Abstract
Introduction
Renal cortical elastography has shown conflicting but promising results in evaluation of chronic kidney disease and other renal disorders. The purpose of this study was to establish a normogram of renal cortical elasticity values and assess their variation between right and left kidney and their relation with age, gender, body mass index, renal dimensions and skin to cortex distance.
Methods
The study was a hospital based cross sectional study performed at Tribhuvan University Teaching Hospital, a tertiary care center in Kathmandu, Nepal. All individuals referred for Ultrasound from General Health Check up clinic were included in the study. Patient with abnormal ultrasound findings and abnormal renal function test were excluded from the study. Renal morphometry including length, cortical thickness, and skin to cortex distance were measured in B mode imaging and renal cortical elastography was measured with region of interest box of 1 × 0.5 cm. All analyses were done using Statistical Package for Social Sciences 20.0 soft ware.
Results
A total of 95 individuals who met the inclusion criteria were included in the study. The mean values of right and left renal cortical shear wave velocity were 1.49 ± 0.19 m/s and 1.54 ± 0.19 m/s respectively. Statistical significant difference was observed between the renal cortical shear wave velocity of right and left kidney. The renal shear wave velocity was seen to decrease with age, however the correlation was not statistically significant. No significant difference was also noted in renal shear wave velocity among various sex or Body mass index groups. Statistically significant negative correlation was noted between skin to cortex distance and renal cortical shear wave velocities. However no statistically significant correlation was noted between renal dimensions and renal cortical shear wave velocities.
Conclusions
The normal cortical elasticity values in terms of shear wave velocity of right and left kidney were established. Renal elasticity is independent of the age, gender, Body mass index and renal dimensions.
Keywords: Renal elastography, Ultrasound, Shear wave elastography
Introduction
Chronic kidney disease (CKD) is one of the common major public health problems in the world. Fibrosis of the renal parenchyma is the main pathologic process leading to progression of CKD. Renal fibrosis comprises of glomerulo-sclerosis, tubular atrophy, interstitial fibrosis and vascular changes [1,2]. Imaging of the kidney is mainly based on the morphologic evaluation of the parenchyma, excretory system and renal vasculature using Ultrasonography (USG), Computed Tomography (CT) and Magnetic Resonance Imaging (MRI). Renal length, parenchymal thickness and resistive index (RI) have showed significant correlation with renal fibrotic changes [3,4]. However these imaging parameters are not sensitive and specific in the evaluation of renal failure, as the renal morphology may still appear normal in early stages of CKD.
Elastography is a non-invasive technique that measures tissue elasticity, which is the capacity to deform and to return to the initial shape when a stress is applied. Most elastography techniques used today are ultrasound based, like shear wave elastography (SWE) using Acoustic Radiation Force Impulse (ARFI) quantification. Shear wave elas-tography is a technique that applies low frequency focused impulse to induce a shear wave in the tissue that is transmitted perpendicularly to the direction of applied impulse. The propagation speed of the shear wave, named shear wave velocity, is proportional to the tissue stiffness. Elas-tography quantification of shear wave velocity has been proposed as an alternative technique for assessing liver fibrosis and has shown promising results [5,6]. There are few studies conducted in determining the role of elastog-raphy in evaluation of renal parenchyma in CKD, Chronic Allograft Nephropathy (CAN), Vesico-ureteral reflux (VUR) and renal tumors which have shown although conflicting but still promising results [7,8,9,10,11,12,13,14,15,16,17,18,19]. Till date no such studies evaluating normal or abnormal renal cortical elasticity values has been done in Nepal.
So the purpose of this study was to establish a normogram of renal cortical elasticity values and assess their variation in between right and left kidney as well as with age, gender, BMI, renal dimensions and skin to cortex distance.
Methods
The study was a hospital based cross sectional study performed at Tribhuvan University Teaching Hospital (TUTH), a tertiary care center in Kathmandu performed as a Thesis, a partial requirement for the fulfillment of the degree of doctor of medicine (MD) in Radiodiagnosis. The study population was all individuals referred for USG from General Health Check up clinic. All individuals who came for routine ultrasound as a part of general health check up from (1st) October, 2014 to (30th) September, 2015 were included in the study after obtaining a written informed consent. Individuals unwilling to participate in the study; individuals with abnormal ultrasound like ascites, renal stone disease, hydronephrosis or any urinary tract pathology or deranged renal function or laboratory or clinical findings of urinary tract pathology were excluded from the study.
Renal ultrasound examinations and measurements were performed by a single observer on Philips iU22 Ultrasound machine, (Philips Medical System, Bothell, WA) using C5-1 (1–5 MHz) convex probe. All measurements were taken with electronic calipers in lateral decubitus position post micturition. All patients were screened in supine position for any residual urine before the scan.
Renal length was measured on coronal plane from superior pole to inferior pole of the kidney. Renal width was measured from the renal hilum to the renal capsule at mid pole on coronal plane. Parenchymal thickness was measured from the renal capsule at mid pole to the outer margin of the renal sinus on coronal plane. Cortical thickness was measured from the renal capsule at mid pole to base of the medullary pyramid. At last, the distance of skin to the outer margin of the renal cortex corresponding to renal capsule at mid pole was measured.
Subsequently point shear wave elastography (pSWE) was performed using ELAST PQ software based on ARFI technology maintaining the same lateral decubitus position and using the same C5-1 (1–5 MHz) convex probe. The probe was placed steadily with minimum compression and the person was asked to hold breath in full inspiration for a few seconds to minimize motion of the kidney with respiration. After that Region of interest (ROI) box of 1.0 × 0.5 cm (predefined by the manufacturer) was positioned in the renal cortex in the middle third of the kidney excluding the medulla as much as possible with the main axis of the ROI box lying as parallel as possible to the main axis of the medullary pyramids in the mid pole and the “Update” button was pressed for quantification and the renal cortical shear wave velocity was obtained in m/s (Fig. 1).
Figure 1.
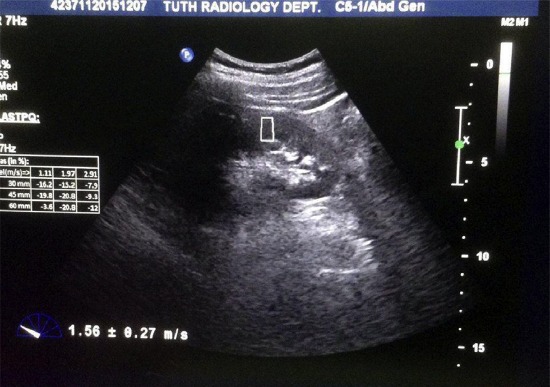
Measurement of renal cortical elasticity. ROI box is seen placed in the renal cortex parallel to the renal pyramids (marked hypoechoic triangular area) and not extending up to the renal sinus (hyperechoic area). The shear wave velocity in m/s is displayed in the lower left corner.
Five valid elasticity measurements in terms of shear wave velocity were made during separate breath holds for the particular kidney and then mean shear wave velocity value for that kidney was obtained. In case of invalid measurement the screen would display 0 m/s, and such measurements were repeated. Then the examination procedure was repeated for the contralateral kidney. Age, gender, height and weight of the subjects were also noted.
Body Mass Index (BMI) was calculated from height and weight of the subject and categorized according to criteria given by World Health Organization (WHO) for Asian population as follows: [20]
Underweight – BMI <18.5 kg/m2.
Normal – BMI 18.5 – <23 kg/m2.
Overweight – BMI 23 – <27.5 kg/m2
Obesity – BMI ≥27.5 kg/m2
Statistical analysis was done using SPSS 20.0 soft ware. Means of continuous variables were calculated. Correlation was calculated using Pearson’s correlation coefficient and t-test and one-way ANOVA was used to calculate the difference in mean. Inter class correlation coefficient was calculated analyze the intra observer variability as a measure of reliability.
Observations and results
A total of 95 individuals who met the inclusion criteria were included in the study. The age ranged from 18 to 69 years with mean age of 38.72 ± 12.02 years. Females (50; 52.63%) were slightly more frequently sampled than males 45 (47.37%) The mean BMI of the study population was 24.78 ± 3.46 kg/m2.
There was significant difference between the length, width, parenchymal and cortical thickness between right and left kidney (LK); left kidney showing larger values as compared to right kidney (RK) (p value < 0.05). However no significant difference was noted in skin to cortex distance in right and left kidney (Table 1).
Table 1.
Kidney dimensions and skin to cortex distance.
| Dimensions (cm) | Right kidney | Left kidney | p-value# |
|---|---|---|---|
| Length | 9.76 ± 0.78 | 10.02 ± 0.90 | 0.021* |
| Width | 4.21 ± 0.55 | 4.64 ± 0.67 | <0.001* |
| Parenchymal thickness | 1.86 ± 0.28 | 2.06 ± 0.34 | <0.001* |
| Cortical thickness | 1.27 ± 0.20 | 1.38 ±0..26 | 0.001* |
| Skin to cortex distance | 3.66 ± 0.96 | 3.52 ± 1.01 | 0.085 |
# Obtained using paired sample t-test, * statistically significant.
The interclass correlation coefficient of the five measurements was 0.52 and 0.35 for right and left kidney respectively. Standard error of measurement was calculated to be 0.66 and 0.76 for right and left kidney respectively.
The range of right and left renal cortical shear wave velocity values were 1.10–2.22 m/s and 1.13–2.23 m/s respectively. The mean values of right and left renal cortical shear wave velocity were 1.49 ± 0.19 m/s and 1.54 ± 0.19 m/s respectively. The median values of right and left renal cortical shear wave velocities were 1.46 m/s and 1.51 m/s respectively. The interquartile range for both right and left renal cortical shear wave velocities was 0.24 m/s. There was significant difference of mean cortical shear wave velocities between right and left kidney (p-value = 0.016).
The renal shear wave velocity was seen to decrease with age, however the correlation was not statistically significant (RK- Pearson’s correlation coefficient (r) = -0.075; p = 0.47 and LK- r = -0.199; p = 0.053). Also no significant difference was noted in mean renal shear wave velocities among various age groups (RK-p = 0.92; LK-p = 0.25) (Fig. 2) No significant difference was also noted in renal shear wave velocity among various BMI groups (Table 2).
Figure 2.
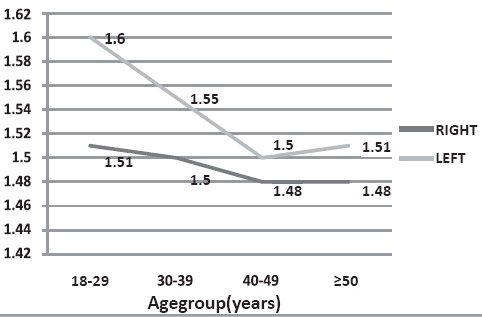
Renal cortical elasticity variation by age group.
Table 2.
Shear wave velocities according to BMI groups.
| BMI groups | 18.5 to <23 (normal BMI) | 23 to <27.5 (overweight) | >27.5 (obese) | P-value# |
|---|---|---|---|---|
| Right kidney SWV (m/s) | 1.54 ± 0.20 | 1.47 ± 0.16 | 1.49 ± 0.24 | 0.284 |
| Left kidney SWV (m/s) | 1.54 ± 0.17 | 1.57 ± 0.18 | 1.48 ± 0.23 | 0.226 |
# Obtained using one way ANOVA.
Mean renal cortical shear wave velocity were high in females measuring 1.53 ± 0.21 m/s, 1.57 ± 0.18 m/s in of right and left kidney respectively. However no statistically significant difference of shear wave velocities was noted between males and females in bilateral kidney (RK-p = 0.079; LK- p = 0.055).
There was statistically significant negative correlation noted between skin to cortex distance of right (r = –0.298, p = 0.003) and left (r = – 0.352, p < 0.001) with renal cortical shear wave velocity. However no statistically significant correlation was noted between renal dimensions of either kidney with renal cortical shear wave velocity in the study population (Table 3).
Table 3.
Shear wave velocities according to renal dimensions and skin to cortex distance.
| Dimensions (cm) | Right kidney | Left kidney | ||
|---|---|---|---|---|
| r-value# | p-value# | r-value# | p-value# | |
| Length | 0.033 | 0.749 | −0.017 | 0.872 |
| Width | −0.070 | 0.500 | −0.009 | 0.927 |
| Parenchymal thickness | 0.101 | 0.331 | −0.034 | 0.743 |
| Cortical thickness | 0.021 | 0.840 | −0.120 | 0.245 |
| Skin to cortex distance | −0.298 | 0.003 | −0.352 | <0.001 |
# Obtained using Pearson’s correlation.
Statistically significant values are represented in bold.
Discussion
The measurement of elasticity value of solid viscera can be used to identify the change in stiffness in disease condition as cirrhosis of liver. However the measurement of Shear wave velocity is challenging in kidneys as compared to liver due to inhomogeneous highly compartmentalized aniso-tropic tissue and offering a small area for quantification, variable in person to person. Sometimes it becomes difficult to orient the long axis of the ROI box parallel to the renal pyramid [21,22]. Due to these difficulties, renal elastog-raphy is a less popular and is not in clinical utility. However renal elastography has the potential to diagnose early chronic kidney disease and also be used to assess damage in chronic vesico-ureteric reflux and hydronephrosis.
The intra observer variability was calculated using inter class correlation coefficient (ICC) in our study which was 0.52 and 0.35 for right and left kidney respectively. The data shows that there is poor reliability (less than 0.4) of renal cortical elastography measurement in left kidney and fair reliability (0.4–0.59) of cortical elastography measurement in right kidney [23]. The ICC value in our study was similar to or slightly better then study done by Grenier et al. [19] while it is low as compared to study done by Bob et al. [24]. Bob et al. also found lower ICC value in normal subject as compared to patients. The technical difficulties, including anisotropy, effect of compression and need for ROI box to be parallel to the renal pyramids, faced during renal elastography might be the result of the lower ICC values [21,22].
The range of right and left renal cortical shear wave velocities were within the range specified by other studies but mean values of right and left kidney were lower in comparison with the other studies [25,26,27,28]. Bota et al. [25] in their study found that the mean cortical shear wave speed values obtained in the right and left kidneys were 2.49 ± 0.81 m/s v/s 2.36 ± 0.75 m/s. Goertz et al. [26] found renal parenchymal ARFI velocities were 2.37 ± 0.59 m/s and 2.37 ± 0.95 m/s with ranges of 1.53–3.98 m/s and 0.81–3.81 m/s in right and left kidney respectively in healthy volunteers. Galloti et al. [27] in their study chose either the left or the right kidney based on the best visualization on the conventional ultrasound image. They found the mean shear wave velocity value of 2.24 m/s with range of 0.52–4.83 m/s in renal parenchyma. Similarly in another study conducted by Bota et al. [28] using ARFI the mean cortical shear wave velocity of right and left renal parenchyma were 2.34 ± 0.75 m/s and 2.30 ± 0.76 m/s. Zheng et al. [9] in their study found the SWV in different parts of kidney in the healthy participants were noted to be as follows: 3.45 ± 0.29 m/s in the cortex, 2.34 ± 0.49 m/s in the medulla and 1.07 ± 0.37 m/s in the sinus, indicating that SWV values gradually decreased from the renal cortex to the medulla and sinus. They also concluded that the best cut off for predicting renal insufficiency at renal cortical shear wave velocity of less than 1.92 m/s.
The difference in shear wave velocity in various studies might be a result of different equipment used to measure the shear wave velocities, the inherent variation in the population characteristics and might as well be due to the low reproducibility of the renal elastography due to the technical difficulties. As shown in previous studies renal blood flow has a major influence on renal elasticity values [11,12,21,29], the lower mean values of renal cortical elasticity may also be attributed to the increase in capillary rarefaction in the renal microvasculature. The functional and/or anatomical loss of microvessels is known as rarefaction. Capillary or microvascular rarefaction has been noted in other tissues like retina and skin along with kidney and is more prevalent among the South Asian individuals as compared to other population. It has also been proposed as an independent risk factor for diabetes mellitus and cardiovascular diseases like hypertension [30,31,32]. A larger population based studies with renal perfusion correlation with standardized protocol needs to be conducted to establish the prevalence of capillary rarefaction in kidney and its role in renal cortical elasticity values in Nepalese population.
The variation of renal cortical elasticity with laterality as seen in our study was not seen in any of the previous studies [25,26,27,28,33]. The difference might only indicate a statistical bias or may be due to the larger dimension including the cortical thickness of left kidney. Though not statistically significant right kidney had a higher mean skin to cortex distance as compared to left, which might also be a reason for reduced renal cortical elasticity in right kidney. The negative correlation of renal cortical elasticity with skin to cortex distance was also seen in our study and has also been seen in previous studies in kidney and liver [7,29,34]. Renal cortical elastography, was not seen to be significantly affected by renal dimensions, age, sex or BMI in our study. Similar findings have also been established in previous studies [7,26,27]. Thus renal elastography values can be used in all age groups, sex and weight groups.
We had certain limitations in our study. First our sample size was small (N = 95). Second the study was hospital based and we took our sample from the individuals coming for general health check up, which is mostly attended by the people from affluent society and less from the poor society which still forms the bulk of the population of Nepal. So the sample may not be representative of the general population. Third only five valid readings were taken for each kidney in our study, which may not be sufficient enough to estimate normal renal cortical elasticity values owing to its less reproducibility. Fourth the fixed dimensions of the ROI box compared to the variable small area of the renal cortex as well as difficulty in placing the long axis of the ROI box in parallel orientation to the medullary pyramids may have sometimes led to estimation of shear wave velocity value from medulla and renal sinus also. Fifth we did not investigate the effect of renal perfusion on renal cortical elasticity values which can greatly influence the renal cortical elasticity.
Conclusions
The normal cortical elasticity values in terms of shear wave velocity of right and left kidney were established. Left kidney has higher cortical shear wave velocity compared to the right kidney and as the skin to cortex distance increases the shear wave velocity decreases. Renal elasticity is independent of the age, gender, BMI and renal dimensions. Future larger population based studies with standardized protocol and correlation with renal perfusion needs to be conducted to establish the normal values of renal cortical elasticity.
Footnotes
Conflicts of Interest: None.
References
- [1].Lopez-Novoa JM, Rodríguez-Peña AB, Ortiz A, et al. Etiopa-thology of chronic tubular, glomerular and renovascular ne-phropathies: clinical implications. J Transl Med. 2011;9(13):13. doi: 10.1186/1479-5876-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Nankivell BJ, Borrows RJ, Fung CL-S, et al. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349(24):2326–33. doi: 10.1056/NEJMoa020009. [DOI] [PubMed] [Google Scholar]
- [3].Moghazi S, Jones E, Schroepple J, et al. Correlation of renal histopathology with sonographic findings. Kidney Int. 2005;67(4):1515–20. doi: 10.1111/j.1523-1755.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- [4].Sugiura T, Nakamori A, Wada A, et al. Evaluation of tubu-lointerstitial injury by Doppler ultrasonography in glomerular diseases. Clin Nephrol. 2004;61(2):119–26. doi: 10.5414/cnp61119. [DOI] [PubMed] [Google Scholar]
- [5].Rizzo L, Calvaruso V, Cacopardo B, et al. Comparison of transient elastography and acoustic radiation force impulse for non-invasive staging of liver fibrosis in patients with chronic hepatitis C. Am J Gastroenterology. 2011;106(12):2112–20. doi: 10.1038/ajg.2011.341. [DOI] [PubMed] [Google Scholar]
- [6].Friedrich-Rust M, Wunder K, Kriener S, et al. Liver fibrosis in viral hepatitis: noninvasive assessment with acoustic radiation force impulse imaging versus transient elastography. Radiology. 2009;252(2):595–604. doi: 10.1148/radiol.2523081928. [DOI] [PubMed] [Google Scholar]
- [7].Guo LH, Xu HX, Fu HJ, et al. Acoustic radiation force impulse imaging for noninvasive evaluation of renal parenchyma elasticity: preliminary findings. PloS one. 2013;8(7):e68925. doi: 10.1371/journal.pone.0068925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hu Q, Wang XY, He HG, et al. Acoustic radiation force impulse imaging for non-invasive assessment of renal histopathology in chronic kidney disease. PloS one. 2014;9(12):e115051. doi: 10.1371/journal.pone.0115051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zheng XZ, Yang B, Fu NH. Preliminary study on the kidney elasticity quantification in patients with chronic kidney disease using virtual touch tissue quantification. Iran J Radiology. 2015;1:12. doi: 10.5812/iranjradiol.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wang L, Xia P, Lv K, et al. Assessment of renal tissue elasticity by acoustic radiation force impulse quantification with histo-pathological correlation: preliminary experience in chronic kidney disease. Eur Radiol. 2014;24(7):1694–9. doi: 10.1007/s00330-014-3162-5. [DOI] [PubMed] [Google Scholar]
- [11].Asano K, Ogata A, Tanaka K, et al. Acoustic radiation force impulse elastography of the kidneys Is shear wave velocity affected by tissue fibrosis or renal blood flow? J Ultrasound Med. 2014;33(5):793–801. doi: 10.7863/ultra.33.5.793. [DOI] [PubMed] [Google Scholar]
- [12].Bota S, Bob F, Sporea I, et al. The influence of glomerular filtration rate on kidney stiffness values assessed by acoustic radiation force impulse (ARFI) elastography. Ultraschall der Medizin-European J Ultrasound. 2013;(S 01):34. WS_SL9_01. [Google Scholar]
- [13].Stock K, Klein B, Vo CM, et al. ARFI-based tissue elasticity quantification in comparison to histology for the diagnosis of renal transplant fibrosis. Clin Hemorheol Microcirc. 2009;46(2-3):139–48. doi: 10.3233/CH-2010-1340. [DOI] [PubMed] [Google Scholar]
- [14].Syversveen T, Brabrand K, Midtvedt K, et al. Assessment of renal allograft fibrosis by acoustic radiation force impulse quantification - a pilot study. Transpl Int. 2011;24(1):100–5. doi: 10.1111/j.1432-2277.2010.01165.x. [DOI] [PubMed] [Google Scholar]
- [15].Bruno C, Caliari G, Zaffanello M, et al. Acoustic radiation force impulse (ARFI) in the evaluation of the renal paren-chymal stiffness in paediatric patients with vesicoureteral reflux: preliminary results. Eur Radiol. 2013;23(12):3477–84. doi: 10.1007/s00330-013-2959-y. [DOI] [PubMed] [Google Scholar]
- [16].Sohn B, Kim MJ, Han SW, et al. Shear wave velocity measurements using acoustic radiation force impulse in young children with normal kidneys versus hydronephrotic kidneys. Ultrasonography. 2014;33(2):116. doi: 10.14366/usg.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Clevert D, Stock K, Klein B, et al. Evaluation of acoustic radiation force impulse (ARFI) imaging and contrast-enhanced ultrasound in renal tumors of unknown etiology in comparison to histological findings. Clin Hemorheol Microcirc. 2008;43(1-2):95–107. doi: 10.3233/CH-2009-1224. [DOI] [PubMed] [Google Scholar]
- [18].Ozkan F, Yavuz YC, Inci MF, et al. Interobserver variability of ultrasound elastography in transplant kidneys: correlations with clinical-Doppler parameters. Ultrasound Med Biol. 2013;39(1):4–9. doi: 10.1016/j.ultrasmedbio.2012.09.013. [DOI] [PubMed] [Google Scholar]
- [19].Grenier N, Poulain S, Lepreux S, et al. Quantitative elastog-raphy of renal transplants using supersonic shear imaging: a pilot study. Eur Radiol. 2012;22(10):2138–46. doi: 10.1007/s00330-012-2471-9. [DOI] [PubMed] [Google Scholar]
- [20].Tang JW, Mason M, Kushner RF, et al. Peer reviewed: South Asian American perspectives on overweight, obesity, and the relationship between weight and health. Prev chronic Dis. 2012:9. doi: 10.5888/pcd9.110284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Grenier N, Gennisson JL, Cornelis F, et al. Ultrasound elastography of kidney. Ultrasound Clin. 2013;8(4):551–64. [Google Scholar]
- [22].Grenier N, Gennisson JL, Cornelis F, et al. Renal ultrasound elastography. Diagn Interv Imaging. 2013;94(5):545–50. doi: 10.1016/j.diii.2013.02.003. [DOI] [PubMed] [Google Scholar]
- [23].Fleiss JL. Reliability of measurement. In: Fleiss JL, editor. The design and analysis of clinical experiments. Toronto: Wiley; 1986. pp. 1–32. [Google Scholar]
- [24].Bob F, Bota S, Sporea I, et al. Kidney shear wave speed values in subjects with and without renal pathology and inter-operator reproducibility of acoustic radiation force impulse elas-tography (ARFI) - preliminary results. PloS one. 2014;9(11):e113761. doi: 10.1371/journal.pone.0113761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bota S, Bob F, Sporea I, et al. Factors that influence kidney shear wave speed assessed by acoustic radiation force impulse elastography in patients without kidney pathology. Ultrasound Med Biol. 2015;41(1):1–6. doi: 10.1016/j.ultrasmedbio.2014.07.023. [DOI] [PubMed] [Google Scholar]
- [26].Goertz R, Amann K, Heide R, et al. An abdominal and thyroid status with acoustic radiation force impulse elastometry - a feasibility study: acoustic radiation force impulse elas-tometry of human organs. Eur journal radiology. 2011;80(3):e226–30. doi: 10.1016/j.ejrad.2010.09.025. [DOI] [PubMed] [Google Scholar]
- [27].Gallotti A, D'Onofrio M, Mucelli RP. Acoustic radiation force impulse (ARFI) technique in ultrasound with virtual touch tissue quantification of the upper abdomen. La Radiol medica. 2010;115(6):889–97. doi: 10.1007/s11547-010-0504-5. [DOI] [PubMed] [Google Scholar]
- [28].Bota S, Bob F, Sporea I, et al. The age influence the kidney stiffness assessed by means of acoustic radiation force impulse (ARFI) elastography? Ultraschall der Medizin-European J Ultrasound. 2013;(S 01):34. WS_SL9_04. [Google Scholar]
- [29].Gennisson JL, Grenier N, Combe C, et al. Supersonic shear wave elastography of in vivo pig kidney: influence of blood pressure, urinary pressure and tissue anisotropy. Ultrasound Med Biol. 2012;38(9):1559–67. doi: 10.1016/j.ultrasmedbio.2012.04.013. [DOI] [PubMed] [Google Scholar]
- [30].Chade AR. Renal vascular structure and rarefaction. Compr Physiol. 2013;3(2):817–31. doi: 10.1002/cphy.c120012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hughes AD, Bathula R, Park C, et al. Microcirculatory rarefaction in South Asians - a potential mechanism for increased cardiovascular risk and diabetes. PloS one. 2013;10:8. doi: 10.1371/journal.pone.0076680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nama V, Onwude J, Manyonda I, et al. Is capillary rarefaction an independent risk marker for cardiovascular disease in South Asians? J Hum Hypertens. 2011;25(7):465–6. doi: 10.1038/jhh.2011.1. [DOI] [PubMed] [Google Scholar]
- [33].Bob F, Bota S, Sporea I, et al. Is Acoustic Radiation Force Impulse (ARFI) elastography a useful method for predicting kidney pathology? Ultraschall der Medizin-European J Ultrasound. 2013;(S 01):34. WS_SL9_06. [Google Scholar]
- [34].Horster S, Mandel P, Zachoval R, et al. Comparing acoustic radiation force impulse imaging to transient elastography to assess liver stiffness in healthy volunteers with and without valsalva manoeuvre. Clin Hemorheol Microcirc. 2009;46(2-3):159–68. doi: 10.3233/CH-2010-1342. [DOI] [PubMed] [Google Scholar]


