Abstract
Silent cerebral infarction is the most common neurological abnormality in children with sickle cell anemia, affecting 30-40% of 14 year olds. There are no known biomarkers to identify children with silent cerebral infarcts, and the pathological basis is also unknown. We used an unbiased proteomic discovery approach to identify plasma proteins differing in concentration between children with and without silent cerebral infarcts. Clinical parameters and plasma samples were analysed from 51 children (mean age 11.8 years, range 6-18) with sickle cell anemia (HbSS). A total of 19 children had silent cerebral infarcts and 32 normal MRI; the children with silent infarcts had lower HbF levels (8.6 vs. 16.1%, P=0.049) and higher systolic blood pressures (115 vs. 108.6, P=0.027). Plasma proteomic analysis showed 13 proteins increased more than 1.3 fold in the SCI patients, including proteins involved in hypercoagulability (α2-antiplasmin, fibrinogen−γ chain, thrombospondin-4), inflammation (α2-macroglobulin, complement C1s and C3), and atherosclerosis (apolipoprotein B-100). Higher levels of gelsolin and retinol-binding protein 4 were also found in the population with silent infarcts, both of which have been linked to stroke. We investigated the genetic basis of these differences by studying 359 adults with sickle cell disease (199 with silent cerebral infarcts, 160 normal MRIs), who had previously undergone a genome-wide genotyping array. None of the genes coding for the differentially expressed proteins were significantly associated with silent infarction. Our study suggests that silent cerebral infarcts in sickle cell anemia may be associated with higher systolic blood pressure, lower HbF levels, hypercoagulability, inflammation and atherosclerotic lipoproteins.
Introduction
Sickle cell anemia (SCA) is the most common cause of stroke in childhood.1 The abnormal sickle hemoglobin (HbS) polymerizes when deoxygenated, damaging red cells, causing vaso-occlusion and vascular endothelial dysfunction.2 A cascade of pathological processes follows, including inflammation, hemolysis, anemia, oxidative stress, reperfusion injury, hypercoagulability, and nitric oxide deficiency.3 Survival is reduced by 20-30 years4,5 and vasculopathic complications include pulmonary hypertension, priapism, and cerebrovascular disease.6 The latter is the major cause of morbidity in children and takes two forms: overt stroke often associated with large vessel disease,7 and silent cerebral infarcts (SCI) of less certain pathology.8 Without intervention, overt stroke has a peak incidence of 1.02/100 patient years between 2 and 5 years,9 although primary prevention using transcranial Doppler (TCD) scanning and blood transfusion reduces this by 90%.10 The implementation of TCD-based stroke prevention has led to a significant fall in overt strokes in many countries.11,12 SCI is more common than overt stroke with MRI showing relevant lesions in 13% of two year olds, 25% of six year olds, and 30-40% of 14 year olds.13
Although by definition SCIs are not associated with overt symptoms, they cause significant morbidity, including a reduction in IQ, defects in executive function, epilepsy, and increased risk of further SCIs and overt strokes.13 Early detection of SCI is useful to allow cognitive assessment, educational support and therapeutic intervention. The Silent Cerebral Infarct Multi-Center Clinical Trial (SIT Trial), a randomised controlled study, showed that regular blood transfusion reduced the incidence of recurrent infarction in children with SCIs compared to the observational arm.14
The pathophysiology of SCI is unclear. It is often stated that SCIs are caused by small vessel disease, although there is no direct evidence for this. There are no postmortem studies looking at the histological changes corresponding to the MRI appearances of SCI, although older studies identified small necrotic lesions in the subcortex of the brain, possibly representing SCIs.15 It is also possible that SCIs are areas of demyelination, or linked to venous sinus thrombosis.13 SCIs may also be caused by difficulties maintaining constant blood flow to the brain, leading to watershed infarction precipitated by acute anemia or hypoxia.16 The lack of pathophysiological understanding makes it difficult to develop new therapeutic strategies. Here, we used an unbiased proteomic approach to identify plasma proteins associated with SCI and potentially linked to the underlying pathophysiology.
It is not easy to identify children with or at increased risk of SCI. Baseline data from the SIT Trial showed that steady state hemoglobin <7.6g/dl, steady state systolic blood pressure >104mmHg and male sex were all associated with increased risk of SCI, although a model combining all three significant factors had weak predictive powers, with a C statistic of about 0.6.8 Abnormal transcranial Doppler velocities are not reliably associated with SCI, although there is a possible association with stenosis of the cervical internal carotid artery.17 Neuropsychometry can detect some children with SCIs, but is time consuming and not easily available. MRI is currently the only way of diagnosing SCI, although before 7 years of age this usually requires sedation or general anesthetic, which carries significant risks in children with SCD and is unsuitable for screening. MRI is also not widely available in many African countries where SCA is most prevalent. It would therefore be beneficial to develop a simple, screening test for SCI, potentially based on blood testing.
Proteomics can detect, identify and quantify very low concentrations of proteins. For example, a recent study suggested that urine proteomics might help in the diagnosis of acute stroke by detecting brain peptides released by ischemia.18 The presence of SCI may suggest small blood vessel disease and a tendency to infarct brain tissue, which is likely to be a chronic process, releasing detectable brain proteins into plasma. Plasma protein profiles could also differ between those with and without SCI because of causative pathological factors, including coagulopathy, inflammation, hypoxia and vasculopathy; these differences may be acquired or inherited, and reflect genetic predisposition to silent cerebral infarction. We investigated the possibility that there might be differences in the plasma proteome between children with and without SCIs, with a view to understanding more about the pathophysiology of SCIs and identifying potentially useful biomarkers. We further investigated the hypothesis that genetic factors account for the differences in concentrations of the various proteins, using data from a genome wide association study (GWAS) in a separate cohort of patients with SCA and SCIs.
Methods
Patients and setting
The National Research Ethics Committee approved the study (reference 13/LO/0709). Children were recruited from clinics at King’s College Hospital and the Evelina Children’s Hospital. Approximately 1000 different children are seen annually in these clinics with a further 500 seen in outreach clinics.19 The aim was to recruit a total of 50 patients, with equal numbers of those with SCIs and controls with normal brain MRIs. Children known to have SCIs from previous MRI scans were selectively recruited. Eligible patients without previous MRIs were recruited sequentially in clinic, on the basis that 20-30% of these children would have SCIs, and combining the two groups would result in approximately 25 children in each group. SCI and control groups were not specifically matched for age and gender. Inclusion criteria were: sickle cell anemia (HbSS), age 8-18 years old, normal or conditional TCD velocities (<200cm/s) (10), able to tolerate MRI scan without sedation. Exclusion criteria were: history of overt stroke, blood transfusion in last 4 months, serious co-existing disorder (renal failure, HIV, hepatitis, malignancy, autoimmune disease, chronic infection, cardiac disease, long-term medication), acute complications requiring hospital attendance in last 3 months. Patients on hydroxyurea (HU) were recruited to both arms. Routine clinical and laboratory data were collected.
Brain MRI and neurological assessment
Brain MRIs were performed to identify the presence of SCIs using standardised definitions20 (see Online Supplementary Materials). On the day of blood sampling, all children underwent standardised neurological examination by experienced clinicians to document the absence of neurological abnormalities suggestive of an overt stroke. All children had routine TCD scans performed at least annually, and these were used in this study.
Proteomic analysis
Blood samples were collected on all children at the time of consent. A data-driven proteomics approach was used to discover protein changes in the plasma associated with SCI. We used an established workflow combining isobaric Tandem Mass Tags (TMT) reagents with off-gel fractionation, followed by high sensitivity rapid throughput mass spectrometry (MS) and LC-MS/MS. (details in Online Supplementary Materials). Relative amounts of each peptide were compared between the SCI and control groups, and P values were calculated for those peptides showing greater than 1.3 fold differences.
Targeted plasma analysis for biomarkers of neurological disease
Plasma samples were also analysed for 92 protein biomarkers known to be implicated in neurological disease, using a multiplexed proximity extension assay21 (see Online Supplementary Materials for full list of biomarkers).
Data analysis
Statistical significance was assessed using appropriate tests to compare means with adjustments for multiple comparisons when appropriate. Statistical analyses were performed in the proteomics laboratory and medical statistics department of Public Health Sciences at King’s College London.
Genetic association of protein biomarkers with SCIs
The genes coding for plasma proteins which differed significantly between children with and without SCIs were identified using standard databases. Genetic association analysis was then undertaken in a separate adult cohort at KCH, evaluating the variants within each gene and their association with SCI22 (details in Online Supplementary Materials).
Results
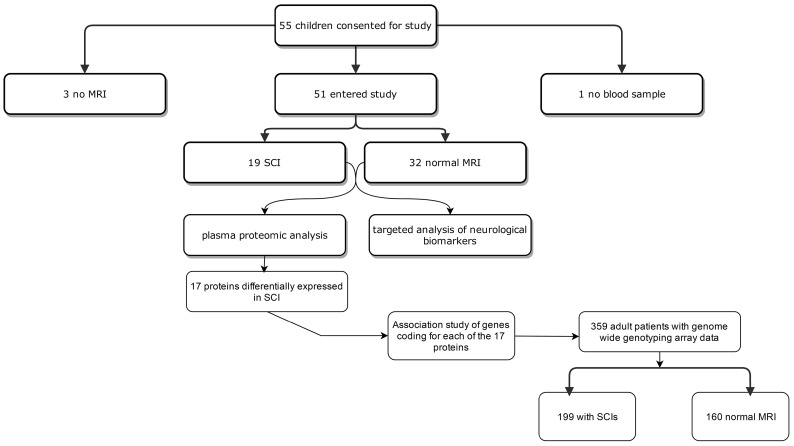
A total of 55 patients consented to take part in the study, although no blood samples were taken from one, and three further patients did not have an MRI scan performed. Plasma from 54 patients underwent proteomic analysis, although only data from the 51 patients (22 female) with MRI results were analysed. In total, 19 (37%) had SCIs and 32 (63%) normal brain MRIs, deviating slightly from the aim of equal numbers in each group because of recruitment of children without previous MRIs, the majority of whom had normal brain imaging (Figure 1). A total of 15 children were identified as having SCIs from scans performed as part of this study, and four children were recruited as a result of previous MRI scans showing SCIs; in these four children, there was a median delay of 20 months (range 9 – 40 months) between MRI scan and blood sampling for proteomic analysis. One child had conditional velocities and 50 normal velocities on TCD scanning.
Figure 1:
Diagram showing the flow of patients through the study.
Basic clinical and laboratory data
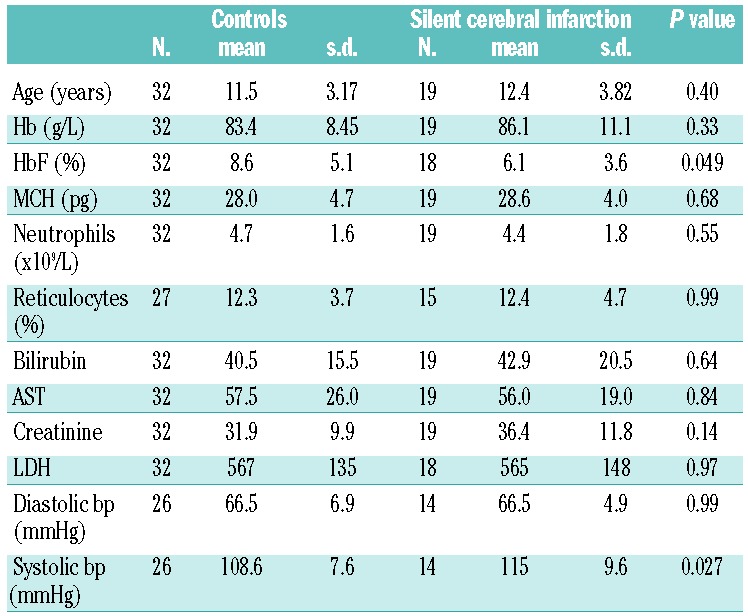
Relatively more males (13/29, 44%) than females (6/22, 27%) were in the SCI group, although this was not significantly different (Chi square, P=0.199). There was no significant difference in HU use between the SCI and control groups: controls 11/32 (36%) on HU, SCI 7/19 (37%) on HU (Chi square, P=0.86). G6PD assays were available for 45 patients, and there was no association with SCI: G6PD deficiency was present in 3/29 (10%) controls and 2/16 (12%) with SCIs (Chi square, P=0.83). Basic laboratory and clinical characteristics are summarized in Table 1. Mean HbF levels were significantly lower (6.1% versus 8.6%, P=0.049), and mean systolic blood pressures significantly higher (115 versus 108.6, P=0.027) in those with SCIs compared to controls.
Table 1.
Comparison of laboratory and clinical measurements of those with and without SCI. P values from t-tests, with significant results shown in bold (P<0.05), not corrected for multiple comparisons.
Plasma proteomic analysis
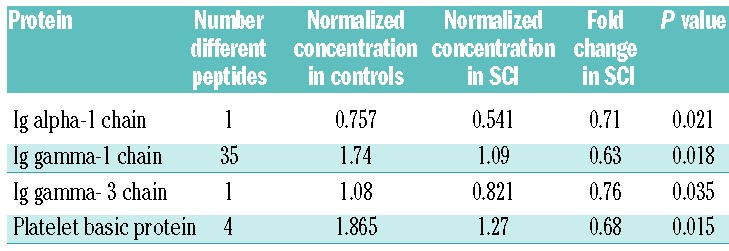
A total of 4662 different peptides were identified, although only 1312 were present in all 51 patient samples, corresponding to 346 different proteins. Forty-four peptides from 13 different proteins were present at more than 1.3 times the concentration in the SCI patients compared to controls (Table 2), whereas 41 peptides from 4 proteins were more than 1.3 higher in the control population (Table 3). Proteins differentially expressed in the SCI group included those implicated in coagulation, lipid and inflammatory pathways.
Table 2.
List of all peptides and proteins present at significantly increased concentrations (>1.3 fold) in children with SCIs compared to controls. Where more than one peptide was identified, the most significant difference is given. Concentrations are normalized against standard controls.
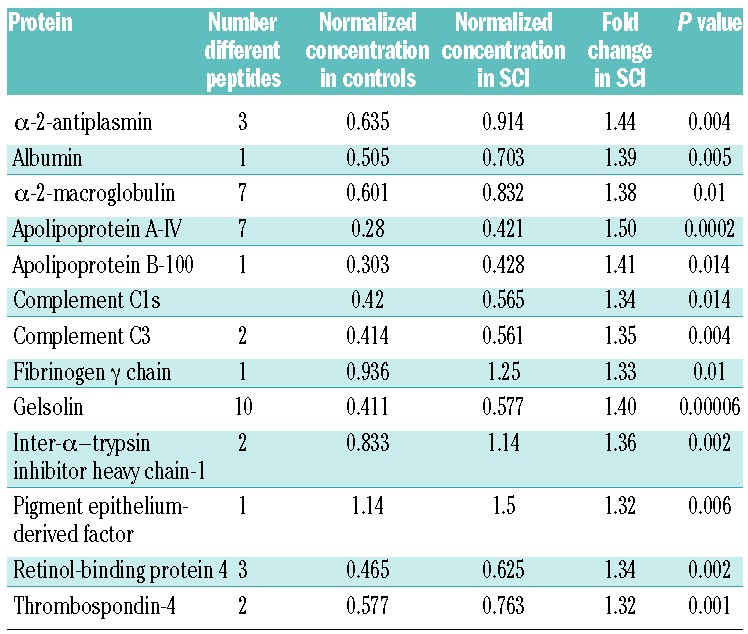
Table 3.
List of all peptides and proteins present at significantly decreased concentrations (>0.77 fold) in children with SCIs compared to controls. Where more than one peptide was identified, the most significant difference is given. Concentrations are normalized against standard controls.
Genetic association of identified biomarkers with SCI
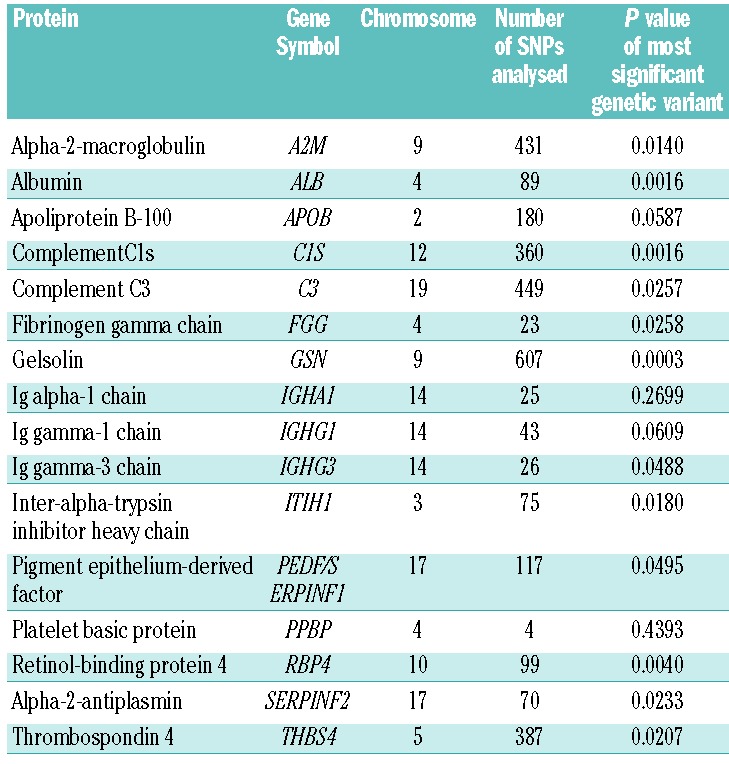
We explored the possibility that differences in concentrations of proteins (Tables 2 and 3) between SCI and control populations were inherited, using a different sample of adult patients who had undergone brain MRI and a genome-wide genotyping array. Of 359 patients with brain MRIs and MEGAchip data, 199 had SCI and 160 did not. Regions of interest were analysed for the candidate genes identified in the proteomic study; the most significant genetic variant from each region is shown in Table 4. No genetic variant in the candidate genes was significantly associated with SCI after correcting for multiple testing. A variant in gelsolin was closest to achieving significance (P=0.00029, threshold for significance 0.00009).
Table 4.
Association of candidate genes for SCI identified in the proteomic part of the study. The most significant genetic variants are shown. None of the variants were significant after adjustment for multiple comparisons.
Targeted plasma analysis
Concentrations of 92 known neurological biomarkers were measured in the plasma of 54 patients who consented to the study and gave blood samples. Fifty-one patients had MRI scans available and were analysed. In order to control for the potential effect of hydroxyurea, a logistic regression model was fitted for each biomarker; the dependent variable was SCI status, and the biomarker and hydroxyurea were included as covariates in each model. Seven biomarkers were significantly associated with SCI, but after correction for multiple hypotheses none of these were significant (Table 5). Several biomarkers were strongly correlated with each other and a heatmap of the correlation matrix indicated a moderate level of redundancy between them. A principal component analysis found that approximately 90% of the variance could be attributed to the first 30 principal components. None of the principal components were found to be differentially expressed with respect to SCI status, either with or without controlling for HU status.
Table 5.
Known biomarkers of neurological disease showing significant differences between SCI and control populations, although none of these maintain significance when correcting for multiple tests.

Discussion
Our study found that the presence of SCI in SCA is associated with higher systolic blood pressures, an association that was also found in the SIT trial.8 Blood pressure is relatively low in SCA,23 possibly related to chronic anemia and subsequent vasodilatation, and the levels found in our study, with a mean of 115mmHg in the SCI group, would not be considered hypertensive. The exact way in which relative systolic hypertension is associated with SCI in children with SCA is unknown, although systolic hypertension is linked with other vascular complications in SCA (overt stroke,23 renal insufficiency,24 and high tricuspid jet velocity24). Systolic hypertension is also a risk factor for SCI in the non-sickle population.25 The association between relative systolic hypertension and SCI in SCA seems robust, and is biologically plausible, although there is currently no evidence on whether it is beneficial to lower blood pressure in children with SCA, and at what level such treatment should be considered.
We also found that higher HbF levels were protective against SCI. Increased HbF levels lessen the severity of SCA, with a clear association with decreased pain and longer life expectancy,2 but a more complex association with cerebrovascular complications. In the SIT Trial, HbF was not associated with SCI in a logistic regression model8 and analysis of data from the Cooperative Study of Sickle Cell Disease also showed no protective effect of higher HbF levels.26 Other studies have suggested that there may be neurological benefit to having higher HbF levels. In France, HbF levels were higher in children without SCIs compared to those with (16.5% vs. 13.1%, P=0.02);27 a Dutch study found that although HbF did not protect against the occurrence of SCIs, higher levels were associated with a smaller volume of white matter hyperintensities;28 a study on Brazilian patients showed protection against stroke was associated with genetic determinants of high HbF levels, particularly BCL11A alleles.29 It seems likely that the inherited ability to make more HbF offers some protection against neurological injury in SCA, although this may not always be reflected in the measured HbF percentage, particularly in small studies.
The unbiased proteomic discovery part of this study identified 44 peptides from 17 different proteins which were significantly up- or down-regulated compared to levels in the control population, without correction for multiplicity (Tables 2 and 3). Many of these proteins can be plausibly implicated in pathogenesis of SCI and fall in to three broad groups.
Prothrombotic proteins
Four proteins which were found at higher levels in the SCI patients are known to be prothrombotic. α2-antiplasmin is a serine protease inhibitor which inactivates plasmin and decreases the rate of fibrinolysis; high levels are associated with increased ischemic stroke in the non-sickle population.30 Similarly, α2-macroglobulin has prothrombotic properties via the inactivation of plasmin, and elevated levels have been linked to stroke and white matter lesions in non-sickle patients.31 The fibrinogen γ chain was increased 1.33 fold in the SCI population, and is at the center of fibrin polymerization, interaction with platelets and regulation of factor XIII activity.32 Increased levels have been associated with vasculopathy, higher levels being increased in inflammation and also possibly inherited.33 Fourthly, thrombospondin-4 was increased 1.32 fold with SCI. Thrombospondins mediate cellular adhesion to other cells and matrix, and are implicated in many thrombotic and vasculopathic processes. Thrombospondin-1 levels have been found to be increased in children with SCA in association with both SCI34 and overt stroke,35 although thrombospondin-4 has not previously been studied in this context. These findings support the potential use of anticoagulant and antiplatelet agents to prevent and treat SCI in SCA.
Pro-inflammatory proteins
Elevated levels of α2-macroglobulin, complement C1s and complement C3 in the SCI patients suggest that there is increased inflammation, which is known to predispose towards vasculopathy and stroke. As mentioned previously, α2-macroglobulin is a prothrombotic protease inhibitor, but is also involved in inflammatory states, as a carrier for interleukin-6.31 Both complements C1s and C3 are elevated in inflammation, and high levels of C3 are a known risk factor for atherosclerosis and stroke in the general population.36 Inflammation is known to be an important component of pathophysiology in SCA, and the role of anti-inflammatory agents in preventing SCIs should be explored further.
Lipoproteins
Apolipoprotein B-100 is the main component of low density lipoprotein cholesterol (LDL-C) and high levels are a well-established risk factor for atherosclerosis. Various studies have shown that drugs which reduce LDL-C also reduce the rate of cardiovascular events, including ischemic stroke,37 suggesting a possible therapeutic option for children with SCA and ischemic stroke for drugs such as statins. We also found that apolipoprotein A-IV levels were higher in the SCI population, which is perhaps paradoxical in that it is thought to be protective against coronary syndromes,38 although it has not been directly implicated in cerebrovascular disease or studied in children. A recent study suggested that higher levels were associated with reduced glomerular filtration rates,39 and this could be one explanation for the differences in our study.
Other interesting candidates
Several other interesting candidates were identified, both shedding light on the pathophysiology of SCI and with the potential to act as useful biomarkers.
Gelsolin binds to and remodels actin by both severing and capping the protein; it is present in both cytoplasm and plasma. Increased plasma levels have been identified as a poor prognostic marker in ischemic stroke.40 Further significant actions in the context of SCI include a role in promoting platelet formation and activation.41 In our genetic analysis, a genetic variant in the gelsolin gene showed a trend towards being associated with SCI.
Retinol binding protein-4 is a lipocalin transporting vitamin A from the liver to other tissues, including the brain; plasma levels have been found to be elevated in atherosclerosis and also ischemic stroke.42,43
Pigment epithelium-derived factor is multifunctional with anti-angiogenic properties, secreted by different tissues including adipocytes. It is potentially significant in the development of SCI because of its ability to induce insulin resistance, and inflammation and proliferation of muscle cells.44 It is harder to know the significance of some other proteins, including the lower levels of immunoglobulin chains and platelet basic protein in SCI, although immunodeficiency has been associated with primary and recurrent stroke in the general pediatric population.45
Several plausible biomarkers for SCI were identified in the targeted plasma analysis, including von Willebrand factor C domain, although none of these reached statistical significance when corrected for multiple assays. Similarly, none of the candidate proteins identified by proteomics showed a convincing genetic association with SCI in a separate cohort of 359 patients with SCA, although the pathophysiology of SCIs seen in the adults used in this part of the study may differ from that in the children used in the proteomic part. This may also reflect the relatively small size of this study, or that the changes seen in proteins of those with SCIs are secondary to other pathological events rather than inherited quantitative trait loci.
The strength of our study is that it used an unbiased proteomics approach to identify proteins involved in the pathogenesis of SCI, together with a combination of targeted plasma analysis and genetic association studies. Our findings suggest that proinflammatory and prothrombotic states contribute to the development of SCI, with possible abnormalities in lipoproteins. Previous studies to identify biomarkers for SCI in SCD have been summarized by Lance et al.35 They identified nine studies measuring a range of different candidates, although no studies used an unbiased proteomic approach. In total, 17 potential candidates were identified in this review, again with a predominance of factors suggesting coagulation activation and increased inflammation.35 None of the identified candidates were the same as our candidates, although one study found increased levels of thrombospondin-1 in children with SCIs and SCA.34
Although our study is relatively small, in keeping with proteomic discovery approaches, some significant factors have emerged. We confirmed the importance of hypertension as a risk factor for SCI, together with support for higher HbF levels being protective, suggesting that anti-hypertensives and hydroxyurea may be of benefit. The utility of individual biomarkers, such as gelsolin or α-2-antiplasmin, to act as clinically useful biomarkers in screening for SCI needs to be confirmed in larger, prospective studies. Previous studies have shown increased inflammation and coagulation in children with SCD,46,47 possibly linked to small blood vessel disease,48 and our study suggests that this is more marked in children who also have SCIs. This relatively prothrombotic and pro-inflammatory state associated with SCIs suggests that it may be useful to study anti-inflammatory and antiplatelet agents in clinical trials of SCI prevention. Ongoing clinical trials of antiplatelet agents,49 statins50 and canakinumab are particularly relevant in this context.
Supplementary Material
Acknowledgements
The authors would like to thank the patients involved in this study and the Stroke Association for funding this research (Grant TSA 2012/06).
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/103/7/1136
References
- 1.Earley CJ, Kittner SJ, Feeser BR, et al. Stroke in children and sickle-cell disease: Baltimore-Washington Cooperative Young Stroke Study. Neurology. 1998;51(1):169–176. [DOI] [PubMed] [Google Scholar]
- 2.Brousse V, Makani J, Rees DC. Management of sickle cell disease in the community. BMJ. 2014;348:g1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rees DC, Gibson JS. Biomarkers in sickle cell disease. Br J Haematol. 2012;156(4):433–445. [DOI] [PubMed] [Google Scholar]
- 4.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330(23):1639–1644. [DOI] [PubMed] [Google Scholar]
- 5.Gardner K, Douiri A, Drasar E, et al. Survival in adults with sickle cell disease in a high-income setting. Blood. 2016;128(10):1436–1438. [DOI] [PubMed] [Google Scholar]
- 6.Kato GJ, Gladwin MT, Steinberg MH. Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev. 2007;21(1):37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helton KJ, Adams RJ, Kesler KL, et al. Magnetic resonance imaging/angiography and transcranial Doppler velocities in sickle cell anemia: results from the SWiTCH trial. Blood. 2014;124(6):891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeBaun MR, Sarnaik SA, Rodeghier MJ, et al. Associated risk factors for silent cerebral infarcts in sickle cell anemia: low baseline hemoglobin, sex, and relative high systolic blood pressure. Blood. 2012;119(16):3684–3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91(1):288–294. [PubMed] [Google Scholar]
- 10.Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339(1):5–11. [DOI] [PubMed] [Google Scholar]
- 11.Bernaudin F, Verlhac S, Arnaud C, et al. Impact of early transcranial Doppler screening and intensive therapy on cerebral vasculopathy outcome in a newborn sickle cell anemia cohort. Blood. 2011;117(4):1130–1140; quiz 1436. [DOI] [PubMed] [Google Scholar]
- 12.Fullerton HJ, Adams RJ, Zhao S, Johnston SC. Declining stroke rates in Californian children with sickle cell disease. Blood. 2004;104(2):336–339. [DOI] [PubMed] [Google Scholar]
- 13.DeBaun MR, Armstrong FD, McKinstry RC, Ware RE, Vichinsky E, Kirkham FJ. Silent cerebral infarcts: a review on a prevalent and progressive cause of neurologic injury in sickle cell anemia. Blood. 2012;119(20):4587–4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeBaun MR, Gordon M, McKinstry RC, et al. Controlled trial of transfusions for silent cerebral infarcts in sickle cell anemia. N Engl J Med. 2014;371(8):699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rothman SM, Fulling KH, Nelson JS. Sickle cell anemia and central nervous system infarction: a neuropathological study. Ann Neurol. 1986;20(6):684–690. [DOI] [PubMed] [Google Scholar]
- 16.Dowling MM, Quinn CT, Plumb P, et al. Acute silent cerebral ischemia and infarction during acute anemia in children with and without sickle cell disease. Blood. 2012;120(19):3891–3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deane CR, Goss D, Bartram J, et al. Extracranial internal carotid arterial disease in children with sickle cell anemia. Haematologica. 2010;95(8):1287–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dawson J, Walters M, Delles C, Mischak H, Mullen W. Urinary proteomics to support diagnosis of stroke. PLoS One. 2012;7(5):e35879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Day TG, Thein SL, Drasar E, et al. Changing pattern of hospital admissions of children with sickle cell disease over the last 50 years. J Pediatr Haematol Oncol. 2011;33(7):491–495. [DOI] [PubMed] [Google Scholar]
- 20.Casella JF, King AA, Barton B, et al. Design of the silent cerebral infarct transfusion (SIT) trial. Pediatr Hematol Oncol. 2010;27(2):69–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Assarsson E, Lundberg M, Holmquist G, et al. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS One. 2014;9(4): e95192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheverud JM, Vaughn TT, Pletscher LS, et al. Genetic architecture of adiposity in the cross of LG/J and SM/J inbred mice. Mamm Genome. 2001;12(1):3–12. [DOI] [PubMed] [Google Scholar]
- 23.Rodgers GP, Walker EC, Podgor MJ. Is “relative” hypertension a risk factor for vaso-occlusive complications in sickle cell disease? Am J Med Sci. 1993;305(3):150–156. [DOI] [PubMed] [Google Scholar]
- 24.Gordeuk VR, Sachdev V, Taylor JG, Gladwin MT, Kato G, Castro OL. Relative systemic hypertension in patients with sickle cell disease is associated with risk of pulmonary hypertension and renal insufficiency. Am J Hematol. 2008;83(1):15–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vermeer SE, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM. Prevalence and risk factors of silent brain infarcts in the population-based Rotterdam Scan Study. Stroke. 2002;33(1):21–25. [DOI] [PubMed] [Google Scholar]
- 26.Kinney TR, Sleeper LA, Wang WC, et al. Silent cerebral infarcts in sickle cell anemia: a risk factor analysis. The Cooperative Study of Sickle Cell Disease. Pediatrics. 1999;103(3):640–645. [DOI] [PubMed] [Google Scholar]
- 27.Bernaudin F, Verlhac S, Arnaud C, et al. Chronic and acute anemia and extracranial internal carotid stenosis are risk factors for silent cerebral infarcts in sickle cell anemia. Blood. 2015;125(10):1653–1661. [DOI] [PubMed] [Google Scholar]
- 28.van der Land V, Mutsaerts HJ, Engelen M, et al. Risk factor analysis of cerebral white matter hyperintensities in children with sickle cell disease. Br J Haematol. 2016;172(2):274–284. [DOI] [PubMed] [Google Scholar]
- 29.Leonardo FC, Brugnerotto AF, Domingos IF, et al. Reduced rate of sickle-related complications in Brazilian patients carrying HbF-promoting alleles at the BCL11A and HMIP-2 loci. Br J Haematol. 2016;173(3):456–460. [DOI] [PubMed] [Google Scholar]
- 30.Reed GL, Houng AK, Singh S, Wang D. alpha2-Antiplasmin: new insights and opportunities for ischemic stroke. Semin Thromb Hemost. 2017;43(2):191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nezu T, Hosomi N, Aoki S, et al. Alpha2-macroglobulin as a promising biomarker for cerebral small vessel disease in acute ischemic stroke patients. J Neurol. 2013;260(10):2642–2649. [DOI] [PubMed] [Google Scholar]
- 32.Mosesson MW. Fibrinogen gamma chain functions. J Thromb Haemost. 2003;1(2): 231–238. [DOI] [PubMed] [Google Scholar]
- 33.Williams SR, Hsu FC, Keene KL, et al. Shared genetic susceptibility of vascular-related biomarkers with ischemic and recurrent stroke. Neurology. 2016;86(4):351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faulcon LM, Fu Z, Dulloor P, et al. Thrombospondin-1 and L-selectin are associated with silent cerebral infarct in children with sickle cell anaemia. Br J Haematol. 2013;162(3):421–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lance EI, Casella JF, Everett AD, Barron-Casella E. Proteomic and biomarker studies and neurological complications of pediatric sickle cell disease. Proteomics Clin Appl. 2014;8(11-12):813–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niculescu F, Rus H. The role of complement activation in atherosclerosis. Immunol Res. 2004;30(1):73–80. [DOI] [PubMed] [Google Scholar]
- 37.Ray KK, Ginsberg HN, Davidson MH, et al. Reductions in atherogenic lipids and major cardiovascular events: a pooled analysis of 10 ODYSSEY trials comparing alirocumab with control. Circulation. 2016;134(24): 1931–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kronenberg F, Stuhlinger M, Trenkwalder E, et al. Low apolipoprotein A-IV plasma concentrations in men with coronary artery disease. J Am Coll Cardiol. 2000;36(3):751–757. [DOI] [PubMed] [Google Scholar]
- 39.Mack S, Coassin S, Vaucher J, Kronenberg F, Lamina C, Apo AIVGC. Evaluating the causal relation of ApoA-IV with disease-related traits - A bidirectional two-sample Mendelian randomization study. Sci Rep. 2017;7(1):8734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garcia-Berrocoso T, Penalba A, Boada C, et al. From brain to blood: new biomarkers for ischemic stroke prognosis. J Proteomics. 2013;94:138–148. [DOI] [PubMed] [Google Scholar]
- 41.Silacci P, Mazzolai L, Gauci C, Stergiopulos N, Yin HL, Hayoz D. Gelsolin superfamily proteins: key regulators of cellular functions. Cell Mol Life Sci. 2004;61(19-20): 2614–2623. [DOI] [PubMed] [Google Scholar]
- 42.Llombart V, Garcia-Berrocoso T, Bustamante A, et al. Plasmatic retinol-binding protein 4 and glial fibrillary acidic protein as biomarkers to differentiate ischemic stroke and intracerebral hemorrhage. J Neurochem. 2016;136(2):416–424. [DOI] [PubMed] [Google Scholar]
- 43.Sasaki M, Otani T, Kawakami M, Ishikawa SE. Elevation of plasma retinol-binding protein 4 and reduction of plasma adiponectin in subjects with cerebral infarction. Metabolism. 2010;59(4):527–532. [DOI] [PubMed] [Google Scholar]
- 44.Famulla S, Lamers D, Hartwig S, et al. Pigment epithelium-derived factor (PEDF) is one of the most abundant proteins secreted by human adipocytes and induces insulin resistance and inflammatory signaling in muscle and fat cells. Int J Obes (Lond). 2011;35(6):762–772. [DOI] [PubMed] [Google Scholar]
- 45.Ganesan V, Prengler M, Wade A, Kirkham FJ. Clinical and radiological recurrence after childhood arterial ischemic stroke. Circulation. 2006;114(20):2170–2177. [DOI] [PubMed] [Google Scholar]
- 46.van der Land V, Peters M, Biemond BJ, Heijboer H, Harteveld CL, Fijnvandraat K. Markers of endothelial dysfunction differ between subphenotypes in children with sickle cell disease. Thromb Res. 2013;132(6):712–717. [DOI] [PubMed] [Google Scholar]
- 47.Chen J, Hobbs WE, Le J, Lenting PJ, de Groot PG, Lopez JA. The rate of hemolysis in sickle cell disease correlates with the quantity of active von Willebrand factor in the plasma. Blood. 2011;117(13):3680–3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Colombatti R, De Bon E, Bertomoro A, et al. Coagulation activation in children with sickle cell disease is associated with cerebral small vessel vasculopathy. PLoS One. 2013;8(10):e78801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heeney MM, Hoppe CC, Abboud MR, et al. A multinational trial of prasugrel for sickle cell vaso-occlusive events. N Engl J Med. 2016;374(7):625–635. [DOI] [PubMed] [Google Scholar]
- 50.Hoppe C, Jacob E, Styles L, Kuypers F, Larkin S, Vichinsky E. Simvastatin reduces vaso-occlusive pain in sickle cell anaemia: a pilot efficacy trial. Br J Haematol. 2017;177(4):620–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.