Abstract
Alkali metal-rare earth polyphosphates LiGd(1-x)Eux(PO3)4 (LGP:Eu3+) (where x= 0, 0.02 and 0.04) were synthesized by solid-state reaction. The Rietveld refinement showed the following cell parameters: I 2/a space group, a=9.635(3) Å, b=7.035(3) Å, c=13.191(3) Å, β=90.082°, V= 894.214Å3, and Z=4. The similarity between RF=4.21% and RB=4.31% indicated that the realized refinement is reliable. The crystal structure consists of infinite zig-zag chains of (PO4)3- tetrahedra, linked by bridging oxygen. The acyclic structure of polyphosphates is confirmed by infrared and Raman (IR) spectroscopies. A good thermal stability up to 940°C and paramagnetic behavior of these compounds were also proved by thermal analyses and magnetic susceptibility measurements, respectively. Excitation spectra revealed the charge transfer phenomenon between O2- and Eu3+ (CTB), the energy transfer from Gd3+ to Eu3+, and the intrinsic 4f-4f transitions of Eu3+ where the electronic transitions were also identified. Moreover, LGP:Eu3+ can emit intense reddish orange light under excitation at 394 nm. The strongest tow at 578 and 601 nm can be attributed to the transitions from excited state 5D0 to ground states 7F1 and 7F2, respectively.
1. Introduction
Condensed alkali metal-rare earth polyphosphates, with the general formula MIREIII(PO3)4 (where MI= are alkali metal and REIII= are rare earth ions), have been extensively investigated thanks to their structural diversity [1–4] and their interesting magnetic [5], optic [6], and electric [7] proprieties. These polyphosphates are generally stable in normal conditions of temperature and humidity [8], which makes them useful for industrial applications. For example, Yamada et al. have used the LiNd(PO3)4 polyphosphate as a solid-state laser material [9, 10]. However, Z. Mua et al. have used LiEu(PO3)4 compound for white light-emitting diodes [11]. They are also used as promising scintillation material such as the Ce3+ doped MGd(PO3)4 compound [12].
In order to enhance the optical properties of polyphosphates, researches were oriented to doping them with metal-rare earths. In recent years, a great interest was accorded to europium earth-rare due to its outstanding photoluminescence feature [13–19]. In fact, the presence of well-defined energy levels in europium allows the emission of monochromatic and coherent radiations in solid laser.
The Gd–Eu couple is well known for its efficient conversion of the absorbed high-energy photons into two visible ones [20–24]. This phenomenon may be followed by a sequence of two steps of energies transfer. The first step is the transition 6GJ→6PJ of Gd3+, involving the 5D0→7F1 transition of the Eu3+ ion. The one red photon related to the Eu3+ 5D0 → 7FJ transition is created. In the second step, the energy of Gd3+ is related to 6P7/2→ 8S transition which is transported over the Gd3+ sublattice and then transferred to another Eu3+ ion. This phenomenon leads to 5DJ emission (J = 0, 1, 2, or 3) [25].
This work describes the synthesis of LiGd(1-x)Eux(PO3)4 polyphosphate, doped with different low percentages of europium (2 and 4%). The structural study of all obtained compounds is carried out with XRD diffraction. The infrared and Raman spectroscopies and magnetic and thermal analyses were recorded at room temperature. Moreover, the optical study through excitation and emission of Eu3+ ions spectra was also undertaken.
2. Experimental
The condensed phosphates LiGd(1-x)Eux(PO3)4 (where x= 0, 0.02 and 0.04) were synthesized by solid-state reaction (methods of Hammami et al. 2017) [26]. A mixture of the reagents, Li2CO3, Gd2O3, Eu2O3, and NH4H2PO4, was prepared with the molar ratio (2.1:1:8) of Li:Gd:P, respectively. First, the raw materials were grounding in an agate mortar for one hour at least to homogenize the solid phase and improve the interatomic diffusion. Second, the mixture was introduced into the oven and submitted to the following thermal program. The first level was at 430°C to eliminate H2O, NH3, and CO2, the second one was at 730°C to get LiGd(1-x)Eux(PO3)4 pure phase. Then, the obtained products were cooled with the rate of 2°C/min to ensure a better crystallinity. Finally, the synthesized polyphosphates were washed with boiling water and nitric acid solution (1mol/L) to eliminate the residual raw materials from the final product.
The proposed chemical reaction for polyphosphate synthesis is
| (1) |
Samples were characterized using an INEL XRG 3000 (D5000T) diffractometer with monochromatic Cu Kα radiation. The diffraction pattern was recorded under 300K over the angular range 10-90° (2θ). The luminescence spectra were performed under ambient atmosphere via Xenius (the fluorescence Genius) spectrophotometer, at 591nm and 394nm for excitation and emission, respectively. The infrared spectra were recorded in the range of 250–1500 cm−1 with a Thermo Scientific Nicolet N10 MX using sample dispersed in KBr pellets. Raman analysis was carried out at room temperature, with 514.5 nm radiation from an argon ion laser as the excitation beam. A microscope allowed a selection of high optical quality regions in the crystalline sample. Thermal stability of Eu3+ doped LGP was measured with differential thermal analysis SETARAM TAG 16 operating from room temperature up to 1000°C with heating rate of 5°C min−1. Magnetic measurements were carried out using Quantum Design MPMSXL magnetometer with detection SQUID (at institute NEEL France).
3. Results and Discussion
3.1. Rietveld Refinement Data Analyses
The Rietveld refinement of X-ray diffraction patterns of synthesized LiGd(PO3)4 samples is shown in Figure 1. The graph presents the experimental and the calculated data as well as the difference between them. As it is shown, the presence of only single phase was checked by Rietveld fitting quality through the reliability R factors: Rexp, Rbrag, profile Rp, and weighted profile Rwp, which should be less than 10%. The final R factors, atomic coordinates, site occupancy, thermal displacement parameters, and their estimated standard deviations in parentheses for LGP are shown in Table 1. Interatomic bond distances and angles are given in Table 2. The new lattice parameters, derived from the Rietveld refinement, are a=9.635(3) Å, b= 7.035(3) Å, c= 13.191(3) Å, and β= 90.082° and with monoclinic space group I 2/a.
Figure 1.

The Rietveld analysis of X-ray diffraction patterns for LiGd(PO3)4.
Table 1.
Refined structure parameters from powder X-ray Rietveld analysis for LiGd(PO3)4 in space group I 2/a.
| Atom | Wyck | x | y | z | B | Occ |
|
| ||||||
| Gd | 4e | 0.75000 | 0.2982(3) | 0.00000 | 1.35(8) | 0.50 |
|
| ||||||
| Li | 4e | 0.75000 | 0.79603(4) | 0.00000 | 1.88(16) | 0.50 |
|
| ||||||
| P1 | 8f | 0.48189(3) | 0.06204(4) | 0.143135(20) | 1.88(16) | 1.00 |
|
| ||||||
| P2 | 8f | 0.54601(3) | 0.66399(4) | 0.15354(2) | 1.88(16) | 1.00 |
|
| ||||||
| OL12 | 8f | 0.5665(6) | 0.87995(6) | 0.1598(14) | 2.7(2) | 1.00 |
|
| ||||||
| OL21 | 8f | 0.4057(4) | 0.0815(16) | 0.2447(3) | 2.7(2) | 1.00 |
|
| ||||||
| OE21 | 8f | 0.6520(11) | 0.59342(6) | 0.0754(8) | 2.7(2) | 1.00 |
|
| ||||||
| OE11 | 8f | 0.5743(13) | 0.2282(14) | 0.1098(11) | 2.7(2) | 1.00 |
|
| ||||||
| OE22 | 8f | 0.3953(6) | 0.626(2) | 0.1220(12) | 2.7(2) | 1.00 |
|
| ||||||
| OE12 | 8f | 0.3696(12) | 0.0106(3) | 0.0652(10) | 2.7(2) | 1.00 |
|
| ||||||
| R p = 1.237 | R wp= 1.598 | R exp = 1.134 | R Bragg= 4.301 | χ 2= 2 | ||
Table 2.
Atomic distances(Å) and angles in LiGd(PO3)4 with standard deviations in parentheses.
| Tetrahedra | aroundP1 | ||||
|
| |||||
| P1-OL12 | 1.534(4) | OL12 -OL21 | 2.381(12) | OL12 i —P1—OE11 | 111.61(38) |
|
| |||||
| P1-OL21 | 1.535(4) | OL12 -OE21 | 2.446(11) | OL12 i —P1—OL21 | 101.76(23) |
|
| |||||
| P1-OE11 | 1.534(11) | OL12 -OL21 | 2.463(14) | OL12 i —P1—OE12 | 105.86(29) |
|
| |||||
| P1-OE12 | 1.535(12) | OL12 -OE11 | 2.538(11) | OL21—P1—OE11 | 117.46(53) |
|
| |||||
| OL12 -OE22 | 2.482(12) | OL21—P1—OE12 | 105.55(40) | ||
|
| |||||
| OL12 -OE12 | 2.448(15) | OE12—P1—OE11 | 113.37(47) | ||
|
| |||||
| Tetrahedra | around P2 | ||||
|
| |||||
| P2 -OL12 | 1.5343(13) | OE22 -OL12 | 2.482(12) | OE22—P2—OE21 | 113.09(39) |
|
| |||||
| P2 -OL21 | 1.534(6) | OE22 -OL21 | 2.619(12) | OE22—P2—OL12 | 108.04(41) |
|
| |||||
| P2 -OE21 | 1.535(10) | OE22 -OE21 | 2.560 | OE22—P2—OL21 vi | 117.27(36) |
|
| |||||
| P2 -OE22 | 1.533(7) | OL21 -OL12 | 2.381(12) | OE21—P2—OL12 | 105.66(4) |
|
| |||||
| OL21 -OL12 | 2.440(11) | OE21—P2—OL21 vi | 105.25(16) | ||
|
| |||||
| OE21 -OE22 | 2.560(13) | OL12—P2—OL21 vi | 106.79(38) | ||
|
| |||||
| Polyhedra | around Gd |
Tetrahedra around Li |
|||
|
| |||||
| Gd -OE21 | 2.489(6) | Li -OE21 | Gd -Gd iv | 5.592(2) | |
|
| |||||
| Gd -OE21 | 2.489(6) | Li -OE21 | Li -Gd ii | 3.533(2) | |
|
| |||||
| Gd -OE11 | 2.283(13) | Li -OE12 | Li -Li v | 5.068(0) | |
|
| |||||
| Gd -OE11 | 2.283(13) | Li -OE12 | P1 - P2 | 2.8710(4) | |
|
| |||||
| Gd-OE22 | 2.197(13) | P2 -P1 | 2.7897(4) | ||
|
| |||||
| Gd-OE22 | 2.197(13) | Gd -P1 iii | 6.256(2) | ||
|
| |||||
| Gd-OE12 | 2.605(7) | ||||
|
| |||||
| Gd -OE12 | 2.605(7) | ||||
|
| |||||
|
Symmetry code |
i: x,1+y,z | ii:x,1+y,z iii:1.5-x,1+y,-z |
iv:2-x,1-y,-z v:2-x,2-y,-z |
vi:1-x,0.5+y,0.5-z | |
The LGP structure can be simply described as three-dimensional framework of GdO8 polyhedra linked to (PO4)3– rings by Gd-O-P bridges. This framework delimits interesting tunnels with Li+ ions which are bonded to four oxygen atoms (LiO4). Each LiO4 tetrahedron shares all four O atoms with two LaO8 polyhedra and four different PO4 tetrahedra. A view of this structure projected along the b axis is shown in Figure 2.
Figure 2.

The structural arrangement of the LiGd(PO3)4 viewed in the (0 1 0) plane.
3.2. X-Ray Powder Diffraction
X-ray diffraction patterns of LiGd(1-x)Eux(PO3)4 (where x= 0, 0.02 and 0.04) are shown in Figure 3. The obtained crystalline phases are isotypes of the mother-phase LiGd(PO3)4 [27]. Mainly, it is shown that XR diffraction peaks of studied solids, with different percentages of europium, are described in a cell with a super space group I 2/a instead of C 2/c usually used in crystallographic data of the old LGP. The same XRD pattern is obtained for almost of synthesized compounds, even at high Eu3+ concentrations. However, a shift of the diffraction peaks to the lower 2θ is observed. This shift can be explained by Bragg's theory “nλ= 2dhklsinθ” (where λ is the X-ray wavelength (Cu Kα =1.5406Å), θ is diffraction angle, and d is interplanar distance of corresponding diffraction peaks). Therefore, λ is constant; it can be concluded that this shift is due to the increase of interplanar distance “d”. Considering the characteristics of Gd and Eu (ionic radius of Gd+3: 1.05 Å, Eu+3: 1.07 Å and atomic volume Gd: 19.9 cm3/mol, Eu: 28.9 cm3/mol), this phenomenon can be attributed to the distortion of the tetrahedra of polyphosphates upon europium insertion [28].
Figure 3.

XRD patterns of LiGd(1-x)Eux(PO3)4 ( x=0, 0.02 and 0.04).
The crystallite size of obtained polyphosphates is calculated using Sherrer's equation below [29] and values are summarized in Table 3. Results show that the range of calculated crystallite size is between 42.49 and 42.79 nm, which prove that the synthesized compounds are nanometric.
| (2) |
where λ is the X-ray wavelength (Cu Kα =1.5406Å), θ is the Bragg diffraction angle, and βe is the full width at half -maximum (FWHM) in radian of the main peak of each XRD pattern.
Table 3.
The size of the crystallite according to percentage of europium.
| Percentages (%) | FWHM | Position(2θ) | D(nm) |
|
| |||
| 0 | 0.189 | 20.630 | 42.49 |
|
| |||
| 2 | 0.189 | 20.628 | 42.79 |
|
| |||
| 4 | 0.189 | 20.621 | 42.79 |
3.3. Infrared and Raman Spectroscopy Investigations
3.3.1. Infrared
Figure 4 shows the IR spectra of all studied compounds. The comparison of spectra (Figure 4) and those obtained in previous works in literature for condensed polyphosphates [30, 31] proves that positions of infrared absorption bands are characteristic of phosphates with chain structures.
Figure 4.

IR spectra of LiGd(1-x)Eux(PO3)4 ( x=0, 0.02 and 0.04).
IR bands attribution is carried out based on (O-P-O)− groups and P-O-P bridges vibrations [32, 33]. The IR absorption spectra show the presence of two bands around 1249 cm−1 which are assigned to the asymmetric stretching vibration (υas) of O-P-O. The weak band observed between 1071 and 1136 cm−1 is attributed to the symmetric stretching vibration υs of O-P-O. The large and intense band around 944 cm−1 is assigned to the asymmetric vibration υas of P-O-P. We can also attribute the few bands at 689-818 cm−1 to the symmetric vibration υs (P-O-P). At low frequencies region, below 600 cm−1 , it is very difficult to distinguish the symmetric and antisymmetric bending modes of the (O-P-O) and (P-O-P) groups. The frequencies of the corresponding bands are given in Table 4.
Table 4.
Attributions of main IR bands (cm−1) of LiGd(1-x)Eux(PO3)4 samples.
| Assignment | x=0 | x=0.02 | x= 0.04 |
|
| |||
| υ as OPO | 1257 | 1241 | 1250 |
| 1137 | 1127 | 1149 | |
|
| |||
| υ s OPO | 1089 | 1089 | 109 |
| υ as POP | 1014 | 1014 | 1014 |
| 944 | 944 | 947 | |
|
| |||
| υ s POP | 689 | 689 | 689 |
| 736 | 736 | 736 | |
| 761 | 758 | 761 | |
| 818 | 818 | 818 | |
|
| |||
| δPOP | 437- | 437 | 437 |
| δPOP | 456 | 456 | 457 |
| 519 | 519 | 521 | |
| 586 | 585 | 585 | |
The major difference between the IR spectra of cyclic polyphosphate and polyphosphate is the absence of vibration bands between 750 and 1000 cm−1 . In this range, IR spectroscopy confirms the structure as long as polyphosphates chains.
3.3.2. Raman
The Raman spectra of LiGd(1-x)Eux(PO3)4 (where x= 0, 0.02 and 0.04) at room temperature are shown in Figure 5. These spectra show the presence of many bands; the first intense band at 1178 cm−1 and the second at 700 cm−1 are assigned to antisymmetric stretching vibration mode υas (O-P-O) and symmetric stretching vibrations mode υs (P-O-P), respectively. The υas (P-O-P) asymmetric and υs (O-P-O) symmetric stretching vibration modes, respectively, appear in the 1000-1100 cm−1 and 1212-1296 cm−1 ranges. The bands under 599 cm−1 are attributed to the symmetric and the asymmetric bending vibrations (δas and δs) of (O-P-O)− and (P-O-P). The intense symmetric stretching vibrations bands around 700 and 1178 cm−1 are characteristic of phosphoric anions (PO3)44− [34].
Figure 5.

Raman spectra of LiGd(PO3)4 (x=0, 0.02 and 0.04) at 300 K.
Distinguishing characteristic cyclotetraphosphates and polyphosphates compounds exit also in the Raman spectrum. The symmetric stretching vibration of the P-O-P (υs (P-O-P)) has a single band at 700 cm−1 . This is the strongest of all the Raman vibration bands. That is because of the monoclinic symmetry of LGP doped Eu and the different positions of the lanthanide and alkali ions. The results of Raman spectroscopy can identify the structure of alkali metal lanthanide polyphosphates.
3.4. DTA (Differential Thermal Analysis)
The thermal stability of lithium polyphosphate is investigated using DTA. The curves of the Eu: LiGd(PO3)4 crystal are given in Figure 6. It is clearly observed that the curves present the same shape (evolution). Indeed, a single sharp endothermic peak is observed between 900 and 1000°C for all samples, which exhibits the characteristics of a first-order phase transition. This signal can be attributed to the decomposition of polyphosphates to GdPO4. The stability of lithium gadolinium polyphosphates can be explained by heavily distorted of PO4 tetrahedra as are the GdO8 polyhedra. We thus conclude that all compounds are stable at high temperatures and it is monophasic.
Figure 6.

DTA of LiGd(1-x)Eux(PO3)4 (x=0, 0.02 and 0.04).
3.5. Magnetic Study
The magnetic susceptibility and inverse magnetic susceptibility versus temperature of LiGd(PO3)4, LiGd0.98Eu0.02(PO3)4, and LiGd0.96Eu0.04(PO3)4 are shown in Figures 7, 8, and 9, respectively. The only other reported type of rare earth polyphosphate structures are those of LGP: Eu3+; and these were chosen because Gd3+ has an effective magnetic moment and the 4fn electrons.
Figure 7.

The magnetic susceptibility (χ) and inverse magnetic susceptibility (1/χ) measurements as a function of temperature of LiGd(PO3)4.
Figure 8.

The magnetic susceptibility (χ) and inverse magnetic susceptibility (1/χ) measurements as a function temperature of LiGd0.98Eu0.02(PO3)4.
Figure 9.

The magnetic susceptibility (χ) and inverse magnetic susceptibility (1/χ) measurements as a function temperature of LiGd0.96Eu0.04(PO3)4.
These curves prove that all three rare earth polyphosphate compounds exhibit a paramagnetic response. The nondoped LGP is the most paramagnetic one; this is explained by their structural stability. Indeed, the addition of europium in the host disturbs samples in crystallinity. The response for LGP obeys Curie's Law very well; this is consistent with the (8S7/2) ground state of Gd3+, which has no orbital angular momentum and so is unaffected by crystal field effects. Fitting, the temperature dependence of the inverse of susceptibility χ−1 in high temperatures is given by the formula [35]
| (3) |
where θP is the Weiss temperature and C is the Curie constant given by
| (4) |
where N is the number of carriers of magnetic moment, μ0 is the vacuum permeability, KB is the Boltzmann constant, μB is the Bohr magnetron, and μeff is effective moment of carriers. Samples' structure consists of one magnetic species (i), possessing each a magnetic moment μeff (i); the magnetic susceptibility is given by the relation:
| (5) |
Generally, the magnetic moment is determined by
| (6) |
where gJ is the Lande factor and J is the total angular moment. The theoretical effective paramagnetic moment μeffthe for the samples can be calculated by
| (7) |
Curves of χ−1 versus temperature allow deducing μeffexp values, which are summarized in Table 5. We can notice that the values of μeffthe decrease with the decrease of Gd percentage in the system, due to the important magnetic moment of Gd3+ ions (7.94μB). The comparison between the theoretical and the experimental effective moment values shows that the former are higher than the latter. This result can be associated with the increase of disorder in the matrix (LGP). On the other side, when the temperature increases to more than 75K, it induces a thermal agitation and causes magnetic moments disorientation of atoms in Eu doped LGP polyphosphates. Consequently, a decrease of paramagnetism is clearly observed (Figure 10).
Table 5.
Values of C, μeffthe (μB), and μeffexp (μB) for the LiGd(1-x)Eux(PO3)4 (x=0, 0.02 and 0.04) compounds.
| x=0 | x=0.02 | x=0.04 | |
|
| |||
| C (μB.KT−1) | 5.26 | 5 | 2.86 |
|
| |||
| μ eff the (μB) | 7.94 | 7.86 | 7.78 |
|
| |||
| μ eff exp (μB) | 6.50 | 6.32 | 4.65 |
Figure 10.
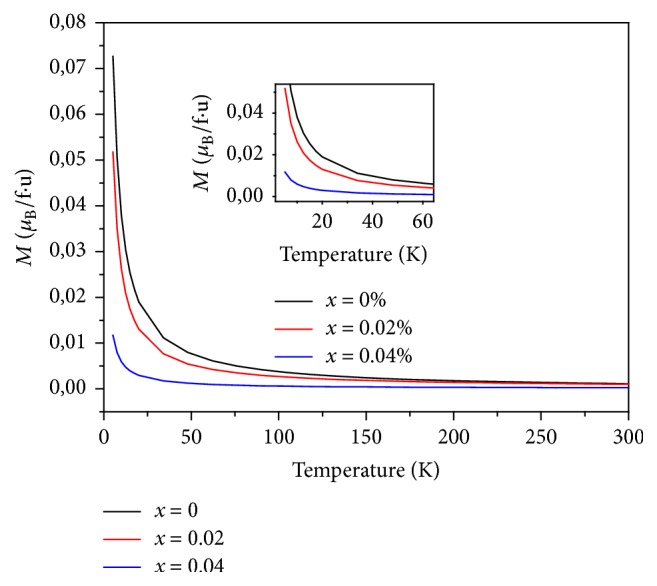
Magnetic measurements of LiGd(1-x)Eux(PO3)4 (x=0, 0.02 and 0.04).
3.6. Luminescence Properties
3.6.1. Excitation
Excitation spectra of LiGd(PO3)4, doped with europium (2, 4%) (Figure 11), are measured at 300K under emission with λem= 591 nm. Figure 11 shows broad band from 254 to 271 nm. These bands are assigned to the charge transfer bands (CTB), resulting from the transfer of an electron from the orbital 2p6 of the ligand O2- to the empty state of the configuration [Xe]4f6 of the Eu3+ ion (Eu3+-O2- transition). The maximum of the CTB is located at 245 nm. The differences between broadening and positions of the maxima intensities of the CTB in polyphosphate indicate their dependence on the host lattices [36]. This is due to the strong binding of the oxygen ligands in the polyphosphate compound [37].
Figure 11.

Excitation spectra with λem=591 nm of LiGd(1-x)Eux(PO3)4 (x=0, 0.02 and 0.04) at 300 K.
At low frequencies, several groups of narrow bands in the spectral region 271-310 nm are clearly observed and assigned to 8S7/2→6HJ, 8S7/2→6GJ, 8S7/2→ 6DJ, 8S7/2→6IJ, and 8S7/2→6PJtransitions of the Gd3+ ion. LiYF4:Gd3+ crystal is used as reference to identify excitation bands, which describe the basis of the detailed energy level scheme proposed for the trivalent gadolinium [38]. The presence of band in the range between 271 and 310 nm indicates the presence of energy transfer between the two rare earths, which occurs from Gd3+ to Eu3+ in the matrix. However, there is no CTB of Eu3+–O2- or energy transfer band Gd3+-Eu3+ above 310 nm. Excitation spectra within the wavelength range of 310–550 nm, show only the intrinsic transitions 4f-4f from the ground state 7F0 to different excited levels (5D, or 5L) of Eu3+ ion. These transitions are assigned as follows: 7F0→5HJ at 316 nm, 7F0→5D4 at 362 nm, 7F0→5G2, 5L7 at 382 nm, 7F0→5L6 at 393 nm, 7F0→5D3 at 417 nm, 7F0→5D2 at 464 nm,and 7F0→5D1 at 502 nm. All these assignments and wavelengths are given in Table 6. Most of the excitation bands are broadened and some of them overlap together to form a strong band, particularly the band between 369 and 409 nm with FWHM of about 18 nm.
Table 6.
Excitation lines attribution of Eu3+ doped LiGd(PO3)4.
| Wavelength (nm) | Attribution |
|
| |
| 287 | 7F0→5I6 |
|
| |
| 294 | 7F0→5F4 |
|
| |
| 297 | 7F0→5F2 |
|
| |
| 6318 | 7F0→5H6 |
|
| |
| 321 | 7F0→5H4 |
|
| |
| 328 | 7F0→5H7 |
|
| |
| 363 | 7F0→5D4 |
|
| |
| 376 | 7F1→5D4 |
|
| |
| 373-390 | 7F0→5GJ(2,4) |
|
| |
| 394 | 7F0→5L6 |
|
| |
| 405 | 7F1→5L6 |
|
| |
| 416 | 7F0→5D3 |
|
| |
| 464 | 7F0→5D2 |
|
| |
| 526 | 7F0→5D1 |
The perfect match of this excitation band with the emission wavelength of NUV In GaN-based LED chips makes these phosphors conveniently useful in white LEDs [39]. Figure 11 shows that the band intensities increase with europium concentration. However, they maintain the same shape and position.
3.6.2. Emission
The emission spectra of condensed phosphates are recorded at ambient temperature (300K) and in the range of 500-750 nm after excitation with λex= 394 nm (Figure 12). These spectra present the same shapes, with bands intensity proportional to Eu3+ active ion concentration. However, we notice that the undoped LiGd(PO3)4 polyphosphate does not emit light. The observed emission bands are attributed to the following transitions: 5D0→7FJ (where J = 0,1,2,3 or 4) of Eu3+ ion in the matrix LiGd(PO3)4 [40, 41].
Figure 12.

Emission spectra with λex=394 nm of LiGd(1-x)Eux(PO3)4 (x=0, 0.02 and 0.04) at 300 K.
Figure 12 proves the presence of five bands in the emission spectra where the most intense ones are those situated at 578-600 nm (5D0→7F1) and 604-634 nm (5D0→7F2). The other emission bands are observed at 554 nm (5D0→7F0), 660 nm (5D0→7F3), and 686-706 nm (5D0→7F4). The corresponding assignments and wavelengths of these emissions are given in Table 7. The relative intensities of the most intense transitions 5D0 →7F0, 7F1, 7F2 7F3 and 7F4 are strongly influenced by the nature of the host and the crystalline environment [42]. Therefore, the dominance of magnetic dipole (MD) transition 5D0→7F1 of Eu3+ means that Eu3+ occupies a site in the crystal lattice with inversion symmetry. However, in the case of absence of symmetry inversion in the site of Eu3+, the main emission would be the electric dipole (ED) transition 5D0→7F2 [43]. The synthesized polyphosphates showed that orange emission transition (5D0→7F1) is slightly dominated. This indicates that Eu3+ occupies a site in the crystal lattice with symmetry inversion.
Table 7.
Emission attribution of Eu3+ doped LiGd(PO3)4.
| Transitions | Wavelengths (nm) |
|
| |
| 5D0→7F0 | 554 |
|
| |
| 5D0→7F1 | 578-601 |
|
| |
| 5D0→7F2 | 604-634 |
|
| |
| 5D0→7F3 | 660 |
|
| |
| 5D0→7F4 | 686-706 |
4. Conclusion
Polyphosphates of rare earth and alkali metal LGP:Eu3+ were successfully synthesized by solid-state reaction at 730°C. XRD patterns proved that the obtained samples crystallize in a monoclinic single phase with space group I 2/a and following cell parameters a= 9.635(3) Å, b= 7.035(3) Å, c= 13.191(3) Å, β= 90.082°, V= 894.214 Å3, and Z= 4. The synthesized polyphosphates showed a good thermal stability until 940°C. Spectroscopic analyses by IR and Raman spectra confirmed the acyclic zig-zag chain of (PO4)3– in LGP structure, involving GdO8 dodecahedra and LiO4 polyhedra. The magnetic susceptibility carried out on single crystals revealed that the title compounds were paramagnetic between 5 and 300 K. An increase in excitation and emission bands intensities was observed with the increase of europium concentration. The presence of band in the range between 271 and 310 nm in excitation spectra proved the energy transfer process from Gd3+ to Eu3+. The dominance of 5D0→7F1 transition in the emission spectra confirms that Eu3+ occupies a site in the crystal lattice with symmetry inversion. The change in transition bands intensity proves that LGP phosphates affect europium environment.
Conflicts of Interest
There are no conflicts of interest regarding the publication of this paper.
References
- 1.Campayo L., Audubert F., Bernache-Assollant D. Synthesis study of alkaline-bearing rare earth phosphates with rhabdophane structure. Solid State Ionics. 2005;176(35-36):2663–2669. doi: 10.1016/j.ssi.2005.07.007. [DOI] [Google Scholar]
- 2.Zhu J., Chen H., Wang Y., Guan H., Xiao X. Structure determination, electronic and optical properties of rubidium holmium polyphosphate RbHo(PO3)4. Journal of Molecular Structure. 2012;1030:204–208. doi: 10.1016/j.molstruc.2012.04.033. [DOI] [Google Scholar]
- 3.Yang S., Chen Z. The Study on Aging and Degradation Mechanism of Ammonium Polyphosphate in Artificial Accelerated Aging. Procedia Engineering. 2018;211:906–910. doi: 10.1016/j.proeng.2017.12.091. [DOI] [Google Scholar]
- 4.El Masloumi M., Imaz I., Chaminade J. P., et al. Synthesis, crystal structure and vibrational spectra characterization of MILa(PO3)4 (MI = Na, Ag) Journal of Solid State Chemistry. 2005;178:3581–3588. [Google Scholar]
- 5.Cole J. M., Lees M. R., Howard J. A. K., Newport R. J., Saunders G. A., Schönherr E. Crystal Structures and Magnetic Properties of Rare-Earth Ultraphosphates, RP5O14 (R=La, Nd, Sm, Eu, Gd) Journal of Solid State Chemistry. 2000;150:377–382. [Google Scholar]
- 6.Gavaldà J. J., Parreu I., Solé R., Solans X., Díaz F., Aguiló M. Growth and Structural Characterization of Rb3Yb2(PO4)3: A New Material for Laser and Nonlinear Optical Applications. J. Chemistry of Materials. 2005;17(26):6746–6754. [Google Scholar]
- 7.Ferid M., Horchani-Naifer K. Structure and ionic conductivity of NaCeP2O7. Journal of Solid State Ionics. 2005;176:1949–1953. [Google Scholar]
- 8.Jaouadi K., Zouari N., Mhiri T., Pierrot M. Synthesis and crystal structure of sodium–bismuth polyphosphate NaBi(PO3)4. Journal of Crystal Growth. 2005;273(3-4):638–645. doi: 10.1016/j.jcrysgro.2004.09.056. [DOI] [Google Scholar]
- 9.Amria M., Zouaria N., Mhiri T., Daoud A., Gravereau P. Crystal structure and conductivity investigation of KDyP4O12: a new potassium dysprosium cyclotetraphosphate. Journal of Molecular Structure. 2006;782:16–23. [Google Scholar]
- 10.Minh D. P., Sane A. R., Semlal N., Sharrock P., Nzihou A. Alkali polyphosphates as new potential materials for thermal energy storage. Journal of Solar Energy. 2017;157:277–283. [Google Scholar]
- 11.Mua Z., Hub Y., Chen L., et al. A reddish orange stoichiometric phosphor LiEu(PO3)4 for white light-emitting diodes. Journal of Ceramics International. 2014;40:2575–2579. [Google Scholar]
- 12.Zhong J., Liang H., Su Q., Zhou J., Khodyuk I. V., Dorenbos P. Radioluminescence properties of Ce3+-activated MGd(PO3)4 (M = Li, Na, K, Cs) Optical Materials. 2009;32(2):378–381. doi: 10.1016/j.optmat.2009.09.006. [DOI] [Google Scholar]
- 13.Gu J., Zhong J., Liang H., Zhang J., Su Q. Competitive absorption of Eu3+ and Tb3+codoped in NaGd(PO3)4 phosphors. Chemical Physics Letters. 2014;592:261–264. doi: 10.1016/j.cplett.2013.12.033. [DOI] [Google Scholar]
- 14.Han B., Liang H., Ni H., et al. Intense red light emission of Eu3+-doped LiGd(PO3)4 for mercury-free lamps and plasma display panels application. J. of Optics Express. 2009;17:7138–7144. doi: 10.1364/oe.17.007138. [DOI] [PubMed] [Google Scholar]
- 15.Shalapska T., Stryganyuk G., Demchenko P., Voloshinovskii A., Dorenbos P. Luminescence properties of Ce3+-doped LiGdP4O12 upon vacuum-ultraviolet and x-ray excitation. Journal of Physics: Condensed Matter. 2009;21(44) doi: 10.1088/0953-8984/21/44/445901.445901 [DOI] [PubMed] [Google Scholar]
- 16.Han B., Liang H., Huang Y., Tao Y., Su Q. Vacuum Ultraviolet−Visible Spectroscopic Properties of Tb3+ in Li(Y, Gd)(PO3)4: Tunable Emission, Quantum Cutting, and Energy Transfer. The Journal of Physical Chemistry. 2010;C114(14):6770–6777. doi: 10.1021/jp100755d. [DOI] [Google Scholar]
- 17.Shi R., Liu G., Liang H., Huang Y., Tao Y., Zhang J. Consequences of ET and MMCT on Luminescence of Ce3+, Eu3+, and Tb3+-doped LiYSiO4. Inorganic Chemistry. 2016;55(15):7777–7786. doi: 10.1021/acs.inorgchem.6b01249. [DOI] [PubMed] [Google Scholar]
- 18.Krasnikov A., Shalapska T., Stryganyuk G., Voloshinovskii A., Zazubovich S. Photoluminescence and energy transfer in Eu3+-doped alkali gadolinium phosphates. Physica Status Solidi (b) – Basic Solid State Physics. 2013;250(7):1418–1425. doi: 10.1002/pssb.201349020. [DOI] [Google Scholar]
- 19.Zhong J., Liang H., Su Q., Zhou J. Luminescence properties of NaGd(PO3)4:Eu3+ and energy transfer from Gd3+ to Eu3+ Applied Physics B: Lasers and Optics. 2010;98:139–147. [Google Scholar]
- 20.Xu W., Dai J.-G., Ding Z., Wang Y. Polyphosphate-modified calcium aluminate cement under normal and elevated temperatures: Phase evolution, microstructure, and mechanical properties. Ceramics International. 2017;43(17):15525–15536. doi: 10.1016/j.ceramint.2017.08.102. [DOI] [Google Scholar]
- 21.Liu B., Chen Y., Shi C., Tang H., Tao Y. Visible quantum cutting in BaF2: Gd, Eu downconversion. Journal of Luminescence. 2003;101(1-2):155–159. doi: 10.1016/S0022-2313(02)00408-8. [DOI] [Google Scholar]
- 22.Kondo H., Hirai T., Hashimoto S. Dynamical behavior of quantum cutting in alkali gadolinium fluoride phosphors. Journal of Luminescence. 2004;108(1-4):59–63. doi: 10.1016/j.jlumin.2004.01.011. [DOI] [Google Scholar]
- 23.Kodama N., Watanabe Y. Visible quantum cutting through downconversion in Eu3+-doped KGd3F10 and KGd2F7 crystals. Applied Physics Letters. 2004;84(21):4141–4143. doi: 10.1063/1.1713038. [DOI] [Google Scholar]
- 24.Ghosh P., Tang S., Mudring A.-V. Efficient quantum cutting in hexagonal NaGdF4:Eu3+nanorods. Journal of Materials Chemistry. 2011;21(24):8640–8644. doi: 10.1039/c1jm10728c. [DOI] [Google Scholar]
- 25.Legendziewicz J., Guzik M., Cybińska J. VUV spectroscopy of double phosphates doped with rare earth ions. Optical Materials. 2009;31(3):567–574. doi: 10.1016/j.optmat.2007.11.035. [DOI] [Google Scholar]
- 26.Hammami S., Sebai S., Jegouso D., Reita V., Boudjada N. C., Megriche A. Magnetic and Photon Cascade Emission of Gd3+ of NaGd(PO3)4 Monocrystal Under Appropriate Synthesis Conditions. Journal of Physical Chemistry & J Biophysics. 2017;7:2161–0398. [Google Scholar]
- 27.Ettis H., Naïli H., Mhiri T. The crystal structure, thermal behaviour and ionic conductivity of a novel lithium gadolinium polyphosphate LiGd(PO3)4. Journal of Solid State Chemistry. 2006;179(10):3107–3113. doi: 10.1016/j.jssc.2006.06.003. [DOI] [Google Scholar]
- 28.Shannon R. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallographica Section A: Foundations of Crystallography. 1976;32:751–767. doi: 10.1107/S0567739476001551. [DOI] [Google Scholar]
- 29.Gholami T., Salavati-Niasari M. Green facile thermal decomposition synthesis, characterization and electrochemical hydrogen storage characteristics of ZnAl2O4nanostructure. International Journal of Hydrogen Energy. 2017;42(27):17167–17177. doi: 10.1016/j.ijhydene.2017.05.215. [DOI] [Google Scholar]
- 30.Sebai S., Hammami S., Megriche A., Zambon D., Mahiou R. Synthesis, structural characterization and VUV excited luminescence properties of LixNa(1-x)Sm(PO3)4 polyphosphates. Optical Materials. 2016;62:578–583. doi: 10.1016/j.optmat.2016.11.015. [DOI] [Google Scholar]
- 31.Sun T., Zhang Y., Shan P., et al. Growth, structure, thermal properties and spectroscopic characteristics of Nd3+- doped KGdP4O12 crystal. PLoS ONE. 2014;9(6) doi: 10.1371/journal.pone.0100922.e100922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rekik W., Naïli H., Mhiri T., Bataille T. [NH3(CH2)2NH3][Co(SO4)2(H2O)4]: Chemical preparation, crystal structure, thermal decomposition and magnetic properties. Materials Research Bulletin. 2008;43(10):2709–2718. doi: 10.1016/j.materresbull.2007.10.024. [DOI] [Google Scholar]
- 33.Zhang J., Zhang Z., Zhang W., et al. Polymorphism of BaTeMo2O9: a new polar polymorph and the phase transformation. Chemistry of Materials. 2011;23(16):3752–3761. doi: 10.1021/cm2015143. [DOI] [Google Scholar]
- 34.Jaouadi K., Naili H., Zouari N., Mhiri T., Daoud A. Synthesis and crystal structure of a new form of potassium–bismuth polyphosphate KBi(PO3)4. Alloys Compds. 2003;354(30):p. 104. doi: 10.1002/chin.200330007. [DOI] [Google Scholar]
- 35.Petrov D., Angelov B., Lovchinov V. Magnetic and XPS studies of lithium lanthanide tetraphosphates LiLnP4O12 (Ln=Nd, Gd, Er) Journal of Rare Earths. 2013;31(5):485–489. doi: 10.1016/S1002-0721(12)60307-X. [DOI] [Google Scholar]
- 36.Hachani S., Moine B., El-akrmi A., Férid M. Luminescent properties of some ortho- and pentaphosphates doped with Gd3+–Eu3+: Potential phosphors for vacuum ultraviolet excitation. Optical Materials. 2009;31(4):678–684. doi: 10.1016/j.optmat.2008.07.011. [DOI] [Google Scholar]
- 37.Ferhi M., Horchani-Naifer K., Férid M. Spectroscopic properties of Eu3+-doped KLa(PO3)4 and LiLa(PO3)4 powders. Optical Materials. 2011;34(1):12–18. doi: 10.1016/j.optmat.2011.07.016. [DOI] [Google Scholar]
- 38.Wegh R. T., Donker H., Meijerink A., Lamminmäki R. J., Hölsä J. Vacuum-ultraviolet spectroscopy and quantum cutting for Gd3+ in LiYF4. Physical Review B. 1997;56(21):13841–13848. doi: 10.1103/PhysRevB.56.13841. [DOI] [Google Scholar]
- 39.Abdelhedi M., Horchani-Naifer K., Dammak M., Ferid M. Structural and spectroscopic properties of pure and doped LiCe(PO3)4. Materials Research Bulletin. 2015;70:303–308. doi: 10.1016/j.materresbull.2015.04.062. [DOI] [Google Scholar]
- 40.Teotonio E. E. S., Brito H. F., Felinto M. C. F. C., Thompson L. C., Young V. G., Malta O. L. Preparation, crystal structure and optical spectroscopy of the rare earth complexes (RE3+=Sm, Eu, Gd and Tb) with 2-thiopheneacetate anion. Journal of Molecular Structure. 2005;751(1-3):85–94. doi: 10.1016/j.molstruc.2005.05.001. [DOI] [Google Scholar]
- 41.Mbarek A. Synthesis, structural and optical properties of Eu3+-doped ALnP2O7 (A = Cs, Rb, Tl; Ln = Y, Lu, Tm) pyrophosphates phosphors for solid-state lighting. Journal of Molecular Structure. 2017;1138:149–154. doi: 10.1016/j.molstruc.2017.03.010. [DOI] [Google Scholar]
- 42.Tuan D. C., Olazcuaga R., Guillen F., Garcia A., Moine B., Fouassier C. Luminescent properties of Eu3+-doped yttrium or gadolinium phosphates. J. of Physics. 2005;123:259–263. [Google Scholar]
- 43.Gaft M., Panczer G., Reisfeld R., Shinno I., Champagnon B., Boulon G. Laser-induced Eu3+ luminescence in zircon ZrSiO4. Journal of Luminescence. 2000;87:1032–1035. doi: 10.1016/S0022-2313(99)00530-X. [DOI] [Google Scholar]


