SUMMARY
Peripheral processes that mediate beneficial effects of exercise on the brain remain sparsely explored. Here we show that a muscle secretory factor, Cathepsin B (CTSB) protein, is important for cognitive benefits of running. Proteomic analysis revealed elevated levels of CTSB in conditioned medium derived from skeletal muscle cell cultures treated with AMP-kinase agonist AICAR. Consistently, running increased CTSB levels in mouse gastrocnemius muscle and plasma. In addition, in male wildtype (WT), but not CTSB knockout (KO) mice running enhanced adult hippocampal neurogenesis and spatial memory. Furthermore, recombinant CTSB application enhanced expression of brain-derived neurotrophic factor (BDNF) and doublecortin (DCX) in adult hippocampal progenitor cells through a multifunctional protein, P11, dependent mechanism. Interestingly, in Rhesus monkeys and humans treadmill exercise elevated CTSB in plasma. In humans CTSB levels correlated with fitness and hippocampus-dependent memory function. Our findings suggest CTSB as a mediator of effects of exercise on cognition.
Keywords: Cathepsin B, Exercise, Muscle, Memory, Hippocampus, Humans, Mice
Graphical abstract

INTRODUCTION
Physical activity benefits human health, including brain function (Voss et al., 2013). In particular, exercise may maintain and improve cognition (Duzel et al., 2016). In rodents running induces changes in brain neurotransmitter, neurotrophin levels, neuronal morphology and vascularization. In addition, hippocampus-dependent memory and adult neurogenesis is enhanced (Voss et al., 2013). In humans, there is a relationship between aerobic capacity, hippocampal plasticity and memory (Duzel et al., 2016). However, peripheral mechanisms that elicits these positive effects of running remain unclear.
Peripheral factors in the blood of young animals can improve brain plasticity in aged animals (Katsimpardi et al., 2014). Given that skeletal muscle plays a pivotal role in exercise (Hawley et al., 2014), myokines (Pedersen and Febbraio, 2008) may influence neural plasticity. Indeed, overexpression of peroxisome proliferator-activated receptor-gamma co-activator (PGC-1α) in muscle increased production of Fibronectin type III domain containing 5 (FNDC5), which is cleaved and secreted as irisin, increasing hippocampal Bdnf expression. Exercise elevates irisin in human plasma (Wrann, 2015). Furthermore, PGC-1α1 overexpression in muscle has an antidepressant-like effect by reducing entry of neurotoxic kynurenine into the brain (Agudelo et al., 2014). Moreover, spatial memory is enhanced by AICAR treatment in WT mice (Kobilo et al., 2011), but not in muscle specific AMPK mutant mice (Kobilo et al., 2014), supporting a link between skeletal muscle and cognition.
In this study, using proteomic and biochemical analyses, we found that secretory CTSB (Lemaire et al., 1997) is a myokine that is increased in plasma and gastrocnemius muscle by exercise in adult male mice. CTSB treatment of neural cells in vitro enhanced expression of DCX and BDNF. CTSB KO mice showed deficits in spatial memory, adult hippocampal neurogenesis, dentate granule cell (GC) physiology, and hippocampal P11 levels. In Rhesus monkeys and humans treadmill exercise increased CTSB plasma levels. Moreover, in humans CTSB plasma levels were positively associated with memory. Overall, CTSB may play an important role in the beneficial effects of exercise on the brain.
RESULTS
Identification and Validation of candidates
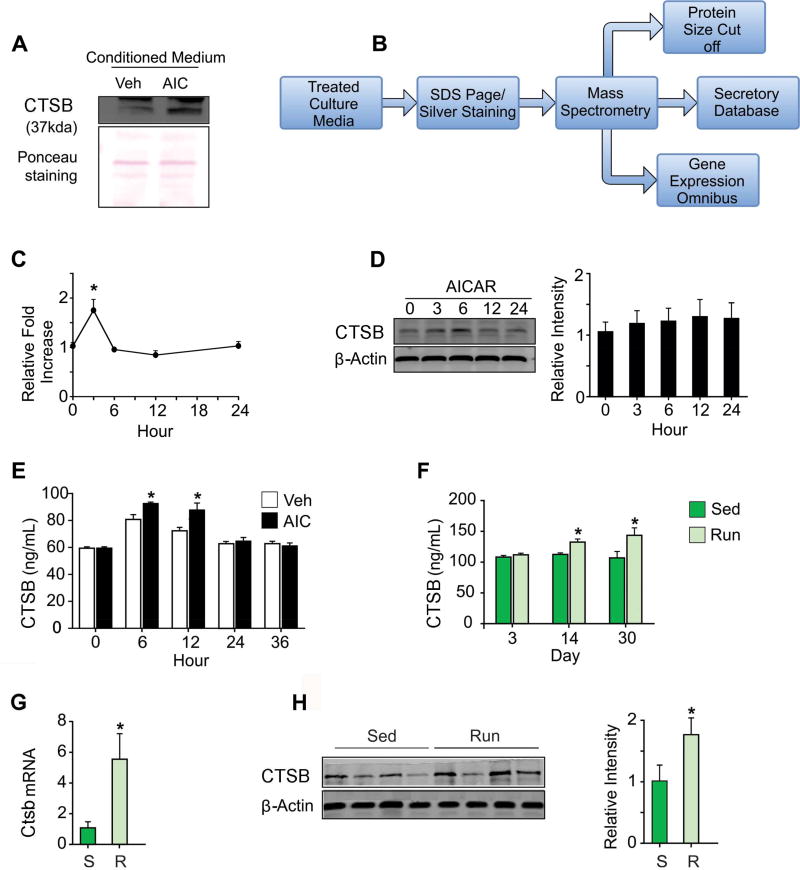
To model effects of exercise in vitro we applied AMPK agonist AICAR (100 µM) to L6 myoblast cells and analyzed the conditioned medium (CM; Figure 1A and 1B). Specifically, L6 myoblast cells were differentiated for 8 days and CM was collected after 6 hours of AICAR or vehicle (0.1% DMSO) treatment. CM was used for silver staining based proteomic analysis (Figure 1B). Interestingly, CM from AICAR treated cells revealed a differential protein expression pattern as compared to vehicle controls (Figure S1A). The differentially expressed bands were excised and eluted. We analyzed the peptide sequence of selected candidate proteins by mass spectrometry (Figure S1B). First, we selected the candidates based on the estimated protein size between 37 to 50 Kilo Dalton (kda) based on size marker (Bio-rad). Further evaluation was performed utilizing QSPEC (http://www.nesvilab.org/qspec.php/) and a secretory database (http://spd.cbi.pku.edu.cn/spd_index.php), exercise microarray datasets (GSD2234) and AICAR treated microarray datasets (GSE50873). The 37 kda, lysosomal cysteine protease, CTSB which has extracellular functions was selected (Figure 1A and Figure S1B).
Figure 1.
CTSB as a candidate myokine. (A) CTSB is present in the conditioned media (CM) of AICAR (AIC, 100µM) and Vehicle (Veh, 0.1% DMSO) treated differentiated L6 myoblast cultures as indicated by WB analysis. PONCEAU staining was used as a loading control. (B) Flowchart indicating how CTSB was identified. (C, D, E) Ctsb gene expression and CTSB protein levels in cells and CM from cultures treated with Vehicle (0.1 % DMSO) or AICAR (100 µM). (C) Time-course analysis showed Ctsb mRNA increased 3 hours after AICAR treatment. (D) WB analysis of intracellular CTSB levels at indicated time-points (hours) after treatment with AICAR (100µM). The graph shows the relative CTSB intensity normalized by β-actin. There was no difference in intracellular CTSB levels. E) CTSB protein levels in the CM of AICAR (100 µM) treated cultures is increased at 6 and 12 hours as compared to control. (F–H) In vivo analyses of Ctsb gene expression and CTSB protein levels in plasma and gastrocnemius muscle tissue of sedentary (Sed) and running (Run) mice. (F) Running increased CTSB plasma levels at 14 and 30 days. (G) Ctsb gene expression was elevated in the mouse gastrocnemius muscle after 30 days of running. (H) CTSB protein levels are elevated in gastrocnemius muscle after 30 days of running. Specifically, WB analysis of CTSB levels and a graph showing the relative intensity normalized by β-actin. Data represent means ± S.E.M. *p < 0.05
Validation of CTSB expression after exercise
To validate CTSB as a candidate myokine that may affect the brain, we studied Ctsb gene expression in differentiated myoblast cells. After 8 days cells were differentiated and starved for 3 hours with serum free media and incubated with vehicle or AICAR (100 µM) at the indicated time points (Figure 1C). There were significant differences in Ctsb gene expression between time-points (F(4,15) =32.91, p<0.0001). Specifically, short-term treatment (3 hours) of AICAR increased Ctsb mRNA levels (p<0.0001; Figure 1C). However, there was no increment in intracellular protein levels after treatment of AICAR as measured by western-blot (WB) analysis (Figure 1D). CTSB is known to be secreted (Lemaire et al., 1997). Therefore, extracellular protein levels were measured by ELISA. CTSB was significantly increased in differentiated L6 muscle cell lines after 100 µM of AICAR treatment at 6 hours (t(6)=3.67, p<0.01) and at 12 hours (t(6) =2.86, p<0.03), (Figure 1E). Furthermore, analysis of plasma samples from mice (n= 6~8 per group) that were running for 3, 14 or 30 days showed changes in CTSB levels (F(5,35)=4.64, p<0.0024). Specifically, plasma CTSB increased after 14 days and 30 days (p<0.044; p<0.0008, respectively) compared to controls (Figure 1F). In addition, Ctsb gene expression and protein amount was evaluated in muscle and other peripheral tissues derived from long-term (30 days) voluntary wheel running mice. Ctsb mRNA (t(14)=2.613, p<0.021) and protein (t(10)=6.429, p<0.03) levels increased in the gastrocnemius skeletal muscle (Figure 1G and 1H). Ctsb mRNA expression was unaltered in soleus, white adipose tissue, liver (Figure S1C), and frontal cortex (Figure S1D), and decreased in the spleen of running mice (t(14)=3.682, p<0.0025; Figure S1C). These findings suggest that running results in CTSB secretion from skeletal muscle.
Behavior in sedentary and running CTSB KO mice
Increased levels of CTSB in plasma and skeletal muscle after running led us to evaluate behavior in male CTSB KO and WT littermate mice, housed under control (WT-S, KO-S) or running (WT-R, KO-R) conditions (n=7–9 per group). The running distances did not differ between groups [WT-R (2735 ± 196 m/day), KO-R (2654 ± 321 m/day), p>0.05].
Motor behavior
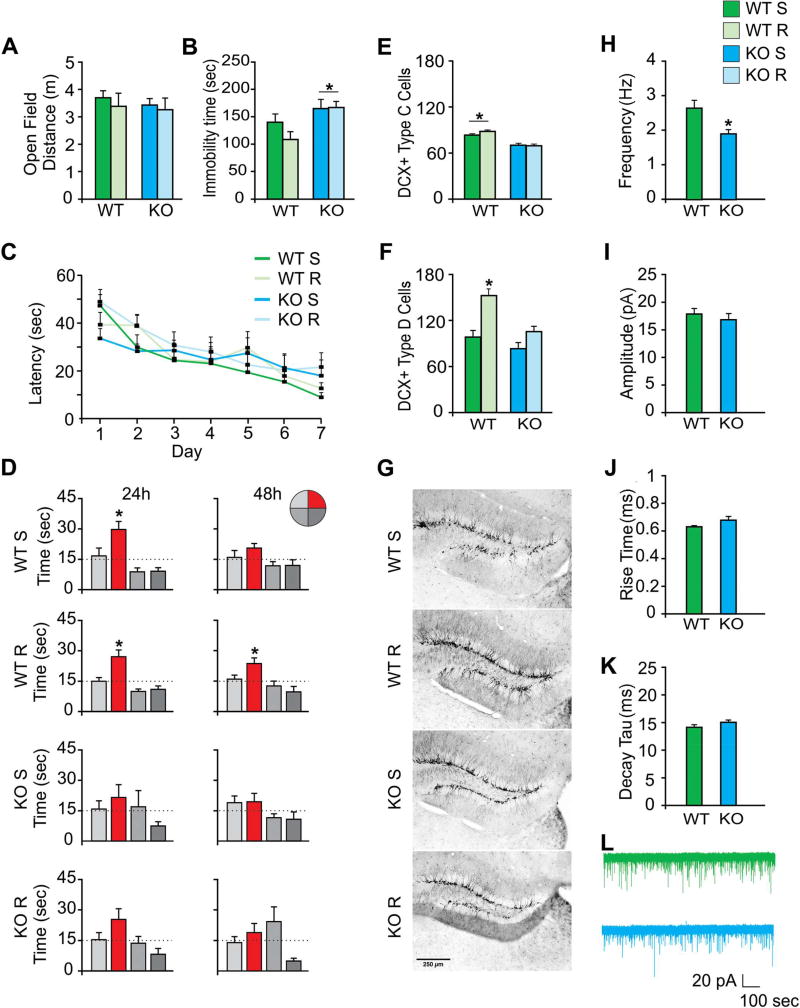
Locomotor activity in the open field was examined over 30 min. Total distance travelled did not differ between groups (Figure 2A). In addition, no difference between the groups was observed in the latency to fall in the rotarod test (Figure S1E).
Figure 2.
Behavioral analyses of CTSB KO mice and WT littermates housed under sedentary or running conditions. (A) Total distance travelled in the open-field test did not differ between the groups (WT sedentary, WT-S; WT runner, WT-R; KO sedentary, KO-S; KO runner, KO-R). (B) Forced swim test showed increased immobility time in KO compared to WT mice. (C) Water maze acquisition over 7 days did not differ between the groups. (D) Probe trials (60-sec) were performed 24 hours and 48 hours after the last training session. WT-R mice preferred the target quadrant as compared to all other quadrants in both probe trials. WT-S mice searched preferentially in the target quadrant at 24 hours but not at 48 hours. Neither KO-S nor KO-R mice showed retention of spatial memory. (E) DCX+ Type C cell number/section was higher in WT than KO groups. (F) DCX+ Type D cell number/section in the WT-R group was greater than in all the other groups. (G) Representative DG images of DCX staining. (H–L) Patch-clamp recordings from mature DG cells in acute slices derived from WT (n=3 mice, 12 cells) and KO (n=3 mice, 15 cells). (H) Averaged data from WT (n=12) and KO (n=15) cells show a significant decrease in mIPSC frequency. (I–K) There was no change in (I) amplitude (J) rise time or (K) decay tau. (L) Representative traces of whole cell patch-clamp recordings of mIPSCs from WT (green trace) and KO (blue trace) mice. Data represent mean ± SEM.*p<0.05
Mood related behaviors
The forced swim test was used to test depression-like behavior. There was a significant main effect of genotype on immobility time (F(1,27)=8.62, p<0.007; Figure 2B). Immobility time was reduced in the WT-R as compared to KO mice (p<0.01). Mice were also tested in the sucrose preference test to evaluate anhedonia (Figure S1F). In addition, the elevated plus maze test was performed to assay anxiety (Figure S1G). There was no difference between the groups consistent with previous research in male KO mice (Czibere et al., 2011).
Spatial memory
Mice (n=7–9 per group) were trained in the Morris water maze. There was no difference between the groups in acquisition of the task (p>0.05), (Figure 2C). After the last training day, probe trials were conducted 24h and 48h later to evaluate retention of spatial memory. The WT-R group preferred the platform quadrant compared to all other quadrants in both probe trials at 24h (F(3,32)=14.38, p<0.0001) and 48h (F(3,32)=7.58, p<0.0006). The WT-S group showed target preference at 24h (F(3,32)=11.54, p<0.0001) but not at 48h (p>0.05). The KO groups did not exhibit target preference (Figure 2D).
Adult hippocampal neurogenesis
Running-induced adult neurogenesis in the dentate gyrus of the hippocampus is positively associated with memory (Voss et al., 2013). Running did not improve memory in the KO mice. There was a reduction in immature adult-born DCX+ Type C cells in KO mice, main effect of genotype (F(1,28)=7.735, p<0.0074; Figure 2E). DCX+ Type D cell counts showed main effects of running (F(1,28)=20.224, p<0.0001) and genotype (F(1,28)=13.438, p<0.0005; Figure 2F). Type D cells were increased in the WT-R group as compared to all other groups (p<0.0002). DCX staining is shown for each group (Figure 2G).
Electrophysiological recordings from dentate GCs
To determine whether CTSB KO affects memory function by changing physiological properties of mature dentate granule GCs, patch-clamp recordings were made of developmentally-born GCs (Nowakowski and Rakic, 1981). To evaluate GABAergic inhibitory transmission, recordings of miniature inhibitory postsynaptic currents (mIPSCs) were performed. mIPSCs frequency was reduced in cells (n=15) derived from KO mice as compared to cells (n=12) from WT (t(25)=3.388, p<0.005), suggesting reduced inhibitory neurotransmission onto GCs (Figure 2H–L). Examination of intrinsic properties of mature GCs revealed a more depolarized resting membrane potential in KO (−82.8 ± 2.2 mV) as compared to WT (−88.4 ± 0.49 mV) cells (t(17)=2.62, p< 0.02). Other intrinsic properties, such as input resistance, membrane time constant and capacitance did not differ between groups (P>0.05).
Permeability of CTSB across the Blood-Brain-Barrier (BBB)
To test whether CTSB can cross the BBB rCTSB (50 µg per mouse) or vehicle (distilled water) was injected intravenously (i.v.) into CTSB KO mice. Fifteen minutes after i.v. CTSB injection, there was a significant increase in blood (104.21 ± 4.11 ng/ml vs control 2.25 ± 2.20 ng/ml; t(4)=21.87, p<0.0001) and tissue derived from whole brain (8.17 ± 0.90 ng/ml vs control 1.005 ± 0.46 ng/ml; t(4)=7.08, p<0.021) CTSB levels.
CTSB enhances DCX and BDNF levels in aNPCs
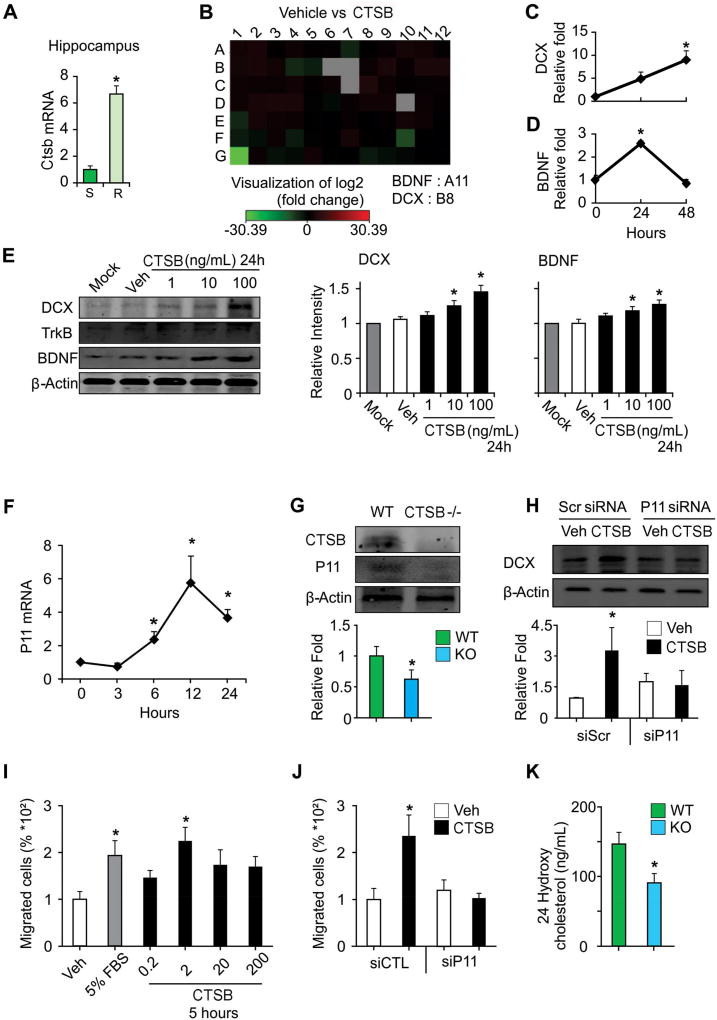
Running increases hippocampal Ctsb mRNA (Figure 3A) and adult neurogenesis. Therefore, we applied exogenous CTSB to hippocampal progenitor cells with different dosages for 24 hours. There was no effect on proliferation (live cell portion) or survival of aNPCs compared to controls (Figure S2A and S2B). We further utilized neurogenesis pathway specific PCR arrays (SAbioscience) to screen for genes regulated by rCTSB treatment in the aNPCs. Of the 86 genes, 15 genes exhibited a consistently changed expression level with 24 hour treatment of 100 ng/ml rCTSB as compared to basal differentiation media (Figure 3B and Table S1). Two genes relevant to neurogenesis, Dcx and Bdnf, were selected for validation of PCR array screening. Dcx mRNA (F(2,9)=5.74, p<0.03) and Bdnf mRNA expression increased (F(2,6)=75.88, p<0.0001) with rCTSB (100ng/ml) treatment, respectively (Figure 3C and 3D). Specifically, Dcx mRNA expression after 48 hours of rCTSB was elevated (p<0.008). Furthermore, 24 hours of rCTSB increased Bdnf mRNA compared to control (p<0.0001; Figure 3C and 3D). Administration of rCTSB (10 and 100 ng/ml) for 24 hours increased DCX (F(4,10)=9.03, p<0.0023) and BDNF (F(4,10)=5.69, p<0.012) levels in aNPCs (Figure 3E).
Figure 3.
Running-induced hippocampal Ctsb gene expression and analysis of CTSB function (A) Thirty days of running (R) increased hippocampal Ctsb mRNA levels compared to sedentary (S) controls. (B) aNPC cultures were treated with rCTSB (100 ng/mL) for 24 h and analyzed by Q-PCR arrays. (C–D) Dcx and Bdnf gene expression were among the top ten most elevated genes. RT-PCR analysis shows a significant increase in Dcx mRNA level after 48 hours and Bdnf mRNA level after 24 hours of rCTSB (100 ng/ml) treatment as compared to control (0 hour). (E) WB analysis of DCX, TrkB and BDNF protein levels in aNPCs treated with rCTSB (10 and 100 ng/ml) or Vehicle (Veh, Distilled water) for 24 h. The graphs show the relative intensity normalized by β-actin. rCTSB treatment significantly increased DCX and BDNF levels, as compared to Mock or Vehicle treatment. (F) P11 mRNA levels in PC12 cells are elevated 6, 12 and 24 hours after treatment with rCTSB, 100 ng/ml. Hsp90 gene was used for normalization. (G) Hippocampal CTSB and P11 levels in CTSB KO and WT littermates measured by WB analysis, β-actin was used as a control. P11 was significantly reduced in the CTSB KO. (H) Knockdown of P11 with si-RNA in PC12 cells decreased DCX protein level elevation by rCTSB (100 ng/ml), (Scr, scramble siRNA transfected cells) measured by WB analysis, β-actin was used as a control. (I, J) Effect of rCTSB (0.2, 2, 20 or 200 ng/ml) on cell migration. (I) Percentage of migrated PC12 cells treated with 5% FBS or 2ng/ml of rCTSB showed increased migration. (J) Knockdown of P11 in PC12 cells suppressed cell migration induced by rCTSB (2ng/ml) treatment for 5 hours as compared to Scr control. (K) Hippocampal 24-hydroxy cholesterol level was reduced in the CTSB KO mice. Data represent mean ± SEM. *p < 0.05. Veh, vehicle (Distilled water).
Involvement of P11 protein in CTSB effects on neuronal cells
Exercise increases hippocampal P11 (S100A10) expression (Sartori et al., 2011). In PC12 cells P11 level was enhanced by rCTSB (F(4,10)=6.72, p<0.007; Figure 3F, Figure S2D), while hippocampal P11was reduced in KO mice (t(7)=2.41, p<0.05; Figure 3G). In addition, 12 (p<0.002) and 24 hours (p<0.041) of rCTSB (100ng/ml) increased Dcx mRNA compared to control (0 hour; Figure S2C). P11 knockdown using siRNA altered rCTSB treatment effect on DCX in PC12 cells (F(1,8)=9.36, p<0.02). rCTSB elevated DCX levels in control but not P11 knockdown conditions (Figure 3H).
CTSB plays a role in cancer cell migration (Olson and Joyce, 2015). Five hours of rCTSB treatment affected PC12 cell migration (F(5,30)=2.55, p<0.048). Specifically, rCTSB (2 ng/ml) increased PC12 cell migration (p<0.003; Figure 3I). There was also a significant interaction between P11 siRNA transfection and rCTSB treatment (F(3,16)=5.01, p<0.012) on PC12 cell mobility (Figure 3I). rCTSB did not enhance migration in P11 knockdown PC12 cells (Figure 3J and Figure S2E).
P11 is associated with cholesterol-rich platforms on endosomal membranes (Morel and Gruenberg, 2007) and CTSB is also involved in peripheral cholesterol absorption (Wong et al., 2013). Hippocampal 24-hydoxycholesterol levels, the form of brain specific cholesterol, was diminished in CTSB KO mice compared to WT (t(10)=2.55, p<0.03; Figure 3K).
Treadmill training increases CTSB plasma levels Rhesus monkeys and humans
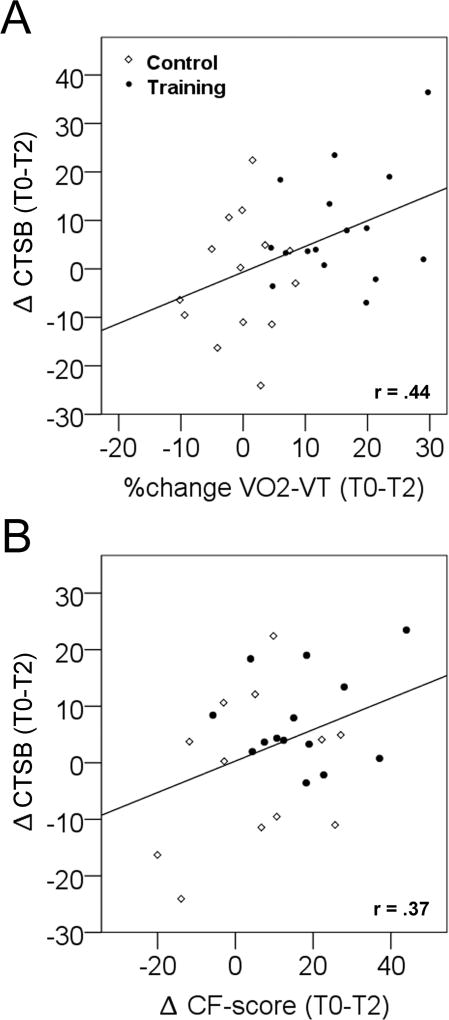
We evaluated CTSB levels in Rhesus monkeys and humans. In monkeys CTSB plasma levels were significantly greater in the exercise group (4 months of treadmill training) compared to control subjects (p<0.02; Table S2A). In humans we analyzed two forms of Cathepsin, L (CTSL) and CTSB. CTSB plasma levels in the training group differed significantly from control after four months of treadmill exercise (p<0.048). There was no effect on CTSL. In addition, Pearson correlation analyses across groups revealed a positive correlation between fitness increase (percentage change of VO2-VT) and changes in CTSB level after four months of treadmill exercise (r=0.44; p<0.016; Figure 4A, Table S2B).
Figure 4.
Effects of four months of treadmill exercise on correlations between plasma CTSB, fitness and complex figure recall (CF) in humans. (A) There is a positive correlation in humans between aerobic fitness change as measured by VO2 ventilatory threshold (VT) and CTSB plasma protein level change after 4 months of treadmill training intervention (p<0.016). (B) There is a positive correlation between change in CF score and CTSB level change (p<0.01, one-tailed; p=0.063, two-tailed) after 4 months of treadmill training.
Cognition and CTSB plasma levels in humans
Human subjects were tested in complex figure (CF) drawing recall test. There was a positive correlation between pre-post differences in CTSB plasma levels and late complex-object recall score (CF-score) changes (r=0.37; p<0.01, one-tailed; p=0.063, two-tailed). Partial correlations with VO2-VT as a control variable eliminated the correlation between CTSB and the CF-score (r=0.296; p=0.16) indicating that the relationship between CTSB and complex-figure recall was dependent on aerobic fitness (Figure 4B, Table S2B).
DISCUSSION
Benefits of exercise for brain function depend on central and peripheral factors. Candidate myokine CTSB may be important for brain plasticity. In vitro, AMPK activation elicited CTSB secretion in skeletal muscle cells. In vivo, exercise elevated CTSB plasma levels and hippocampal Ctsb gene expression, suggesting both direct and indirect CTSB effects on brain function. Ctsb gene KO precluded exercise induced enhancement of retention of spatial memory and adult neurogenesis, and reduced inhibitory transmission onto dentate GCs and decreased hippocampal P11, a protein needed for CTSB effects on neuronal differentiation and migration. In primates, treadmill training elevated CTSB plasma levels, and may contribute to exercise-induced memory benefits in humans.
Lysosomal cysteine protease CTSB is ubiquitously expressed throughout the body (Turk et al., 2012). High levels are found in multiple human cancers (Aggarwal and Sloane, 2014). The role of CTSB in normal physiology has remained unexplored. CTSB was increased in plasma after long-term training in mice, Rhesus monkeys and humans. Voluntary wheel running in mice elevated CTSB in plasma and gastrocnemius muscle, but not in other organs. Long-term exercise can cause muscle injury and inflammation. Transport and breakdown of amino acids and activation of the immune response are required for muscle repair. Indeed, gastrocnemius tenotomy upregulated CTSB and L activity (Harris and Baillie, 1990). Our time-course of CTSB increase is consistent with studies indicating that muscle regeneration after exercise-induced lysosomal activation takes several weeks (Salminen et al. 1984).
Our study suggests that CTSB is a myokine that can cross the BBB. However, the role of CTSB in brain function has been controversial. In a transient ischemia model, a CTSB inhibitor prevented neuronal cell death (Yoshida et al., 2002). In addition, CTSB was considered as a protease involved in cell death after brain injury (Bannerjee et al., 2015) and onset of Alzheimer’s Disease (Hook et al., 2008). However, CTSB is also reportedly neuroprotective (Bendiske and Bahr, 2003) with anti-amyloidogenic properties (Mueller-Steiner et al., 2006). Furthermore, in double-KO mice lacking both CTSB and Cathepsin L (CTSL) there is brain atrophy (Felbor et al., 2002).
CTSB may mediate the benefits of exercise for brain function through several pathways. Running increased whole hippocampus Ctsb gene expression. Running induces hypoxia (Radak et al., 2013) which in turn may elevate brain CTSB levels (Yakovlev and Gulyaeva, 2015). This could promote clearance of neural debris (Devi and Kiran, 2004) and adult neurogenesis, a process implicated in memory function (Abrous and Wojtowicz, 2015). Running increased neurogenesis in WT but not CTSB KO mice. In vitro analyses are consistent with in vivo observations. Dcx and Bdnf levels increased after rCTSB administration in aNPCs. Consistently, inhibition of both CTSB and CTSL reduced hippocampal Bdnf expression (Bednarski et al., 1998). BDNF regulates synaptic plasticity, cell survival and differentiation (Chao et al., 2006). DCX is important for neuronal migration, a process critical for brain development (Kawauchi, 2015).
In WT, but not KO, mice running improved spatial memory. These observations are compatible with the human exercise results. Plasma CTSB, but not CTSL (Felbor et al., 2012), levels increased after 4 months of exercise and were positively correlated with fitness levels. Humans were also tested for complex figure (CF) recall, a task that is strongly dependent on the hippocampus (Vargha-Khadem et al., 1997). The positive relationship of CTSB with complex-spatial object recall (CF) was dependent on exercise-induced changes in aerobic fitness. Aerobic activity is also associated with an increase in hippocampal volume (Duzel et al., 2016). It will be of interest to measure whether CTSB levels are correlated with hippocampal gray matter volume.
In the periphery multifunctional adaptor protein P11, Annexin A II light chain, is a known binding partner of CTSB in caveolae of human umbilical vein endothelial cells (Cavallo-Medved et al., 2009). Annexin A2 down-regulation decreased CTSB expression in human lung adenoma cells (Wang et al., 2012). In the brain, exercise increases hippocampal P11 (Sartori et al., 2011). P11 regulates serotonin (Svenningsson et al., 2013), important for exercise-induced neurogenesis (Klempin et al., 2013), as well as glutamate and GABA (Eriksson et al., 2013). Hippocampal P11 expression and inhibitory neurotransmission onto dentate granule was reduced was reduced in CTSB KO mice. Consistently, hippocampal GABA is lower in P11 KO mice (Eriksson et al., 2013). In vitro, DCX and migration of PC12 cells (Westerink and Ewing, 2008), induced by CTSB treatment was diminished by P11 knockdown. P11 is also associated with the cholesterol formation (Morel and Gruenberg, 2007) and GABAergic signaling is affected by membrane cholesterol amount (Sooksawate and Simmonds, 2001). Reduced 24-hydroxycholesterol in CTSB KO hippocampi may affect cognition.
Exercise increases CTSB in mouse and primate plasma. CTSB deficiency in mice precludes benefits of running on spatial memory. In humans there is a positive correlation between CTSB levels, fitness and hippocampus-dependent memory. These findings expand our understanding of how exercise-induced peripheral factors boost brain function.
EXPERIMENTAL PROCEDURES
Subjects
For animal experiments, one-month-old C57Bl/6 male (n=64) mice were purchased from Jackson Labs. Mice were individually housed in standard conditions with food and water ad libitum.
For Rhesus monkey studies, monkeys [n=13 (3 female, 10 male), 6.9~ 20.7 years old] are housed individually in standard nonhuman primate caging on a 12h light/12h dark cycle.
For human studies, healthy young adults [n=43 (24 female), 19~ 34 years old] were enrolled in the study. Subjects were randomly assigned to either the training or control group, matched by gender, age and body mass index.
Cell culture
Rat L6 skeletal myoblast cells (ATCC CRL-1458, VA) were grown in Dulbecco's Modified Eagle's Medium (DMEM; Gibco, NY) supplemented with 10% fetal bovine serum (FBS).
The aNPCs were grown in Neurobasal medium with B27 supplement (1:50), L-glutamine (1:100), EGF (20 ng/ml), bFGF (20 ng/ml) and heparin (20 ng/ml).
PC12 cells were grown in DMEM (Gibco, NY) supplemented with 10% FBS.
Molecular analyses
For qPCR analysis, total RNA was extracted from L6, aNPC, PC12 cells and various tissues using a total RNA extraction kit (Ribozol, Amresco) according to the manufacturer’s manual.
For western blotting, equal amounts of protein lysates from cells and tissues were subjected to SDS-PAGE and transferred to a NC membrane (Millipore, MA). Specific signals were visualized by Odyssey (Amersham Bio-sciences).
For the ELISA assays, species-specific ELISA kits were used according to the manufacturer’s specifications. The plate was read using SpectraMax Plus 384 Microplate Reader (Molecular Devices, Sunnyvale, CA).
Behavioral testing
Mice were housed individually in standard or running wheel cages. After four weeks mice were tested in the Open Field, Rotarod, Morris Water Maze, Forced Swim Test, Sucrose Preference Test and Elevated Plus Maze.
For detailed methods, see Supplemental Experimental Procedures.
Supplementary Material
Highlights.
Proteomics of AICAR treated L6 muscle cells revealed myokine Cathepsin B (CTSB).
Exercise increases CTSB levels in mouse, monkey, and human plasma.
Running does not improve memory or adult neurogenesis in CTSB Knockout mice.
CTSB enhances neurotrophin levels in adult hippocampal progenitor cells.
In humans plasma CTSB levels are positively correlated with fitness and memory.
Acknowledgments
This work was supported in part by the National Institute on Aging, Intramural Research Program, the Korean Visiting Scientist Training Award (KVSTA to H.Y.M.) and the Deutsche Forschungs Gemeinschaft (SFB 779 TP A7). We thank Drs. Young Ah Goo and Hyung Won Choi for Proteomic analysis, Yang An for statistical advice and Linda R. Kitabayashi for image preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
H.Y.M., E.D., H.v.P. designed experiments. M.H.Y, A.B., D.B., B.B., N.S., G.B., E.J., S.L., N.G., J.M. performed experiments. M.H.Y., A.B., D.B., B.B., N.S., E.J., E.D., H.v.P. analyzed the data. H.Y.M. and H.v.P. wrote the manuscript.
References
- Abrous DN, Wojtowicz JM. Interaction between neurogenesis and hippocampal memory system: new vistas. CSH Persp. Biol. 2015;7 doi: 10.1101/cshperspect.a018952. pii:a018952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal N, Sloane BF. CTSB: multiple roles in cancer. Proteomics Clin. Appl. 2014;8:427–437. doi: 10.1002/prca.201300105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agudelo LZ, Femenia T, Orhan F, Porsmyr-Palmertz M, Goiny M, Martinez-Redondo V, Correia JC, Izadi M, Bhat M, Schuppe-Koistinen I, et al. Skeletal muscle PGC-1alpha1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell. 2014;159:33–45. doi: 10.1016/j.cell.2014.07.051. [DOI] [PubMed] [Google Scholar]
- Banerjee M, Sasse VA, Wang Y, Maulik M, Kar S. Increased levels and activity of cathepsins B and D in kainate-induced toxicity. Neuroscience. 2015;284:360–373. doi: 10.1016/j.neuroscience.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Bednarski E, Lauterborn JC, Gall CM, Lynch G. Lysosomal dysfunction reduces brain-derived neurotrophic factor expression. Exp. Neurol. 1998;150:128–135. doi: 10.1006/exnr.1997.6747. [DOI] [PubMed] [Google Scholar]
- Bendiske J, Bahr BA. Lysomal activation is a compensatory response against protein accumulation and associated synaptopathogenesis – an approach for slowing Alzheimer Disease? J. Neuropath. and Exp. Neurol. 2003;62:451–463. doi: 10.1093/jnen/62.5.451. [DOI] [PubMed] [Google Scholar]
- Cavallo-Medved D, Rudy D, Blum G, Bogyo M, Caglic D, Sloane BF. Live-cell imaging demonstrates extracellular matrix degradation in association with active Cathepsin B in caveolae of endothelial cells during tube formation. Exp. Cell Res. 2009;315:1234–1246. doi: 10.1016/j.yexcr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MV, Rajagopal R, Lee FS. Neurotrophin signaling in health and disease. Clin. Sci. 2006;110:167–173. doi: 10.1042/CS20050163. [DOI] [PubMed] [Google Scholar]
- Czibere L, Baur LA, Wittmann A, Gemmeke K, Steiner A, Weber P, Putz B, Ahmad N, Bunck M, Graf C, et al. Profiling trait anxiety: transcriptome analysis reveals cathepsin B (Ctsb) as a novel candidate gene for emotionality in mice. PloS ONE. 2011;6:e23604. doi: 10.1371/journal.pone.0023604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi SA, Kiran TR. Regional responses in antioxidant system to exercise training and dietary vitamin E in aging rat brain. Neurobiol. of Aging. 2004;25:501–508. doi: 10.1016/S0197-4580(03)00112-X. [DOI] [PubMed] [Google Scholar]
- Duzel E, van Praag H, Sendtner M. Can physical exercise in old age improve memory and hippocampal function? Brain. 2016;139:662–673. doi: 10.1093/brain/awv407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson TM, Alvarsson A, Stan TL, Zhang X, Hascup KN, Hascup ER, Kehr J, Gerhardt GA, Warner-Schmidt J, Arango-Lievano M, Kaplitt MG, et al. Bidirectional regulation of emotional memory by 5-HT1B receptors involves hippocampal p11. Mol. Psych. 2013;18:1096–105. doi: 10.1038/mp.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felbor U, Kessler B, Mothes W, Goebel HH, Ploegh HL, Bronson RT, Olsen BR. Neuronal loss and brain atrophy in mice lacking cathepsins B and L. PNAS. 2002;99:7883–7888. doi: 10.1073/pnas.112632299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris CI, Baillie AG. The localized elevation of cathepsins B and L in rat gastrocnemius muscle following tenotomy. Biochem. Soc. Trans. 1990;18:1254–5. doi: 10.1042/bst0181254. [DOI] [PubMed] [Google Scholar]
- Hawley JA, Hargreaves M, Joyner MJ, Zierath JR. Integrative biology of exercise. Cell. 2014;159:738–749. doi: 10.1016/j.cell.2014.10.029. [DOI] [PubMed] [Google Scholar]
- Hook VY, Kindy M, Hook G. Inhibitors of cathepsin B improve memory and reduce beta-amyloid in transgenic Alzheimer disease mice expressing the wild-type, but not the Swedish mutant, beta-secretase site of the amyloid precursor protein. J. Biol. Chem. 2008;283:7745–7753. doi: 10.1074/jbc.M708362200. [DOI] [PubMed] [Google Scholar]
- Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, Chen JW, Lee RT, Wagers AJ, Rubin LL. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014;344:630–634. doi: 10.1126/science.1251141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi T. Cellullar insights into cerebral cortical development: focusing on the locomotion mode of neuronal migration. Front. Cell. Neurosci. 2015;7:394. doi: 10.3389/fncel.2015.00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempin F, Beis D, Mosienko V, Kempermann G, Bader M, Alenina N. Serotonin is required for exercise-induced adult hippocampal neurogenesis. J. Neurosci. 2013;33:8270–5. doi: 10.1523/JNEUROSCI.5855-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobilo T, Guerrieri D, Zhang Y, Collica SC, Becker KG, van Praag H. AMPK agonist AICAR improves cognition and motor coordination in young and aged mice. Learn. Mem. 2014;21:119–126. doi: 10.1101/lm.033332.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobilo T, Yuan C, van Praag H. Endurance factors improve hippocampal neurogenesis and spatial memory in mice. Learn. Mem. 2011;18:103–107. doi: 10.1101/lm.2001611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire R, Flipo RM, Migaud H, Fontaine C, Huet G, Dacquembronne E, Lafyatis R. Alternative splicing of the 5' region of cathepsin B pre-messenger RNA in rheumatoid synovial tissue. Arthritis Rheum. 1997;40:1540–1542. doi: 10.1002/art.1780400824. [DOI] [PubMed] [Google Scholar]
- Morel E, Gruenberg J. The p11/S100A10 light chain of annexin A2 is dispensable for annexin A2 association to endosomes and functions in endosomal transport. PloS ONE. 2007;2:e1118. doi: 10.1371/journal.pone.0001118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller-Steiner S, Zhou Y, Arai H, Roberson ED, Sun B, Chen J, Wang X, Yu G, Esposito L, Mucke L, et al. Anti-amyloidogenic and neuroprotective functions of cathepsin B: implications for Alzheimer's disease. Neuron. 2006;51:703–714. doi: 10.1016/j.neuron.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Nowakowski RS, Rakic P. The site of origin and route and rate of migration of neurons to the hippocampal region of the rhesus monkey. J. Comp. Neurol. 1981;196:129–154. doi: 10.1002/cne.901960110. [DOI] [PubMed] [Google Scholar]
- Olson OC, Joyce JA. Cysteine cathepsin proteases: regulators of cancer progression and therapeutic response. Nat. Rev. Cancer. 2015;15:712–729. doi: 10.1038/nrc4027. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol. Rev. 2008;88:1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- Radak Z, Zhao Z, Koltai E, Ohno H, Atalay M. Oxygen consumption and usage during physical exercise: the balance between oxidative stress and ROS-dependent adaptive signaling. Antioxid. & Redox Signal. 2013;18:1208–1246. doi: 10.1089/ars.2011.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A, Hongisto K, Vihko V. Lysosomal changes related to exercise injuries and training-induced protection in mouse skeletal muscle Acta. Physiol. Scand. 1984;120:15–9. doi: 10.1111/j.1748-1716.1984.tb07367.x. [DOI] [PubMed] [Google Scholar]
- Sartori CR, Vieira AS, Ferrari EM, Langone F, Tongiorgi E, Parada CA. The antidepressive effect of the physical exercise correlates with increased levels of mature BDNF, and proBDNF proteolytic cleavage-related genes, p11 and tPA. Neuroscience. 2011;180:9–18. doi: 10.1016/j.neuroscience.2011.02.055. [DOI] [PubMed] [Google Scholar]
- Sooksawate T, Simmonds MA. Effects of membrane cholesterol on the sensitivity of the GABA(A) receptor to GABA in acutely dissociated rat hippocampal neurons. Neuropharm. 2001;40:178–184. doi: 10.1016/s0028-3908(00)00159-3. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Kim Y, Warner-Schmidt J, Oh YS, Greengard P. p11 and its role in depression and therapeutic responses to antidepressants. Nat. Rev. Neurosci. 2013;14:673–680. doi: 10.1038/nrn3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk V, Stoka V, Vasiljeva O, Renko M, Sun T, Turk B, Turk D. Cysteine cathepsins: from structure, function and regulation to new frontiers. Biochim. Biophys. Acta. 2012;1824:68–88. doi: 10.1016/j.bbapap.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargha-Khadem F, Gadian DG, Watkins KE, Connelly A, Van Paesschen W, Mishkin M. Differential effects of early hippocampal pathology on episodic and semantic memory. Science. 1997;277:376–380. doi: 10.1126/science.277.5324.376. [DOI] [PubMed] [Google Scholar]
- Voss MW, Vivar C, Kramer AF, van Praag H. Bridging animal and human models of exercise-induced brain plasticity. TICS. 2013;17:525–544. doi: 10.1016/j.tics.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YX, Lv H, Li ZX, Li C, Wu XY. Effect of shRNA mediated down-regulation of Annexin A2 on biological behavior of human lung adenocarcinoma cells A549. Pathology oncology research: Pathol. Oncol. Res. 2012;18:183–190. doi: 10.1007/s12253-011-9427-2. [DOI] [PubMed] [Google Scholar]
- Westerink RH, Ewing AG. The PC12 cell as model for neurosecretion. Acta Physiol. 2008;192:273–285. doi: 10.1111/j.1748-1716.2007.01805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong WP, Altemus JB, Hester JF, Chan ER, Cote JF, Serre D, Sehayek E. Cathepsin B is a novel gender-dependent determinant of cholesterol absorption from the intestine. J. Lipid Res. 2013;54:816–822. doi: 10.1194/jlr.M034579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrann CD. FNDC5/Irisin – Their role in the nervous system and as a mediator for beneficial effects of exercise on the brain. Brain Plasticity. 2015;1:55–61. doi: 10.3233/BPL-150019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovlev AA, Gulyaeva NV. Possible Role of Proteases in Preconditioning of Brain Cells to Pathological Conditions. Biochemistry. 2015;80:163–171. doi: 10.1134/S0006297915020030. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Yamashima T, Zhao L, Tsuchiya K, Kohda Y, Tonchev AB, Matsuda M, Kominami E. Primate neurons show different vulnerability to transient ischemia and response to cathepsin inhibition. Acta. Neuropathol. 2002;104:267–272. doi: 10.1007/s00401-002-0554-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.