Primary central nervous system lymphoma (PCNSL) is a rare pure extra nodal lymphoma accounting for 2% of all primary brain tumors and 1% of non-Hodgkin lymphoma.1 Long-term progression free survival (PFS) and overall survival (OS) may be achieved in more than half of the patients with current strategies involving high-doses of methotrexate and rituximab, frequently combined with various blood-brain crossing agents.2–13 Consolidation is generally offered to young/fit responding patients, with either whole brain radiotherapy (WBRT),4,5 autologous stem cell transplantation (ASCT)6–10 or intensive chemotherapy.11–13 While the trend of recent studies is to favor ASCT over WBRT after methotrexate-based induction due to the risks of long-term cognitive decline associated with radiotherapy,10 intensive consolidative chemotherapy without ASCT has been little studied in PCNSL.11–13 Recent studies, including the CALGB50202 prospective phase II trial, suggested a favorable safety/efficacy balance of a consolidation response regimen combining high-dose etoposide and cytarabine (EA) in patients of up to 84 years old after rituximab, methotrexate and temozolomide (RMT) induction.11,12 We enrolled 28 consecutive newly diagnosed PCNSL patients treated at a single center (Cochin Hospital, Paris, France) into a retrospective study with a focus on safety data. Patients were treated with RMT induction, and half of them with EA consolidation, from November 2013 to January 2017. Patients with concurrent human immunodeficiency virus (HIV) infection (n=1), localized intraocular lymphoma (n=1) and secondary central nervous system (CNS) lymphoma (screened by a systematic 18F-2-fluoro-2-deoxy-D-glucose positron emission tomography computed tomography/scan [18F-FDG PET CT/scan] at diagnosis; n=2) referred to our center during the same period were excluded from this analysis.
Induction consisted of four 28-day cycles of 375 mg/m2 rituximab and 8 g/m2 methotrexate at days one and 14, and oral temozolomide 150mg/m2/d on days seven through 11. Compared to the initial report, the schedule of rituximab administration was simplified in our study to be delivered together with methotrexate. Prophylactic dose-reduction of methotrexate was applied based on age and renal function status, at the discretion of the physician. Corticosteroids, which were used during the perioperative period in nearly all patients, were tapered within a few days after RMT onset. In patients achieving complete response (CR) or partial response (PR), EA consolidation was given based on performance status (PS), age and comorbidities. Treatment consisted of intravenous etoposide 40mg/kg for a continuous 96 hours infusion on days one through four, and cytarabine 2 g/m2/12 hours on days one through four. All patients were monitored continuously on an inpatient unit, from the day before EA initiation to neutrophil recovery (absolute neutrophil count [ANC] > 0.5×109/l). Supportive care included granulocyte-colony stimulating factor (G-CSF; starting on day eight after EA onset) and multi-agent prophylaxis with amoxicillin, posaconazole, valacyclovir, and trimethoprim/sulfamethoxazole (or atovaquone) until neutrophil recovery. Response to therapy was assessed by magnetic resonance imagery (MRI) after the second and fourth cycle of induction, then every three months for two years, and then every six months. Response was evaluated according to International PCNSL Collaborative Group (IPCG) criteria.14
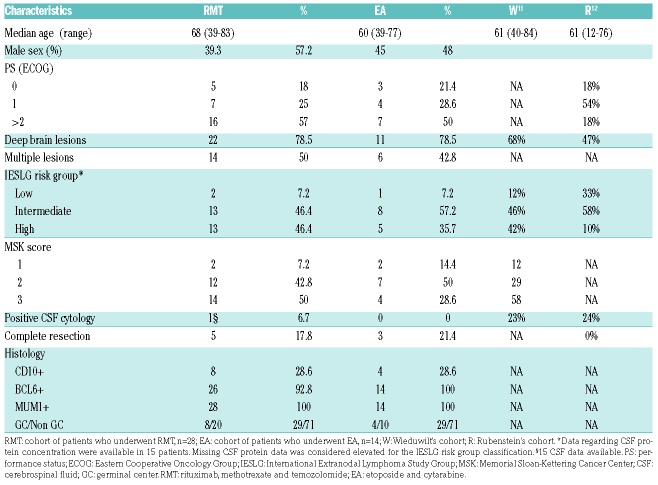
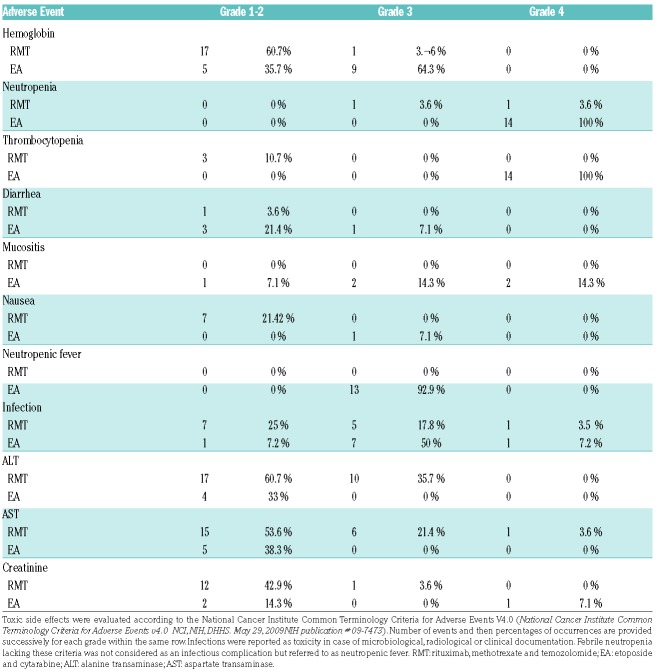
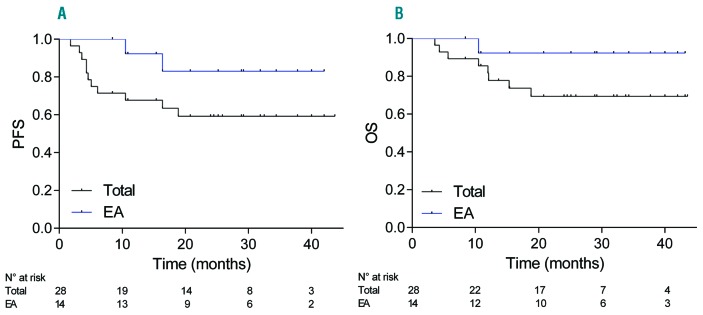
Patient’s characteristics are listed in Table 1. The median age was 68, and only 29% of the patients were under 60. Median time from diagnosis to treatment onset was eight (2-14) days. The mean per cycle dose of methotrexate was 6 g/m2 (1-8 g/m2), and patients received a mean of 6.7 (1-8) injections of methotrexate. Frontline methotrexate dose reduction occurred for 39% of the patients. Subsequent dose reductions or discontinuation of methotrexate were decided during induction therapy in 43% of the patients due to delayed clearance (41.6%), decreased glomerular filtration rate (41.6%), therapy-related hepatitis (27.2%) and/or acute pulmonary edema (9%). In four patients, methotrexate was discontinued for delayed clearance (n=2), altered general condition (n=1) and repeated grade 3 hepatitis (n=1). For the 21 patients who completed the induction therapy, the mean interval between cycles was 29.5 (24.5-33.25) days. Toxicities of the RMT regimen appeared frequent but manageable, and mostly encompassed grade 3/4 infections in 21% of the patients, and grade 3/4 hepatitis in 37% of the patients (Table 2). We observed a single case of grade 3 renal dysfunction, probably induced by methotrexate, who recovered fully. Best responses achieved during induction were CR, PR, stable disease, progressive disease (PD), and non-evaluated disease in 18 (64%), five (18%), three (11%), one (3,5%) and one (3.5%) patient, respectively. Twenty patients (71%, 16 in CR and four in PR) were eligible for consolidation therapy. Among them, four received WBRT, two were assigned to a watchful waiting strategy due to altered general condition, and 14 received the EA consolidation. After a median follow-up of 29 (9-44) months, the two-year PFS and OS for the whole cohort were 59% (confidence interval [CI]95%, 39-73%) and 69% (CI95%, 48-83%), respectively (Figure 1A,B). During the follow-up, two patients relapsed from CR and eight (29.6%) died, four from disease progression (including one relapsing patient) and four from causes unrelated to lymphoma (suicide, n=1; on-therapy cardiac failure, n=1; lung adenocarcinoma, n=1; pneumonitis, n=1). The median hospital stays and the duration of severe neutropenia (ANC < 0.5×109/l) among the 14 patients receiving EA therapy were 22 (20-183) and 11 (9-14) days, respectively. All EA recipients developed grade 4 neutropenia and thrombocytopenia, and 61% had grade 3 anemia. Neutropenic fever was observed in 92.9% of patients. Grade 3/4 mucositis occurred in 28.6% of patients. We observed 11 grade 3-4 documented infections in eight patients (57%), including implantable port infection (n=5), septicemia (n=3), Clostridium difficile colitis (n=1), probable invasive aspergillosis (n=1) and confirmed lung mucormycosis (n=1) (Table 2). The two-year PFS and OS for the EA cohort were 83% (CI95%, 44-95%) and 92% (CI95%, 54-99%), respectively, (Figures 1A,B). With a median follow-up of 29 (12-43) months for EA-treated patients, 12 patients were alive and disease-free, one patient died from relapse, and one relapsing patient achieved long-lasting CR after salvage therapy and ASCT.
Table 1.
Characteristics of the patients from the whole cohort, and the EA cohort.
Table 2.
Common toxicities by grade observed during RMT (n=28) or EA (n=14) therapies.
Figure 1.
Survival analyses. (A) Progression-free survival (PFS) and (B) overall survival (OS) for the entire population and the subset of patients who received EA consolidation; PFS was defined as the time from diagnosis until progression, relapse from CR, or death. OS was defined as the time from diagnosis until death from any cause. PFS and OS were evaluated using the Kaplan-Meier method. EA: etoposide and cytarabine.
Our retrospective study on 28 consecutive PCNSL patients confirmed, in the context of routine practice, the results of Wieduwilt and Rubenstein’s studies in terms of efficacy. Indeed, we observed a near 60% CR rate after four cycles of RMT, in line with most currently used induction regimens using high-dose methotrexate.2–7 Moreover, our survival results were similar to these earlier reports. We observed a similar two-year PFS/OS in our global cohort (45%/58% and 57%/70%, in Wieduwilt and Rubenstein’s studies, respectively, compared to 59%/69% herein), and a very favorable outcome among EA-treated patients.11,12 However, these results must be moderated due to the exclusion from EA of six unfit patients in CR (20% of the initial cohort, similar to the work by Wieduwilt et al.11). Using the same 8g/m2 dosing of methotrexate during induction, we observed frequent but manageable toxicities, as anticipated. Strikingly, a higher rate of EA-induced toxicity was observed in the two real-life studies (Wieduwilt’s and ours) compared to the prospective trial by Rubenstein et al., particularly regarding the occurrence of neutropenic fever, documented infections and mucositis, observed in 16%, 18% and 8%, respectively, of the patients in the prospective trial, as compared to 82%/93%, 30%/57% and 35%/29% in Wieduwilt’s and our cohorts, respectively.12 Due to the expected lengthy neutropenia period following EA therapy, all our patients were hospitalized in laminar flow clean rooms, received prophylactic anti-bacterial and anti-fungal therapies, and underwent close clinical and biological monitoring by trained staff. Hence, we believe that these discrepancies were not due to clinical management, but may reflect the inclusion of less selected patients in retrospective daily practice-based studies, as exemplified by a higher number of patients with an Eastern Cooperative Oncology Group (ECOG) performance status ≥2 in the study herein compared to that of Rubenstein et al. (57% versus 18%, respectively). Recent years has seen the completion of many phase I/II trials in PCNSL, but few were externally validated in daily care practice non-selected patients, which we believe is an important objective to work on, as exemplified by our current study regarding the outbreak of infections compared to the ALLIANCE/CALGB 50202 trial.
In our opinion, EA consolidation might represent an interesting front-line therapeutic option for immunocompetent PCNSL patients; although apparently more toxic than previously reported, particularly regarding the length of neutropenia and related infectious complications. In paticular, we did not observe treatment-related mortality (TRM), as compared to 3-14% in PCNSL ASCT studies.7,10,15 While ASCT is offered to selected patients of up to 75 years of age in recent ASCT trials such as IELSG (clinicaltrials.gov identifier: 02531841) or CALBG-51101 (clinicaltrials.gov identifier: 01511562), our experience with EA suggests that this option might be more broadly offered to less selected patients of up to 75 years of age. For younger patients, this strategy may delay ASCT, preserving most patients from ASCT-induced TRM, which may nevertheless offer a valid therapeutic option in case of relapse. However, considering the high toxicity of the procedure, EA may be as resource consuming as ASCT, requiring inpatient monitoring and high-cost supportive care. Results from the aforementioned IELSG and CALBG-51101 trials are expected to better define the role of intensive consolidation strategies compared to frontline ASCT in PCNSL patients.
Our data confirmed that the RMT regimen represents a suitable first-line option in PCNSL. A chemotherapy-based consolidation using the EA combination appeared efficient, but with more toxic side effects in routine practice than those previously reported in clinical trials.
Supplementary Material
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro-Oncol 2014;16 Suppl 4iv 1–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferreri AJM, Cwynarski K, Pulczynski E, et al. Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol 2016;3(5):e217–227. [DOI] [PubMed] [Google Scholar]
- 3.Fritsch K, Kasenda B, Schorb E, et al. High-dose methotrexate-based immunochemotherapy for elderly primary CNS lymphoma patients (PRIMAIN study). Leukemia 2017;31(4):846–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glass J, Won M, Schultz CJ, et al. Phase I and II study of induction chemotherapy with methotrexate, rituximab, and temozolomide, followed by whole-brain radiotherapy and postirradiation temozolomide for primary CNS lymphoma. J Clin Oncol Off J Am Soc Clin Oncol 2016;34(14):1620–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morris PG, Correa DD, Yahalom J, et al. Rituximab, methotrexate, procarbazine, and vincristine followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in newly diagnosed primary CNS lymphoma: final results and long-term outcome. J Clin Oncol Off J Am Soc Clin Oncol 2013;31(31):3971–3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Illerhaus G, Kasenda B, Ihorst G, et al. High-dose chemotherapy with autologous haemopoietic stem cell transplantation for newly diagnosed primary CNS lymphoma: a prospective, single-arm, phase 2 trial. Lancet Haematol 2016;3(8):e388–397. [DOI] [PubMed] [Google Scholar]
- 7.Omuro A, Correa DD, DeAngelis LM, et al. R-MPV followed by high-dose chemotherapy with TBC and autologous stem-cell transplant for newly diagnosed primary CNS lymphoma. Blood 2015;125(9):1403–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y-B, Batchelor T, Li S, et al. Phase 2 trial of high-dose rituximab with high-dose cytarabine mobilization therapy and high-dose thiotepa, busulfan, and cyclophosphamide autologous stem cell transplantation in patients with central nervous system involvement by non-Hodgkin lymphoma. Cancer 2015;121(2):226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schorb E, Fox CP, Fritsch K, et al. High-dose thiotepa-based chemotherapy with autologous stem cell support in elderly patients with primary central nervous system lymphoma: a European retrospective study. Bone Marrow Transplant 2017;52(8):1113–1119. [DOI] [PubMed] [Google Scholar]
- 10.Ferreri AJM, Cwynarski K, Pulczynski E, et al. Whole-brain radiotherapy or autologous stem-cell transplantation as consolidation strategies after high-dose methotrexate-based chemoimmunotherapy in patients with primary CNS lymphoma: results of the second randomisation of the International Extranodal Lymphoma Study Group-32 phase 2 trial. Lancet Haematol 2017;4(11):e510–e523. [DOI] [PubMed] [Google Scholar]
- 11.Wieduwilt MJ, Valles F, Issa S, et al. Immunochemotherapy with intensive consolidation for primary CNS lymphoma: a pilot study and prognostic assessment by diffusion-weighted MRI. Clin Cancer Res Off J Am Assoc Cancer Res 2012;18(4):1146–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubenstein JL, Hsi ED, Johnson JL, et al. Intensive chemotherapy and immunotherapy in patients with newly diagnosed primary CNS lymphoma: CALGB 50202 (Alliance 50202). J Clin Oncol Off J Am Soc Clin Oncol 2013;31(25):3061–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Houillier C, Ghesquières H, Chabrot C, et al. Rituximab, methotrexate, procarbazine, vincristine and intensified cytarabine consolidation for primary central nervous system lymphoma (PCNSL) in the elderly: a LOC network study. J Neurooncol 2017;133(2):315–320. [DOI] [PubMed] [Google Scholar]
- 14.Abrey LE, Batchelor TT, Ferreri AJM, et al. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol Off J Am Soc Clin Oncol 2005;23(22):5034–5043. [DOI] [PubMed] [Google Scholar]
- 15.Ferreri AJM, Illerhaus G. The role of autologous stem cell transplantation in primary central nervous system lymphoma. Blood 2016;127(13):1642–1649. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.