Abstract
This analysis explored factors influencing survival of patients with primary refractory and relapsed peripheral T-cell lymphomas enrolled in the prospective International T-cell Project. We analyzed data from 1020 patients with newly diagnosed disease, enrolled between September 2006 and December 2015. Out of 937 patients who received first-line treatment, 436 (47%) were identified as refractory and 197 (21%) as relapsed. Median time from the end of treatment to relapse was 8 months (range 2-73). Overall, 75 patients (8%) were consolidated with bone marrow transplantation, including 12 refractory and 22 relapsed patients. After a median follow up of 38 months (range 1-96 months) from documentation of refractory/relapsed disease, 440 patients had died. The median overall survival (OS) was 5.8 months; 3-year overall survival rates were 21% and 28% for refractory and relapsed patients, respectively (P<0.001). Patients receiving or not salvage bone marrow transplantation had a 3-year survival of 48% and 18%, respectively (P<0.001). In a univariate Cox regression analysis, refractory disease was associated with a higher risk of death (HR=1.43, P=0.001), whereas late relapse (>12 months, HR 0.57, P=0.001) and salvage therapy with transplantation (HR=0.36, P<0.001) were associated with a better OS. No difference was found in OS with respect to histology. This study accurately reflects outcomes for patients treated according to standards of care worldwide. Results confirm that peripheral T-cell lymphomas patients had dismal outcome after relapse or progression. Patients with chemotherapy sensitive disease who relapsed after more than 12 months might benefit from consolidation bone marrow transplantation.
Introduction
The mature or peripheral T-cell lymphomas (PTCL) encompass a biologically and clinically heterogeneous group of rare neoplasia arising from post-thymic lymphocytes. They represent 10-15% of all lymphomas in the Western hemisphere.1
Peripheral T-cell lymphoma patients, except for anaplastic large cell lymphoma (ALCL), anaplastic lymphoma kinase (ALK)-positive, have a poor prognosis.2,3 Current treatment strategies are largely unsatisfactory both in first-line and in the refractory/relapsed settings. First-line therapy relies on CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) and CHOP-like regimens, with a remission rate of 50-65%.4–6 Phase II studies demonstrated that early consolidation with high-dose chemotherapy and stem cell rescue could improve outcome, but this approach is restricted to good performance status patients and chemotherapy responsive disease. For the majority of patients, risk of relapse remains quite high, and relapsed or refractory patients have been shown to have a dismal outcome.7,8 In recent years, there have been several studies testing novel therapies in this subset of patients.9
Two recent observational, population-based studies focusing on the outcome of relapsed or refractory PTCL patients have been published.7,8 The first, conducted by the British Columbia Cancer Agency, Canada, included 208 refractory or relapsed patients diagnosed between 1976 and 2010. The second study included 53 patients identified from the Modena Cancer Registry, Italy, with diagnosis confirmed between 1997 and 2010. Both showed extremely poor outcome with short remissions (median survival after relapse of 5.5 months and 2.5 months, respectively), and they confirmed that the outcome was superior in patients able to go forward for transplant.
The International T-cell Project is an international prospective cohort study that enrolled patients at 74 academic centers on four continents. Data on epidemiology, clinical features, treatments and outcomes were collected. The purpose of the present study was to analyze clinical features and explore factors influencing survival of patients with primary refractory or relapsed PTCL.
Methods
The T-Cell Project (TCP; registered at clinicaltrials.gov identifier: 01142674), sponsored by the International T-Cell Lymphoma Project (ITCLP), was set up in 2006, and builds on the retrospective study carried on by the network.2 Patients with different PTCL subtypes according to World Health Organization (WHO) 2001 or 2008 classifications1,10 were registered in the TCP at initial diagnosis. The T-Cell Project is a prospective cohort study that collected clinical and diagnostic information to better define clinical characteristics, therapies and prognosis for the most frequent subtypes of PTCL: PTCL not otherwise specified (PTCL-NOS) and angioimmunoblastic T-cell lymphoma (AITL). Further aims were to better outline clinical characteristics and outcome of the less common PTCL subtypes: extranodal NK/T-cell lymphoma, enteropathy-type T-cell lymphoma, hepatosplenic T-cell lymphoma, peripheral gamma-delta T-cell lymphoma, subcutaneous panniculitis-like T-cell lymphoma.11 Data were collected on front-line treatment, response evaluation and up-dated follow up for at least five years. Patients who did not receive any kind of treatment were also to be registered on the study. Data management was performed at the Trial Office in Modena, Italy (Department of Diagnostic, Clinical and Public Health Medicine, University of Modena and Reggio Emilia). Registration was based on locally established histological diagnosis. A panel of expert hematopathologists reviewed the diagnosis of 70% of all patients. Approximately 4% could not be adequately classified by central reviewers and were retained in the study with the diagnosis made by a local pathologist. Finally, for 26% of cases, samples were not centralized and these cases were evaluated on the basis of the local diagnosis.
The TCP was conducted in compliance with the Declaration of Helsinki and was approved by the appropriate research ethics committees or institutional review boards at each participating institution. Each patient was required to provide written informed consent before registration.
End points
The principal end point of the analysis was survival after relapse (SAR) for patients with primary refractoriness and those who relapsed, measured from the date refractoriness was documented/date of relapse until last follow up or death from any cause.
Conventional response assessment after the first treatment has been adapted from the Standardized Response Criteria for Non-Hodgkin’s Lymphoma and from Recommendations for Revised Response Criteria for Malignant Lymphoma.12,13 Assessments were made by computed tomography (CT) scan or positron emission tomography (PET) scan according to physician’s discretion; responses were determined by the treating physician.
For the present analysis, primary refractory disease was defined as no response or progression to initial treatment within one month from the end of initial therapy or unsatisfactory partial remission (PR), i.e. a PR that according to the physician’s judgment was considered to be inadequate for the patient, and thus requiring salvage therapy immediately after completion of front-line treatment. Relapsed disease was defined as progression at least one month from completion of front-line therapy in patients who achieved a complete remission (CR) or a satisfactory PR.
Statistical analysis
Standard descriptive analyses were carried out. For a crude association analysis, categorical data were analyzed using the χ2 or Fisher’s exact test (two-sided) for data analysis. Survival curves were estimated using the Kaplan-Meier method, and compared using the log-rank test.
Univariate regression analyses were conducted to identify prognostic factors associated with SAR. Odds ratios with their 95% confidence intervals (95%CI) were computed. Two-tailed P<0.05 was considered statistically significant. The Stata software, version 14·0 or greater (StataCorp, LLC, College Station, TX, USA; www.stata.com) was used for data analysis.
Results
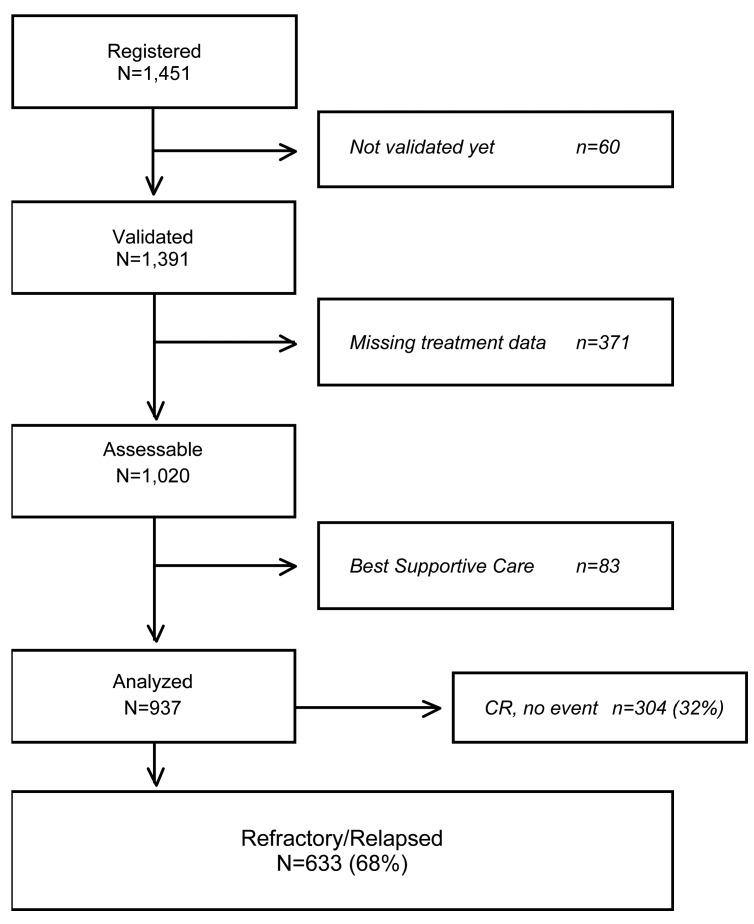
From September 2006 to July 2016, 1451 patients have been registered by 74 institutions worldwide. Among them, 1020 had baseline clinical data, information on first-line treatment, response to initial therapy, time to relapse and salvage treatment available for evaluation. At the time of diagnosis, 83 patients (8%) received only best supportive care and were excluded from this analysis. Out of 937 patients who received an active treatment, 633 (68%) were identified as refractory or relapsed patients, while 304 (32%) patients remained in complete remission.
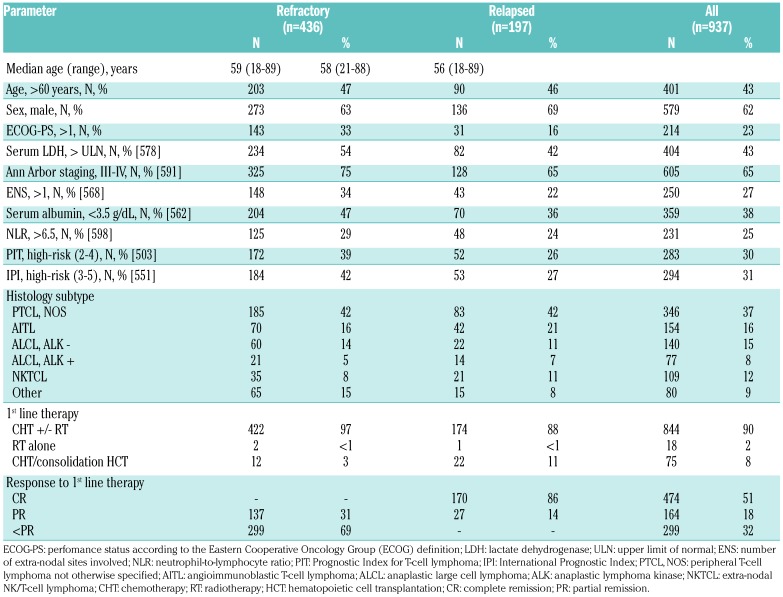
Among the 633 refractory/relapsed patients, 436 (69%) were classified as refractory and 197 (31%) as relapsed patients. The median time to relapse was 8 months (range 2-73 months). Among the relapsed patients, 125 (63%) presented with an early relapse (≤12 months) and 72 (37%) presented with late relapse (>12 months). Main baseline patients’ characteristics of refractory/relapsed patients and all of the analyzed subset are shown in Table 1. The median age at diagnosis of refractory patients was 59 years (range 18-89 years) and that of relapsed patients was 58 years (range 21-88 years). Thirty-three percent of refractory patients and 16% of relapsed patients had ECOG performance status over 1 at diagnosis, respectively. A similar number of patients with AITL and PTCL had refractory (AITL: 16%, PTCL-NOS: 42%) or relapsed (AITL: 21%, PTCL-NOS: 42%) disease (Table 1). Patients with ALCL ALK− were more likely to have refractory disease than ALCL ALK+ (14% vs. 5%), but the frequency of relapsed disease was similar between both groups (11% vs. 7%).
Table 1.
Main characteristics at diagnosis of 436 refractory and 197 relapsed patients, and of all 937 patients analyzed.
Figure 1.
Flow chart of patients included in the analysis. CR: complete remission.
The majority of patients (n=844, 90%) received chemotherapy +/− radiotherapy as first-line treatment and 75 (8%) were consolidated with high-dose therapy (HDT) and hematopoietic cell transplantation (HCT) (Table 1). HCT was considered to be part of first-line therapy when it was given within six weeks from the end of the induction chemotherapy; in addition, patients who received HCT after six weeks from the end of initial chemotherapy, but for whom the clinician specified in the planned treatment schedule that HCT was going to be given as consolidation, and who did not receive additional salvage therapies, were also considered to have received HCT as part of first-line therapy. Of those who received HCT, 41 patients were in first remission, 12 (3%) had refractory and 22 (11%) had relapsed disease.
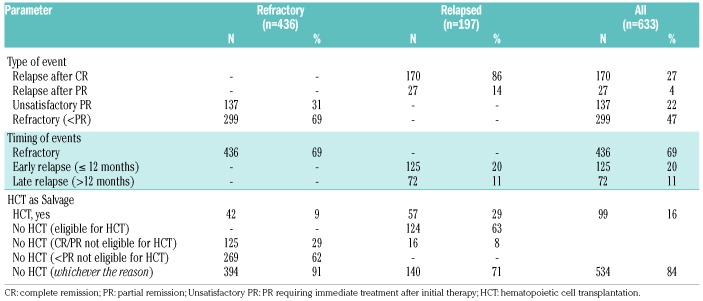
Details of salvage treatments are shown in Table 2. Overall, 99 patients (16%) received HCT as part of salvage treatment. Of those with refractory disease, 62% did not achieve at least a PR with salvage therapy and were therefore not eligible to undergo transplantation. Twenty-nine percent responded well to salvage therapy but were not considered candidates for transplantation. In the relapsed group, likewise, most of the patients (71%) did not undergo transplantation. Data on the reason why patients eligible for transplant were not referred to HCT consolidation was not collected; the choice as to whether the patient should go forward for transplant was at the physician’s discretion.
Table 2.
Details of treatment and events for the refractory/relapsed patients (n=633).
Survival after relapse
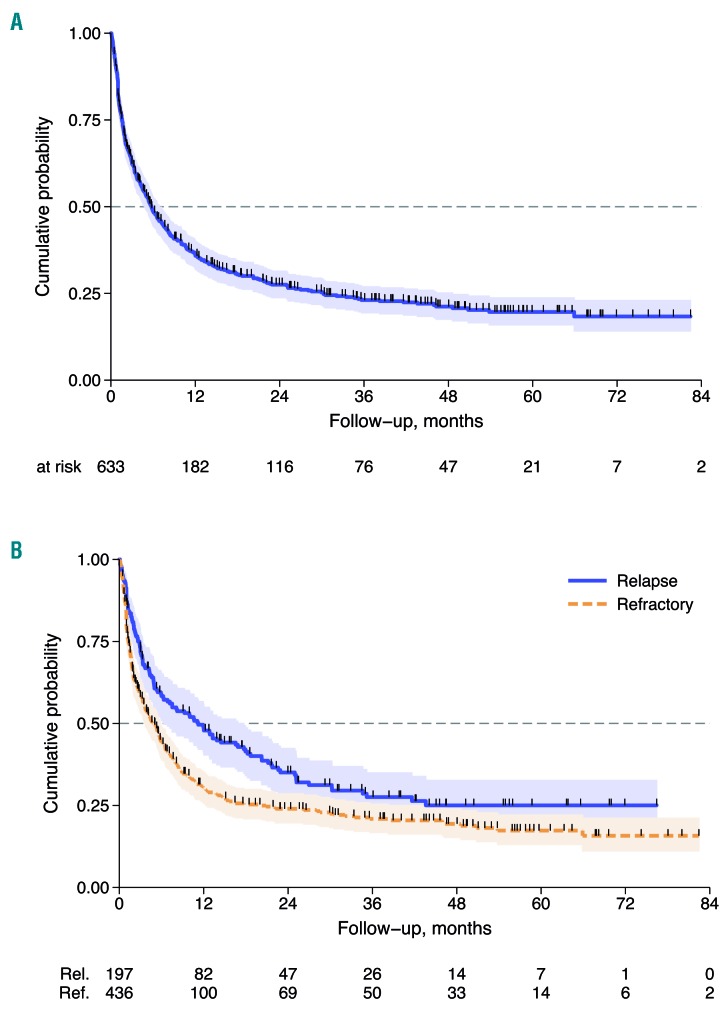
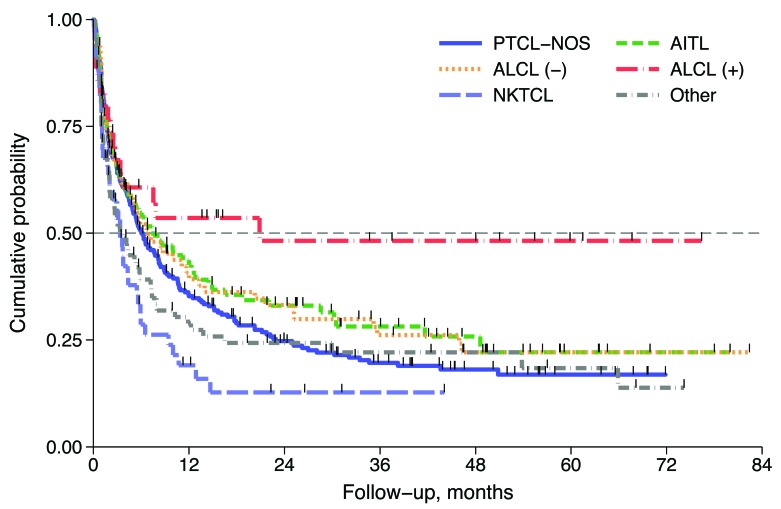
After a median follow up of 38 months (range 1-96 months) from documentation of refractory/relapsed disease, 440 (70%) patients had died. The median survival after relapse (SAR) was 5.8 months (95%CI: 4.9-7.2 months) and 3-year SAR was 23% (95%CI: 19-27) (Figure 2A). Median SAR for refractory and relapsed patients were 5 and 11 months, respectively, and 3-year SAR rates were similar for both groups at 21% (95%CI: 17-25) and 28% (95%CI: 21-35), respectively (Figure 2B). Univariate analysis showed that in the first 24 months refractory patients had a poorer outcome with respect to relapsed patients [Hazard Ratio (HR) HR 1.50, 95%CI: 1.12-1.86; P<0.001], while after 24 months their outcome became similar to that of the relapsed group (HR 0.75, 95%CI: 0.34-1.64; P=0.470). (Figure 2B). No difference was found in outcomes for refractory/relapsed patients with respect to PTCL subtype, with the exception of ALCL ALK+ (Figure 3).
Figure 2.
Survival after relapse (SAR). (A) SAR curve of 633 refractory/relapsed patients. (B) SAR by status: refractory versus relapse. Refractory patients are those with primary refractoriness.
Figure 3.
Outcomes for refractory/relapsed patients depending on histological subtypes. PTCL-NOS: peripheral T-cell lymphoma not otherwise specified; AITL: angioimmunoblastic T-cell lymphoma; ALCL (−): anaplastic large cell lymphoma, anaplastic lymphoma kinase negative; ALCL (+): anaplastic large cell lymphoma, anaplastic lymphoma kinase positive; NKTCL: extranodal NK/T-cell lymphoma.
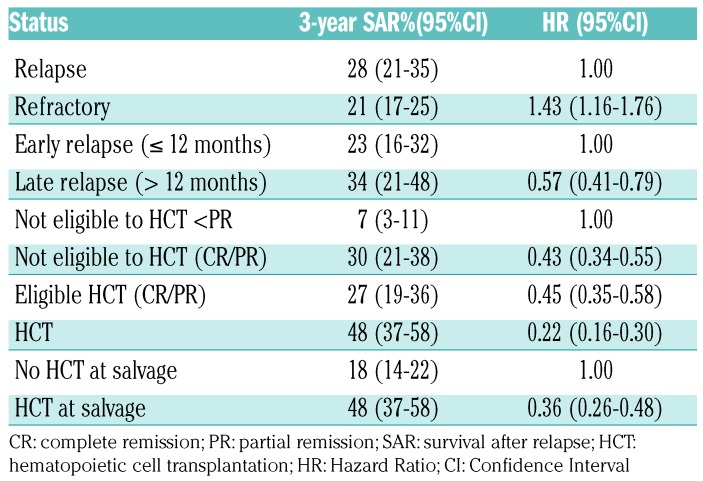
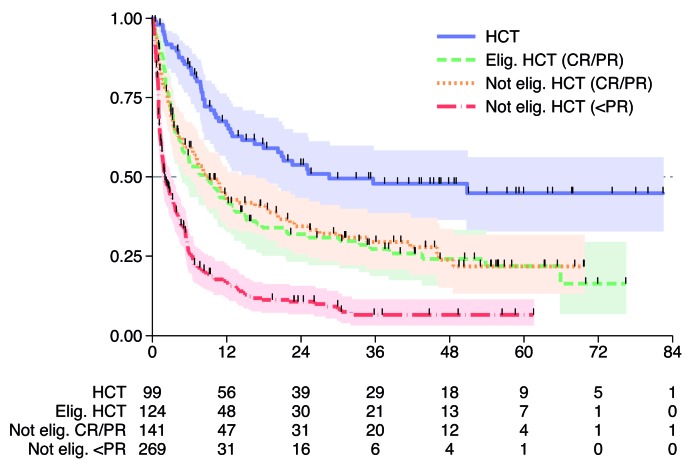
As expected, patients responding to salvage therapy who proceeded to HCT had a better outcome compared to patients with no response (and therefore, ineligible for HCT) and to patients in CR/PR not eligible for HCT (for any reason), with 3-year survival rates of 48%, 7% and 30%, respectively. Similarly, patients proceeding to HCT had significantly better outcome than patients who were eligible but did not undergo HCT for any reason (3-year SAR 48% and 27%). Overall, patients who received HCT had a better outcome with respect to the subset of patients who did not (3-year SAR 48% and 18%, respectively) (P<0.001) (Table 3 and Figure 4).
Table 3.
Univariate Cox regression analysis for SAR.
Figure 4.
Survival after relapse (SAR) by salvage therapy including or not hematopoietic cell transplantation (HCT). Elig: eligible; Not elig: not eligible; CR: complete remission; PR: partial remission.
In a univariate Cox regression analysis, refractory disease was associated with a higher risk of death compared to relapsed patients (HR 1.43, 95%CI: 1.16-1.76; P=0.001), whereas late relapse compared to early relapse (HR 0.57, 95%CI: 0.41-0.79; P=0.001) and salvage therapy with HCT compared to no HCT (HR 0.36, 95%CI: 0.26-0.48; P<0.001) were associated with a longer SAR (Table 3).
Discussion
The International TCP represents the largest cohort of prospectively collected data on patients with aggressive T-cell lymphomas and accurately reflects outcomes for patients treated according to standards of care around the world. In the present study, we sought to analyze the outcomes of patients with relapsed and refractory disease after failure of first-line therapy and to explore potential prognostic factors influencing survival, retrieved from this database. We demonstrated that the outcomes are worse for patients with refractory disease and that the SAR at three years for these patients was only 21%. We also found that late relapse and consolidation with HCT were associated with a longer survival in chemotherapy sensitive patients.
In our analysis, refractory/relapsed patients presented a poor risk profile. Sixty-nine percent of failing patients were refractory to first-line treatment and 80% of refractory and 70% of relapsed patients had advanced stage disease at diagnosis. Fifty percent of refractory and 33% of relapsed patients were at high risk according to the Prognostic Index for T-cell lymphoma (PIT); 50% and 32% of relapsed and refractory patients, respectively, were at high-risk according to the International Prognostic Index (IPI).
Survival was poor in our cohort, with a median SAR for refractory and relapsed patients of only 5 and 11 months, respectively. The results from this prospective cohort confirm findings from other reports that refractory disease is a poor prognostic factor.7,8 While late relapse occurring after 12 months versus early relapse at less than 12 months from front-line therapy was associated with a longer survival, only 11% of patients were in the late relapse category as most relapses occurred within one year from front-line therapy. Surprisingly, there was no difference in outcomes for refractory/relapsed patients with respect to PTCL subtype, suggesting that significant improvements are needed in treatment strategies for all subtypes of PTCL. We were surprised to find similar survival rates between relapsed and refractory PTCL patients at three years post completion of therapy. However, further analysis showed that within the first 24 months of follow up relapsed patients had superior survival, and it is only past that time point that the advantage disappeared. These results suggest that within a category of relapsed patients there is a subgroup with biologically refractory disease and current definitions based on clinical responses are not sensitive enough to identify individuals that would benefit from alternative approaches rather than standard salvage protocols. Furthermore, only about half of the refractory PTCL patients exhibited clinical high risk based on IPI or PIT scores at diagnosis. Emerging genome-wide analysis at diagnosis and/or relapse might overcome these restrictions and provide a better guide for initial and salvage therapy in the near future.
In the relapsed and refractory setting, the best chance of long-term remission and best outcomes occurred in patients with late (>12 months) relapse who were able to undergo HDT followed by HCT, with SAR at three years of 48%. However, a major problem remains: only 16% of the patients could proceed to this strategy as part of the salvage treatment due to refractoriness to induction therapy, early relapses, ineffective salvage therapies, and overall poor performance status and patient-specific factors. Two recent population-based retrospective studies focusing on the outcome of relapsed or refractory PTCL patients have been published and they reported a similar poor outcome (median survival after relapse of 5.5 months and 2.5 months).7,8 Likewise, the outcome was far superior in patients able to receive a transplant. Taken together, the current challenge remains to increase the response rates of induction therapies to raise the number of eligible patients for the most effective available intent-to-treat treatment. Moreover, these results highlight the urgent need for novel agents and more effective salvage therapies.
Advances in understanding the biology and genetics of T-cell lymphomas have led to the identification of several potential novel targets.14–17 Recently, four new-generation drugs have been approved in the USA in refractory/relapsed TCL: pralatrexate (antifolate), romidepsin and belinostat (histone deacetylase inhibitor), and brentuximab vedotin. In addition to these approved drugs, a number of novel drugs with different mechanisms are under investigation: crizotinib (oral tyrosine kinase inhibitor of ALK), mogamulizumab, duvelisib, plitidepsin, and selinexor.9,18 Hopefully, these agents will have an impact both when combined with front-line chemotherapy as well as in the relapsed and refractory setting.
Although the patient cohort could be not completely homogeneous (Investigators were requested to register consecutive cases satisfying the inclusion criteria without selection), the amplitude of the TCP reflects the real-world scenario, obtained through a multi-national database describing the distribution of PTCL subtypes and therapeutic outcomes with standard therapies. Our results complement those of the COMPLETE registry (clinicaltrials.gov identifier: 01110733), a similar prospective study of PTCL patients in the US. These results will provide a useful baseline on which to assess the efficacy of novel agents and therapies for refractory/relapsed patients with T-cell lymphomas. Clinical trials are underway exploring the activity of novel agents in combination with chemotherapy to improve overall response in the front line, and single agent and combination studies of novel agents are underway for patients with refractory/relapsed disease.
Supplementary Material
Acknowledgments
For supporting this study, the authors would like to thank the Fondazione Cassa di Risparmio di Modena, Modena, Italy, the Associazione Angela Serra per la Ricerca sul Cancro, Modena, Italy, the Fondazione Italiana Linfomi (FIL), Alessandria, Italy, Allos Therapeutics, Inc., Westminster, CO, USA, and Spectrum Pharmaceuticals Inc., Henderson, NV, USA, AIRC (Associazione Italiana per la Ricerca sul Cancro) 5×1000 (grant n. 10007 to Stefano Pileri), the NIH/NCI CCSG P30 CA008748 (grant to Steven Horwitz).
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/103/7/1191
References
- 1.Swerdlow S, Campo E, Harris N, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: IARC Press; 2008. [Google Scholar]
- 2.Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124–4130. [DOI] [PubMed] [Google Scholar]
- 3.Adams SV, Newcomb PA, Shustov AR. Racial Patterns of Peripheral T-Cell Lymphoma Incidence and Survival in the United States. J Clin Oncol. 2016;34(9):963–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Melnyk A, Rodriguez A, Pugh WC, Cabannillas F. Evaluation of the Revised European-American Lymphoma classification confirms the clinical relevance of immunophenotype in 560 cases of aggressive non-Hodgkin’s lymphoma. Blood. 1997;89(12):4514–4520. [PubMed] [Google Scholar]
- 5.Lopez-Guillermo A, Cid J, Salar A, et al. Peripheral T-cell lymphomas: initial features, natural history, and prognostic factors in a series of 174 patients diagnosed according to the R.E.A.L. Classification. Ann Oncol. 1998;9(8):849–855. [DOI] [PubMed] [Google Scholar]
- 6.Gisselbrecht C, Gaulard P, Lepage E, et al. Prognostic significance of T-cell phenotype in aggressive non-Hodgkin’s lymphomas. Groupe d’Etudes des Lymphomes de l’Adulte (GELA). Blood. 1998;92(1):76–82. [PubMed] [Google Scholar]
- 7.Mak V, Hamm J, Chhanabhai M, et al. Survival of Patients With Peripheral T-Cell Lymphoma After First Relapse or Progression: Spectrum of Disease and Rare Long-Term Survivors. J Clin Oncol. 2013;31(16):1970–1976. [DOI] [PubMed] [Google Scholar]
- 8.Biasoli I, Cesaretti M, Bellei M, et al. Dismal outcome of T-cell lymphoma patients failing first-line treatment: results of a population-based study from the Modena Cancer Registry. Hematol Oncol. 2015;33(3):147–151. [DOI] [PubMed] [Google Scholar]
- 9.Zinzani PL, Bonthapally V, Huebner D, Lutes R, Chi A, Pileri S. Panoptic clinical review of the current and future treatment of relapsed/refractory T-cell lymphomas: Peripheral T-cell lymphomas. Crit Rev Oncol Hematol. 2016;99214–227. [DOI] [PubMed] [Google Scholar]
- 10.Jaffe ES, Harris NL, Stein H, Vardiman JW. WHO classification of Tumours of Haematopoietic and Lymphoid Tissues: Pathology and Genetics. Lyon, France: IARC Press; 2001. [Google Scholar]
- 11.Federico M, Bellei M, Pesce E, et al. T-Cell Project: an international, longitudinal, observational study of patients with aggressive peripheral T-cell lymphoma. Rev Bras Hematol Hemoter. 2009;31(2):21–25. [Google Scholar]
- 12.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17(4):1244. [DOI] [PubMed] [Google Scholar]
- 13.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–586. [DOI] [PubMed] [Google Scholar]
- 14.Piccaluga PP, Fuligni F, De Leo A, et al. Molecular profiling improves classification and prognostication of nodal peripheral T-cell lymphomas: results of a phase III diagnostic accuracy study. J Clin Oncol. 2013;31(24):3019–3025. [DOI] [PubMed] [Google Scholar]
- 15.Iqbal J, Wright G, Wang C, et al. Gene expression signatures delineate biological and prognostic subgroups in peripheral T-cell lymphoma. Blood. 2014;123(19):2915–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Connor OA, Bhagat G, Ganapathi K, et al. Changing the paradigms of treatment in peripheral T-cell lymphoma: from biology to clinical practice. Clin Cancer Res. 2014;20(20):5240–5254. [DOI] [PubMed] [Google Scholar]
- 17.Inghirami G, Chan WC, Pileri S. Peripheral T-cell and NK cell lymphoproliferative disorders: Cell of origin, clinical and pathological implications. Immunol Rev 2015;263(1):124–159. [DOI] [PubMed] [Google Scholar]
- 18.Coiffier B, Federico M, Caballero D, et al. Therapeutic options in relapsed or refractory peripheral T-cell lymphoma. Cancer Treat Rev. 2014;40(9):1080–1088. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.