Differentiation of O-negative Rhesus factor D negative (O-neg) human induced pluripotent stem cells (hiPSCs) can potentially generate universal donor red blood cells (RBCs) that may be useful for transfusion applications. Among the approaches described for RBCs generation, embryoid body (EB)-mediated differentiation approaches developed with xeno-free and defined conditions appear to be most feasible for future clinical development. However, conventional approaches for EB generation such as by forced aggregation have not yet been successfully demonstrated on large-scale in suspension culture. Culture of hiPSC as 3-dimensional (3D)-aggregates1 or on defined extracellular matrix (ECM)-coated microcarriers (MCs)2 are possible means for scaling up human pluripotent stem cells (hPSC) and EB expansion in suspension culture. We have previously shown that hPSC-MC aggregates could be differentiated into hematopoietic precursors3 and erythroblasts4 when differentiated with a BMP4-based protocol.4 However, repeated attempts to differentiate multiple hPSC lines initially expanded under continuous agitation condition demonstrated variability in erythroid differentiation. It has been hypothesized that agitation shear stress could induce expression of SMAD75,6 which is known to have inhibitory effects7 on phosphorylation of SMAD 1, 5 and 8, components of the TGF-β signaling pathway activated by BMP4 during the initial stages of mesodermal differentiation.8 Thus, inhibition of BMP4 signaling in agitated cultures could be a possible reason for poor mesoderm induction and variability in differentiation outcomes.
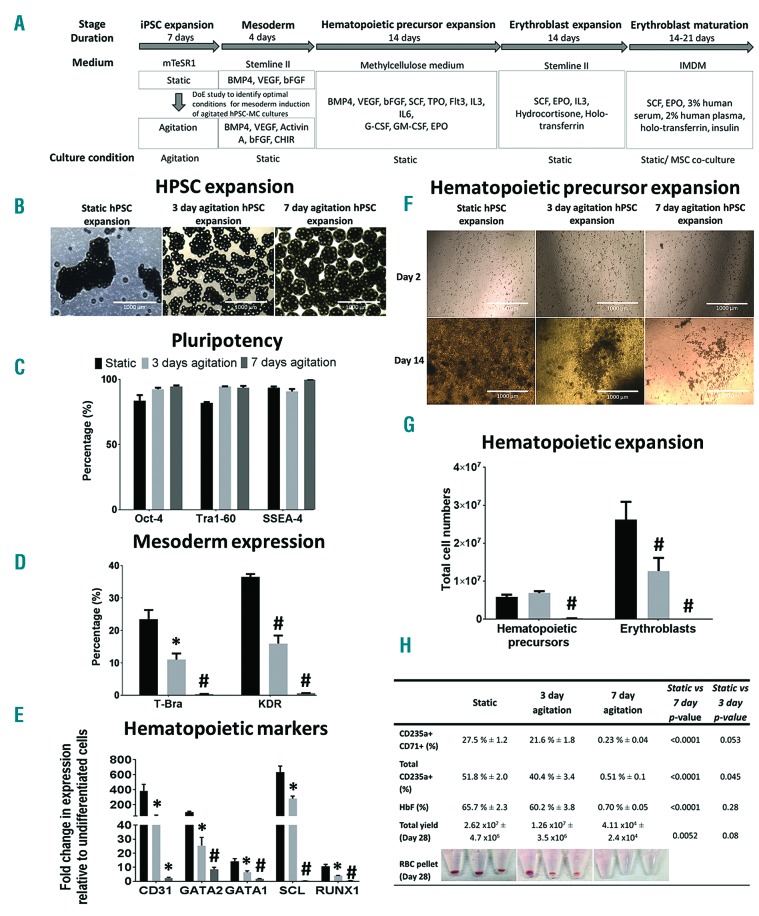
Figure 1A summarizes the experimental study performed to evaluate effects of agitation on mesoderm induction and subsequent erythroid differentiation. By comparing hPSC-MC aggregates derived from static, 3-days or 7-days agitation condition during the hPSC expansion stage (Figure 1B) of an human embryonic stem cell (hESC) line, hES-3, which maintained pluripotency (Figure 1C), we show that continuous agitation for 7 days impedes the expression of the primitive streak/mesoderm marker, T-Bra9 and hematopoietic mesoderm marker, KDR10 (Figure 1D) as well as subsequent hematopoietic precursors (Figure 1E–G) and erythroblasts differentiation (Figure 1G–H) as compared to cultures derived from static condition when differentiated with the BMP4-based protocol.4 In line with our hypothesis, agitation cultures showed increased levels of inhibitory SMAD7 as compared to static cultures. BMP4 signaling was adversely affected in agitation cultures, with phosphorylation of SMAD1/5 evident only in static cultures (Online Supplementary Figure S1A and C).
Figure 1.
Expansion of hPSC in agitated MC cultures result in reduced BPM4-based hematopoietic differentiation as compared to non-agitated static cultures. (A) Schematic showing the entire differentiation process starting from hPSC expansion on MCs (under static/agitation) to differentiation and expansion of hematopoietic precursors and erythroblasts followed by terminal maturation. All subsequent steps were performed under static conditions. (B) Images of hES-3 MC aggregates following 7 days of static or 3 and 7 days of agitated culture. Flow cytometry evaluation of (C) pluripotency markers (3 or 7 days post hPSC expansion), (D) T-Bra (48 hrs post differentiation) and KDR (4 days post differentiation) expression in hES-3-MC aggregates initially expanded under static (static hPSC expansion) or agitated (3 or 7 days agitated hPSC expansion) condition during the pluripotent expansion stage. *P<0.05; #P<0.001 as compared to static hPSC expansion condition. (E) Real-time RT-PCR data showing mean fold change in expression (relative to undifferentiated hES-3) of early hematopoietic specification markers (CD31, GATA2, GATA1, SCL, RUNX1) from hES-3-MC aggregates differentiated for 4 days. *P<0.05; #P<0.001 as compared to static hPSC expansion condition. (F) Images of hematopoietic precursors on day 2 and day 14 post expansion in methylcellulose-based medium. Scale bar =1000 micron. (G) Total counts of hematopoietic precursors (day 14 post expansion in BGM medium following initial seeding of 1 × 105 cells) and erythroblasts (Day 14 post seeding in erythroblast expansion medium) differentiated from hES-3-MC aggregates derived from static and 3 or 7 days agitation cultures (#P<0.001 as compared to static hPSC expansion condition). (H) Table summarizing flow cytometry expression (%) of CD235a+CD71+, total CD235a+ and fetal hemoglobin (HbF) expressing cells and total yield of viable cells 28 days post differentiation of hES3-MC aggregates. Corresponding P-values for comparison between static and agitation cultures as well as images of erythroblast cell pellets at termination of experiment (day 28) are shown. All data are mean ± SEM, n=3.
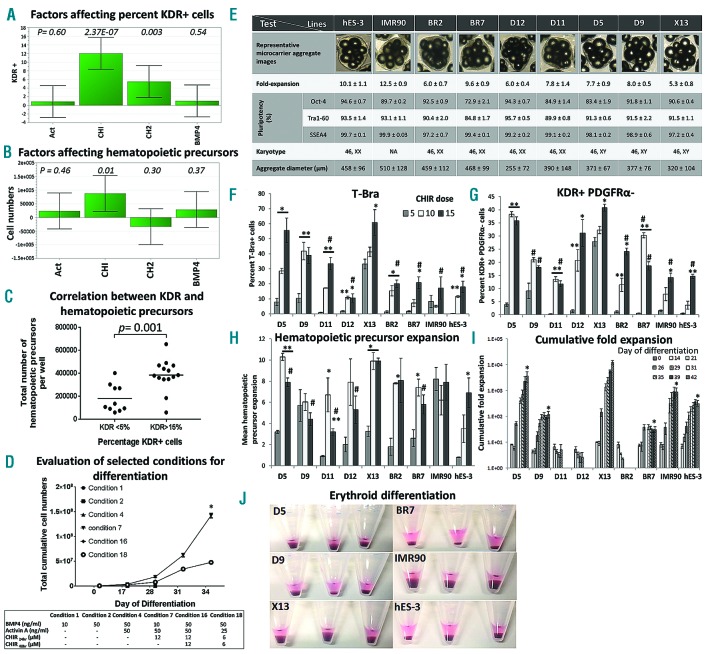
In order to improve the poor differentiation outcomes from agitation culture, we used a multifactorial Design of Experiment (DoE) approach to screen for combination of factors that could improve hematopoietic mesoderm induction of hPSC-MC-aggregate cultures derived from continuous agitation of hES-3 (Figure 2A, B and Online Supplementary Table S1) and an O-neg hiPSC line, D5 (Online Supplementary Figure S2). Our DoE study identified CHIR-99021 (CHIR), a selective inhibitor of glycogen synthase kinase 3-beta (GSK-3β) and a potent activator of canonical Wnt/β-Catenin signaling,11 as the most significant factor for improved development of KDR+ cells (Figure 2A and Online Supplementary Figure S2C) and subsequent generation of hematopoietic precursors (Figure 2B,C), when used in combination with BMP4 and Activin A. Consistent with earlier studies,12 we could correlate higher initial percent KDR+ cell population with higher total number of hematopoietic precursors generated (P=0.001) (Figure 2C). CHIR-99021, has been shown to induce primitive streak/mesoderm development of hPSCs for cardiomyocyte6,11 and hematopoietic differentiation.13 Components of the Wnt/β-catenin signaling pathway14 such as TCF-1 and LEF-1 as well as direct target of Wnt signaling such as T-BRA were detected as early as 24 hours upon induction with CHIR (Online Supplementary Figure S1A and B). CHIR-based protocol resulted in similar erythroblasts output per hPSC seeded (P>0.05) following differentiation of hES-3 MC-aggregates derived from static (11.5±2.6) or agitation (8.0±2.1) condition, whereas for BMP4-based differentiation, efficient expansion was observed only with static (7.7±1.9) and not agitation (1.2±0.7) condition (P<0.05) (Online Supplementary Figure S3).
Figure 2.
Multifactorial DoE analysis identifies CHIR-99021 as a significant factor for improved hematopoietic mesoderm induction and hematopoietic differentiation from hPSC-MC cultures of multiple cell lines initially expanded under agitation condition. (A) hES-3-MC agitation culture: DoE analysis (refer to Online Supplementary Table S1) identified CHIR-99021 (maintained for 24 hrs only) as a significant factor for achieving higher % KDR+ cells on day 4 of differentiation (P=2.3E-07) and (B) greater expansion of hematopoietic precursors (P=0.01). Act=Activin; CHI=CHIR-99021 for 24 hrs, CH2= CHIR-99021 from 24-48 hr; BMP4. Significant P-values are indicated in the chart. (C) hES-3-MC agitation culture: Correlation between high (>15%) and low (<5%) KDR expression on day 4 of differentiation and the corresponding total number of hematopoietic precursors per well derived following 14 days of culture in BGM (data from Online Supplementary Table S1). (D) D5 O-neg hiPSC-MC agitation culture: Total cell expansion from day 0 to day 34 for selected conditions (identified from Online Supplementary Figure S2A): Day 0–4 (mesoderm induction of hPSC-MC aggregates), day 4–17 (expansion of hematopoietic precursors in BGM medium), day 17–34 (expansion of erythroblasts in suspension culture) (*P<0.05 as compared to condition #18). Note that cells from conditions #1, #2, #4 and #16 failed to expand and were terminated at day 28. All data are mean ± SEM, n=3. (E) Expansion of 9 different hPSC lines in agitated-MC culture for 7 days. Table shows: cell-MC aggregate images, cell fold-expansion, pluripotency (Oct-4, Tra1-60 and SSEA4 expression), karyotypes and average aggregate diameters. NA: Not available. Effect of CHIR dose (μM) on (F) the percentage of T-Bra+ cells (as determined by flow cytometry) 48hr post differentiation, (G) percentage of KDR+ PDGFRα-cells (as determined by flow cytometry) 96 hr post differentiation and (H) mean-fold expansion of hematopoietic precursors 14 days post differentiation of 9 different hPSC-MC lines (*P<0.05, **P<0.01 as compared to 5 μM CHIR dose for each line; #P<0.05 for comparison of 15 μM CHIR dose of each line to 15 μM CHIR dose of X13). (I) For each cell line, hematopoietic precursors that gave maximal expansion (from CHIR dose tested) were further differentiated into erythroblasts. Cumulative fold-expansion of total viable cell numbers was determined following differentiation of different hPSC lines (*P<0.05 cumulative fold-expansion of each line (except D11, D12 and BR2) on day 42 as compared to X13). Note that cells derived from D11, D12 and BR2 failed to expand and were terminated at day 26. All data are mean ± SEM, n=3. (J) Corresponding RBC pellets from the different hPSC lines on day 35 of experiment.
In order to validate the effect of CHIR on improved erythroid differentiation of hPSCs expanded in agitated MC culture, we evaluated 29 conditions from a DoE study (Online Supplementary Figure S2A) on D5 (hiPSC line). Six were then chosen for detailed study; conditions 1, 2 and 4 were based on BMP4/Activin A protocols, while conditions 7, 16 and 18 were CHIR-mediated protocols (Online Supplementary Table S2). The BMP4/Activin A protocol resulted in very little primitive streak/mesoderm induction (T-Bra+ cells: 6.66 – 8.36%) 48 hr post induction and low hematopoietic mesoderm induction (5.33 – 13.8 % of KDR+ cells) 96 hr post induction (Online Supplementary Table S2). These conditions also resulted in very low induction of the hematopoietic transcription factors SCL and RUNX1 and had little or no expansion of hematopoietic precursors following 2 weeks of culture in methylcellulose-based blast growth medium (BGM) and subsequently failed to expand as erythroblasts (Online Supplementary Table S2). On the other hand, the CHIR-based protocol resulted in high primitive streak/mesoderm induction (44.9 – 89.6 % T-Bra+ cells on day 2), high hematopoietic mesoderm induction (18.6 – 29.4 % KDR+ cells on day 4), higher fold-induction of CD31, SCL/Tal-1 and RUNX1 (master regulators of hematopoiesis)15 and improved expansion of hematopoietic precursors 14 days post differentiation (Online Supplementary Table S2). Among the CHIR-based conditions tested, condition #7 (12 μM CHIR for 24 hr) and #18 (6 μM CHIR for 48 hr) resulted in erythroid differentiation and expansion (Day 34 fold-expansion: 284.4±9.2 vs. 95.8±1.2, respectively) (Online Supplementary Table S2) with condition #7 showing significantly higher number of erythroblasts than condition #18 [1.42 × 108 as compared to 4.31 × 107 cells, 34 days post differentiation, P=0.0003) (Figure 2D). Erythroblasts differentiated using condition #7 could achieve a cumulative fold-expansion of 62343 ± 15070 by day 56 of culture.
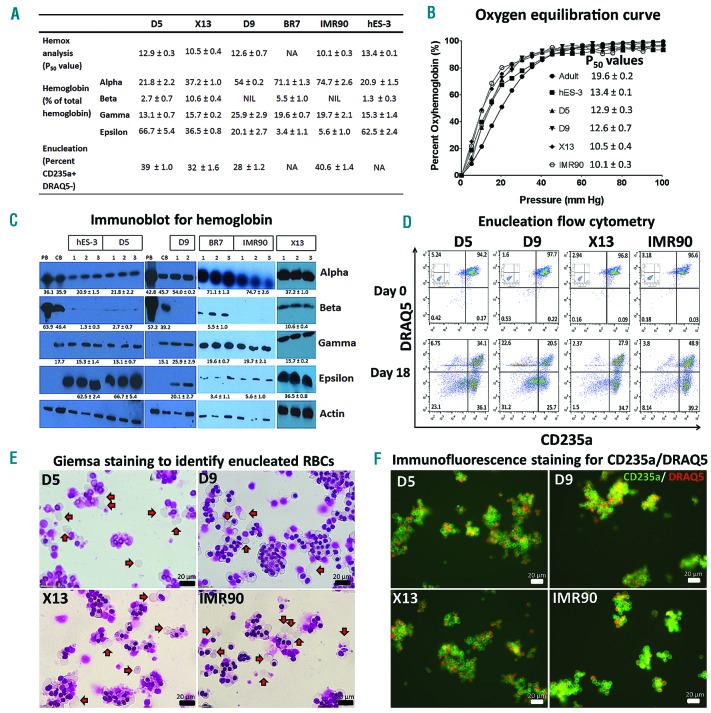
Having optimized conditions for erythroid differentiation of hPSC-MC aggregates derived from agitation condition, we proceeded to test differentiation of 7 karyotypically normal O-neg hiPSC lines (BR2, BR7, D5, D9, D11, D12, X13), 1 commercial hiPSC line (IMR90) and a hESC line (hES-3). HPSC lines expanded on MC for 7 days under agitation condition maintained pluripotency and achieved 5.3 to 12.5-fold expansion, with mean aggregate diameters ranging from 255 to 510 μm (Figure 2E). HPSC-MC aggregates generated from different hPSC lines were differentiated using 3 different CHIR doses (5, 10 and 15 μM for 24 hr). For all of the lines tested, 15 μM CHIR resulted in significantly higher (P<0.05) T-Bra+ cells as compared to 5 μM CHIR (Figure 2F). Expression of KDR+PDGFRα− cells, indicative of hematovascular progenitors, mirrored the trend of T-Bra+ cells, with 15 μM CHIR showing significantly higher (P<0.05) KDR+PDGFRα− cells as compared to 5 μM CHIR (Figure 2G). Hematopoietic induction of differentiated cells evaluated by RT-PCR for expression of CD31 and hematopoietic transcription factors SCL, GATA2, RUNX1 and LMO2 (day 4 post differentiation) showed higher fold up-regulation with increasing dose of CHIR in most of the lines tested (Online Supplementary Figure S4). With the exception of D9 and IMR90, all other hPSC lines had significantly increased (P<0.05) fold-expansion of hematopoietic precursors at day 14 post differentiation when induced with 15 μM CHIR dose as compared to 5 μM CHIR (Figure 2H). Following erythroid differentiation, X13 achieved a cumulative fold-expansion of 12605±2126 which was significantly higher than all other lines tested (Figure 2I). Six of 9 lines (D5, D9, X13, BR7, IMR90, hES-3) successfully differentiated into erythroblasts (Figure 2J) and had expression of CD235a and high levels of HbF (Online Supplementary Table S3). For the best performing line, X13, 7607±1016 CD235a+ erythroid cells could be derived per hPSC seeded (Online Supplementary Table S3). Immunoblot evaluation of hemoglobin subtypes showed that majority of hPSC-differentiated erythroblasts had expression of alpha, gamma and epsilon with very little beta-hemoglobin as compared to adult erythroblasts (Figures 3A and C). Intriguingly, erythroblasts differentiated from BR7 and X13 also showed some expression of beta-hemoglobin subtype (Figure 3C). Oxygen equilibrium curves of hPSC-differentiated erythroblasts indicated higher oxygen binding affinity (P50 values ranging from 10.1 to 13.4) as compared to adult RBCs (P50− 19.6±0.2) (Figures 3A and B). Following 18-day co-culture with primary human MSCs, 28–40.6 % of erythroblasts were CD235a+ and DRAQ5 (cell permeable nuclear dye) negative, indicating enucleated erythroid cells (Figures 3A and D). This was further corroborated by Giemsa staining of cells (Figure 3E) and immunofluorescence staining of terminally matured erythroblasts which showed CD235a+ erythrocytes lacking nuclear staining (Figure 3F).
Figure 3.
Functional characterization and terminal maturation of hPSC derived erythroblasts. (A) Table summarizing P50 values (hemox analysis), percentage of hemoglobin subtypes relative to total hemoglobin (based on densitometric measurements of immunoblots) and percentage of enucleated cells (CD235a+ DRAQ5−) following 18 days of MSC co-culture. NA=Not available; NIL=Not expressed. (B) Oxygen equilibrium curves [percent oxyhemoglobin vs. oxygen pressure (mm Hg)] of adult RBCs (●), hES-3 differentiated erythroblasts (■) and hiPSC differentiated erythroblasts D5 (▲), D9 (▼), X13 (♦) and IMR90 (○). Corresponding p50 values (mean ± SD, n=2) are presented. (C) Cell lysates from peripheral blood (PB), cord-blood (CB) and erythroblasts differentiated from X13, D5, D9, BR7, IMR90 and hES-3 (day 35 post differentiation) were immunoblotted with antibodies specific to alpha, beta, gamma, epsilon human hemoglobin subtypes and the house-keeping control human β-actin. White lines demarcate regions of gel images that were merged together. Densitometric measurement of immunoblot bands was done using ImageJ software. Based on densitometric measurements, the percentage of hemoglobin (relative to total hemoglobin expressed) after normalization with actin loading control are shown (mean ± SEM). (D) Flow cytometry evaluation of CD235a and DRAQ5 expression of hiPSC derived erythroblasts cultured in expansion medium (Day 0) or co-cultured with human MSCs for 18 days under terminal maturation conditions (Day 18). Erythroblasts stained with isotype antibodies served as controls (shown inset of Day 0 FACS plots). (E) Giemsa staining of hiPSC differentiated erythroblasts following 18 days of maturation on MSC co-culture. Red arrows indicate enucleated erythrocytes. (F) Terminally matured hiPSC differentiated erythroblasts were stained with anti-human CD235a-FITC antibody and DRAQ5. Merged fluorescence images of CD235a and DRAQ5 are shown. Enucleated RBCs can be identified in the merged image as CD235a positive cells (green) lacking nuclear staining (red). Scale bar = 20 micron.
HPSC-MC aggregate cultures have previously been successfully scaled-up in spinner culture platforms and bioreactors for differentiation of cardiac progenitor cells.2,6 Thus, there are potentials to use hPSC-MC aggregates for developing large scale-erythroid differentiation processes as well. However, in order to scale-up in bioreactors, the process has to be modified so that the initial stages of differentiation and expansion of hematopoietic precursors can be performed entirely in liquid suspension culture. Moving forward, scale-up of the process would also require the development of efficient enucleation protocols with defined media formulations.
In conclusion, through up-stream process optimization, we report an optimized protocol for efficient erythroid differentiation of hPSC-MC aggregates initially expanded under agitation. This serves as an improvement that could allow us to further develop processes for large-scale generation of universal RBCs.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Filip Laco for his critical review of this manuscript.
Footnotes
Funding: this work was supported by a Joint Council Office DP grant from Agency for Science, Technology and Research, Singapore (1331AFG075).
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Lei Y, Jeong D, Xiao J, Schaffer DV. Developing defined and scalable 3D culture systems for culturing human pluripotent stem cells at high densities. Cell Mol Bioeng. 2014;7(2):172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lam AT, Chen AK, Ting SQ, Reuveny S, Oh SK. Integrated processes for expansion and differentiation of human pluripotent stem cells in suspended microcarriers cultures. Biochem Biophys Res Commun. 2016;473(3):764–768. [DOI] [PubMed] [Google Scholar]
- 3.Lu SJ, Kelley T, Feng Q, et al. 3D microcarrier system for efficient differentiation of human pluripotent stem cells into hematopoietic cells without feeders and serum [corrected]. Regen Med. 2013;8(4):413–424. [DOI] [PubMed] [Google Scholar]
- 4.Sivalingam J, Lam AT, Chen HY, et al. Superior red blood cell generation from human pluripotent stem cells through a novel microcarrier-based embryoid body platform. Tissue Eng Part C Methods. 2016;22(8):765–780. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Nakayama N. WNT and BMP signaling are both required for hematopoietic cell development from human ES cells. Stem Cell Res. 2009;3(2-3):113–125. [DOI] [PubMed] [Google Scholar]
- 6.Ting S, Chen A, Reuveny S, Oh S. An intermittent rocking platform for integrated expansion and differentiation of human pluripotent stem cells to cardiomyocytes in suspended microcarrier cultures. Stem Cell Res. 2014;13(2):202–213. [DOI] [PubMed] [Google Scholar]
- 7.Larsson J, Karlsson S. The role of Smad signaling in hematopoiesis. Oncogene. 2005;24(37):5676–5692. [DOI] [PubMed] [Google Scholar]
- 8.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113(6):685–700. [DOI] [PubMed] [Google Scholar]
- 9.Showell C, Binder O, Conlon FL. T-box genes in early embryogenesis. Dev Dyn. 2004;229(1):201–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lugus JJ, Park C, Ma YD, Choi K. Both primitive and definitive blood cells are derived from Flk-1+ mesoderm. Blood. 2009;113(3):563–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lian X, Hsiao C, Wilson G, et al. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci USA. 2012;109(27): E1848–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu SJ, Feng Q, Park JS, et al. Biologic properties and enucleation of red blood cells from human embryonic stem cells. Blood. 2008;112(12):4475–4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sturgeon CM, Ditadi A, Awong G, Kennedy M, Keller G. Wnt signaling controls the specification of definitive and primitive hematopoiesis from human pluripotent stem cells. Nat Biotechnol. 2014;32(6):554–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hodar C, Assar R, Colombres M, et al. Genome-wide identification of new Wnt/beta-catenin target genes in the human genome using CART method. BMC Genomics. 2010;11:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ran D, Shia WJ, Lo MC, et al. RUNX1a enhances hematopoietic lineage commitment from human embryonic stem cells and inducible pluripotent stem cells. Blood. 2013;121(15):2882–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.