With the international prognostic index for patients with chronic lymphocytic leukemia (CLL-IPI), we have a tool to identify patients with a high risk of progression.1,2 Despite improved supportive care, infections are a significant cause of morbidity and mortality prior to treatment; one third of patients with CLL will die following an infection.3
Though infections in CLL patients have been a subject of interest in many studies, reporting incidences between 0.13-0.19 per patient-year, there is still no consensus on how to identify patients who are at an increased risk of infection. Studies on infectious risk prior to treatment in CLL are scarce and very heterogeneous in terms of study populations.4 Consequently, the identification of prognostic markers for risk of infections are warranted.
Infections in patients with CLL are believed to correlate with the progressive immune dysfunction. The progressive immune dysfunction in CLL is characterized by a cell-mediated and an antibody-mediated dysfunction.5 The CLL-microenvironmental interaction may play a major role in this progressive immune dysfunction. The neoplastic B cells co-evolve together with the microenvironmental changes, which promotes the leukemia cell survival and growth while inhibiting normal B-cell and T-cell function as well as causing hypogammaglobulinemia and cytokine changes.6
The rate, severity and prognostic factors for infections in CLL prior to treatment constitute a gap in our knowledge of the infection-prone nature of CLL. By means of the unique Danish National CLL registry, a nationwide cohort of all patients diagnosed with CLL in Denmark between 2008 and 2016, and a national Danish Microbiology database, we conducted a retrospective study to address this issue.7,8
All patients diagnosed with CLL in Denmark between January 1st 2010 and July 1st 2016, which was also the end of follow-up, were included. The CLL-IPI variables and data on treatment and survival were retrieved from the Danish National CLL registry.7 Information on immunoglobulin levels was included if data were available within six months of diagnosis. Information on blood cultures was retrieved from the Danish Microbiology Database.
The event of the first blood culture prior to CLL therapy was used as a proxy for severe infection, regardless of whether an infectious agent was identified. The time to event was calculated from the date of diagnosis or the first date of the measurement of immunoglobulin at or after diagnosis, whichever came last. Patients were followed until the date of infection, initiation of CLL-specific treatment, death or end of follow-up, whichever came first. Estimates of cumulative incidence for each of the competing risks were calculated using the Aalen-Johansen estimator. We examined the difference in the cumulative incidence of infection using Gray’s test. We fitted a cause-specific hazard model and a Fine-Gray model.9 All models were compared to the non-parametric Aalen-Johansen curves. Martingale residuals and Schoenfeld residuals were visualized for diagnostic purposes. Statistical tests were two-sided and P-values of 0.05 were considered significant. All analyses were performed in R. The source code is available on request.
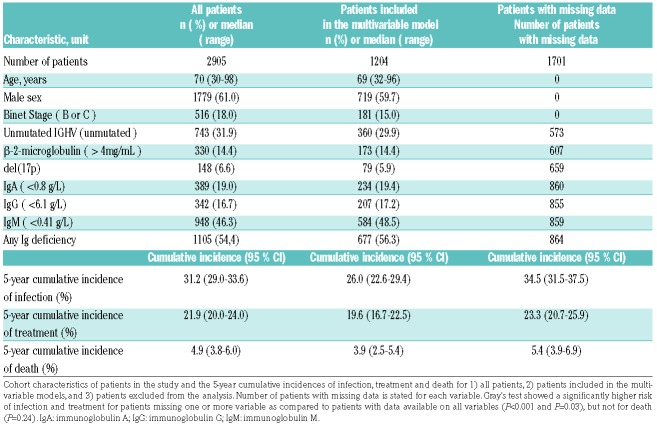
In total, 2905 CLL patients were diagnosed in Denmark between 2010 and 2016. Of these, 1204 patients had complete data on CLL-IPI variables, sex and immunoglobulin levels available within six months of diagnosis. The cohort characteristics of the study population are summarized in Table 1. The median age was 70 years old at time of diagnosis, 61% were male. Eighty-two percent were Binet A, 13% Binet B and 4.7% Binet C stage. Based on the patients with available CLL-IPI variables, 55% were low risk (CLL-IPI score 0-1), 30% were intermediate risk (CLL-IPI score 2-3), 12% were high risk (CLL-IPI score 4-6) and 2.6% were very high risk (CLL-IPI score 7-10). Regarding the immunoglobulin levels, 54% of the patients had immunoglobulin deficiency, with 19%, 17% and 46% having low levels of Immunoglobulin A (IgA), Immunoglobulin G (IgG) and Immunoglobulin M (IgM), respectively.
Table 1.
Cohort description.
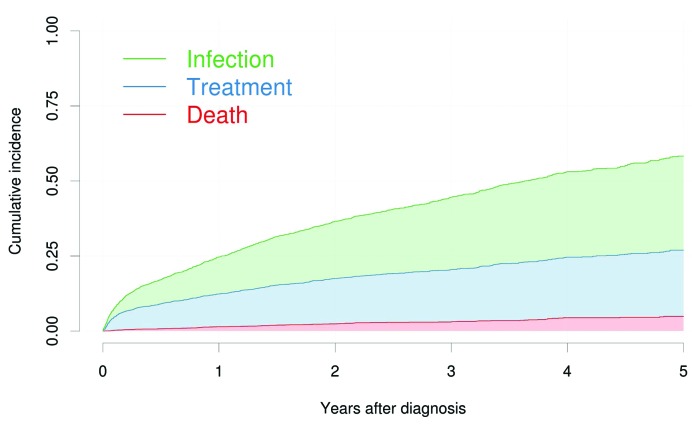
In the entire cohort of 2905 patients, a total of 1239 patients had an event; an infection, treatment initiation or death. The median follow-up time was 2.9 years (IQR: 1.53-4.57). Having a blood culture drawn prior to treatment or death was the most common event, which occurred in 662 patients; 482 patients received treatment without first having had an infection, and 95 patients died without having had an infection or having received treatment. The cumulative incidence of infection prior to treatment was 12% after one year. For treatment initiation prior to having a blood culture drawn, the cumulative incidence was 11% after one year, and 1% after one year for death prior to treatment or infection (Table 1 and Figure 1). Of the patients with an infection, 65 patients (9.8%) died within 30 days after the infection, 163 (25%) died within one year after the infection and 74 (11%) received treatment for CLL within one year.
Figure 1.
Cumulative incidence for all outcomes. Aalen-Johansen cumulative incidence estimates for the three outcomes stacked on top of each other. Each patient could only have one event, that being whichever came first. Thus, infections subsequent to treatment and vice versa were not included. Time zero is the time of diagnosis for all patients.
A significantly higher risk of infection was demonstrated for the following variables: 1) older compared to younger patients (P<0.001), 2) male patients (P=0.02), 3) patients with Binet stage B or C (P=0.04), 4) patients with unmutated IGHV (P=0.04), 5) patients with high levels of β-2-microglobulin (B2M>4mg, P<0.001), 6) patients with low levels of IgA (P=0.004), 7) patients with low levels of IgG (P=0.009), and 8) patients with low levels of IgM (P=0.008) (Online Supplementary Figure S1). We fitted multivariate models on data from 1204 patients with all variables available. The cumulative incidences of infection, treatment and death for these patients were somehow lower than for patients with missing variables (Table 1).
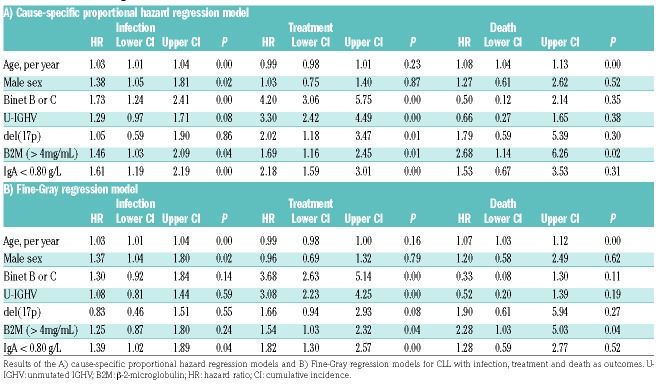
The cause-specific hazard and Fine-Gray models are summarized in Table 2 for all three competing outcomes. From the cause-specific model, we found that patients with low IgA levels compared to those with normal/high IgA levels had a hazard rate for infection of 1.61; 95 % cumulative incidence (CI; 1.19-2.19); P<0.001. For the variables age, sex, Binet stage and B2M the hazard rates were estimated as 1.03 per year; 95% CI (1.01-1.04); P< 0.001, 1.38; 95% CI (1.05-1.81); P=0.02, 1.73; 95% CI (1.24-2.41); P<0.001 and 1.46; 95% CI (1.03-2.09); P=0.04, respectively.
Table 2.
Results of the regression models.
In this nationwide cohort, we assessed the incidence and prognostic factors for infections prior to any CLL treatment as well as the outcomes after such infections in patients prior to treatment of CLL. Severe infections were very common prior to treatment, with a five-year cumulative incidence of 31% among 2905 CLL patients. Age, sex, Binet stage, B2M and IgA levels were significantly associated with the cause-specific hazards rate of infection.
Compared to previously published studies, we present herein the largest and most homogeneous cohort for analysis of prognostic factors for risk of infection. We did not find del(17p) nor unmutated IGHV to be associated with the rate of infection. This might be due both to the small numbers of patients with del(17p) and the fact that these prognostic factors are highly associated with the risk of treatment, thus, in a competing risk scenario they do not correlate with infection before treatment (Table 2).
For this registry-based study, we used the event of a blood culture as a proxy for the event of a severe infection. According to the Danish national guidelines, a blood culture is drawn when a sepsis is suspected, and the patients should always receive intravenous antibiotics afterwards.10 The herein demonstrated cumulative incidence of severe infection in patients with untreated CLL within the first year after diagnosis of 12% is within the range of previously reported 0.13-0.19 infections per patient-year.5 The previously reported rates allow multiple events per patient-year while the cumulative incidence only counts one event per patient, which may explain the slightly lower rate in our study. Moreover, we only included untreated patients, who have a lower infectious burden.5 The 30-day post-infection mortality rate of 9.8% demonstrated herein is comparable to a previous study on hematological malignancies.11
Immunoglobulin deficiency is common at diagnosis and occurs spontaneously throughout the natural course of the disease and is related to disease duration and stage.12 In this study we found IgA to be the best predictor of the rate of infections, however IgG and IgM were also associated with the rate of infection in the univariate analysis. IgA is especially important on mucosal surfaces where it binds and prevents translocations of pathogens.13 Most patients with IgA deficiency are asymptomatic, and the IgA mucosal immunity is often considered superfluous.13 When multiple facets of the immune system are dysfunctional, e.g., in CLL, IgA may either play a greater role itself in protection, or it may merely serve as a proxy for the degree to which the CLL cells inhibit the immune system. Sun et al. showed that upon treatment with ibrutinib, patients experienced an increase in IgA. After six months, patients had fewer infections although their levels of IgG remained low, while the decrease in infectious risk correlated with improved IgA levels in the study herein.14
Due to the registry-based design, we did not have any information on comorbidities. As multiple medical conditions add to the infectious risk of patients with CLL, we were unable to assess whether this confounded our results. The lack of data on comorbidities may overestimate the role of aging rather than recognizing the relevance of comorbidities.15
The data presented herein thus represent the first step for identification of further risk factors for infection at time of CLL diagnosis. Exploration of prophylactic or preemptive treatment strategies for this population in clinical trials is warranted. This should also include investigation of risk factors for infections in treated patients. Validation of the herein presented data in independent cohorts is encouraged.
Online Supplementary Information is available on Haematologica’s website.
Supplementary Material
Acknowledgments
The authors thank the Danish hematology centers that participated with data submission to the Danish National CLL Registry. The following physicians contributed to data collection and represent the Danish Hematology centers participating in the Danish National CLL Registry: Christian Hartmann Geisler, Lisbeth Enggaard, Christian Bjørn Poulsen, Peter de Nully Brown, Henrik Frederiksen, Olav Jonas Bergmann, Elisa Jacobsen Pulczynski, Robert Schou Pedersen, and Linda Højberg Nielsen.7
Footnotes
Funding: this work was supported in part by Danish National Research Foundation grant 126 through the PERSIMUNE project and by the Novo Nordisk Foundation (NNF16OC0019302).
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Bahlo J, Kutsch N, Byrd JC, et al. The International Prognostic Index for patients with CLL (CLL-IPI): an international meta-analysis. J Clin Oncol. 2015;33(15S):374. [Google Scholar]
- 2.Cunha-Bang C da, Christiansen I, Niemann CU. The CLL-IPI applied in a population-based cohort. Blood. 2016;128(17):2181–2183. [DOI] [PubMed] [Google Scholar]
- 3.Cunha-Bang C da, Simonsen J, Rostgaard K, Geisler CH, Hjalgrim H, Niemann C. Improved survival for patients with CLL in the era of combination chemoimmunotherapy - a Danish population based study. Blood. 2015;126(23):1740–1740. [Google Scholar]
- 4.Andersen MA, Vojdeman FJ, Andersen MK, et al. Hypogammaglobulinemia in newly diagnosed chronic lymphocytic leukemia is a predictor of early death. Leuk Lymphoma. 2016; 57(7):1592–1598. [DOI] [PubMed] [Google Scholar]
- 5.Morrison VA. Infectious complications in patients with chronic lymphocytic leukemia: pathogenesis, spectrum of infection, and approaches to prophylaxis. Clin Lymphoma Myeloma. 2009. 9(5):365–370. [DOI] [PubMed] [Google Scholar]
- 6.Ghia P, Caligaris-Cappio F. Monoclonal B-cell lymphocytosis: right track or red herring? Blood. 2012;119(19):4358–4362. [DOI] [PubMed] [Google Scholar]
- 7.da Cunha-Bang C, Geisler CH, Enggaard L, et al. The Danish National Chronic Lymphocytic Leukemia Registry. Clin Epidemiol. 2016;8:561–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gubbels S, Nielsen J, Voldstedlund M, et al. Utilization of blood cultures in Danish hospitals: a population-based descriptive analysis. Clin Microbiol Infect. 2015;21(4):344.e13–21. [DOI] [PubMed] [Google Scholar]
- 9.Andersen PK, Geskus RB, de Witte T, Putter H. Competing risks in epidemiology: possibilities and pitfalls. Int J Epidemiol. 2012; 41(3):861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer CP. Guidelines og retningslinier - Dansk Selskab for Infektionsmedicin. Available from: http://www.infmed.dk/guidelines#sepsis_flowchart_2017.pdf (Last accesed 4th April 2017).
- 11.Leibovici L, Samra Z, Konigsberger H, Drucker M, Ashkenazi S, Pitlik SD. Long-term survival following bacteremia or fungemia. JAMA. 1995;274(10):807–812. [PubMed] [Google Scholar]
- 12.Rozman C, Montserrat E, Vinolas N. Serum immunoglobulins in B-chronic lymphocytic leukemia. Natural history and prognostic significance. Cancer. 1988;61(2):279–283. [DOI] [PubMed] [Google Scholar]
- 13.Yel L. Selective IgA deficiency. J Clin Immunol. 2010;30(1):10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun C, Tian X, Lee YS, et al. Partial reconstitution of humoral immunity and fewer infections in patients with chronic lymphocytic leukemia treated with ibrutinib. Blood. 2015;19(19):2213–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moreira J, Rabe KG, Cerhan JR, et al. Infectious complications among individuals with clinical monoclonal B-cell lymphocytosis (MBL): a cohort study of newly diagnosed cases compared to controls. Leukemia. 2013;27(1):136–141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.