA volume was measured using the 2D biplane area—length method [19] to obtain the maximal endsystolic LA volume (LAVs) and the minimal end-diastolic LA volume (LAVd). LA total emptying fraction (LAEF) was calculated as [(LAVs - LAVd)/LAVs] × 100%. LAVi was derived from LAVs indexed to the body surface area.
The method for LA deformation analysis by STE has been described in detail in our previous studies [18]. The endo-cardial border of the LA was manually defined using a point-and-click technique. After the region of interest (≥8 mm) was automatically generated, manual editing was done to fit the full thickness of the myocardium. Then, a cine loop preview was used to confirm the appropriateness of the region of interest throughout the cardiac cycle. By dividing the LA wall into eight segments (basal septal, middle septal, basal lateral, and middle lateral segments on a 4-chamber view; basal inferior, middle inferior, basal anterior, and middle anterior segments on a 2-chamber view), we recorded the peak value from the time—strain and time—strain rate plots for each individual segment (QRS-timed analysis according to the electrocardiogram). The average value from eight segments were used for analysis (LAεR and LASRR; Figure 2). The measurements were repeated three times for each individual, and the average was used for analysis.
Figure 2.
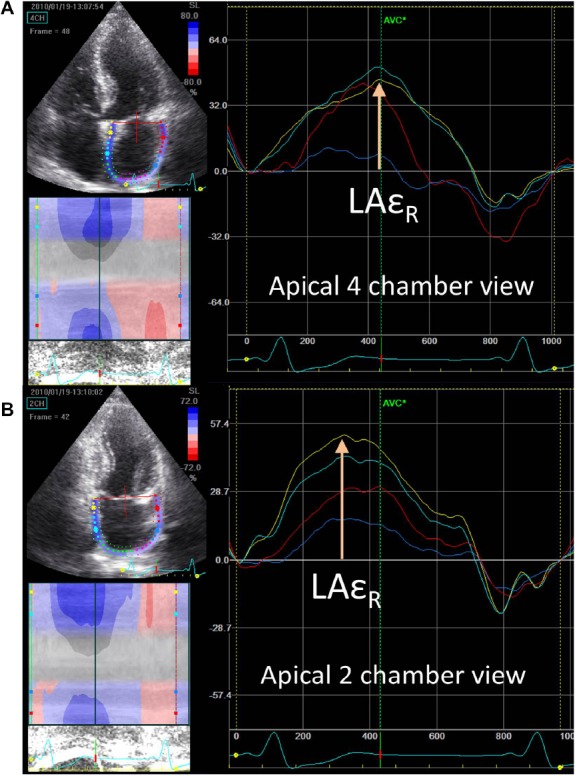
Left atrial strain curve derived from speckle-tracking echocardiographyd–the peak reservoir strain (LAεR) can be obtained from both apical four-chamber and apical two-chamber views. The white arrows indicate LAεR of the (A) basal septal and (B) basal inferior walls. LAεR = left atrial reservoir strain.
Reproducibility
Two independent investigators performed the analysis. Twenty patients were randomly selected for the evaluation of the interobserver variability of LAεR and LASRR measurements by two independent observers. As for intra-observer variability, the same measurements were repeated 1 week apart. The mean percentage error was calculated as the absolute difference divided by the average of the two observations.
Statistical analysis
All data were presented as mean ± standard deviation unless otherwise stated.
Using the independent t test for categorical variables and Pearson’s correlation coefficients for continuous variables, we identified factors correlated with a poor LA reservoir function (decreased LAR and LASRR) and the interval change of LASRR (Δ LASRR = follow-up LASRR - baseline LASRR; a minus value indicated declined LA reservoir function). Multiple linear regression analysis was performed to identify the determinants of LAεR or LASRR.
The median value of ALAVi (follow-up LAVi - baseline LAVi) was used to stratify patients into groups with or without accelerated LA remodeling. Using the chi-square (χ2) test for categorical variables and independent-sample t test for continuous variables, we identified factors (p < 0.05) related to accelerated LA remodeling. Then, we conducted multivariate logistic regression analysis by adjusting for significant [age, E/e’, pulmonary arterial systolic pressure (PASP), and ALASRR] and important factors (AF, LVEF, and LAVi). A p value < 0.05 was considered to be statistically significant. All analyses were performed with SPSS 18.0 for Windows (SPSS Institute, Chicago, IL, USA).
Results
A total of 159 patients (mean age 57.2 ± 15.7 years, 68% men) with Carpentier II severe MR formed our study group (Figure 1, Table 1). All of them were asymptomatic (NYHAI) or mildly symptomatic (NYHA II) who refused operation. Among them, 55 patients (mean age 56.2 ± 15.8 years, 57% men) underwent a second nonpostoperative follow-up echocardiography with the same vendor at a mean period of 15.3 ± 8.3 months (interquartile range 8—20 months), from which analysis for LA remodeling was performed. This subgroup (n = 55) and the remainder of the patients (n = 104) were alike with regard to clinical characteristics, chamber dimension, LAEF, LVEF, LAVi, LV mass index, LV GLS, and the severity of MR. By comparison of the baseline and follow-up echocardiography, severe MR rendered a progressively enlarged left atrium represented by increased LAVi(48.6 ± 33.1 mL/m2 vs. 64.8 ± 67.1 mL/m2, p < 0.001).
Table 1.
Baseline characteristics of 159 patients.
| Variable | |
|---|---|
| Age (y) | 57.3 ± 15.7 |
| Men | 108 (68) |
| Heart rate (beats/min) | 75.1 ± 14.3 |
| Hypertension | 93 (58) |
| Diabetes mellitus | 17 (11) |
| Atrial fibrillation | 25 (16) |
| Coronary artery disease | 10 (6) |
| Smoking | 21 (13) |
| Hyperlipidemia | 58 (36) |
| LVEDD (cm) | 5.7 ± 0.6 |
| LVESD (cm) | 3.3 ± 0.6 |
| LVESDi (cm/m2) | 1.9 ± 0.4 |
| LVMi (g/m2) | 115.7 ±31.0 |
| LVEF (%) | 72.7 ±6.7 |
| LV GLS (%) | -20.4 ±4.7 |
| E/e′ | 12.1 ±4.0 |
| LAVi (mL/m2) | 48.6 ±33.1 |
| LAEi | 1.2 ± 0.2 |
| LAEF (%) | 44.6 ± 14.7 |
| LAεR (%) | 27.5 ± 10.4 |
| LASRR (1/s) | 2.25 ±0.64 |
| PASP (mmHg) | 37.2 ±19.0 |
| EROA (cm2) | 0.92 ±0.6 |
| Regurgitant fraction (%) | 58.1 ± 17.8 |
| Regurgitant volume (mL/beat) | 125.9 ±66.0 |
Data are expressed as mean ± SD or n (%).
E/e′ = early transmitral velocity to tissue Doppler mitral annular early diastolic velocity ratio; EROA = effective regurgitant orifice area; LAEF = left atrial ejection fraction; LAEi - = left atrial eccentricity index; LAVi = left atrial volume index; LAɛR = left atrial reservoir strain; LASRR = left atrial reservoir strain rate; LVEDD = left ventricular end-diastolic dimension; LVEF = left ventricular ejection fraction; LVESD = left ventricular end-systolic dimension; LVESDi = left ventricular endsystolic dimension index; LV GLS = global left ventricular peak systolic longitudinal strain; LVMi = left ventricular volume index; PASP = pulmonary arterial systolic pressure; SD = standard deviation.
Determinants of impaired LA reservoir function
Correlates for impaired LA reservoir function (denoted by both LAεR and LASRR) are the presence of AF, hypertension, older age, elevated E/e’, LV mass index, LAVi, pulmonary pressure, decreased GLS and LAEF, as well as more elongated left atrium (Table 2). Patients with NYHA I without diabetes mellitus had better LAεR but not LASRR (Table 2). After multivariate regression analysis, independent factors for impaired baseline LAεR and LASRR are older age, elongated left atrium, and declined LAEF (Table S1). In addition, depressed GLS and increased LAVi were related to a diminished LASRR.
Table 2.
Correlates for decreased left atrial reservoir function.
| LA ɛR | LASRR | ||
|---|---|---|---|
| Continuous variables | Pearson correlation | coefficient (r) | |
| Age | −0.507** | −0.392** | |
| Heart rate | −0.146 | −0.090 | |
| LVESDi | -0.018 | −0.007 | |
| LVEF | 0.019 | 0.071 | |
| LV GLS | −0.379** | −0.462** | |
| LVMi | −0.285** | −0.394** | |
| E/e′ | −0.373** | −0.235** | |
| LAEi | −0.292** | −0.257** | |
| LAEF | 0.514** | 0.602** | |
| LAVi | −0.287** | −0.463** | |
| PASP | −0.341** | −0.319** | |
| EROA | −0.071 | −0.091 | |
| Regurgitant volume | −0.118 | −0.190 | |
| Regurgitant fraction | 0.107 | 0.042 | |
| Categorical variables | |||
| Atrial fibrillation | yes | 19.7 ± 10.3*** | 1.68 ± 0.6*** |
| No | 28.9 ± 9.8 | 2.35 ± 0.6 | |
| NYHA | |||
| I (n = 71) | 29.5 ± 10.6* | 2.26 ± 0.6 | |
| II (n = 88) | 25.9 ±10.1 | 2.24 ± 0.7 | |
| Diabetes | Yes | 21.2 ± 7.7** | 1.97 ± 0.5 |
| Mellitus | No | 28.4 ± 10.5 | 2.30 ± 0.6 |
| Hypertension | Yes | 24.8 ± 9.2*** | 2.11 ± 0.6** |
| No | 31.2 ± 11.0 | 2.44 ± 0.7 |
* p < 0.05.
** p < 0.01.
*** p < 0.001.
E/e′ = early transmitral velocity to tissue Doppler mitral annular early diastolic velocity ratio; EROA = effective regurgitant orifice area; LAEF= left atrial ejection fraction; LAEi - = left atrial eccentricity index; LAVi = left atrial volume index; LA ɛR= left atrial reservoir strain; LVEF = left ventricular ejection fraction; LVESDi = left ventricular end-systolic dimension index; LV GLS = global left ventricular peak systolic longitudinal strain; LVMi = left ventricular volume index; NYHA = New York Heart Association functional classification; PASP = pulmonary arterial systolic pressure.
Differences between patients with or without accelerated LA remodeling
The clinical characteristics did not differ significantly between the patients with or without accelerated LA remodeling (ΔLAVi ≥ median value) except for age (Table 3). As for the echocardiographic parameters, elevated PASP, E/e’, and negative Δ LASRR (implying a deteriorated LASRR during follow-up) were associated with more rapid LA remodeling. After multivariate logistic regression analysis controlling age, status of AF, LVEF, LAVi, E/e’, and PASP, only ΔLASRr (odds ratio 0.037, 95% confidence interval 0.003—0.496, p = 0.013) exhibited a strong correlation with accelerated LA remodeling (Table 4).
Table 3.
Clinical and echocardiographic parameters between patients with or without accelerated LA remodeling.
| Available follow-up echo N = 55 | Rapid LA remodeling N = 27 | Slower LA remodeling N = 28 | |
|---|---|---|---|
| Age (y) | 56.2 ± 15.8 | 60.6 ± 17.3* | 51.2 ± 12.3 |
| Men | 34 (57) | 14 (52) | 19 (68) |
| Hypertension | 34 (57) | 14 (52) | 18 (64) |
| Diabetes mellitus | 6 (12) | 2 (7) | 3 (11) |
| Atrial fibrillation | 6 (10) | 5 (19)* | 1 (4) |
| Coronary artery disease | 4 (7) | 2 (7) | 2 (7) |
| Hyperlipidemia | 26 (43) | 10 (37) | 15 (54) |
| NYHA I | 31 (52) | 11 (41) | 14 (50) |
| Heart rate (beats/min) | 73.6 ± 9.6 | 71.6 ± 10.9 | 75.4 ± 7.4 |
| LVEDD (mm) | 5.8 ± 0.7 | 5.7 ± 0. | 5.8 ± 0.5 |
| LVESD (mm) | 3.4 ± 0.6 | 3.2 ± 0.5 | 3.4 ± 0.5 |
| LVESDi (mL/m2) | 2.0 ± 0.3 | 1.9 ± 0.3 | 1.9 ± 0.3 |
| LVEF (%) | 72.7 ± 7.4 | 74.1 ± 8.3 | 71.4 ± 6.6 |
| LV GLS (%) | −20.6 ± 4.2 | −20.4 ± 5.0 | −20.7 ± 3.5 |
| LVMi (g/m2) | 114.3 ± 32.5 | 121.3 ± 39.3 | 106.2 ± 22.8 |
| E/e′ | 12.0 ± 4.2 | 13.4 ± 4.5* | 10.6 ± 3.1 |
| LAVi (mL/m2) | 49.5 ± 46.6 | 58.2 ± 63.5 | 40.7 ± 15.5 |
| LAEi | 1.2 ± 0.1 | 1.24 ± 0.15 | 1.16 ± 0.13 |
| LAEF (%) | 45.5 ± 15.5 | 41.5 ± 17.4 | 49.4 ± 11.1 |
| LA ɛR (%) | 29.1 ± 9.6 | 26.4 ± 11.0 | 31.2 ± 7.7 |
| LASRR (1/s) | 2.39 ± 0.55 | 2.30 ± 0.66 | 2.40 ± 0.41 |
| ΔLA′RɛR (%) | −0.88 ± 8.4 | −1.41 ± 7.78 | −1.35 ± 9.03 |
| ΔLASRR (1/s) | −0.15 ± 0.62 | −0.41 ± 0.45* | 0.14 ± 0.59 |
| PASP (mmHg) | 32.6 ± 16.0 | 38.0 ± 18.0* | 26.3 ± 10.1 |
| EROA (cm2) | 0.89 ± 0.4 | 0.74 ± 0.39 | 0.97 ± 0.49 |
| Regurgitant fraction (%) | 56.9 ± 16.5 | 53.7 ± 17.3 | 59.8 ± 14.9 |
| Regurgitant volume (mL/beat) | 121.3 ± 62.4 | 110.2 ± 52.9 | 126.1 ± 75.1 |
The symbol Δ represents the change between baseline and follow-up echocardiography (follow-up minus baseline parameters) * p < 0.05 between those with and without accelerated LA remodeling.
E/e′ = early transmitral velocity to tissue Doppler mitral annular early diastolic velocity ratio; EROA = effective regurgitant orifice area; LA = left atrial; LAEF = left atrial ejection fraction; LAEi = left atrial eccentricity index; LAVi = left atrial volume index; LA ɛR = left atrial reservoir strain; LASRR = left atrial reservoir strain rate; LVEDD = left ventricular end-diastolic dimension; LVEF = left ventricular ejection fraction; LVESD = left ventricular end-systolic dimension; LVESDi = left ventricular end-systolic dimension index; LV GLS = global left ventricular peak systolic longitudinal strain; LVMi = left ventricular volume index; NYHA = New York Heart Association functional classification; PASP = pulmonary arterial systolic pressure.
Table 4.
Multivariate logistic regression analysis for determinants of accelerated left atrial remodeling.
| OR (95% CI) | p | |
|---|---|---|
| Age (y) | 0.985 (0.916e1−058) | 0.671 |
| Atrial fibrillation | 7.079 (0.514e97−471) | 0.144 |
| E/e′ | 1.001 (0.771e1−300) | 0.995 |
| PASP (mmHg) | 1.088 (0.989e1−196) | 0.082 |
| LVEF(%) | 1.082(0.955e1−225) | 0.216 |
| LAVi (mL/m2) | 1.007 (0.978e1−037) | 0.627 |
| ΔLASR (1/s) | 0.037 (0.003e0−496) | 0.013 |
The symbol Δ represents the change between baseline and follow-up echocardiography (follow-up minus baseline parameters).
CI = confidence interval; E/e′ = early transmitral velocity to tissue Doppler mitral annular early diastolic velocity ratio; LAVi = left atrial volume index; LASRR = left atrial reservoir strain rate; LVEF = left ventricular ejection fraction; OR = odds ratio; PASP = pulmonary arterial systolic pressure.
Determinants of worse LASRR during follow-up
Table S2 shows factors that were related to hastened LASRR deterioration during the follow-up period. Patients with a lower baseline LASRR (r= -0.424; p= 0.002) were more vulnerable to such aggravation.
The intra- and interobserver mean percentage errors for LAεR were 2.02% and 7.9%, respectively. As for LASRR, the intra- and inter-observer mean percentage errors were 2.0% and 2.4%, respectively.
Discussion
In chronic severe primary MR, we demonstrated that older age, declined LAEF, depressed GLS, elongated left atrium, and increased LAVi, are independent factors for a diminished LASRR. A lower baseline LASRR leads to its faster reduction and is able to predict accelerated LA remodeling.
LA reservoir function and its determinants in severe MR
Using 2D STE LA deformation analysis, we are able to evaluate LA phasic function (reservoir, conduit, and active contractile functions). The reservoir indexes (LAεR and LASRR) are most commonly utilized [14,20]. In severe primary MR, reservoir function has prognostication in the occurrence of heart failure, paroxysmal AF, and postoperative outcomes (survival, LA reverse remodeling, and postoperative AF) [11,12,13,14,21,22,23,24,25]. LA reservoir function reflects atrial resilience and elasticity, and is influenced by the loading condition and longitudinal function of the left ventricle. Therefore, it is not surprising that reservoir parameters closely link to other LA parameters (LAEF and LAVi) and LV GLS. Interestingly, we showed that an elongated left atrium is related to a lower reservoir function, while Maiello et al [18] showed that a spherical left atrium is associated with a poor outcome (higher risk of AF). More studies are needed before drawing a definitive conclusion regarding the impact of LA sphericity in severe MR.
LA remodeling in severe MR
In the present study, patients may be in a relatively early stage of severe MR (average LAVi <60 mL/m2). Their left atrium enlarged as MR proceeded. This phenomenon is a compensatory response and may indicate a poor outcome in severe MR. LAVi is a known predictor of mortality, cardiac events, as well as postoperative LA reverse remodeling [2,26,27]. It is utilized to guide surgical timing to improve life expectancy and outcomes [2]. Consequently, it is important to recognize contributors for accelerated LA remodeling. We showed that rapid LA remodeling is associated with high pulmonary pressure and E/e’, suggesting its general link to volume overload in MR [2]. However, only the reduction of reservoir function (a negative Δ LASRR) closely links to rapid LA remodeling in the follow-up period. An LASRR may, therefore, serve as an early marker to identify patients at risk of rapid LA enlargement.
Influence of LA reservoir function on accelerated LA remodeling
Our findings suggest that in severe primary MR, LA reservoir function forecasts a rapid expansion of the left atrium, while baseline LAVi and other currently used parameters (age, AF, LVEF, GLS, and PASP) do not. Histologically, impaired LA deformation in severe MR is associated with atrial interstitial fibrosis, resulting in impaired LA elastance and compliance [24]. Hence, it is likely that patients with poor reservoir deformation are prone to having accelerated LA remodeling. This early and subtle ultrastructural and histological LA change could be best detected by a sensitive tool such as deformation analysis, but not by volumetric and sphericity parameters. To the best of our knowledge, this is the first report of this interesting finding.
Clinical implications
The current study shows a close relationship between baseline LA reservoir function and future accelerated LA remodeling in chronic severe primary MR. In asymptomatic or mildly symptomatic patients choosing a wait-and-see strategy, LA reservoir deformation analysis may provide additional information for physicians to determine optimal surgical timing. Factors contributing to a poor baseline reservoir function may also serve as warning signs and could be considered in the follow-up assessment of chronic severe MR. To extend the generalizability, future outcome studies incorporating LA reservoir indexes in conjunction with existing markers in asymptomatic severe primary MR are of interest.
Study limitations
This is a single-center study with a relatively small case number. We included patients with mild symptoms (NYHA II) and AF; however, this population is closer to the real-world circumstances. In addition, functional classification and AF status showed no significance in the analysis. Besides, we used only LA reservoir indexes, and disregarded conduit and contractile parameters. Second, the exact duration of MR was not known due to difficulties in verifying true exposure. However, in patients with or without rapid LA remodeling, there were no significant differences regarding parameters for MR severity as well as LA and LV size. It may imply a comparable duration of severe MR. Large multicenter studies are needed to confirm our results in severe primary MR.
Conclusion
Among patients with severe chronic Carpentier II MR and preserved LVEF, an attenuated LASRR during follow-up is associated with accelerated LA remodeling. Patients with a poor baseline LASRR are more vulnerable to its future deterioration.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jmu.2016.09.003.
Footnotes
Conflicts of interest: There is no conflict of interest.
References
- [1].Kihara Y, Sasayama S, Miyazaki S, et al. Role of the left atrium in adaptation of the heart to chronic mitral regurgitation in conscious dogs. Circ Res. 1988;62:543–53. doi: 10.1161/01.res.62.3.543. [DOI] [PubMed] [Google Scholar]
- [2].Le Tourneau T, Messika-Zeitoun D, Russo A, et al. Impact of left atrial volume on clinical outcome in organic mitral regurgitation. J Am Coll Cardiol. 2010;56:570–8. doi: 10.1016/j.jacc.2010.02.059. [DOI] [PubMed] [Google Scholar]
- [3].Tsang TS, Barnes ME, Bailey KR, et al. Left atrial volume: important risk marker of incident atrial fibrillation in 1655 older men and women. Mayo Clin Proc. 2001;76:467–75. doi: 10.4065/76.5.467. [DOI] [PubMed] [Google Scholar]
- [4].Takemoto Y, Barnes ME, Seward JB, et al. Usefulness of left atrial volume in predicting first congestive heart failure in patients >or Z 65years of age with well-preserved left ventricular systolic function. Am J Cardiol. 2005;96:832–6. doi: 10.1016/j.amjcard.2005.05.031. [DOI] [PubMed] [Google Scholar]
- [5].Messika-Zeitoun D, Bellamy M, Avierinos JF, et al. Left atrial remodeling in mitral regurgitationdmethodologic approach, physiological determinants, and outcome implications: a prospective quantitative Doppler-echocardiographic and electron beam-computed tomographic study. Eur Heart J. 2007;28:1773–81. doi: 10.1093/eurheartj/ehm199. [DOI] [PubMed] [Google Scholar]
- [6].Grigioni F, Avierinos JF, Ling LH, et al. Atrial fibrillation complicating the course of degenerative mitral regurgitation: determinants and long-term outcome. J Am Coll Cardiol. 2002;40:84–92. doi: 10.1016/s0735-1097(02)01922-8. [DOI] [PubMed] [Google Scholar]
- [7].Kernis SJ, Nkomo VT, Messika-Zeitoun D, et al. Atrial fibrillation after surgical correction of mitral regurgitation in sinus rhythm: incidence, outcome, and determinants. Circulation. 2004;110:2320–5. doi: 10.1161/01.CIR.0000145121.25259.54. [DOI] [PubMed] [Google Scholar]
- [8].Cameli M, Lisi M, Giacomin E, et al. Chronic mitral regurgitation: left atrial deformation analysis by two-dimensional speckle tracking echocardiography. Echocardiography. 2011;28:327–34. doi: 10.1111/j.1540-8175.2010.01329.x. [DOI] [PubMed] [Google Scholar]
- [9].Moustafa SE, Alharthi M, Kansal M, et al. Global left atrial dysfunction and regional heterogeneity in primary chronic mitral insufficiency. Eur J Echocardiogr. 2011;12:384–93. doi: 10.1093/ejechocard/jer033. [DOI] [PubMed] [Google Scholar]
- [10].Mondillo S, Cameli M, Caputo ML, et al. Early detection of left atrial strain abnormalities by speckle-tracking in hypertensive and diabetic patients with normal left atrial size. J Am Soc Echocardiogr. 2011;24:898–908. doi: 10.1016/j.echo.2011.04.014. [DOI] [PubMed] [Google Scholar]
- [11].Debonnaire P, Leong DP, Witkowski TG, et al. Left atrial function by two-dimensional speckle-tracking echocardiography in patients with severe organic mitral regurgitation: association with guidelines-based surgical indication and postoperative (long-term) survival. J Am Soc Echocardiogr. 2013;26:1053–62. doi: 10.1016/j.echo.2013.05.019. [DOI] [PubMed] [Google Scholar]
- [12].Yang LT, Shih JY, Liu YW, et al. Effects of left atrial strain on functional capacity in chronic severe mitral regurgitation. Int J Cardiol. 2013;168:e151–3. doi: 10.1016/j.ijcard.2013.08.070. [DOI] [PubMed] [Google Scholar]
- [13].Yang LT, Liu YW, Shih JY, et al. Predictive value of left atrial deformation on prognosis in severe primary mitral regurgitation. J Am Soc Echocardiogr. 2015;28:1309–17. doi: 10.1016/j.echo.2015.07.004. [DOI] [PubMed] [Google Scholar]
- [14].Vieira MJ, Teixeira R, Gonc¸alves L, et al. Left atrial mechanics: echocardiographic assessment and clinical implications. J Am Soc Echocardiogr. 2014;27:463–78. doi: 10.1016/j.echo.2014.01.021. [DOI] [PubMed] [Google Scholar]
- [15].Lancellotti P, Moura L, Pierard LA, et al. Part 2: mitral and tricuspid regurgitation (native valve disease) Eur J Echocardiogr. 2010;11:307–32. doi: 10.1093/ejechocard/jeq031. [DOI] [PubMed] [Google Scholar]
- [16].Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- [17].Tsai WC, Liu WY, Huang YY, et al. Diagnostic value of segmental longitudinal strain by automated function imaging in coronary artery disease without left ventricular dysfunction. J Am Soc Echocardiogr. 2010;23:1183–9. doi: 10.1016/j.echo.2010.08.011. [DOI] [PubMed] [Google Scholar]
- [18].Maiello M, Sharma RK, Matteo CM, et al. Differential left atrial remodeling in LV diastolic dysfunction and mitral regurgitation. Echocardiography. 2009;26:772–8. doi: 10.1111/j.1540-8175.2008.00889.x. [DOI] [PubMed] [Google Scholar]
- [19].Ujino K, Barnes ME, Cha SS, et al. Two-dimensional echocardiographic methods for assessment of left atrial volume. Am J Cardiol. 2006;98:1185–8. doi: 10.1016/j.amjcard.2006.05.040. [DOI] [PubMed] [Google Scholar]
- [20].Ersbøll M, Andersen MJ, Valeur N, et al. The prognostic value of left atrial peak reservoir strain in acute myocardial infarction is dependent on left ventricular longitudinal function and left atrial size. Circ Cardiovasc Imaging. 2013;6:26–33. doi: 10.1161/CIRCIMAGING.112.978296. [DOI] [PubMed] [Google Scholar]
- [21].Shih JY, Tsai WC, Huang YY, et al. Association of decreased left atrial strain and strain rate with stroke in chronic atrial fibrillation. J Am Soc Echocardiogr. 2011;24:513–9. doi: 10.1016/j.echo.2011.01.016. [DOI] [PubMed] [Google Scholar]
- [22].Candan O, Ozdemir N, Aung SM, et al. Atrial longitudinal strain parameters predict left atrial reverse remodeling after mitral valve surgery: a speckle tracking echocardiography study. Int J Cardiovasc Imaging. 2014;30:1049–56. doi: 10.1007/s10554-014-0433-9. [DOI] [PubMed] [Google Scholar]
- [23].Candan O, Ozdemir N, Aung SM, et al. Left atrial longitudinal strain parameters predict postoperative persistent atrial fibrillation following mitral valve surgery: a speckle tracking echocardiography study. Echocardiography. 2013;30:1061–8. doi: 10.1111/echo.12222. [DOI] [PubMed] [Google Scholar]
- [24].Cameli M, Lisi M, Righini FM, et al. Usefulness of atrial deformation analysis to predict left atrial fibrosis and endocardial thickness in patients undergoing mitral valve operations for severe mitral regurgitation secondary to mitral valve prolapse. Am J Cardiol. 2013;111:595–601. doi: 10.1016/j.amjcard.2012.10.049. [DOI] [PubMed] [Google Scholar]
- [25].Cameli M, Lisi M, Righini FM, et al. Left atrial speckle tracking analysis in patients with mitral insufficiency and history of paroxysmal atrial fibrillation. Int J Cardiovasc Imaging. 2012;28:1663–70. doi: 10.1007/s10554-011-9987-y. [DOI] [PubMed] [Google Scholar]
- [26].Marsan NA, Maffessanti F, Tamborini G, et al. Left atrial reverse remodeling and functional improvement after mitral valve repair in degenerative mitral regurgitation: a real-time 3-dimensional echocardiography study. Am Heart J. 2011;161:314–21. doi: 10.1016/j.ahj.2010.10.029. [DOI] [PubMed] [Google Scholar]
- [27].Hylle´n S, Nozohoor S, Meurling C, et al. Determinants of left atrial reverse remodeling after valve surgery for degenerative mitral regurgitation. J Heart Valve Dis. 2013;22:2–10. [PubMed] [Google Scholar]


