Introduction
Prenatal investigation of the fetal venous system is becoming an increasingly important aspect of the examination of fetal circulation. This is because isolated venous anomalies can be identified not only as a lethal disease but also as a part of complex heart diseases or genetic syndromes. Among cyanotic heart diseases, total anomalous pulmonary venous connection (TAPVC) is the only condition involving a venous system malformation [1] and is easily misdiagnosed. The key characteristic of this uncommon congenital heart disease (CHD) is that all four pulmonary veins (PVs) fail to form a direct connection to the left atrium (LA); instead, they drain into the right heart through different routes of systemic venous return. The incidence of this condition is approximately 7–9 per 100,000 live births [2,3], and it accounts for 0.7–1.5% of all CHDs [4]. Patients with CHD who were born from 2000 to 2006 in Taiwan also exhibited a similar prevalence of TAPVC (0.11/1000) [5].
Over the past decades, TAPVC has been challenging for obstetricians to recognize in utero. According to a retrospective study of birth records from 1998 to 2004, only 1.9% of TAPVC (8 in 424) cases were prenatally diagnosed [6]. The diagnosis rate gradually improved through both awareness regarding the importance of assessing the venous system during routine screening and the application of new scanning technologies such as spatiotemporal image correlation (STIC) and color flow Doppler imaging. A prospective study identified 14 TAPVC fetuses before birth without missing single one (but one false positive reported) through a step-by-step, careful examination of one major and several minor sonographic features [7].
In addition to identifying echocardiographic marks related to a TAPVC diagnosis, understanding the embryonic development of the venous system is fundamental and crucial. This helps in recognizing abnormal features during anatomical screening and prenatal counseling.
Embryology
The fetal cardiovascular system is the first embryonically developed organ, which begins to develop at the gesta-tional age of 6 weeks (fourth week of embryonic development). The embryonic formation of the pulmonary venous system is not as complicated as that of the systemic venous circulation, which involves the fusion and degeneration of dual cardinal veins, umbilical veins, and vitelline veins. However, whether the PV becomes connected to the LA directly [8] or through the tributaries of the sinus venosus remains disputed [9].
The former theory prescribes that a single embryonic common PV evaginates from the dorsal wall of the LA and develops as an outgrowth of the LA, adjacent to the septum primum. Meanwhile, the lung buds that have arisen from the lung parenchyma canalize as a vessel and gradually connect to the developing PV directly [10]. Other experts have suggested that the pulmonary bud is initially enmeshed in the splanchnic plexus, which drains into the cardinal and umbilicovitelline veins (systemic venous system) [11]. By the gestational age of 4 weeks, the primordial common PV derived from the posterior LA connects to the pulmonary portion of the splanchnic plexus and forms the pulmonary plexus. Ultimately, the well-established pulmonary plexus loses its connection with the splanchnic plexus; in other words, the pulmonary venous system separates from the systemic veins [12]. The incorporated PV subsequently divides into four branches, two left and two right tributaries, with each having an orifice at the posterior LA.
Both theories are supported by different lines of evidence such as vascular markers or animal embryological studies [13,14]. The latter theory can offer a better understanding of the failure of PVs in emptying into the LA and for joining with the systemic venous system instead.
Definition and classification system
The normal pulmonary venous system consists of right and left pairs of PVs delivering the blood from both lungs to the LA. In patients with TAPVC, the anomalous veins either directly empty into the right atrium (RA) of the heart or empty through other routes of systemic venous return. Several classification systems based on anatomy, physiology, or perinatal outcomes exist.
Darling’s classification
Darling’s classification, first introduced in 1957, is the most commonly used system. TAPVC is classified into four categories according to the sites where the abnormal connection occurs [15] (Fig. 1).
Figure 1.
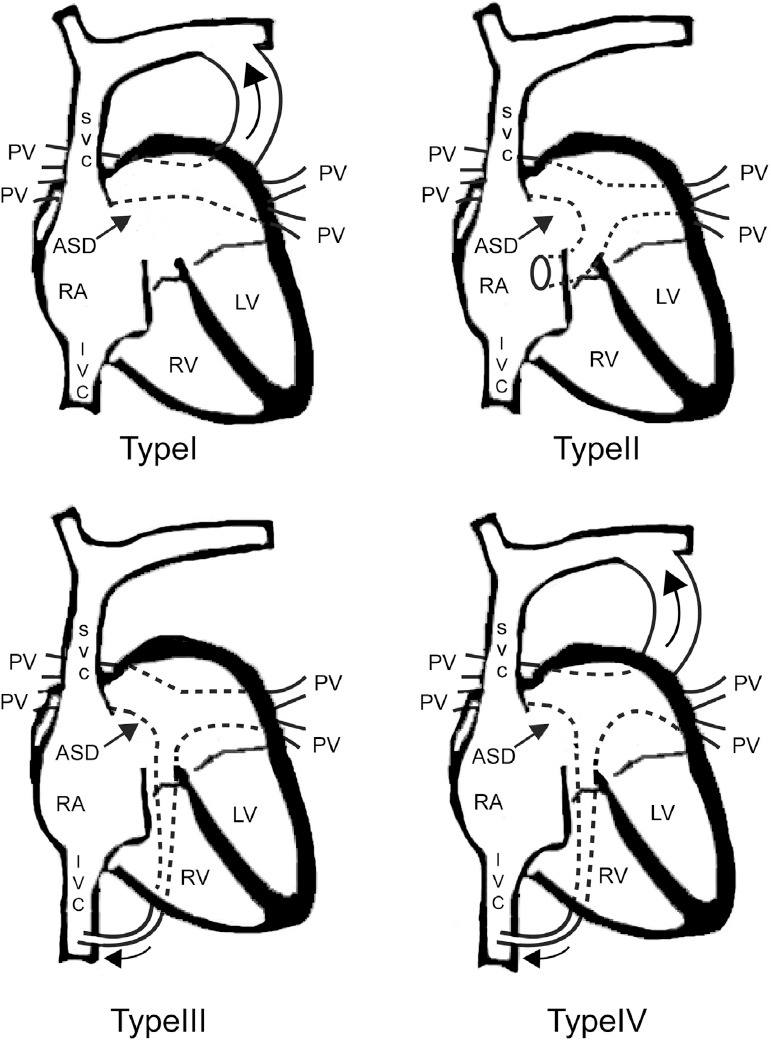
Darling classification of total anomalous pulmonary venous connection (TAPVC). Type I: supracardiac type. Four pulmonary veins connect to a vertical vein which subsequently drains into the left brachiocephalic vein and superior vena cava (curved arrow). Type II: intracardiac type. The pulmonary veins empty into the coronary sinus or directly into the right atrium. Type III: infracardiac type. The pulmonary veins connect to a vertical vein passes through diaphragm. The pulmonary flow ultimately drains to the systemic venous system, such as portal vein or inferior vena cava (curved arrow). Type IV: mixed type. ASD, atrial septum defect; LV, left antrum; IVC, inferior vena cava; PV, pulmonary vein; RA, right antrum; SVC, superior vena cava.
Type I: Supracardiac type, which is the most common type. The entrance of the pulmonary blood flow into the systemic venous system is cranial to the RA. This accounts for 45–55% of TAPVC cases, in which the confluent vessel usually empties into the innominate vein or the right or left superior vena cava (SVC) [16,17]. Type II: Cardiac type, which is diagnosed when the PVs converge on a confluent vessel and then horizontally connect to the RA through the coronary sinus (CS) or at the posterior wall of the RA. Approximately 20–30% of TAPVC patients exhibit the cardiac type. Type III: Infracardiac type. The PVs conjoin and form a vertical vessel that travels caudally into the portal vein or its branches such as the ductus venosus, hepatic vein, and inferior vena cava (IVC). This type accounts for 13–25% of cases. Type IV: Mixed type. Less than 10% patients belong to this subtype, in which the right and left pulmonary tributaries drain at two or more different levels. In Taiwan, the frequency of these four types are 42.3% (Type I), 39.8% (Type II), 12.8% (Type III), and 5.1% (Type IV), according to a 15-year cohort study [18].
Smith classification
TAPVC cases are simply subdivided into two groups, supradiaphragmatic without pulmonary venous obstruction and infradiaphragmatic with pulmonary venous obstruction. The distinct features of these two groups are how the abnormal drainage anastomosis is related to the diaphragm and the presence of venous obstruction [19].
Although supradiaphragmatic TAPVC is mainly non-obstructive, obstructions have been noted in some cases [20]. Nevertheless, the infradiaphragmatic type is almost always obstructive.
Another classification system
Herlong and colleagues announced a more detailed classification system. This system is fundamentally related to the anatomical and physiological changes in TAPVC, which involve the following parameters: (1) the level of connections: supracardiac, cardiac, infracardiac, and mixed; (2) presence or absence of obstruction; and (3) cause of obstruction: extrinsic, intrinsic, or obstructive atrial septal communication [21].
Pathophysiology and presentation
The hemodynamic changes in TAPVC primarily result from the mixing of oxygen-rich blood from the pulmonary system and deoxygenated blood from the systemic venous circulation. This leads to cyanosis and hypoxia in neonates. Hence, TAPVC is the fifth common cause of cyanotic heart disease.
Several essential factors have a considerable effect on the pathophysiology and presentation of TAPVC. The presence or absence of obstruction at any level of the venous route is the most critical factor. Obstructions may occur in different situations: (1) the confluent vein passing through tissue, which causes extrinsic compression, similar to that by intrathoracic structures (supracardiac type) or at the entry of the diaphragm (infracardiac type); (2) intrinsic compression resulting from narrowing of the lumen; and (3) at the site where the confluent blood enters the route of systemic venous return.
The infracardiac type is almost always associated with obstruction, which usually occurs when the confluent vein vertically enters the diaphragm through the esophageal orifice. The intracardiac type is rarely associated with obstruction. However, obstruction still can be detected at the CS or at the entry to the RA. Half of the supracardiac TAPVC cases are associated with venous obstruction.
Furthermore, the lumen narrowing of the left innominate vein, SVC, or azygos vein can lead to obstructive TAPVC. In addition, passing of the vertical vein between the left pulmonary artery and left bronchus, leading to external compression, is a possible cause of obstruction [17].
Among patients with unobstructed TAPVC, the size of interatrial communication plays an important role. After birth, pulmonary resistance decreases and an adequate amount of blood enters the pulmonary bed for adequate oxygen exchange. The mixture of saturated and desatu-rated blood occurs at the RA. In cases of nonrestrictive interatrial communication (such as a large atrial septal defect [ASD]), the blood enters the left heart and supplies the systemic circulation of infants. In spite of the right-to-left shunt, 3–5 times more blood enters the pulmonary bed and increases the pulmonary artery pressure gradually. Overcirculation leads to right ventricular hypertrophy, right heart failure, and subsequent desaturation in neonates.
In the obstructed types, high pulmonary venous pressure leads to an increase of hydrostatic pressure in the capillaries, leading to the development of pulmonary edema. Simultaneously, the elevated pulmonary artery pressure results in insufficient pulmonary flow. Severe desaturation occurs without immediate relief of the obstructed vessel.
The presentation of TAPVC varies widely and depends on the severity of the obstruction and the resistance of the pulmonary vessels. If severe obstruction is present, acute illness with tachypnea, tachycardia, dyspnea, hypoxemia, and metabolic acidosis manifests as early as within the first 12 h of life. Early death occurs within the first few days if surgical correction cannot be performed. At the other end of the spectrum, patients without venous obstruction are usually asymptomatic at birth, followed by the development of tachypnea, mild cyanosis, and feeding difficulties in the first few weeks. Profound failure to thrive and recurrent respiratory tract infection are noticed gradually, and only a small number of patients can survive up to late childhood or adolescence without treatment [22].
Associated anomalies and genetic mechanism
Approximately 30% TAPVC cases are associated with het-erotaxy syndrome, according to an analysis of patients receiving postnatal surgical repair at a hospital [23]. This is commonly observed in cases of right atrial isomerism (RAI), owing to the lack of a functional LA for PV connection. In non-heterotaxy cases, TAPVC can be detected as an isolated anomaly or with other complex heart/great vessel lesions such as atrioventricular septal defect, transposition of the great arteries, pulmonary stenosis, double outlet right ventricle, and coarctation of the aorta. When TAPVC is diagnosed through prenatal echocardiography, a trend of more fetuses accompanied with heterotaxy syndrome or complex CHD and less fetuses with isolated TAPVC has been noted [24]. TAPVC genetic etiology is remaining vogue and previous study reported some possible disease-driven genes (e.g., ACVRL1, SGCD, 4p13-q12, ANKRD1, etc.) by whole-exome sequencing and linkage mapping with polymorphic microsatellite markers [25].
Perinatal outcomes
An increasing trend in TAPVC research has been observed in the last decade, and most of these studies are related to the postnatal management, radiological diagnosis, and surgical outcomes of TAPVC [26,27,28]. The surgical outcomes have considerably improved from a mortality rate of 42.1% in 1970 to 7.4% after 2010 [29]. Despite the advances in perioperative cardiovascular care, TAPVC remains one of the true surgical emergencies after diagnosis. The goal of surgical repair is to establish the normal anastomosis of the PVs to the LA. Patients with heterotaxy syndrome and a single functional ventricle have a poorer prognosis [30]. Other nonpreferable factors are obstructed PVs, coexistent complex cardiac defects [2], pulmonary atresia [23], and younger age at surgery [26].
Pearls in prenatal sonographic diagnosis
Postnatal ultrasound diagnosis has a sensitivity and specificity of >97% [28], whereas prenatal diagnosis is more challenging. In a large (n = 424) TAPVC case series implemented from 1998 to 2004, only 1.9% cases were identified in utero [6]. France reported a 10% prenatal diagnosis rate for 95 isolated TAPVC neonates born between 2001 and 2011 [31].
The prenatal diagnosis rate is believed to be increased through a systemic, step-by-step ultrasound examination [7]. Here we describe the characteristics of TAPVC in prenatal ultrasonography based on the International Society of Ultrasound in Obstetrics and Gynecology guideline for cardiac screening in midgestation [32].
Step 1: Identifying the normal connection of the PV to the LA and inspecting for indirect signs (Table 1).
Table 1.
Indirect imaging features of prenatal echocardiography for TAPVC.
| View | Description of the abnormal image | Comment | Sensitivity (from small case series) |
|---|---|---|---|
| Situs | Situs ambiguous | TAPVC is associated with heterotaxy syndrome, especially RAI | – |
| Four-chamber view | |||
| Atrium | Failure to visualize the normal PV connection to the LA | -First clue to suspect TAPVC -diagnostic triad |
60-100% [7,24,31] |
| Smooth posterior wall of the LA | Additional feature | 79% [7] | |
| Ventricle | Ventricular disproportion or asymmetry (RV > LV) | Inconsistent feature for fetuses before 28 weeks of gestation; observed in a large VSD and obstructive type of TAPVC | 19–60% [24,31] |
| Retrocardiac space | Increased space between the LA and DAo | Post-LA space index cut-off of 1.27 Post-LA Space index cut-off of 1.27 The cut-off value of “post-LA space index” is 1.27 [32] |
50–100% [7,35] |
| Three-vessel view | A fourth vessel adjacent to the pulmonary trunk | Supracardiac type | 33%a [24] |
| SVC dilatation | Supracardiac type | 72–100%a [7,24,31] | |
| Abdominal view | An extra vessel between the IVC and aorta | Infracardiac type | 100%b [24] |
TAPVC, total anomalous pulmonary venous connection; RAI, right atrium isomerism; PV, pulmonary vein; LA, left atrium; RV, right ventricle; LV, left ventricle; VSD, ventricular septal defect; Dao, descending aorta; SVC, superior vena cava; IVC, inferior vena cava.
a The sensitivity is solely calculated for the supracardiac type.
b The sensitivity is solely calculated for the infracardiac types.
A. General aspects: Situs
TAPVC is part of the heterotaxy syndrome in both RAI and left atrial isomerism (LAI) subtypes. This abnormal venous return can be observed in 50% of fetuses with RAI and 5% of those with LAI. Therefore, careful attention should be paid to the examination of PV connections when the situs is ambiguous.
B. Four-chamber view
(a) Demonstrate normal PV connections into the LA
The PVs can often be seen entering the LA in the axial four-chamber view (Fig. 2). An inability to demonstrate this normal connection is an important sign for suspecting TAPVC. Recent studies have described this as the first clue to suspect the disease [7,24]. A smooth posterior wall of the LA can be viewed as an accessory feature to this abnormal connection of PVs and is observed in some cases.
Figure 2.

On an apical four-chamber view, right and left inferior pulmonary vein enters left antrum posteriorly and forms a “horn-like” insertion. Note the posterior wall of left antrum is not as round and smooth as right antrum.
(b) Ventricular disproportion: RV > LV
The discrepancy in bilateral ventricles is not always observed and tends to be recognized after the gestational age of 7 months [24]. This is because the flow of pulmonary circulation increases in the latter stage of fetal life. Therefore, the extra abnormal flow from the PV appears to affect the size of the heart chambers in the late second trimester. Furthermore, dilatation of the right ventricle is not obvious among cases of prominent ASD or obstructive TAPVC [33]. A recent study recommended that this feature should not be enrolled into the diagnostic criteria because routine second trimester obstetric ultrasonography is executed at the gestational age of 18–22 weeks [7].
(c) Retrocardiac space examination: wide space behind the heart
Evaluation of the area behind the heart offers important information for diagnosing a fetal CHD, including TAPVC [34]. The diagnostic marker is a longer-than-usual distance between the LA and aorta [7]. A Japanese group introduced a post-LA space index to objectively examine this area. This index, defined as the ratio of the LA-descending aorta distance to the descending aorta diameter, was significantly higher in eight TAPVC fetuses than in 101 non-TAPVC fetuses (mean, 1.51 versus 0.71 ± 0.23) [35].
C. Three-vessel view
(a) A fourth vessel adjacent to the pulmonary trunk (supracardiac type)
The three-vessel view is a section for surveying the alignment, position, and size of the pulmonary artery, aorta, and SVC. In supracardiac TAPVC, the PV empties into a common vertical vein, which then connects to the RA. In this view, the vertical vein can be observed as a fourth vessel to the left or posterior left of the pulmonary artery [36].
(b) SVC dilatation (supracardiac type)
In the three-vessel view, the SVC usually appears smaller than both the pulmonary artery and aorta. In some cases of supracardiac TAPVC, the SVC is either as large as or more prominent than the aorta [37]. In supracardiac TAPVC, SVC dilatation is more frequently identified than is the extra vertical vein [24].
D. Abdominal view
(a) An extra vessel between the IVC and aorta (infra-cardiac type)
Similar to the fourth vessel in the supracardiac type, the descending vertical vein in infracardiac TAPVC can be seen between the IVC and aorta in the transverse view of abdominal images [36].
Step 2: Actively looking for the confluent vessel and vertical vein and identifying the TAPVC type.
A. Confluent vein
When the four PVs fail to develop a normal connection with the LA, they usually drain into a confluent vein before emptying into the RA. Carefully searching for the confluent vein is important when a normal PV connection is not identified, and this can be achieved in most cases [38]. This tubular vessel is usually located between the LA and aorta in types I, II, and III TAPVC [24]. Among those with highly suspected TAPVC but without a visible confluent vein, focus on the CS or mixed-type TAPVC should be emphasized [36,24] (see description below).
B. Dilatation of the CS (intracardiac type)
The diameter of the CS is usually <3 mm in a normal fetal heart, and an enlarged CS is associated with CHD, including left SVC and coarctation [39]. In intracardiac TAPVC, the PV empties into the CS through PV confluence or direct drainage. Therefore, dilatation of the CS raises the suspicion of this type of TAPVC [31].
C. Vertical vein (infracardiac and supracardiac types)
Following the recognition of the confluence vein, the sonographer can sweep the transducer along the transverse axis or change to the coronal/sagittal view to identify the site of PV confluence joining the vertical vein [38]. The ascending or descending vertical vein can usually be visualized in both infracardiac and supracardiac cases through two-dimensional ultrasound or Doppler flow examination [24] (see below).
D. Color flow Doppler and spectra Doppler
The application of color Doppler imaging is very useful to detect the aforementioned direct and indirect signs, especially the (1) normal connection of the PVs to the LA, (2) confluent vein, and (3) vertical vein [24,36]. The fetal heart should be placed apically or transversely, and then the color box should be narrowed to the LA for depiction of the PV entry. Using low pulse-repetition frequency and high sensitivity settings, the PV can be rapidly identified in most pregnant woman [7] (Fig. 3). A careful survey of the vertical vein through color flow mapping is essential for both the diagnosis and outcomes. Color Doppler shows flow turbulence if an obstruction is present at the connection site, which leads to a poor prognosis [38].
Figure 3.

Four-chamber view of the right pulmonary vein empty into left antrum in color Doppler image. Note the color box should be narrowed to the LA with low pulse-repetition frequency (0.8–2.0 Hz) and high sensitivity setting.
The normal PV Doppler waveform is pulsatile and biphasic, with distinct systolic and diastolic peaks, followed by little or no forward flow at the end of diastole [36] (Fig. 4). Abnormal PV spectral Doppler findings include a continuous monophasic pattern or abnormal pulsatility [24]. A low velocity monophasic waveform in the vertical vein with a high velocity (>0.5 m/s) flow over the connection site indicates the presence of obstruction [24,38].
Figure 4.

Doppler waveform across the inferior pulmonary vein. This triphasic pattern is similar to the waveform observed in the ductus venosus. Note the Doppler sample gate placed on the pulmonary vein is within the lung parenchyma (s, systolic velocity; d, diastolic velocity; a, atrial reversal flow).
E. Four-dimensional ultrasound
One study applied four-dimensional ultrasound with B-flow imaging and STIC to obtain more information regarding abnormal venous connections. The advantage of this technique is the demonstration of the entire route and anatomic correlation of the PV confluence, vertical vein, and anomalous venous connection site, which sometimes cannot be clearly identified through two-dimensional examination [37].
Conclusion
Over the past few years, an increasing number of studies on the prenatal diagnosis and postnatal management of TAPVC have been published. Through awareness of this rare disease and a good understanding of the embryology/anatomy of the venous system, the recognition of anomalous venous return can be achieved in mid-trimester anatomical screening examinations or through echocardiography. Failure to identify a normal PV connection and a demonstration of the vertical vein and confluent vessel are essential for a confirmation of the diagnosis. Advanced sonographic techniques offer further detailed information for postnatal care and surgical preparation.
Footnotes
Conflict of interest: The authors have no conflicts of interest relevant to this article.
References
- [1].Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002 Jun 19;39(12):1890–900. doi: 10.1016/s0735-1097(02)01886-7. Review. [DOI] [PubMed] [Google Scholar]
- [2].Seale AN, Uemura H, Webber SA, et al. British Congenital Cardiac Association. Total anomalous pulmonary venous connection: morphology and outcome from an international population-based study. Circulation. 2010 Dec 21;122(25):2718–26. doi: 10.1161/CIRCULATIONAHA.110.940825. [DOI] [PubMed] [Google Scholar]
- [3].Hoffman JI, Kaplan S, Liberthson RR. Prevalence of congenital heart disease. Am Heart J. 2004 Mar;147(3):425–39. doi: 10.1016/j.ahj.2003.05.003. [DOI] [PubMed] [Google Scholar]
- [4].Reller MD, Strickland MJ, Riehle-Colarusso T, et al. Prevalence of congenital heart defects in metropolitan Atlanta, 1998–2005. J Pediatr. 2008 Dec;153(6):807–13. doi: 10.1016/j.jpeds.2008.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wu MH, Chen HC, Lu CW, et al. Prevalence of congenital heart disease at live birth in Taiwan. J Pediatr. 2010 May;156(5):782–5. doi: 10.1016/j.jpeds.2009.11.062. [DOI] [PubMed] [Google Scholar]
- [6].Seale AN, Carvalho JS, Gardiner HM, et al. British Congenital Cardiac Association. Total anomalous pulmonary venous connection: impact of prenatal diagnosis. Ultrasound Obstet Gynecol. 2012 Sep;40(3):310–8. doi: 10.1002/uog.11093. [DOI] [PubMed] [Google Scholar]
- [7].Tongsong T, Luewan S, Jatavan P, et al. A simple rule for prenatal diagnosis of total anomalous pulmonary venous return. J Ultrasound Med. 2016 Jul;35(7):1601–7. doi: 10.7863/ultra.15.08016. [DOI] [PubMed] [Google Scholar]
- [8].Anderson RH, Brown NA, Moorman AFM. Development and structures of the venous pole of the heart. Dev Dyn. 2006;235:2–9. doi: 10.1002/dvdy.20578. [DOI] [PubMed] [Google Scholar]
- [9].Jongbloed MR, Mahtab EA, Blom NA, et al. Development of the cardiac conduction system and the possible relation to predilection sites of arrhythmogenesis. Scientific World Journal. 2008 Mar 3;8:239–69. doi: 10.1100/tsw.2008.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Review of medical embryology, Ch.127 text and illustrations by Ben Pansy, Ph.D., M.D [Google Scholar]
- [11].Lucas RV, Jr, Anderson RC, Amplatz K, et al. Congenital causes of pulmonary venous obstruction. Pediatr Clin North Am. 1963 Aug;10:781–836. doi: 10.1016/s0031-3955(16)31451-1. [DOI] [PubMed] [Google Scholar]
- [12].Neill CA. Development of the pulmonary veins;with reference to the embryology of anomalies of pulmonary venous return. Pediatrics. 1956 Dec;18(6):880–7. [PubMed] [Google Scholar]
- [13].Webb S, Kanani M, Anderson RH, et al. Development of the human pulmonary vein and its incorporation in the morphologically left atrium. Cardiol Young. 2001 Nov;11(6):632–42. doi: 10.1017/s1047951101000993. [DOI] [PubMed] [Google Scholar]
- [14].van den Berg G, Moorman AF. Development of the pulmonary vein and the systemic venous sinus: an interactive 3D overview. PLoS One. 2011;6(7):e22055. doi: 10.1371/journal.pone.0022055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Darling RC, Rothney WB, Craig JM. Total pulmonary venous drainage into the right side of the heart: report of 17 autop-sied cases not associated with other major cardiovascular anomalies. Lab Invest. 1957;6:44–64. [PubMed] [Google Scholar]
- [16].Karamlou T, Gurofsky R, Al Sukhni E, et al. Factors associated with mortality and reoperation in 377 children with total anomalous pulmonary venous connection. Circulation. 2007 Mar 27;115(12):1591–8. doi: 10.1161/CIRCULATIONAHA.106.635441. [DOI] [PubMed] [Google Scholar]
- [17].Satpathy M, Mishra BR. Clinical diagnosis of congenital heart disease. 2015;(Ch 35):325. [Google Scholar]
- [18].Fu CM, Wang JK, Lu CW, et al. Total anomalous pulmonary venous connection: 15 years'experience of a tertiary care center in Taiwan. Pediatr Neonatol. 2012 Jun;53(3):164–70. doi: 10.1016/j.pedneo.2012.04.002. [DOI] [PubMed] [Google Scholar]
- [19].Smith B, Thomas RF, Newton WA., Jr Total anomalous pulmonary venous return: diagnostic criteria and a new classification. Am J Dis Child. 1961 Jan;101:37–40. [Google Scholar]
- [20].Rao PS, Silbert DR. Superior vena caval obstruction in total anomalous pulmonary venous connexion. Br Heart J. 1974 Feb;36(2):228–32. doi: 10.1136/hrt.36.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Herlong JR, Jaggers JJ, Ungerleider RM. Congenital Heart Surgery Nomenclature and Database Project: pulmonary venous anomalies. Review Ann Thorac Surg. 2000 Apr;69(4 Suppl):S56–69. doi: 10.1016/s0003-4975(99)01237-0. [DOI] [PubMed] [Google Scholar]
- [22].Douglas M. Clinical management of congenital heart disease from infancy to adulthood. 2003:80. [Google Scholar]
- [23].Morales DL, Braud BE, Booth JH, et al. Heterotaxy patients with total anomalous pulmonary venous return: improving surgical results. Ann Thorac Surg. 2006 Nov;82(5):1621–7. doi: 10.1016/j.athoracsur.2006.05.053. discussion 1627–1628. [DOI] [PubMed] [Google Scholar]
- [24].Ganesan S, Brook MM, Silverman NH, et al. Prenatal findings in total anomalous pulmonary venous return: a diagnostic road map starts with obstetric screening views. J Ultrasound Med. 2014 Jul;33(7):1193–207. doi: 10.7863/ultra.33.7.1193. [DOI] [PubMed] [Google Scholar]
- [25].Li J, Yang S, Pu Z, et al. Whole-exome sequencing identifies SGCD and ACVRL1 mutations associated with total anomalous pulmonary venous return (TAPVR) in Chinese population. Oncotarget. 2017 Apr 25;8(17):27812–9. doi: 10.18632/oncotarget.15434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Shi G, Zhu Z, Chen J, et al. Total anomalous pulmonary venous connection: the current management strategies in a pediatric cohort of 768 patients. Circulation. 2017 Jan 3;135(1):48–58. doi: 10.1161/CIRCULATIONAHA.116.023889. [DOI] [PubMed] [Google Scholar]
- [27].Yoshimura N, Fukahara K, Yamashita A, et al. Current topics in surgery for isolated total anomalous pulmonary venous connection. Surg Today. 2014 Dec;44(12):2221–6. doi: 10.1007/s00595-014-0877-5. [DOI] [PubMed] [Google Scholar]
- [28].Zhang Z, Zhang L, Xie F, et al. Echocardiographic diagnosis of anomalous pulmonary venous connections: experience of 84 cases from 1 medical center. Medicine (Baltimore) 2016 Nov;95(44):e5389. doi: 10.1097/MD.0000000000005389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lemaire A, DiFilippo S, Parienti JJ, et al. Total anomalous pulmonary venous connection: a 40 years' experience analysis. Thorac Cardiovasc Surg. 2016 Sep 16; doi: 10.1055/s-0036-1588007. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [30].Hancock Friesen CL, Zurakowski D, Thiagarajan RR, et al. Total anomalous pulmonary venous connection: an analysis of current management strategies in a single institution. Ann Thorac Surg. 2005 Feb;79(2):596–606. doi: 10.1016/j.athoracsur.2004.07.005. [DOI] [PubMed] [Google Scholar]
- [31].Laux D, Fermont L, Bajolle F, et al. Prenatal diagnosis of isolated total anomalous pulmonary venous connection: a series of 10 cases. Ultrasound Obstet Gynecol. 2013 Mar;41(3):291–7. doi: 10.1002/uog.11186. [DOI] [PubMed] [Google Scholar]
- [32].International Society of Ultrasound in Obstetrics and Gyne-cology. Carvalho JS, Allan LD, et al. ISUOG Practice Guidelines (updated): sonographic screening examination of the fetal heart. Ultrasound Obstet Gynecol. 2013 Mar;41(3):348–59. doi: 10.1002/uog.12403. [DOI] [PubMed] [Google Scholar]
- [33].Allan LD, Sharland GK. The echocardiographic diagnosis of totally anomalous pulmonary venous connection in the fetus. Heart. 2001 Apr;85(4):433–7. doi: 10.1136/heart.85.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Berg C, Georgiadis M, Geipel A, et al. The area behind the heart in the four-chamber view and the quest for congenital heart defects. Ultrasound Obstet Gynecol. 2007 Oct;30(5):721–7. doi: 10.1002/uog.5152. [DOI] [PubMed] [Google Scholar]
- [35].Kawazu Y, Inamura N, Shiono N, et al. 'Post-LA space index'as a potential novel marker for the prenatal diagnosis of isolated total anomalous pulmonary venous connection. Ultrasound Obstet Gynecol. 2014 Dec;44(6):682–7. doi: 10.1002/uog.13357. [DOI] [PubMed] [Google Scholar]
- [36].Patel CR, Lane JR, Spector ML, et al. Totally anomalous pulmonary venous connection and complex congenital heart disease: prenatal echocardiographic diagnosis and prognosis. J Ultrasound Med. 2005 Sep;24(9):1191–8. doi: 10.7863/jum.2005.24.9.1191. [DOI] [PubMed] [Google Scholar]
- [37].Volpe P, Campobasso G, De Robertis V, et al. Two- and four-dimensional echocardiography with B-flow imaging and spatio-temporal image correlation in prenatal diagnosis of isolated total anomalous pulmonary venous connection. Ultrasound Obstet Gynecol. 2007 Nov;30(6):830–7. doi: 10.1002/uog.5145. Erratum in: Ultrasound Obstet Gynecol. 2008 Mar;31(3): 365. [DOI] [PubMed] [Google Scholar]
- [38].Valsangiacomo ER, Hornberger LK, Barrea C, et al. Partial and total anomalous pulmonary venous connection in the fetus: two-dimensional and Doppler echocardiographic findings. Ultrasound Obstet Gynecol. 2003 Sep;22(3):257–63. doi: 10.1002/uog.214. [DOI] [PubMed] [Google Scholar]
- [39].Chaoui R, Heling KS, Kalache KD. Caliber of the coronary sinus in fetuses with cardiac defects with and without left persistent superior vena cava and in growth-restricted fetuses with heart-sparing effect. Prenat Diagn. 2003 Jul;23(7):552–7. doi: 10.1002/pd.626. [DOI] [PubMed] [Google Scholar]


