Abstract
Although outstanding progress has been made in understanding the pathophysiology of thrombotic thrombocytopenic purpura (TTP), knowledge of the immunopathogenesis of the disease is only at an early stage. Anti-ADAMTS13 auto-antibodies were shown to block proteolysis of von Willebrand factor and/or induce ADAMTS13 clearance from the circulation. However, it still remains to identify which immune cells are involved in the production of anti-ADAMTS13 autoantibodies, and therefore account for the remarkable efficacy of the B-cell depleting agents in this disease. The mechanisms leading to the loss of tolerance of the immune system towards ADAMTS13 involve the predisposing genetic factors of the human leukocyte antigen class II locus DRB1*11 and DQB1*03 alleles as well as the protective allele DRB1*04, and modifying factors such as ethnicity, sex and obesity. Future studies have to identify why these identified genetic risk factors are also frequently to be found in the healthy population although the incidence of immune-mediated thrombotic thrombocytopenic purpura (iTTP) is extremely low. Moreover, the development of recombinant ADAMTS13 opens a new therapeutic era in the field. Interactions of recombinant ADAMTS13 with the immune system of iTTP patients will require intensive investigation, especially for its potential immunogenicity. Better understanding of iTTP immunopathogenesis should, therefore, provide a basis for the development of novel therapeutic approaches to restore immune tolerance towards ADAMTS13 and thereby better prevent refractoriness and relapses in patients with iTTP. In this review, we address these issues and the related challenges in this field.
Introduction
Thrombotic thrombocytopenic purpura (TTP) is a devastating disease resulting from a severe deficiency in the von Willebrand factor (VWF)-cleaving protease ADAMTS13. This deficiency causes the accumulation of ultra-large VWF multimers in the circulation and the formation of thrombi in the microvasculature under high shear stress conditions. When left untreated, these microthrombi cause multi-organ failure and lead to death. In the acquired immune-mediated form of TTP (iTTP), patients develop antibodies (Abs) against ADAMTS13 that enhance its clearance or inhibit its VWF processing activity.1 Therapeutic plasma exchange (TPE) greatly improved the fatal outcome of iTTP leading to survival rates of more than 80%.1 As iTTP is an autoimmune disease, steroids were used together with TPE.1 Over the last few years, the use of the B-cell depleting agent rituximab (Mabthera®, Roche) as a more targeted immunomodulator led to a reduction in TPE duration and to efficient prevention of 1-year relapses. The pre-emptive administration of rituximab is also increasingly used in patients with a persistently severe acquired ADAMTS13 deficiency, and otherwise in remission, to prevent long-term relapses.2–4 However, the efficacy of rituximab is only transient, and in up to 50% of cases, additional courses of rituximab are required to maintain a detectable ADAMTS13 activity. Moreover, 10-15% of patients are primarily unresponsive to rituximab, or experience a subsequent refractoriness after an initial response.3 The pathophysiological mechanisms underlying these different scenarios in iTTP, as well as the specific B- and T-cell and plasmacytic subpopulations involved in the reoccurrence of anti-ADAMTS13 Abs after rituximab, still remain unknown. Besides the increasing use of immunomodulators, a recombinant form of ADAMTS13 has passed through a phase I clinical trial5 and should soon be available for the treatment of the congenital form of TTP. The use of recombinant ADAMTS13 in iTTP to over-ride inhibitory Abs, in combination with the anti-VWF nanobody caplacizumab, probably represents the next breakthrough in the management of this disease.6 However, the use of a recombinant ADAMTS13 in iTTP may involve the potential risk of boosting inhibitor titers by the activation of ADAMTS13 specific memory B and T cells, as suggested previously.7
Taking into consideration these challenges, it is crucial to understand in more detail the mechanisms leading to the loss and re-establishment of self-tolerance of the immune system towards ADAMTS13. In this review, we address the current knowledge on the immunopathogenesis of iTTP and the potential forthcoming challenges in the field. We also provide evidence that iTTP represents an illustrative model of multistep disease resulting from the combination of genetic risk factors for autoimmunity and environmental precipitating factors.
Anti-ADAMTS13 Abs: physical and functional features, mechanisms of pathogenicity
The presence of anti-ADAMTS13 Abs in plasma of iTTP patients with an inhibitory activity towards the VWF cleavage activity of normal plasma was first demonstrated by isolating IgGs via protein A-Sepharose, as well as on protein G-Sepharose column chromatography.8,9 Isolated IgGs were later shown to bind to ADAMTS13 in ELISA.10 It is now known that anti-ADAMTS13 Abs result in a profound deficiency in ADAMTS13 activity by two main mechanisms: inhibitory (neutralizing) Abs block the proteolytic activity of ADAMTS13 towards VWF, whereas non-inhibitory Abs increase ADAMTS13 clearance from the circulation by forming immune-complexes.11–13 ADAMTS13 antigen (Ag) levels are decreased in most patients, suggesting that Ab-mediated ADAMTS13 depletion is an important pathogenic mechanism underlying severe loss of enzyme activity.11–13 It is likely that both inhibitory and non-inhibitory Abs promote ADAMTS13 clearance. Inhibitory IgGs that account for the majority of auto-Abs found in patients with iTTP are mainly directed against the spacer domain of ADAMTS13, while those solely targeting the carboxy-terminal domains are in vitro non-inhibitory.14 Whether immune complexes may in addition activate the complement system and bind to cellular Fc receptors, thereby promoting inflammation, endothelial activation with thrombosis, and relapse, represent attractive mechanisms requiring further exploration.15–17
Retrospective studies based on large series of iTTP patients have shown that, during the first acute event, anti-ADAMTS13 Abs of IgG type (detected either by functional or immunological methods) were identified in approximately 75-90% of patients.18–27 Of all subclasses of anti-ADAMTS13 IgG detected in patients with iTTP, IgG4 was the most prevalent (90%) followed by IgG1 (53%), IgG2 (50%), and IgG3 (33%). There was an inverse correlation between the frequency and abundance of IgG4 and IgG1 Abs.17,28 Anti-ADAMTS13 Abs of IgM and IgA type are found in only approximately 10% of iTTP patients, mainly in association with IgG.21,29,30
Interestingly, anti-ADAMTS13 IgG have been reported to be present in 5% of healthy individuals, possibly sharing some linear epitopes with iTTP patients.21,22,31 Importantly, these Abs in healthy individuals are non-inhibitory against ADAMTS13, which is likely due to a lower affinity towards the protein.31 However, whether individuals with non-pathogenic anti-ADAMTS13 Abs are prone to develop iTTP still has to be established. It is nevertheless tempting to speculate that non-pathogenic anti-ADAMTS13 IgG Abs could precede clinical disease onset in iTTP, as reported for other autoimmune diseases such as systemic lupus erythematosus (SLE).32 In this scenario, the mechanisms turning non-pathogenic anti-ADAMTS13 Abs into pathogenic Abs could involve an enhanced frequency of somatic hypermutation of IgG memory B cells, as well as epitope spreading, underlining the need for future studies in this direction. It would also be of interest to assess whether non-pathogenic anti-ADAMTS13 Abs occur more frequently in healthy individuals carrying the HLA susceptibility alleles DRB1*11 and DQB1*03, and if these individuals are more prone to develop inhibitory autoantibodies from non-pathogenic antibodies. In a study including 160 patients with miscellaneous diseases but no severe ADAMTS13 deficiency,21 anti-ADAMTS13 Abs of IgG type were also found in 20% of patients with a thrombotic microangiopathy (TMA) other than iTTP, in 8% of patients with various causes of thrombocytopenia, in 13% of patients with SLE, and in 5% of patients with the anti-phospholipid syndrome (APS). In addition, anti-ADAMTS13 Abs of IgM type were also detected in 18% of SLE and APS patients.21 Indeed, conventional inhibitor and ELISA assays allow for assessment of anti-ADAMTS13 Abs in a significant proportion of patients with various TMAs. Therefore, attribution of the mechanistic role and clinical significance of anti-ADAMTS13 Abs alone remains challenging.21
The Abs directed against ADAMTS13 spacer domain use the heavy chain (VH) gene segment VH1-69 in 75% of cases.14,33,34 The clinical significance of the restricted VH1-69 germline gene segment has been observed in neutralizing Abs directed toward a highly conserved region in the hemagglutinin ectodomain of the influenza virus35 and in patients with B-cell lymphoma after chronic hepatitis C infection.36 Sequence analysis of anti-ADAMTS13 IgGs revealed unique heavy-chain complementary determining region 3 motifs, of which some were shared by unrelated patients, suggesting that the autoimmune response in iTTP is antigen-driven.37
Several animal models of iTTP have been developed by directly injecting ADAMTS13 inhibiting Abs.38–41 Importantly, some of these models highlight the role of auto-Abs in the pathology of iTTP, as administration of the antibody alone (passive transfer) was enough to trigger the hallmarks of iTTP, as described for the baboon model.38
Clinical relevance of anti-ADAMTS13 Abs in iTTP treatment
The identification of anti-ADAMTS13 antibodies as the main mechanism of ADAMTS13 deficiency in iTTP prompted an evaluation of B-cell depleting therapies in the management of the disease. Several studies showed that rituximab at the acute phase of the disease, in association with TPE and steroids, shortens the time to response.2,33 Upon treatment with rituximab, TPE duration usually does not exceed 30 days, whereas before the era of rituximab, 25% of patients required TPE for more than one month.2,42 Moreover, the administration of rituximab during the acute phase results in fewer relapses in the 12-18 months following remission. Beyond this time, however, relapses can reoccur as a consequence of the reappearance of anti-ADAMTS13 Abs along with peripheral B-cell recovery.2,42,43 The persistence of a severe ADAMTS13 deficiency following the acute phase has been associated with a high risk of relapse,3,29,44 and this has led investigators to propose pre-emptive B-cell depleting strategies. Although based on comparative studies, this strategy proved its efficiency in protecting patients from full-blown relapses by maintaining ADAMTS13 activity within normal values.3 This strategy implies, therefore, a regular assessment of ADAMTS13 activity during long-term follow up.
Prognostic value of anti-ADAMTS13 Abs
The role of anti-ADAMTS13 Abs in iTTP prognosis has still not been fully defined. Anti-ADAMTS13 Abs with a strong inhibitory activity were typically associated with more plasma volume to achieve remission and with an increased risk of relapse, as well as with a lower survival rate in some studies (but not all).19,27,29,45 Also, the combination of several anti-ADAMTS13 Abs isotypes (IgG, IgM, and IgA), including very high IgA titers, and the presence of high levels of IgG1 combined with undetectable levels of IgG4, has been associated with poor prognosis and an increased risk of mortality.17,28 Recently, higher mortality rates were found in individuals with higher anti-ADAMTS13 Abs and lower ADAMTS13 antigen levels; patients with anti-ADAMTS13 IgG Abs in the upper quartile and ADAMTS13 antigen in the lowest quartile had the highest mortality (27.3%).46
Epitope mapping studies have shown that patients typically display auto-Abs against the cysteine-rich/spacer domains of ADAMTS13, while more than 50% of patients have auto-Abs against the CUB domains or the TSP2-8 domains.47 A major antibody epitope in the spacer domain has been resolved at the amino acid level; residues R568, F592, R659, R660, Y661 and Y665 in the spacer exosite-3 constitute a conformational epitope that is targeted by the majority of anti-spacer Abs.30,48–52 Auto-Abs directed toward the terminal TSP2-8 repeats (+/− CUB domains) have been associated with lower platelet counts;53 however, others have found no correlation between these parameters, suggesting that the “specificity profile” of patients’ auto-Abs is not a major determinant of their pathophysiology.13 Furthermore, the “specificity profile” of these auto-Abs may change with time (“epitope spreading”), even in patients subjected to immunomodulatory treatments. Importantly, anti-ADAMTS13 Abs have been shown to persist during iTTP remission, either in free form or within immune complexes, in spite of a detectable ADAMTS13 activity.16
Human leukocyte antigen molecules as predisposing factors to iTTP; from serotype to immunochip
Immune-mediated thrombotic thrombocytopenic purpura occurs after the development of a specific adaptive immune response targeting ADAMTS13. The human leukocyte antigen (HLA) system has an important role as a genetic risk factor in autoimmune diseases, and a similar association could thus be assumed for iTTP, especially considering cases of iTTP in siblings.54,55 The presence of class-switched high-affinity anti-ADAMTS13 Abs implies co-operation between T and B lymphocytes. Activation of ADAMTS13-specific CD4+ T cells requires recognition of ADAMTS13 peptides bound to HLA molecules by T-cell receptors, thereby potentially implicating HLA molecules as predisposing factors for iTTP.
In the 90’s, the first possible association between iTTP/the hemolytic-uremic syndrome (HUS) and HLA was reported.56 More specifically, study of the frequency of supertypic antigens DR52 and DR53, which are linked to the HLA-DRB3 and HLA-DRB4 genes respectively, indicated under-representation of DR53 in patients with iTTP compared to healthy individuals. Interestingly, molecular testing did not reveal any association with specific HLA-DRB3 alleles, suggesting the association was not with DR52 but with absence of the DR53 antigen, which could, therefore, be protective against iTTP.56
In 2010, two studies reported for the first time an association of HLA class II with iTTP.57,58 In a cohort of European patients, HLA-DRB1*04 and its associated HLA-DRB4-encoded serotype HLA-DR53 were protective against the development of iTTP. The decreased frequency of HLA-DRB4 in iTTP patients as compared to healthy controls appeared to be attributable to the specific reduction in the frequency of the linked HLA-DRB1*04 allele. In addition, the frequencies of HLA-DRB1*11 and HLA-DQB1*0301 were found to be higher in patients with iTTP when compared to healthy controls, suggesting these alleles to be risk factors for the onset of iTTP, although a primary role for HLA-DQB1*0301 could not be excluded.57 Reconstruction of HLA-haplotypes resulted in statistically higher frequency of DQB1*03-DRB1*11 haplotype in iTTP when compared to healthy controls. High-resolution typing of HLA-DRB1*11 revealed that both DRB1*1101 and DRB1*1104 were over-represented in iTTP, suggesting that the generic DRB1*11 is the predisposing factor.58 Other groups reported comparable findings,59,60 and provided additional protective haplotypes (DRB1*07-DQB1*02 and DRB1*13-DQB1*06) or haplotypes associated with susceptibility (DRB1*15-DQB1*06) for iTTP.60 Recently, additional evidence for the involvement of the HLA-DRB1*11 and HLA-DQB1*03 haplotype in the development of iTTP was provided from a case of familial iTTP on 2 first-degree relatives (mother and daughter) who both carried HLA-DRB1*1101/DRB1*1104 and the linked HLA-DQB1*03 allele.
Interestingly, previous reports had shown associations between DRB1*11 and certain clinical conditions such as systemic sclerosis, early-onset juvenile chronic arthritis and sarcoidosis. In accordance with these findings, such associated conditions could be observed in our iTTP registry.61,62 The wide spectrum of autoimmune diseases sharing DRB1*11 clearly argues for the existence of additional risk factors, which may determine the specific clinical features of those diseases.
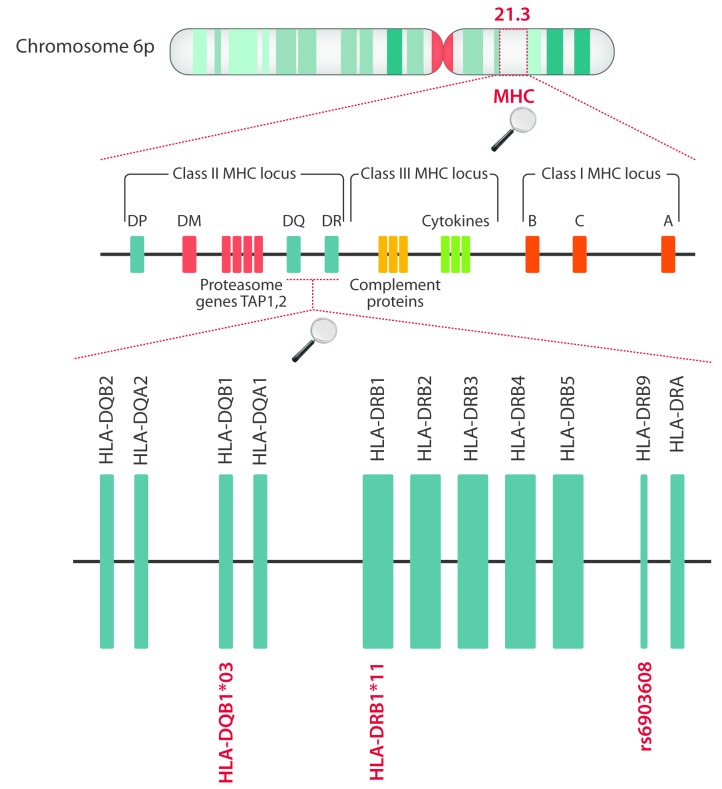
Mancini et al. employed immunochip analysis to identify susceptibility loci in the HLA region for iTTP.63 A common variant rs6903608 was shown to confer 2.6-fold increased risk of development of iTTP.63 The rs6903608 is an intron of pseudogene HLA-DRB9 that maps to an inter-genic region between HLA-DRA and HLA-DRB5 (Figure 1). Unexpectedly, HLA-DRB1*11 and HLA-DQB1*03 were not identified as risk factors in this study. It was proposed that rs6903608 is in linkage disequilibrium with HLA-DRB1*11, thereby masking a potential contribution of this allele.63 Interestingly, imputation analysis suggested that HLA-DQB1*0503 was also associated with iTTP.63 This particular allele had not been linked to iTTP in previous studies.
Figure 1.
The genes of the human MHC locus involved in immune-mediated thrombotic thrombocytopenic purpura (iTTP) susceptibility. Localization of genes of the two HLA alleles, HLA-DQB1*03 and HLA-DRB1*11, previously described as risk factors for iTTP, as well as the approximate localization of single polymorphism rs6903608, which appears to be in linkage disequilibrium with HLA-DRB1*11 and which has also been linked to the onset of iTTP.97
Potential mechanisms of HLA association with onset of iTTP
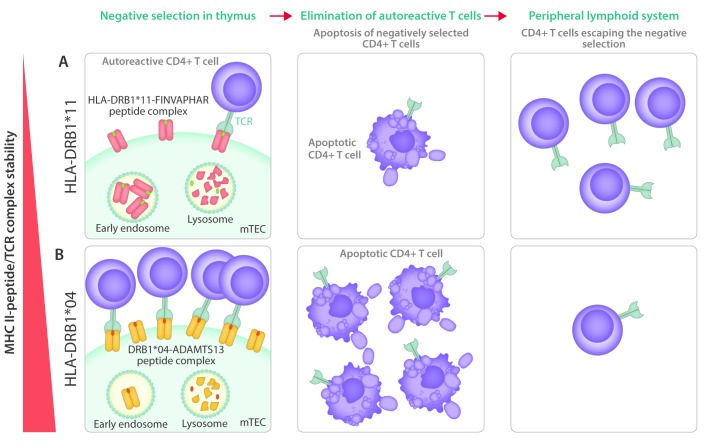
The observed association between HLA alleles and the development of autoimmunity has been considered to arise from recognition of self-peptides by low affinity CD4+ T cells that have escaped negative selection in the thymus.64 It has recently been shown that differences in the intrinsic stability of HLA-DQ proteins may be linked to the onset of autoimmunity.65 Several HLA-DQ proteins have been shown to be poorly expressed on the cell surface; immuno-dominant epitopes that may potentially bind to these HLA-DQ proteins may, therefore, not be sufficiently presented, allowing CD4+ T cells to escape the negative selection in the thymus. This would result in the appearance of potentially auto-reactive CD4+ T cells in the periphery that could contribute to the onset of autoimmunity.65 It is possible that the increased frequency of HLA-DQB1*03 in patients with iTTP is caused by the inability of HLA-DQB1*03-containing DQ proteins to efficiently present immune-dominant self-peptides. This would result in a defective elimination of self-reactive CD4+ T cells during negative selection in the thymus. In this respect, the single nucleotide polymorphism (rs6903608) that has been linked to the onset of iTTP (see above), is located in the HLA locus that affects expression of a number of MHC class II subunits.63 More precisely, this single nucleotide polymorphism is located in close proximity to the DRB9 pseudogene, upstream of DRA gene. Although this has not been established, the rs6903608 single nucleotide polymorphism may affect the expression of the DRA gene, thereby modulating the amount of MHC class II on antigen-presenting cells. As outlined by Miyadera et al., increased stability of MHCII-peptide complexes may also confer protection for autoimmunity by a more rigorous elimination of self-reactive CD4+ T cells recognizing immune-dominant self-epitopes in the thymus (Figure 2).65 This mechanism may potentially provide an explanation for the protective effect of HLA-DRB1*04 on iTTP development. Future studies are needed to address whether HLA-DR or HLA-DQ stability modulates CD4+ T-cell responses in iTTP.
Figure 2.
The possible role of MHC class II stability in the onset of immune-mediated thrombotic thrombocytopenic purpura (iTTP). (A) Intrinsically unstable HLA-DRB1*11 molecules are rapidly endocytosed, thus limiting the exposure of HLA-DRB1*11-loaded peptides on medullary thymic epithelial cells (mTEC) to CD4+ T cells. The proposed instability of HLA-DRB1*11/peptide complexes results in inefficient removal of self-reactive CD4+ T cells (through induction of apoptosis) in the thymus, and the appearance of potentially self-reactive CD4+ T cells in the peripheral lymphoid system.98,99 (B) Intrinsically stable HLA-DRB1*04 molecules are retained for prolonged periods of time on the surface of mTEC, thereby efficiently eliminating self-reactive CD4+ T cells. Consequently, the number of self-reactive CD4+ T cells escaping the negative selection in thymus will be very limited, which could account for the protective role of HLA-DRB1*04 in the development of iTTP.
Presentation of ADAMTS13-derived peptides to CD4+ T cells
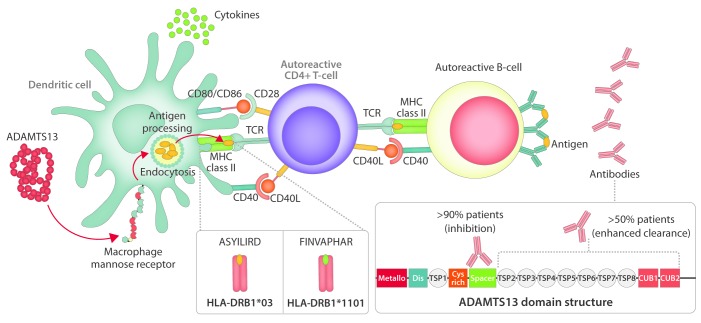
Activation of ADAMTS13-specific CD4+ T cells requires uptake of ADAMTS13 by antigen-presenting cells (APCs) and presentation of ADAMTS13-derived peptides on HLA molecules. As the HLA-DRB1*11 was identified as a risk factor for the development of iTTP, investigation of ADAMTS13-derived peptides that are preferentially presented on MHC class II of healthy individuals positive for this allele was performed.66 The results of this study revealed peptides with a core sequence FINVAPHAR to be presented on MHC class II domains of HLA-DRB1*11 positive individuals. A number of individuals positive for HLA-DQB1*03 were also included in the study and peptides with core sequence LIRDTHSLR were found to be presented by APCs. Interestingly, both peptide sequences originate from the CUB2 domain of ADAMTS13. However, when the APCs of these patients were pulsed with higher concentrations of ADAMTS13 (500 nM), the presented peptides within HLA-DRB1*11 or DQB1*03 donors remained essentially the same, but in patients with other alleles, some presented peptides belonging to other ADAMTS13 domains (i.e. TSP2-8 repeats; the metalloprotease or the spacer domains), in addition to the above-mentioned core peptides FINVAPHAR and LIRDTHSLR. The results of this study thus suggest that FINVAPHAR and LIRDTHSLR-containing peptides contribute to the onset of iTTP.66 The next step was to investigate whether CD4+ T cells from HLA-typed patients with iTTP would recognize these peptides.67 In vitro stimulated peripheral blood mononuclear cells (PBMCs) of healthy donors and iTTP patients with FINVAPHAR (NAGGCRLFINVAPHARIAIH) or ASYILIRD (EGANASYILIRDTHSLRT-TA) peptides were analyzed by flow cytometry. The results confirmed the initial assumption of FINVAPHAR peptide being able to activate CD4+ T cells of HLA-DRB1*11 expressing patients, whereas ASYILIRD resulted in activation of CD4+ T cells of HLA-DQB1*03 positive patients (Figure 3).67 In a separate study, Gilardin et al. identified another CUB2 domain-derived peptide with sequence GDMLLLWGRLTWRKM (1239-1253) as a potential DRB1*01 and DRB1*11 restricted T-cell epitope.68 This peptide was previously found to be presented by APCs from a DRB1*0401/DRB1*1301 positive donor which were pulsed with 500 nM ADAMTS13.66 In a recent study, we explored the repertoire of ADAMTS13-derived peptides that were presented on HLA-DQ.69 In 4 of 9 donors analyzed, ADAMTS13-derived peptides presented on HLA-DQ were identified. Three HLA-DQB1*0301 positive donors were included in this study, and ADAMTS13-derived peptides were only found in one donor.69 The identified HLA-DQ-presented ADAMTS13 peptides originated from different domains; a CUB1 domain-derived peptide was presented in 3 of 8 donors. In the same study, the re-evaluation of HLA-DR-presented peptide repertoires confirmed the presentation of FIN-VAPHAR-derived peptides on HLA-DRB1*11.69 A large collection of ADAMTS13 derived peptides appeared to be presented on HLA-DR when compared to a previous study.66 Overall, the presented peptides were derived from multiple domains of ADAMTS13, with, however, a dominant contribution of CUB1/2-derived peptides to the total repertoire.69
Figure 3.
The onset of immune-mediated thrombotic thrombocytopenic purpura (iTTP). ADAMTS13 is endocytosed by antigen-presenting cell [in this figure, dendritic cell (DC)] and processed to peptides that are subsequently loaded on MHC-II molecules. As described previously, ADAMTS13-derived peptides FINVAPHAR and ASYILIRD were found to be presented on HLA-DRB1*1101 and HLA-DQB1*03, respectively. In the case of presence of specific autoreactive CD4+ T cells, the complex MHC-II/peptide will be recognized by TCR, which will cause the activation of the CD4+ T cell. Such activated CD4+ T cells will then provide help to autoreactive B cells that will result in a production of ADAMTS13-specific auto-Abs.
To summarize, the specific peptides of ADAMTS13 recognized preferentially by HLA-DRB1*11 and DQB1*03 have now been identified. However, we need to understand how the recognition of these peptides by specific HLA molecules leads to the production of pathogenic anti-ADAMTS13 antibodies. Comparison of autoantibody profiles in animal models possessing/lacking risk MHC II alleles for iTTP and immunized with ADAMTS13 might help address this question.
Patients with iTTP are prone to develop autoimmunity
Immune-mediated thrombotic thrombocytopenic purpura is associated with another autoimmune disease in up to 20% of cases. Other autoimmune diseases can occur years before iTTP, during long-term follow up, or con comitantly with iTTP. SLE can be observed in up to 10% of iTTP patients and represents the most common associated autoimmune disease, followed by Sjogren’s syndrome, presented by 3% of iTTP patients. Antinuclear Abs can be identified in 50% of cases. The presence of additional autoimmune diseases has no impact on the outcome of an acute iTTP episode. The presence of anti-double stranded DNA Abs or anti-SSA Abs at iTTP diagnosis is significantly associated with the development of an additional autoimmune disease during follow up.25,61,62,70
Modifying factors: role of ethnicity, sex, obesity, and others
The over-representation of women and blacks in most iTTP registries in Western countries highlighted sex and ethnic disparities in this disease, further suggesting the involvement of specific genetic risk factors.23,71,72 In black patients, iTTP is typically associated with an increased risk of exacerbations at the acute phase but with less fatal outcomes (death rate 2.7% vs. 11.6% white patients in our experience), although initial presentation and prognosis is comparable to that of white patients,72,73 raising the hypothesis of a differential tolerance and/or adaptation to tissue ischemia between both ethnic groups.74 The increased prevalence of iTTP in blacks could at least in part result from the naturally low prevalence of the protective allele DRB1*04 in this ethnic group.72
The striking predominance of iTTP in women of childbearing age and during pregnancy raises the hypothesis that estrogen may favor the occurrence of iTTP. As reported in SLE, it is likely that estrogen increases the risk of iTTP in genetically predisposed women by elevating type-1 interferon production and favoring the survival of autoreactive B cells.75 Similarly, obesity could represent a risk factor for iTTP as a result of increased peripheral aromatization of androgens to estrogens, but also higher leptin levels, in this population of patients.71,76 In addition, obese healthy individuals have an increased prevalence of non-inhibitory ADAMTS13 auto-Abs, although no differences in ADAMTS13 activity and antigen levels were found with lean people.77 Taken together, these findings stress the role of modifying factors including hormones, cytokines and other mediators involved in the loss of tolerance against ADAMTS13; these require further study to be better understood mechanistically. There is also a need to specify whether iTTP patients with associated conditions, including pregnancy, connective tissue diseases or obesity, also have specific genetic risk factors as compared to patients with idiopathic iTTP.
Von Willebrand Factor (VWF) is another important modifier of TTP susceptibility, either in the congenital or in the autoimmune form. Raised VWF levels are required to induce TTP in Adamts13−/− mice, but varying the concentration between 20 and 120 U/mL does not appear to affect the occurrence or severity of the disease, suggesting that a threshold level of VWF is sufficient, and that higher levels confer little additional risk. However, humans appear to be more sensitive to changes in VWF levels than mice. Women with inherited ADAMTS13 deficiency frequently develop TTP during pregnancy, which probably results from the progressive increase in VWF levels throughout the gestation period. Moreover, obese individuals have higher levels of VWF, providing further evidence for an association between obesity and TTP. Thus, changes in VWF secretion, multimer distribution, and plasma level may trigger TTP.78 Among additional modifiers of TTP, blood group O was found over-represented in iTTP cohorts, suggesting that blood group O could be a risk factor for TTP.79
ADAMTS13 conformation in iTTP
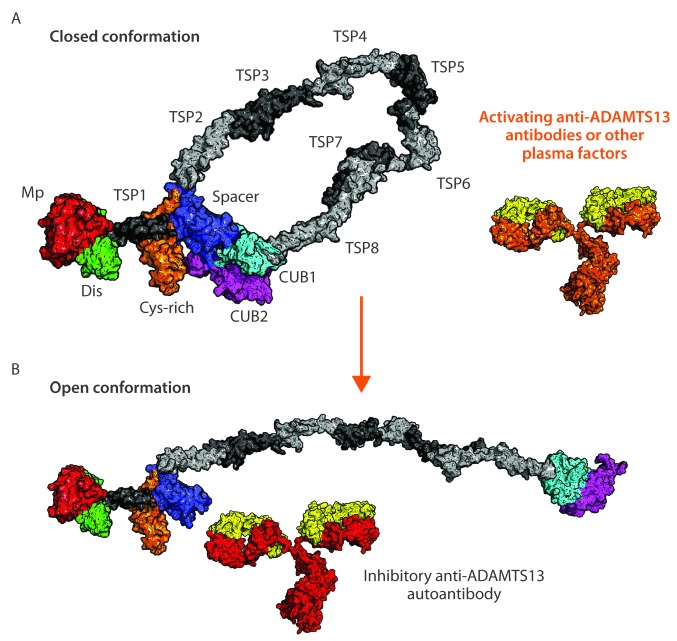
The precise mechanism triggering the formation of anti-ADAMTS13 Abs in previously healthy individuals still has to be identified. As mentioned earlier, the majority of anti-spacer auto-Abs that develop in patients with iTTP is directed toward an exosite composed of residues R568, F592, R660, Y661 and Y665.30 Under quiescent conditions, the exosite within the spacer domain is covered by the CUB1/2 domains maintaining ADAMTS13 in a so-called closed conformation.80–82 Binding of VWF to the carboxy-terminal CUB domains induces a conformational activation of ADAMTS13 which results in the exposure of the spacer domain exosite composed of residues R568, F592, R660, Y661 and Y665 which now becomes available for binding to the unfolded VWF A2 domain.83 Apart from VWF, murine monoclonal Abs can induce a conversion of ADAMTS13 from a closed to an open conformation.80,82 Conservative mutations of the spacer domain exosite created a variant with increased proteolytic activity toward its substrate VWF.84 Recently, it was shown that this so-called gain-of-function (GoF) variant is present in an open conformation, which promotes binding of the spacer domain exosite to the unfolded A2 domain of VWF.81 These results imply that the major B-cell epitope in the spacer domain is not accessible for binding of auto-Abs when ADAMTS13 circulates in a so-called closed conformation. Because the knowledge on conformational state of ADAMTS13 in patient plasma could be of help in the diagnosis and prognosis of iTTP, Roose et al. designed an assay to monitor this.85 Their results showed that ADAMTS13 adopts an altered (more open) conformation in iTTP patients in the acute phase of the disease while ADAMTS13 was closed in the majority of iTTP patients in remission, in healthy donors, and patients with sepsis or HUS. Hence, acute iTTP is not only characterized by severe thrombocytopenia, hemolytic anemia, ADAMTS13 activity less than 10%, and presence of anti-ADAMTS13 Abs, but also by the presence of an open ADAMTS13 conformation. It now remains to be determined which factors (anti-ADAMTS13 Abs and/or other plasma factors) open ADAMTS13. However, once the conformation of ADAMTS13 is changed during acute iTTP, anti-ADAMTS13 Abs recognizing not only non-cryptic epitopes but also cryptic epitopes in the spacer domain can now bind, leading to the inhibition and/or clearance of ADAMTS13 (Figure 4). Whether the conformational change in ADAMTS13 also leads to an (additional) immune response, remains to be determined.85
Figure 4.
Conformational changes of ADAMTS13. [Mp: metalloprotease (red); Dis: disintegrin-like domain (green); Cys-rich: cysteine-rich domain (orange); TSRs: thrombospondin-type 1 repeats (light and dark gray); CUB1 domain (cyan); CUB2 domain (magenta)]. (A) In the blood circulation of healthy individuals and immune-mediated thrombotic thrombocytopenic purpura (iTTP) patients in remission ADAMTS13 is present in closed conformation with CUB1/2 domains covering the spacer domain. Binding of plasma factors (e.g. activating anti-ADAMTS13 auto-Abs against the TSR5-8-CUB1/2 domains80,82) (orange part of the anti-ADAMTS13 antibodies) results in a conformational change: the opening of the ADAMTS13.83,100 (B) Open conformation of ADAMTS13 exposes the B-cell epitope localized in the spacer domain, making it accessible for additional anti-ADAMTS13 Abs.
The autoimmune response against ADAMTS13: an infection-driven event?
Many studies have suggested a link between infections and the onset of iTTP. First, infectious agents can be precipitating factors in patients with a severe ADAMTS13 deficiency, through endothelial activation. No specific agents could be identified,86 as opposed to HUS, which was typically associated to specific strains of the bacteria Escherichia coli. Endothelial activation in this context may involve nucleosomes that derive at least in part from neutrophil extracellular traps (NETS), networks made of nuclear DNA, histones, granular and cytoplasmic proteins that are released by neutrophils in response to infections.87 In response to these agonists, endothelial cells release high molecular weight VWF multimers leading to thrombi in the microvasculature of most organs. Human neutrophil peptides released from activated and degranulated neutrophils can also alter ADAMTS13 activity.88 This scenario, termed the “two-hit model”, was evidenced from animal models and illustrates the interaction between a genetic background (a severe constitutive ADAMTS13 deficiency or a propensity to develop an immune-mediated ADAMTS13 deficiency) and environmental factors, especially infections and inflammation.89 Second, infections may potentially result in the presentation of pathogen-derived peptides that are homologous to ADAMTS13-derived peptides such as the CUB2 domain-derived peptide FINVAPHAR.90,91 CD4+ T cells developing in response to a challenge by pathogens may, therefore, cross-react with ADAMTS13 peptides presented on MHC class II, resulting in their activation and proliferation. This phenomenon has been designated molecular or epitope mimicry.92 As discussed earlier, our group identified CD4+ T cells reactive to ADAMTS13 peptides derived from the CUB2 domain in patients with iTTP.67 These peptides with amino acid sequence FINVAPHAR and ASYILIRD (Figure 5) may share epitope mimicry with microbiome-derived peptides. CD4+ T cells targeting these microbiome-derived peptides may cross-react with ADAMTS13 peptides presented on risk alleles for iTTP, thereby contributing to the onset of iTTP.
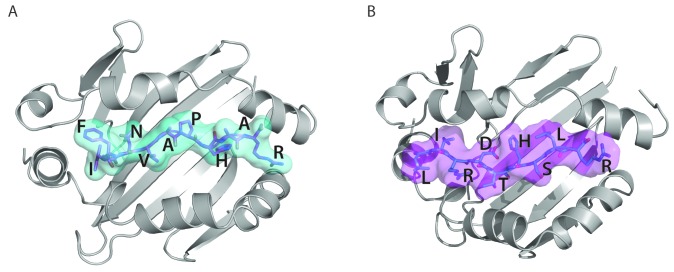
Figure 5.
Presentation of ADAMTS13 peptides on MHC class II. (A) Three-dimensional model structure of HLA-DRB1*11 (graphic representation in gray) with FINVAPHAR peptide (stick representation in magenta with cyan surface) from ADAMTS13 CUB2 domain. (B) Three-dimensional model structure of HLA-DQB1*03 (graphic representation in gray) with LIRDTHSLR peptide (stick representation in cyan with magenta surface) from ADAMTS13 CUB2 domain.
Another hypothesis developed from the observed association between infections and iTTP is a gene-environment interaction process involving microbial effectors that activate endothelial and polymorphonuclear cells. Therefore, functionally relevant molecules belonging to the innate immune response pathways could be important modulators of iTTP initiation in the context of infection. Variants of the anti-infectious innate immunity sensor Toll-like receptor (TLR)-9 were reported to be more represented in iTTP patients negative for the HLA-DRB1*11 Class II susceptibility allele.86 TLR-9 harboring specific variants could, therefore, be more prone to activate endothelial and polymorphonuclear cells and produce Th1 cytokines in a context of infection, precipitating an iTTP episode.86 Similarly, anecdotal responses in patients with severe iTTP following the administration of the complement blocker eculizumab suggest a role for complement deregulation in iTTP pathophysiology.93 Unusually large VWF multimers may activate the alterna tive complement pathway to promote the generation of terminal complement complexes (C5b-9).94 These observations therefore deserve systematic explorations to unravel the role of the alternative complement pathway and its regulators (CFH, CFI, MCP and thrombomodulin) in iTTP pathophysiology.
Interestingly, HLA-DRB1*11 was reported to be protective against tuberculosis, whereas HLA-DRB1*04 was associated with an increased risk of tuberculosis and severe malaria.95,96 These observations raise the intriguing possibility that autoimmunity against ADAMTS13 leading to iTTP could represent the cost of an efficient ancestral immune response selected to fight against historically harmful infectious agents.
Perspectives: future directions
Despite the considerable progress made in unravelling the role of ADAMTS13 in primary hemostasis, our understanding of the immunopathogenesis of iTTP is still not complete. Future studies will have to reveal a reason for the paradoxically extremely low incidence of the disease considering the frequent occurrence of identified genetic risk factors within the HLA-class II locus in the healthy population. Another field of investigation involves the mechanisms determining the reoccurrence of autoreactive lymphocytes in patients following immunomodulation with B-cell depleting therapies in order to better anticipate relapses. Moreover, a new therapeutic area is being opened in the field with the development of the recombinant ADAMTS13; its interactions with the immune system of iTTP patients will require further investigation.
Supplementary Material
Acknowledgements
The authors would like to thank the Horizon 2020 Framework program for Research and Innovation of the European Union for funding this work under 675746 (PROFILE).
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/103/7/1099
References
- 1.Kremer Hovinga JA, Coppo P, Lämmle B, Moake JL, Miyata T, Vanhoorelbeke K. Thrombotic thrombocytopenic purpura. Nat Rev Dis Prim 2017;317020. [DOI] [PubMed] [Google Scholar]
- 2.Scully M, McDonald V, Cavenagh J, Hunt BJ. A phase 2 study of the safety and efficacy of rituximab with plasma exchange in acute acquired thrombotic thrombocytopenic purpura. Blood. 2011;118(7):1746–1754. [DOI] [PubMed] [Google Scholar]
- 3.Hie M, Gay J, Galicier L, et al. Preemptive rituximab infusions after remission efficiently prevent relapses in acquired thrombotic thrombocytopenic purpura. Blood. 2014;124(2):204–210. [DOI] [PubMed] [Google Scholar]
- 4.Westwood J-P, Thomas M, Alwan F, et al. Rituximab prophylaxis to prevent thrombotic thrombocytopenic purpura relapse: outcome and evaluation of dosing regimens. Blood Adv. 2017;1(15):1159 LP–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scully M, Knobl P, Kentouche K, et al. Recombinant ADAMTS-13: first-in-human pharmacokinetics and safety in congenital thrombotic thrombocytopenic purpura. Blood. 2017;130(19):2055–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peyvandi F, Scully M, Kremer Hovinga JA, et al. Caplacizumab reduces the frequency of major thromboembolic events, exacerbations and death in patients with acquired thrombotic thrombocytopenic purpura. J Thromb Haemost. 2017;15(7):1448–1452. [DOI] [PubMed] [Google Scholar]
- 7.Kremer Hovinga JA, Voorberg J. Improving on nature: redesigning ADAMTS13. Blood. 2012;119(16):3654–3655. [DOI] [PubMed] [Google Scholar]
- 8.Furlan M, Robles R, Solenthaler M, Lä B. Acquired Deficiency of von Willebrand Factor-Cleaving Protease in a Patient With Thrombotic Thrombocytopenic Purpura. Blood. 1998;91(8):2839–2846. [PubMed] [Google Scholar]
- 9.Tsai HM, Lian EC. Antibodies to von Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura. N Engl J Med. 1998;339(22):1585–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scheiflinger F, Kno P, Trattner B, Plaimauer B, Mohr G, Dockal M. Nonneutralizing IgM and IgG antibodies to von Willebrand factor – cleaving protease (ADAMTS-13) in a patient with thrombotic thrombocytopenic purpura. Blood. 2003;102(9):3241–3243. [DOI] [PubMed] [Google Scholar]
- 11.Crawley JTB, Scully MA. Thrombotic thrombocytopenic purpura: basic pathophysiology and therapeutic strategies. Hematology Am Soc Hematol Educ Program. 2013;2013:292–299. [DOI] [PubMed] [Google Scholar]
- 12.Sadler JE. What’s new in the diagnosis and pathophysiology of thrombotic thrombocytopenic purpura. Hematology Am Soc Hematol Educ Program. 2015;2015:631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas MR, de Groot R, Scully MA, Crawley JTB. Pathogenicity of Anti-ADAMTS13 Autoantibodies in Acquired Thrombotic Thrombocytopenic Purpura. EBioMedicine. 2015;2(8):942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ostertag EM, Kacir S, Thiboutot M, et al. ADAMTS13 autoantibodies cloned from patients with acquired thrombotic thrombocytopenic purpura: 1. Structural and functional characterization in vitro. Transfusion. 2016;56(7):1763–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lotta LA, Valsecchi C, Pontiggia S, et al. Measurement and prevalence of circulating ADAMTS13-specific immune complexes in autoimmune thrombotic thrombocytopenic purpura. J Thromb Haemost. 2014;12(3): 329–336. [DOI] [PubMed] [Google Scholar]
- 16.Ferrari S, Palavra K, Gruber B, et al. Persistence of circulating ADAMTS13-specific immune complexes in patients with acquired thrombotic thrombocytopenic purpura. Haematologica. 2014;99(4):779–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrari S, Knöbl P, Kolovratova V, et al. Inverse correlation of free and immune complex-sequestered anti-ADAMTS13 antibodies in a patient with acquired thrombotic thrombocytopenic purpura. J Thromb Haemost. 2012;10(1):156–158. [DOI] [PubMed] [Google Scholar]
- 18.Bianchi V, Robles R, Alberio L, Furlan M, Lammle B. Von Willebrand factor-cleaving protease (ADAMTS13) in thrombocytopenic disorders: a severely deficient activity is specific for thrombotic thrombocytopenic purpura. Blood. 2002;100(2):710–713. [DOI] [PubMed] [Google Scholar]
- 19.Zheng XL, Kaufman RM, Goodnough LT, Sadler JE. Effect of plasma exchange on plasma ADAMTS13 metalloprotease activity, inhibitor level, and clinical outcome in patients with idiopathic and nonidiopathic thrombotic thrombocytopenic purpura. Ann Intern Med. 2004;103(11):4043–4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peyvandi F, Ferrari S, Lavoretano S, Canciani MT, Mannucci PM. Von Willebrand factor cleaving (ADAMTS-13) and ADAMTS-13 neutralizing autoantibodies in 100 patients with thrombotic thrombocytopenic purpura. Br J Haematol. 2004;127(4):433–439. [DOI] [PubMed] [Google Scholar]
- 21.Rieger M, Mannucci PM, Kremer Hovinga JA, et al. ADAMTS13 autoantibodies in patients with thrombotic microangiopathies and other immunomediated diseases. Blood. 2005;106(4):1262–1267. [DOI] [PubMed] [Google Scholar]
- 22.Tsai HM, Raoufi M, Zhou W, et al. ADAMTS13-binding IgG are present in patients with thrombotic thrombocytopenic purpura. Thromb Haemost 2006;95(5):886–892. [PMC free article] [PubMed] [Google Scholar]
- 23.Scully M, Yarranton H, Liesner R, et al. Regional UK TTP Registry: Correlation with laboratory ADAMTS 13 analysis and clinical features. Br J Haematol. 2008;142(5):819–826. [DOI] [PubMed] [Google Scholar]
- 24.Fujimura Y, Matsumoto M. Registry of 919 patients with thrombotic microangiopathies across Japan: database of Nara Medical University during 1998-2008. Intern Med. 2010;49(1):7–15. [DOI] [PubMed] [Google Scholar]
- 25.Mariotte E, Azoulay E, Galicier L, et al. Epidemiology and pathophysiology of adulthood-onset thrombotic microangiopathy with severe ADAMTS13 deficiency (thrombotic thrombocytopenic purpura): a cross-sectional analysis of the French national registry for thrombotic microangiopathy. Lancet Haematol. 2016;3(5):e237–245. [DOI] [PubMed] [Google Scholar]
- 26.Joly BS, Stepanian A, Leblanc T, et al. Child-onset and adolescent-onset acquired thrombotic thrombocytopenic purpura with severe ADAMTS13 deficiency: a cohort study of the French national registry for thrombotic microangiopathy. Lancet Haematol. 2016;3(11):e537–e546. [DOI] [PubMed] [Google Scholar]
- 27.Kremer Hovinga JA, Vesely SK, Terrell DR, Lammle B, George JN. Survival and relapse in patients with thrombotic thrombocytopenic purpura. Blood. 2010;115(8):1500–11; quiz 1662. [DOI] [PubMed] [Google Scholar]
- 28.Ferrari S, Mudde GC, Rieger M, Veyradier A, Kremer Hovinga JA, Scheiflinger F. IgG subclass distribution of anti-ADAMTS13 antibodies in patients with acquired thrombotic thrombocytopenic purpura. J Thromb Haemost 2009;7(10):1703–1710. [DOI] [PubMed] [Google Scholar]
- 29.Ferrari S, Scheiflinger F, Rieger M, et al. Prognostic value of anti-ADAMTS13 antibody features (Ig isotype, titer, and inhibitory effect) in a cohort of 35 adult French patients undergoing a first episode of thrombotic microangiopathy with undetectable ADAMTS13 activity. Blood. 2007;109(7): 2815–2822. [DOI] [PubMed] [Google Scholar]
- 30.Pos W, Sorvillo N, Fijnheer R, et al. Residues arg568 and phe592 contribute to an antigenic surface for anti-adamts13 antibodies in the spacer domain. Haematologica. 2011;96(11):1670–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grillberger R, Casina VC, Turecek PL, Long Zheng X, Rottensteiner H, Scheiflinger F. Anti-ADAMTS13 IgG autoantibodies present in healthy individuals share linear epitopes with those in patients with thrombotic thrombocytopenic purpura. Haematologica. 2014;99(4):2–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arbuckle MR, McClain MT, Rubertone MV, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349(16):1526–1533. [DOI] [PubMed] [Google Scholar]
- 33.Luken BM, Kaijen PHP, Turenhout EA, et al. Multiple B-cell clones producing antibodies directed to the spacer and disintegrin/thrombospondin type-1 repeat 1 (TSP1) of ADAMTS13 in a patient with acquired thrombotic thrombocytopenic purpura. J Thromb Haemost. 2006;4(11):2355–2364. [DOI] [PubMed] [Google Scholar]
- 34.Pos W, Luken BM, Kremer Hovinga JA, et al. VH1-69 germline encoded antibodies directed towards ADAMTS13 in patients with acquired thrombotic thrombocytopenic purpura. J Thromb Haemost. 2009;7(3):421–428. [DOI] [PubMed] [Google Scholar]
- 35.Sui J, Hwang WC, Perez S, et al. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat Struct Mol Biol. 2009;16(3):265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marasca R, Vaccari P, Luppi M, et al. Immunoglobulin gene mutations and frequent use of VH1-69 and VH4-34 segments in hepatitis C virus-positive and hepatitis C virus-negative nodal marginal zone B-cell lymphoma. Am J Pathol. 2001;159(1):253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schaller M, Vogel M, Kentouche K, Lämmle B, Kremer Hovinga JA. The splenic autoimmune response to ADAMTS13 in thrombotic thrombocytopenic purpura contains recurrent antigen-binding CDR3 motifs. Blood 2014;124(23):3469–3479. [DOI] [PubMed] [Google Scholar]
- 38.Vanhoorelbeke K, De Meyer SF. Animal models for thrombotic thrombocytopenic purpura. J Thromb Haemost. 2013;11 (Suppl 1):2–10. [DOI] [PubMed] [Google Scholar]
- 39.Ostertag EM, Bdeir K, Kacir S, et al. ADAMTS13 autoantibodies cloned from patients with acquired thrombotic thrombocytopenic purpura: 2. Pathogenicity in an animal model. Transfusion. 2016;56(7): 1775–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tersteeg C, Schiviz A, De Meyer SF, et al. Potential for Recombinant ADAMTS13 as an Effective Therapy for Acquired Thrombotic Thrombocytopenic Purpura. Arterioscler Thromb Vasc Biol. 2015;35(11):2336–2342. [DOI] [PubMed] [Google Scholar]
- 41.Deforche L, Tersteeg C, Roose E, et al. Generation of Anti-Murine ADAMTS13 Antibodies and Their Application in a Mouse Model for Acquired Thrombotic Thrombocytopenic Purpura. PLoS One. 2016;11(8):e0160388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Froissart A, Buffet M, Veyradier A, et al. Efficacy and safety of first-line rituximab in severe, acquired thrombotic thrombocytopenic purpura with a suboptimal response to plasma exchange. Experience of the French Thrombotic Microangiopathies Reference Center. Crit Care Med. 2012;40(1):104–111. [DOI] [PubMed] [Google Scholar]
- 43.Page EE, Kremer Hovinga JA, Terrell DR, Vesely SK, George JN. Rituximab reduces risk for relapse in patients with thrombotic thrombocytopenic purpura. Blood. 2016;127(24):3092–3094. [DOI] [PubMed] [Google Scholar]
- 44.Peyvandi F, Lavoretano S, Palla R, et al. ADAMTS13 and anti-ADAMTS13 antibodies as markers for recurrence of acquired thrombotic thrombocytopenic purpura during remission. Haematologica. 2008;93(2): 232–239. [DOI] [PubMed] [Google Scholar]
- 45.Coppo P, Wolf M, Veyradier A, et al. Prognostic value of inhibitory anti-ADAMTS13 antibodies in adult-acquired thrombotic thrombocytopenic purpura. Br J Haematol. 2006;132(1):66–74. [DOI] [PubMed] [Google Scholar]
- 46.Alwan F, Vendramin C, Vanhoorelbeke K, et al. Presenting ADAMTS13 antibody and antigen levels predict prognosis in immune-mediated thrombotic thrombocytopenic purpura. Blood. 2017;130(4):466–471. [DOI] [PubMed] [Google Scholar]
- 47.Klaus C, Plaimauer B, Studt JD, et al. Epitope mapping of ADAMTS13 autoantibodies in acquired thrombotic thrombocytopenic purpura. Blood. 2004;103(12):4514–4519. [DOI] [PubMed] [Google Scholar]
- 48.Luken BM, Turenhout EAM, Kaijen PHP, et al. Amino acid regions 572-579 and 657-666 of the spacer domain of ADAMTS13 provide a common antigenic core required for binding of antibodies in patients with acquired TTP. Thromb Haemost. 2006;96(3):295–301. [DOI] [PubMed] [Google Scholar]
- 49.Pos W, Crawley JTB, Fijnheer R, Voorberg J, Lane DA, Luken BM. An autoantibody epitope comprising residues R660, Y661, and Y665 in the ADAMTS13 spacer domain identifies a binding site for the A2 domain of VWF. Blood. 2010;115(8):1640–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamaguchi Y, Moriki T, Igari A, et al. Epitope analysis of autoantibodies to ADAMTS13 in patients with acquired thrombotic thrombocytopenic purpura. Thromb Res. 2011;128(2):169–173. [DOI] [PubMed] [Google Scholar]
- 51.Jin S-Y, Skipwith CG, Zheng XL. Amino acid residues Arg(659), Arg(660), and Tyr(661) in the spacer domain of ADAMTS13 are critical for cleavage of von Willebrand factor. Blood. 2010;115(11): 2300–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Casina VC, Hu W, Mao J-H, et al. High-resolution epitope mapping by HX MS reveals the pathogenic mechanism and a possible therapy for autoimmune TTP syndrome. Proc Natl Acad Sci. 2015;112(31):9620–9625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Long Zheng X, Wu HM, Shang D, et al. Multiple domains of ADAMTS13 are targeted by autoantibodies against ADAMTS13 in patients with acquired idiopathic thrombotic thrombocytopenic purpura. Haematologica. 2010;95(9):1555–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Studt JD, Hovinga JAK, Radonic R, et al. Familial acquired thrombotic thrombocytopenic purpura: ADAMTS13 inhibitory autoantibodies in identical twins. Blood. 2004;103(11):4195–4197. [DOI] [PubMed] [Google Scholar]
- 55.Wallace DC, Lovric A, Clubb JS, Carseldine DB. Thrombotic thrombocytopenic purpura in four siblings. Am J Med. 1975;58(5):724–734. [DOI] [PubMed] [Google Scholar]
- 56.Joseph G, Smith KJ, Hadley TJ, et al. HLA-DR53 protects against thrombotic thrombocytopenic purpura/adult hemolytic uremic syndrome. Am J Hematol. 1994;47(3):189–193. [DOI] [PubMed] [Google Scholar]
- 57.Scully M, Brown J, Patel R, Mcdonald V, Brown CJ, Machin S. Human leukocyte antigen association in idiopathic thrombotic thrombocytopenic purpura: Evidence for an immunogenetic link. J Thromb Haemost. 2010;8(2):257–262. [DOI] [PubMed] [Google Scholar]
- 58.Coppo P, Busson M, Veyradier A, et al. HLA-DRB1*11: A strong risk factor for acquired severe ADAMTS13 deficiency-related idiopathic thrombotic thrombocytopenic purpura in Caucasians. J Thromb Haemost. 2010;8(4):856–859. [DOI] [PubMed] [Google Scholar]
- 59.John M-L, Hitzler W, Scharrer I. The role of human leukocyte antigens as predisposing and/or protective factors in patients with idiopathic thrombotic thrombocytopenic purpura. Ann Hematol. 2012;91(4):507–510. [DOI] [PubMed] [Google Scholar]
- 60.Lombardi AM, Pulini S, Passeri C, et al. Familial acquired thrombotic thrombocytopenic purpura: immunogenetic link with HLA-DRB1*11 and DQB1*03 antigens. Br J Haematol. 2017. October 26 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 61.Coppo P, Bengoufa D, Veyradier A, et al. Severe ADAMTS13 deficiency in adult idiopathic thrombotic microangiopathies defines a subset of patients characterized by various autoimmune manifestations, lower platelet count, and mild renal involvement. Medicine (Baltimore). 2004;83(4):233–244. [DOI] [PubMed] [Google Scholar]
- 62.Coppo P, Schwarzinger M, Buffet M, et al. Predictive features of severe acquired ADAMTS13 deficiency in idiopathic thrombotic microangiopathies: the French TMA reference center experience. PLoS One. 2010;5(4):e10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mancini I, Ricaño-Ponce I, Pappalardo E, et al. Immunochip analysis identifies novel susceptibility loci in the human leukocyte antigen region for acquired thrombotic thrombocytopenic purpura. J Thromb Haemost. 2016;14(12):2356–2367. [DOI] [PubMed] [Google Scholar]
- 64.Bergseng E, Dørum S, Arntzen MØ, et al. Different binding motifs of the celiac disease-associated HLA molecules DQ2.5, DQ2.2, and DQ7.5 revealed by relative quantitative proteomics of endogenous peptide repertoires. Immunogenetics. 2015;67(2):73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miyadera H, Ohashi J, Lernmark Å, Kitamura T, Tokunaga K. Cell-surface MHC density profiling reveals instability of autoimmunity-associated HLA. J Clin Invest 2015;125(1):275–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sorvillo N, van Haren SD, Kaijen PH, et al. Preferential HLA-DRB1*11-dependent presentation of CUB2-derived peptides by ADAMTS13-pulsed dendritic cells. Blood. 2013;121(17):3502–3510. [DOI] [PubMed] [Google Scholar]
- 67.Verbij FC, Turksma AW, de Heij F, et al. CD4+ T cells from patients with acquired thrombotic thrombocytopenic purpura recognize CUB2 domain-derived peptides. Blood. 2016;127(12):1606–1609. [DOI] [PubMed] [Google Scholar]
- 68.Gilardin L, Delignat S, Peyron I, et al. The ADAMTS13(1239-1253) peptide is a dominant HLA-DR1-restricted CD4+ T-cell epitope. Haematologica. 2017;102(11):1833–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hrdinova J, Verbij FC, Kaijen PHP, et al. Mass spectrometry-assisted identification of ADAMTS13-derived peptides presented on HLA-DR and HLA-DQ. Haematologica. 2018. March 22 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roriz M, Landais M, Desprez J, et al. Risk Factors for Autoimmune Diseases Development After Thrombotic Thrombocytopenic Purpura. Medicine (Baltimore). 2015;94(42):e1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.George JN, Vesely SK, Terrell DR. The Oklahoma Thrombotic Thrombocytopenic Purpura-Hemolytic Uremic Syndrome (TTP-HUS) Registry: A Community Perspective of Patients with Clinically Diagnosed TTP-HUS. Semin Hematol. 2004;41(1):60–67. [DOI] [PubMed] [Google Scholar]
- 72.Martino S, Jamme M, Deligny C, et al. Thrombotic thrombocytopenic purpura in Black people: Impact of ethnicity on survival and genetic risk factors. PLoS One. 2016;11(7):3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cataland SR, Yang S Bin, Witkoff L, et al. Demographic and ADAMTS13 biomarker data as predictors of early recurrences of idiopathic thrombotic thrombocytopenic purpura. Eur J Haematol. 2009;83(6):559–564. [DOI] [PubMed] [Google Scholar]
- 74.Sheikh N, Sharma S. Impact of ethnicity on cardiac adaptation to exercise. Nat Rev Cardiol. 2014;11(4):198–217. [DOI] [PubMed] [Google Scholar]
- 75.Hughes GC, Choubey D. Modulation of autoimmune rheumatic diseases by oestrogen and progesterone. Nat Rev Rheumatol. 2014;10(12):740–751. [DOI] [PubMed] [Google Scholar]
- 76.Diamanti-Kandarakis E, Bergiele A. The influence of obesity on hyperandrogenism and infertility in the female. Obes Rev. 2001;2(4):231–238. [DOI] [PubMed] [Google Scholar]
- 77.Lombardi AM, Fabris R, Scarda A, et al. Presence of anti-ADAMTS13 antibodies in obesity. Eur J Clin Invest. 2012;42(11):1197–1204. [DOI] [PubMed] [Google Scholar]
- 78.Sadler JE. Pathophysiology of thrombotic thrombocytopenic purpura. Blood. 2017;130(10):1181–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Terrell DR, Motto DG, Kremer Hovinga JA, Lammle B, George JN, Vesely SK. Blood group O and black race are independent risk factors for thrombotic thrombocytopenic purpura associated with severe ADAMTS13 deficiency. Transfusion. 2011;51(10):2237–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Muia J, Zhu J, Gupta G, et al. Allosteric activation of ADAMTS13 by von Willebrand factor. Proc Natl Acad Sci USA. 2014;111(52):18584–18589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.South K, Luken BM, Crawley JTB, et al. Conformational activation of ADAMTS13. Proc Natl Acad Sci USA. 2014;111(52): 18578–18583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Deforche L, Roose E, Vandenbulcke A, et al. Linker regions and flexibility around the metalloprotease domain account for conformational activation of ADAMTS-13. J Thromb Haemost. 2015;13(11):2063–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gao W, Anderson PJ, Sadler JE. Extensive contacts between ADAMTS13 exosites and von Willebrand factor domain A2 contribute to substrate specificity. Blood. 2008;112(5):1713–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jian C, Xiao J, Gong L, et al. Gain-of-function ADAMTS13 variants that are resistant to autoantibodies against ADAMTS13 in patients with acquired thrombotic thrombocytopenic purpura. Blood. 2012;119(16):3836–3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Roose E, Schelpe AS, Joly BS, et al. An open conformation of ADAMTS13 is a hallmark of acute acquired thrombotic thrombocytopenic purpura. J Thromb Haemost. 2018;16(2):378–388. [DOI] [PubMed] [Google Scholar]
- 86.Morgand M, Buffet M, Busson M, et al. High prevalence of infectious events in thrombotic thrombocytopenic purpura and genetic relationship with toll-like receptor 9 polymorphisms: experience of the French Thrombotic Microangiopathies Reference Center. Transfusion. 2014;54(2):389–397. [DOI] [PubMed] [Google Scholar]
- 87.Fuchs TA, Kremer Hovinga JA, Schatzberg D, Wagner DD, Lammle B. Circulating DNA and myeloperoxidase indicate disease activity in patients with thrombotic microangiopathies. Blood. 2012;120(6):1157–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pillai VG, Bao J, Zander CB, et al. Human neutrophil peptides inhibit cleavage of von Willebrand factor by ADAMTS13: a potential link of inflammation to TTP. Blood. 2016;128(1):110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Motto DG, Chauhan AK, Zhu G, et al. Shigatoxin triggers thrombotic thrombocytopenic purpura in genetically susceptible ADAMTS13-deficient mice. J Clin Invest. 2005;115(10):2752–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Verbij FC, Fijnheer R, Voorberg J, Sorvillo N. Acquired TTP: ADAMTS13 meets the immune system. Blood Rev. 2014;28(6):227–234. [DOI] [PubMed] [Google Scholar]
- 91.Sorvillo N, Van Haren SD, Kaijen PH, et al. Preferential HLA-DRB1*11–dependent presentation of CUB2-derived peptides by ADAMTS13-pulsed dendritic cells. Blood. 2013;121(17):3502–3510. [DOI] [PubMed] [Google Scholar]
- 92.Tersteeg C, Verhenne S, Roose E, et al. ADAMTS13 and anti-ADAMTS13 autoantibodies in thrombotic thrombocytopenic purpura – current perspectives and new treatment strategies. Expert Rev Hematol. 2016;9(2):209–221. [DOI] [PubMed] [Google Scholar]
- 93.Chapin J, Weksler B, Magro C, Laurence J. Eculizumab in the treatment of refractory idiopathic thrombotic thrombocytopenic purpura. Br J Haematol. 2012;157(6):772–774. [DOI] [PubMed] [Google Scholar]
- 94.Feng S, Kroll MH, Nolasco L, Moake J, Afshar-Kharghan V. Complement activation in thrombotic microangiopathies. Br J Haematol. 2013;160(3):404–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Osafo-Addo AD, Koram KA, Oduro AR, Wilson M, Hodgson A, Rogers WO. HLA-DRB1*04 allele is associated with severe malaria in Northern Ghana. Am J Trop Med Hyg. 2008;78(2):251–255. [PubMed] [Google Scholar]
- 96.Tong X, Chen L, Liu S, et al. Polymorphisms in HLA-DRB1 gene and the risk of tuberculosis: a meta-analysis of 31 studies. Lung. 2015;193(2):309–318. [DOI] [PubMed] [Google Scholar]
- 97.Mancini I, Ricaño-Ponce I, Pappalardo E, et al. Immunochip analysis identifies novel susceptibility loci in the human leukocyte antigen region for acquired thrombotic thrombocytopenic purpura. J Thromb Haemost. 2016;14(12):2356–2367. [DOI] [PubMed] [Google Scholar]
- 98.Liu GY, Fairchild PJ, Smith RM, Prowle JR, Kioussis D, Wraith DC. Low avidity recognition of self-antigen by T cells permits escape from central tolerance. Immunity. 1995;3(4):407–415. [DOI] [PubMed] [Google Scholar]
- 99.James EA, Kwok WW. Low-affinity major histocompatibility complex-binding peptides in type 1 diabetes. Diabetes. 2008;57(7):1788–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.South K, Freitas MO, Lane DA. A model for the conformational activation of the structurally quiescent metalloprotease ADAMTS13 by von willebrand factor. J Biol Chem. 2017;292(14):5760–5769. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.