Abstract
Low-count monoclonal B-cell lymphocytosis is defined by the presence of very low numbers of circulating clonal B cells, usually phenotypically similar to chronic lymphocytic leukemia cells, whose biological and clinical significance remains elusive. Herein, we re-evaluated 65/91 low-count monoclonal B-cell lymphocytosis cases (54 chronic lymphocytic leukemia-like and 11 non-chronic lymphocytic leukemia-like) followed-up for a median of seven years, using high-sensitivity flow cytometry and interphase fluorescence in situ hybridization. Overall, the clone size significantly increased in 69% of low-count monoclonal B-cell lymphocytosis cases, but only one subject progressed to high-count monoclonal B-cell lymphocytosis. In parallel, the frequency of cytogenetic alterations increased over time (32% vs. 61% of cases, respectively). The absolute number of the major T-cell and natural killer cell populations also increased, but only among chronic lymphocytic leukemia-like cases with increased clone size vs. age- and sex-matched controls. Although progression to chronic lymphocytic leukemia was not observed, the overall survival of low-count monoclonal B-cell lymphocytosis individuals was significantly reduced vs. non-monoclonal B-cell lymphocytosis controls (P=0.03) plus the general population from the same region (P≤0.001), particularly among females (P=0.01); infection and cancer were the main causes of death in low-count monoclonal B-cell lymphocytosis. In summary, despite the fact that mid-term progression from low-count monoclonal B-cell lymphocytosis to high-count monoclonal B-cell lymphocytosis and chronic lymphocytic leukemia appears to be unlikely, these clones persist at increased numbers, usually carrying more genetic alterations, and might thus be a marker of an impaired immune system indirectly associated with a poorer outcome, particularly among females.
Introduction
Chronic lymphocytic leukemia (CLL) is the most common leukemia in adults in the Western world, typically affecting older patients, particularly males, with a median age at diagnosis of 70 years (y) old.1 It is characterized by the accumulation of mature B cells in peripheral blood (PB), bone marrow (BM) and also secondary lymphoid tissues, with a uniquely aberrant CD19+ CD20+lo CD5+/++ CD23+ sIgM−/+lo phenotype and restricted immunoglobulin (Ig) light chain usage.2,3 Typically, CLL shows a heterogeneous clinical outcome; thus, whereas in some patients the disease remains stable and they will never require treatment, in around 70% of cases treatment is required and results in variable outcomes, from complete response and prolonged survival to refractory disease and death.3–5
Currently, it is well established that virtually every CLL case is preceded by monoclonal B-cell lymphocytosis (MBL) defined by smaller numbers of circulating PB clonal CLL-like B-cells (<5,000 clonal B-cells/μL) in the absence of any clinical symptoms or signs of disease.6 In 2010, MBL was further subdivided into low-count (MBLlo) and high-count MBL (MBLhi), depending on the number of PB clonal B cells (lower vs. higher than 0.5×109/L, respectively).7 While MBLhi has been reported to progress to overt CLL requiring treatment at a rate of 1–2% cases per year,8,9 no information is available at present regarding the ≥5-year risk of progression of MBLlo to MBLhi and CLL.10
The detection of MBLlo has become routinely feasible due to the use of highly sensitive flow cytometry (FCM) approaches for the screening of subjects from the general population who present normal blood cell counts. Of note, the prevalence of MBLlo is significantly higher than that of MBLhi and CLL, with a frequency that ranges between 3% and 14% of the general adult (≥40y) population, depending on the sensitivity of the FCM technique used.11 Independently of the method, it is well-established that the incidence of MBLlo progressively increases with age, with a prevalence >20% among individuals of more than 70 years of age.12 Whether MBLlo represents the normal counterpart of CLL (e.g., some studies suggest that MBLlo clones are more likely related to immunosenescence)13 or a very early stage of development of CLL, remains an open question. This is partially because, in contrast to MBLhi, long-term follow-up studies in large series of MBLlo cases have not been reported thus far, which limits our understanding of the biological and clinical significance of very low numbers of circulating CLL-like clones, as well as those factors and mechanisms involved in potential long-term progression of (conceivably) a minor proportion of all MBLlo cases to MBLhi and CLL; likewise, little information is available about the evolution of non CLL-like MBL. Such information is critical to a better understanding of the ontogenesis of CLL from the very early stages of the disease, and to better identify MBL patients with stable vs. progressive B-cell lymphocytosis who might benefit from a closer clinical follow-up.
Herein, we report on a cohort of 91 MBLlo (CLL-like and non CLL-like) subjects identified in a population-based screening study and followed for a minimum of five years (median >seven years). Our primary goal was to determine the rate of medium-term progression of MBLlo to MBLhi and CLL, and to identify the most relevant clinical and biological characteristics of PB lymphocytes associated with progression.
Methods
Subjects and samples
The baseline study was conducted from December 2007 to October 2009, when PB samples from 639 healthy adult (≥40y) volunteers (54% females/46% males) from the general population of the same geographical area (Salamanca, Northwest of Spain) were screened for the presence of small B-cell clones, using highly sensitive FCM.12,14 At inclusion, all subjects had normal PB cell counts and did not suffer from any hematological/immunological disease, as described elsewhere.1,6 In 91/639 subjects studied (14.2%), ≥1 PB clonal B-cell population was detected at recruitment; in the vast majority of them (80/91; 88%) clonal B cells were consistent with CLL-like MBLlo (<0.5×109clonal B cells/L showing a CLL-like phenotype), whereas the remaining 11 individuals (12%) were classified as non CLL-like MBLlo.12,14 MBLlo subjects were re-evaluated at a median time of seven years after recruitment (range: 61 to 95 months). All subjects gave their written informed consent at baseline for both the initial and the follow-up studies, and they filled out an epidemiological questionnaire with demographic and (self-reported) medical information, under the supervision of his/her primary care doctor.15 The study was approved by the Ethics Committee of the University Hospital of Salamanca (Spain).
Flow cytometry immunophenotypic studies
Overall, 1-4 mL of ethylenediamine tetraacetic acid (EDTA)-anticoagulated PB was collected per case and follow-up time-point; subsequently it was processed and analyzed using previously reported highly sensitive FCM approaches12,14,16,17 (Online Supplementary Methods and Online Supplementary Table S1).
Interphase fluorescence in situ hybridization (iFISH) studies studies
The most common CLL - i.e., del(13q14), trisomy 12, del(11q)(ATM) and del(17p)(TP53) - along with other B-cell chronic lymphoproliferative disorders (B-CLPD)-associated cytogenetic alterations were investigated by iFISH on fluorescence-activated cell sorting (FACS)-purified (sorted) single clonal B cells (≥95% purity), as previously described18 (Online Supplementary Table S2). A total of 31/91 PB samples studied at baseline and 56/65 at follow-up (year +7) were analyzed by iFISH; in 21 cases (18 CLL-like and three non CLL-like MBLlo) paired samples were analyzed by iFISH at both baseline and year +7. The potential presence of del(13q14) was also tested in non-clonal B-cells from 5/7 MBLlo cases found to have del(13q14)+ MBL cells.
Statistical analyses
All conventional statistical analyses (i.e., descriptive statistics, univariate analyses, including overall survival (OS) analysis, as well as multivariate analyses to predict the variables independently associated with a greater/lower risk of death), were performed with SPSS 19.0 software (SPSS-IBM, Armonk, NY, USA), using the tests, databases and statistical significance values detailed in Online Supplementary Methods. Appropriate tests were further used to objectively evaluate real changes in the size of the B-cell clones studied during follow-up (resampling bootstrap method)19 and to build a predictive linear regression model to estimate the time CLL-like MBLlo clones might potentially take to progress to MBLhi and CLL, using MATLAB R2015a (Mathworks, Natick, MA, USA) (Online Supplementary Methods).
Results
Follow-up of the MBLlo cohort
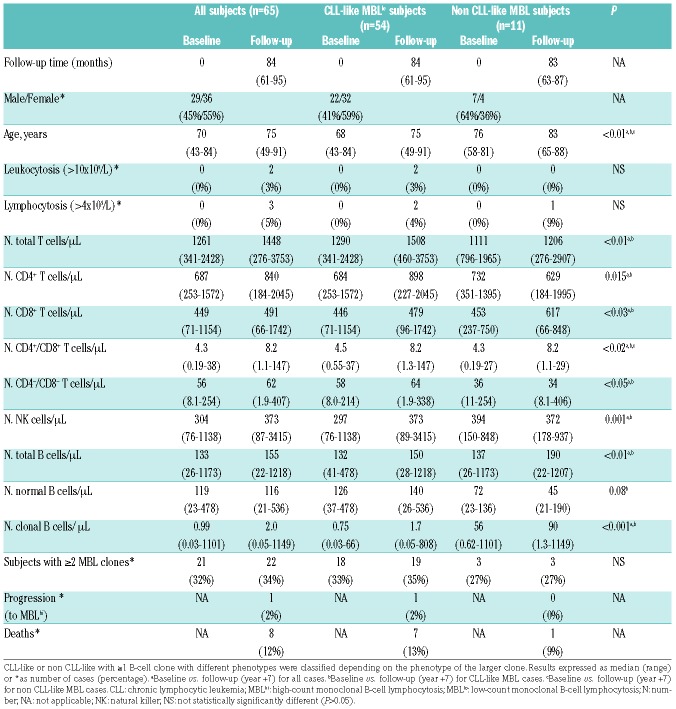
From those 91 MBLlo individuals identified in the screening study performed in the general population of Salamanca between 2007 and 2009,12,14 65–71% of MBLlo cases from the original series; 29 males and 36 females; median age at baseline 70 (range: 43–84 years old)-; were re-evaluated after a median follow-up of seven years (range: 61 to 95 months) (Table 1). These 65 individuals were representative of the original MBLlo cohort for all variables analyzed, except for a significantly lower age (P=0.02) vs. those 26 individuals that could not be followed - median age of 75 (range: 48–95 years)-. These later subjects could only be re-evaluated for their death vs. alive status at the end of the study because of: i) 12/26 (46%) died before the fifth year of follow-up; ii) 2 subjects declined continuing their participation in the study; and iii) the remaining 12 cases were lost to follow-up after >5y from recruitment. Eight of 65 cases followed for >5y (12%) died afterward, making a total of 21 (26%) deaths among MBLlo cases included in OS analyses.
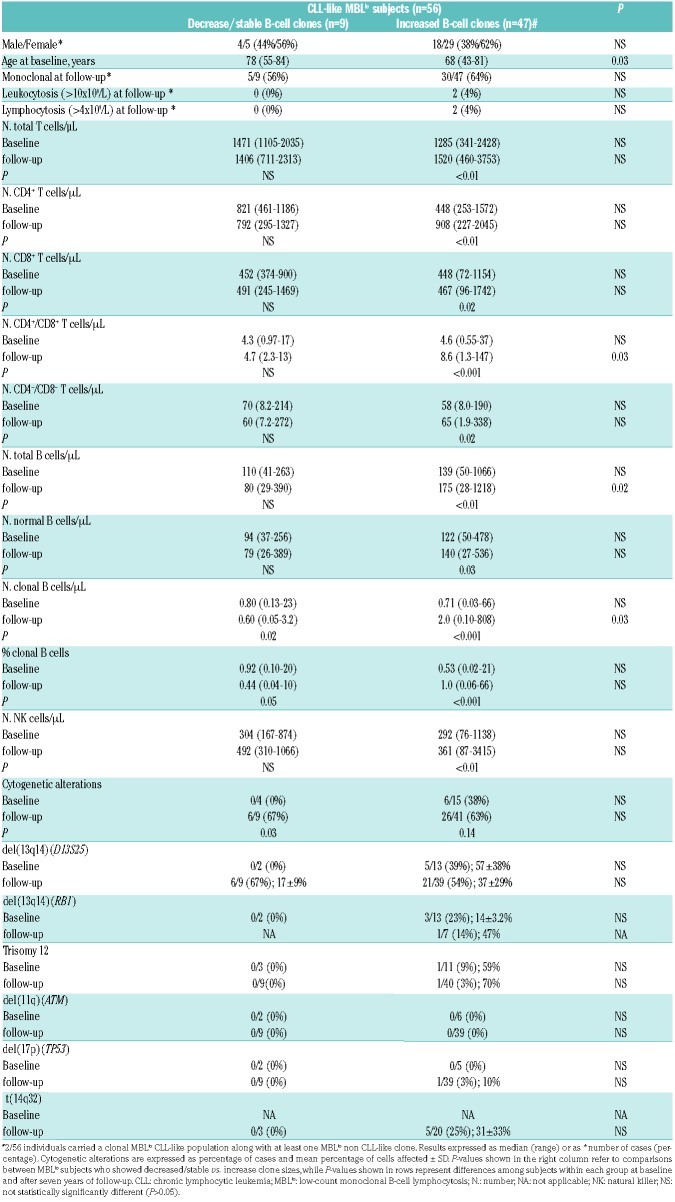
Table 1.
Clinical and biological characteristics of MBLlo subjects at baseline and after follow-up (year +7).
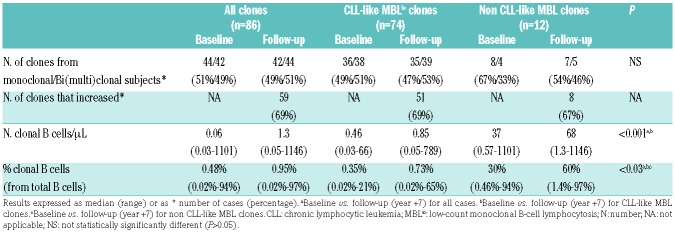
In all 65 individuals who were evaluated after five years, ≥1 clonal B-cell population was reliably identified in PB at follow-up. In 22/65 (34%) cases ≥2 clones were detected (vs. 32% at baseline), resulting in a total of 86 MBLlo clones detected (Table 1 and Table 2). All MBLlo clones showed an identical phenotype at both time-points (Table 2). Thus, 74/86 B-cell clones (86%) showed a classical CLL-like phenotype and 12 (14%) were classified as non CLL-like MBL clones. At year +7, 35/74 CLL-like clones (47%) corresponded to monoclonal cases and the remaining 39 (53%), to 19 subjects with bi(multi)clonal CLL-like MBLlo (Table 2); in two subjects, CLL-like and non CLL-like clones coexisted. Of note, two individuals carrying two CLL-like B-cell clones became “monoclonal” while a second clone emerged in one monoclonal CLL-like MBLlo case at seven years follow-up. In turn, non CLL-like clones (n=12) showed phenotypic profiles identical to those observed at baseline and comparable to those of different B-CLPD, as detailed in Online Supplementary Table S3.6
Table 2.
Biological characteristics of MBLlo clones at baseline and at follow-up (year +7).
Clonal B-cell load in PB at re-evaluation (year +7)
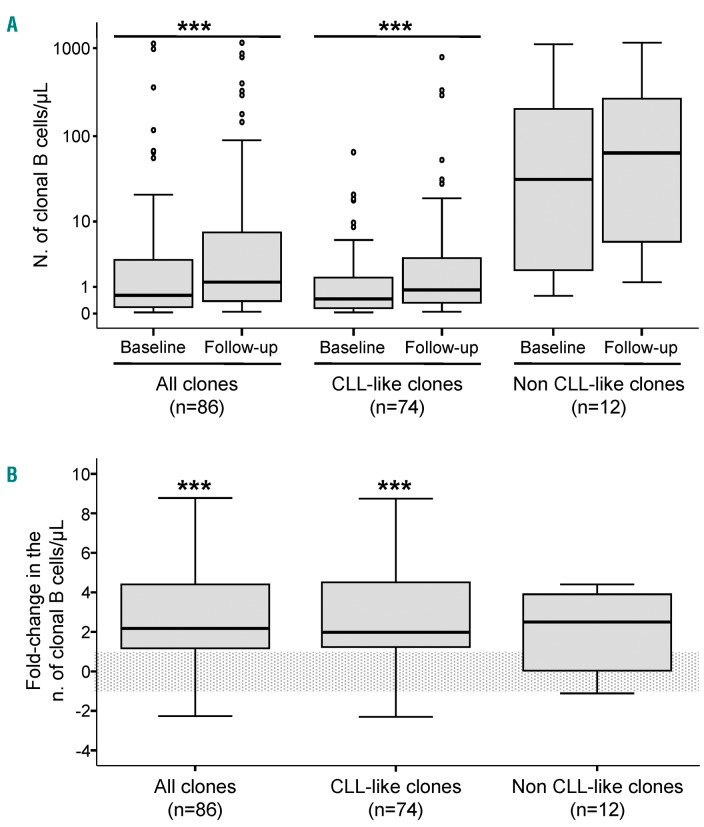
Overall, a significant (P≤0.001) increase in the median size of MBLlo clones was found at follow-up, both for CLL-like (≈2-fold median increase) and for non CLL-like MBLlo clones (≈3-fold median increase) (Table 2 and Figure 1A,B). Such increased absolute number of clonal B-cells over time was associated with a significantly increased (P≤0.001) percentage of clonal B cells from all PB B cells (Table 2). In detail, most MBLlo clones (59/86; 69%) showed significantly increased numbers at re-evaluation vs. baseline, while the remaining 27 B-cell clones persisted at similar (16%) or lower levels (15%); this behavior was very similar for CLL-like and non CLL-like clones (Table 2). Of note, 30/35 (86%) CLL-like clones from (mono)clonal cases increased in size at follow-up vs. only 21/39 (54%) clones from bi(multi)clonal cases (P=0.004). Interestingly, among non CLL-like clones, most marginal zone lymphoma-like clones increased (5/6; 83%), while the two mantle cell lymphoma-like B-cell clones decreased significantly in number (Online Supplementary Table S3).
Figure 1.
Changes in the number of clonal B cells during follow-up. Panel A shows the absolute number of PB clonal B cells/μL detected in MBLlo individuals at baseline and at follow-up, according to the phenotype of the clonal population. Panel B represents the fold-change in the number of clonal B cells/μL from baseline, which is represented by the horizontal light gray box. Notched boxes represent 25th and 75th percentile values; the lines in the middle correspond to median values (50th percentile) and vertical lines represent the highest and lowest values that are neither outliers nor extreme values, which are represented as single dots. ***P-value <0.001. N: number; CLL: chronic lymphocytic leukemia.
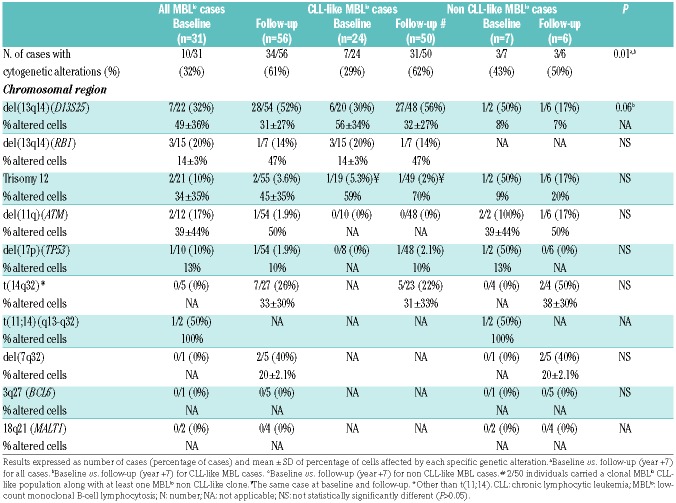
Cytogenetic alterations of MBLlo clonal B cells at baseline and follow-up
The overall frequency of CLL-like MBLlo cases carrying CLL-associated cytogenetic alterations, for example del(13q14), trisomy 12, del(11q)(ATM) and del(17p)(TP53), at baseline was of 29% (7/24 cases tested). At recruitment del(13q14)(D13S25) was found in 56%±34% cells from 6/20 cases evaluated (30%), the RB1 gene was additionally involved in 3 of them, and trisomy 12 was present in the remaining case (59% of cells), both as single alterations. After seven years of follow-up, the percentage of cytogenetic altered cases augmented to 62% of MBLlo cases (31/50 cases, including 15 cases studied at baseline). Interestingly, all cytogenetic alterations observed at baseline also remained at follow-up; in addition, 4/15 (27%) individuals studied at both time-points further acquired del(13q14)(D13S25) (Online Supplementary Table S4). Overall, del(13q14)(D13S25) remained the most frequent alteration at follow-up (27/48; 56%), affecting 32±27% of CLL-like cells. Of note, in five cases in which clonal B cells showed del(13q14)(D13S25), non-clonal B cells were also studied for this alteration, and was found to be absent in all of them. RB1 gene involvement was identified in only 1/7 cases tested; furthermore, trisomy 12 was restricted to one patient who had the same abnormality at baseline (Table 3). Clonal B cells from one individual in whom del(17p)(TP53) was not investigated at baseline was found to carry this cytogenetic alteration in 10% of cells at follow-up. Alterations involving 14q32 were investigated only at follow-up in a subset of 20 CLL-like MBLlo cases, being found in five (20%) patients (Table 3).
Table 3.
Frequency of cases with CLL-associated cytogenetic alterations and percentage of cells affected by each genetic abnormality.
Regarding non CLL-like clones, t(11;14)(q13-q32) was detected in 100% of clonal B cells from one of the two MCL-like cases studied, while del(7q32) was detected in 2/5 splenic marginal zone lymphoma (SMZL)-like cases (Table 3). None of the cases investigated showed t(14;18) (data not shown).
Distribution of normal residual T-, B- and NK-cell populations
The PB counts of total T cells and their CD4+CD8−, CD8+CD4− and CD4−CD8−/lo subsets, as well as NK cells and normal residual polyclonal B cells was significantly increased (P<0.05) in CLL-like MBLlo at follow-up vs. baseline (Table 1). In contrast, among non CLL-like MBL cases, CD4+CD8+ T cells were the only lymphoid subset significantly increased (P=0.02) at the seven year follow-up. To rule out a potential age-related bias and further confirm these findings, we compared the number of PB normal lymphocyte subsets at seven years follow-up vs. a large series of non-MBL healthy donors matched per age and sex distribution to the CLL-like MBLlo cases at seven years (Online Supplementary Table S5) and the same differences were found, ruling out an impact of sex or more advanced age on the increased PB residual lymphocyte counts. No significant correlation (P>0.05) was revealed between the absolute number of clonal B cells and any of the normal residual PB lymphocyte subsets analyzed (data not shown).
Clinical and biological characteristics of CLL-like MBLlo at baseline vs. follow-up, according to the kinetics of the B-cell clone
Upon comparing CLL-like MBLlo cases with increased vs. stable/decreased clonal B-cell numbers at seven years follow-up, the former had a similar male/female distribution, but they were significantly younger (median age: 68y vs. 78y; Table 4).
Table 4.
Clinical and biological characteristics of CLL-like MBLlo subjects at baseline and at follow-up (+7 years) according to the kinetics of the MBL clone in PB (decreased/stable vs. increased size).
Strikingly, MBLlo cases who showed larger CLL-like clone sizes over time also showed significantly higher (P<0.05) numbers of the distinct normal residual T-, B- and NK-cell subsets at follow-up (vs. baseline) (Table 4). Moreover, in these subjects a direct correlation was observed between the absolute number of clonal B cells and CD4+CD8− T cells (r2=0.5; P=0.001). In contrast, no significant (P>0.05) association was found between higher numbers of clonal CLL-like B cells in PB over time, and an increased frequency of cytogenetic alterations. Interestingly, del(13q14) was the sole genetic alteration detected at the seven year follow-up within cases with stable/decreased CLL-like B-cell clones, while those cases with increased CLL-like B-cell clones at year +7 showed cytogenetic alterations other than del(13q14), e.g., trisomy 12 (1/40), del(17p)(TP53) (1/39) and t(14q32) (5/20 cases tested) (Table 4).
Clinical outcome of MBLlo cases
Three subjects developed absolute lymphocytosis after seven years of follow-up (median: 5.3×109 lymphocytes/L; range: 4.1×109–5.9×109/L) in the absence of signs of disease. Two had CLL-like B-cell clones carrying del(13q14), while the remaining case had a non CLL-like clone. In one of the two CLL-like MBLlo cases, the size of the B-cell clone increased over the threshold for MBLhi (>500 clonal B cells/μL), while the other two cases remained as MBLlo. Remarkably, these three subjects displayed the highest increase in clone size at re-evaluation: this translated into a significantly lower (estimated) time to progression into CLL (median: 95y; range: 54–128y) according to the predictive mathematical model used. In turn, the estimated time to progression to CLL for the other MBLlo individuals was far beyond a normal life expectancy (median: 54,767y; range: 54–>63 million years).
Overall survival of MBL vs. non-MBL individuals
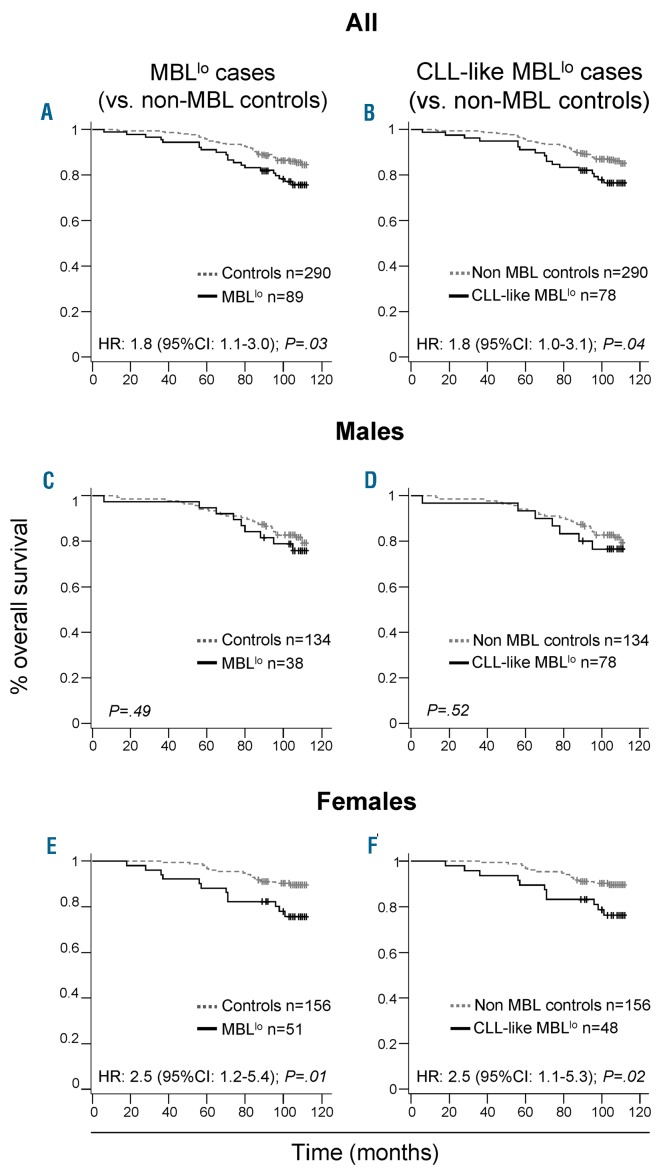
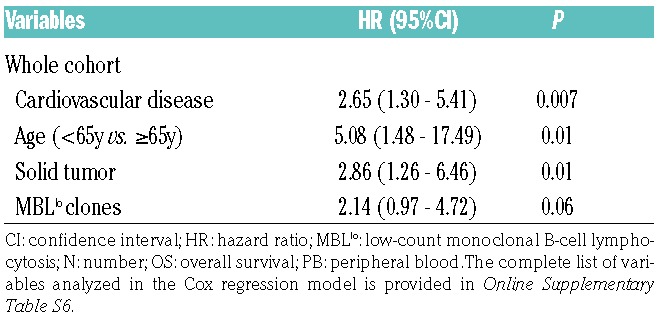
At the end of the study (January 2017), the clinical records and epidemiological questionnaires from all individuals recruited at baseline were reviewed. During follow-up, 21/89 (24%) MBLlo cases and 41/290 (14%) age- and sex-matched non-MBLlo subjects from the original cohort had died (P=0.03). Though the median OS for the two groups had not been reached yet, a significantly shorter OS was observed for MBLlo individuals vs. age- and sex-matched non-MBL controls from the same cohort and geographical area (10y OS rates of 76% vs. 86%, respectively; P=0.03) (Figure 2A,B). Moreover, MBLlo subjects also showed a significantly shortened survival vs. age-matched individuals of the general population from the same geographical region (8.0% vs. 1.8% in the period 2015-2016, respectively; P<0.001) (Online Supplementary Figure S1). Interestingly, such differences in OS were at the expense of a lower OS of CLL-like MBLlo females, who showed a significantly (P=0.01) higher risk of death (hazard ratio (HR) of 2.5; 95% confidence interval (CI) of 1.2-5.4) than non-MBL females of the same age (Figure 2C,F). Infections (21%; mostly respiratory infections and sepsis), cancer (36%; all solid tumors except for an essential thrombocythemia) and cardiovascular diseases (29%; i.e., myocardial infarction and acute ischemic stroke) were the main causes of death among MBLlo subjects. Overall, infections were overrepresented among the MBLlo cohort vs. age- and sex-matched subjects from the general population of the same geographical area (21% vs. 1.4%, respectively; P≤0.001). In contrast, the proportion of deaths caused by tumors (36% vs. 26%, respectively; P>0.05) and by cardiovascular diseases (29% vs. 33%, respectively; P>0.05) were similar in both groups. In turn, no MBLlo subjects died as a cause of non-infectious respiratory tract diseases or genitourinary diseases, diabetes, dementia or other nervous system disorders, which accounted for ≈30% of deaths in the age- and sex-matched general population cohort living in the same geographical area. In order to identify those variables independently associated with OS, a multivariate Cox regression analysis, including laboratory, epidemiological and medical information, was carried out. Advanced age- HR of 5.1; 95% CI: 1.5-17.5; P=0.01-, co-existing cardiovascular diseases (HR: 2.7; 95%CI: 1.3-5.4; P=0.01), solid tumors (HR: 2.9; 95%CI: 1.3-6.5; P=0.007) and, to a lesser extent, the presence of MBLlo clones (HR: 2.1, 95%CI: 0.97-4.7; P=0.06), were independently associated with a shorter OS in the whole cohort (Table 5 and Online Supplementary Table S6).
Figure 2.
Overall survival from baseline (mortality rates) of MBLlo individuals vs. age- and sex-matched non-MBL controls. Left column panels represent comparisons of overall survival curves from MBLlo subjects (black) and age- and sex-matched non-MBL controls (dotted gray). The same comparison is depicted for all individuals (Panel A) and separately for males (Panel C) and females (Panel E). In the right column, overall survival curves comparing all MBLlo subjects with a CLL-like phenotype vs. all age-and sex-matched non-MBL controls (Panel B). The same subjects distributed according to sex are shown in Panel D (males) and in Panel F (females). MBLlo: low-count monoclonal B-cell lymphocytosis.
Table 5.
Variables studied in the Cox regression multivariate analysis showing an independent impact (P<0.1) on OS for the whole MBLlo plus non-MBL cohort.
Discussion
Several preceding studies have shown that virtually all CLL cases are preceded by MBLhi;8,20,21 in contrast, such a relationship has not been demonstrated for MBLlo cases, its role as a preleukemic condition still remaining to be confirmed.9,21 In fact, there exist very few studies with short-term follow-up (i.e., ≤3y) which have investigated the progression rate from MBLlo to MBLhi and CLL thus far.10,22,23 Hence, Fazi et al. showed persistent MBLlo clones over time in 90% of CLL-like MBLlo and only 67% of non CLL-like clones, after a median follow-up of ≈3y.10 Herein, we demonstrate the systematic persistence of both CLL-like and non CLL-like MBLlo B-cell clones with an identical phenotype to baseline after seven years follow-up in 65/65 MBLlo cases, confirming that MBLlo is not a transient condition. Similarly, Matos et al. also found the persistence of B-cell clones in their limited series of CLL-like MBLlo cases (n=5) after a median follow-up of ≈7y.23 Interestingly, in 3/56 CLL-like MBLlo cases, the number of clones identified at seven years follow-up changed, which might suggest the emergence of MBLlo from an oligoclonal background that mirrors competition and natural selection among multiple coexisting clones.24 Changes observed in the VDJ sequences of the expanded B cells from most of these cases (data not shown), together with the progressively decreasing rate of oligoclonality from MBLlo (12-19%) to MBLhi (2.9-13%) and CLL (0.7-3.4%), would further support this hypothesis.9,12,25–27 The significance of such oligoclonal B-cell expansions in MBLlo remains unknown, but might be the consequence of the early stages of altered oligoclonal immune responses against multiple antigens, in which a single clone had not yet emerged as dominant vs. the others, as might occur at the latter, e.g., CLL stage.
Most importantly, over two thirds of all CLL-like MBLlo clones showed a significantly increased size in PB after seven years, while for non CLL-like clones more variable kinetics were observed, depending on the specific phenotype of clonal B-cells. Interestingly, we also observed a significant increase in the frequency of cytogenetic alterations over time, evidencing that B-cell clones are not only dynamic in terms of clone size, but also regarding their capacity to acquire new cytogenetic alterations. Of note, del(13q14), which has been found to be a common mosaicism in the general population,28,29 was absent in non-clonal B cells from 5/5 cases investigated in which CLL-like clonal cells did carry this alteration, indicating that the emergence of this alteration in MBLlo is specific for the clonal population. Altogether, these findings suggest that cytogenetic alterations are a relatively early, but not primary, event in the natural history of MBL/CLL, and might have a potential role in the progression of MBLlo to MBLhi and CLL.
The presence and type of cytogenetic lesions, the IGHV mutational status, or the presence of stereotyped receptors are some of the most important prognostic factors in CLL, which also define the outcome of MBLhi individuals; furthermore, it might identify a subset of cases in whom the presence of the B-cell clonal population influences OS.30–33 Unfortunately, in the present study, the mutational status and VDJ rearrangements were only assessed (both baseline and follow-up) in 8/65 MBLlo individuals (data not shown), making it impossible to validate solid conclusions regarding the potential association with the risk for progression into MBLhi and CLL. To the best of our knowledge, the frequency and impact on disease progression of recurrent mutations (i.e., NOTCH1, SF3B1, MYD88, etc.) found in CLL, and also in MBLhi, to a lesser extent, has not been elucidated for MBLlo.34–36 Therefore, analysis of these CLL-related mutations in MBLlo cases might further contribute to an improvement in better delineating intrinsic tumor cell factors associated to disease progression.
In addition, the environment in which CLL-like MBLlo clones develop might be influenced by chronic immune responses against e.g., host viruses, that might play a critical role in the expansion of clonal B cells, as recently suggested.37 In line with this hypothesis, herein we also show that the expansion of CLL-like MBLlo clones after seven years of follow-up (vs. baseline) is accompanied by a significant increase of all T-cell (but CD4+CD8+cytotoxic T-cells) and NK-cell populations in PB.
Controversial results have been reported regarding PB T-cell numbers in MBLlo. Hence, while te Raa et al. found normal CD4+ and CD8+ T-cell counts in PB of MBLhi,38 other studies have demonstrated that around half of the MBLlo individuals show ≥1 clonal/oligoclonal CD4+CD8+ T-cell population, with an overall increased frequency of clonal T-cell populations vs. age-matched individuals from the general population.10,39 However, the presence of clonal (CD4+CD8+ and other) T-cell expansions has also been described as a common event in older individuals, and has been associated with the ageing of the immune system.39 In this respect, we demonstrate herein that changes in the number of circulating PB T-cell and NK-cell populations among our CLL-like MBLlo subjects were not age-related, via a parallel analysis of a large group of 250 age- and sex-matched non-MBL controls (Online Supplementary Table S5). From a pathophysiological point of view, the increase in most PB T- and NK-cell populations could be associated with either a potentially protective or activating effect of these cellular components of the immune system (microenvironment) on the expanded clonal B-cells.40,41 Therefore, on one hand, increased numbers of (functionally impaired) T cells have been described in CLL38,42,43 while on the other hand, we have recently shown increased titers of plasma antibodies against CMV and EBV in MBLhi and CLL patients vs. MBLlo and non-MBL controls, despite their antibody (immune)deficient state.37 Taken together, these latter findings might further support the existence of additional signals coming from immune cells other than clonal B cells, that could already contribute to the expansion of (cyto)genetically altered CLL-like clones at the earliest stages of disease, by promoting activation, proliferation and/or survival of specific B-cell clones.
A major goal of our study was to investigate the medium-term rate of progression of MBLlo to MBLhi and (potentially also) CLL. Overall, only one subject evolved from MBLlo to MBLhi, and none transformed to CLL, which would translate into a progression rate from MBLlo to MBLhi of 1.8% after seven years of follow-up. Despite the fact that the rate of progression of MBLlo to MBLhi and CLL appears to be extremely low, one of the most astonishing findings of our follow-up study was the significantly higher frequency of deaths among MBLlo subjects, associated with a significant adverse impact on OS vs. both non-MBL controls, particularly among females, and the general population (of similar age and sex distribution) living in the same region in Spain. However, comparisons with the general population must be considered with care, since the conditions of this population might differ from that of non-MBL individuals recruited at the Primary Health Services. Multivariate analysis showed a borderline significant association between the presence of MBLlo clones and a shorter survival. Despite this, the specific mechanisms responsible for the higher frequency of infections and deaths observed, particularly among women, are unknown, and further studies are required to validate and clarify these results. In this regard, controversial results have been reported on MBLhi subjects in the literature. Thus, while Shanafelt et al. showed no differences in OS of MBLhi vs. the general population,33 Shim et al. pointed out a higher frequency of deaths in their MBLlo cohort (4/11; 36%), albeit no statistically significant differences were found vs. non-MBL controls in the latter study, probably due to the small sample size.22 In addition, Fazi et al. also reported that 16/137 (12%) CLL-like MBLlo subjects died before re-evaluation after a median time of three years, which is a high proportion of their whole cohort.10 However, in the aforementioned report no information about the age of the deceased subjects is provided, and therefore, if it is the case they were older (than those subjects remaining alive) such high mortality rates might have been expected. Even more strikingly is the overrepresentation of infections as causes of death in MBLlo compared to that of our non-MBL cohort. Impaired immune responses and higher frequencies of infection have been recurrently reported in both MBLhi and CLL,44–47 but so far very little information exists in MBLlo, and such an association deserves further investigations. Several groups pointed out that the frequency of clonal hematopoiesis dramatically increases with age in the general population, especially among the elderly, in a similar way to the increased frequency of MBLlo, reflecting a clear relationship between clonal hematopoiesis and a higher risk of death.48,49 Whether or not both phenomena are related to MBLlo deserves future investigations.
In summary, we show herein that although MBLlo is a persistent and dynamic condition with a progressive acquisition of cytogenetic alterations, usually associated with an increased clone size and higher T- and NK-cell numbers in PB over time, progression of MBLlo to MBLhi and CLL is extremely rare in the medium-term. Despite this, the MBLlo subjects analyzed herein, particularly women, showed a shortened OS associated with an increased risk of death, particularly due to infections, further supporting the notion that MBLlo could be a marker of an impaired immune system, indirectly associated with a poorer outcome. Additional studies are necessary to confirm these findings and shed light onto the specific immune defects and microenvironmental factors involved in MBLlo.
Supplementary Material
Acknowledgments
The authors would like to thank “The Primary Health Care Group of Salamanca for the Study of MBL” for their contribution to the study; a complete list of members is included in the Online Supplementary Information.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/103/7/1198
Funding
This work was supported by the RD06/0020/0035 and RD12/0036/0048 grants from Red Temática de Investigación Cooperativa en Cáncer (RTICC), Instituto de Salud Carlos III, Ministerio de Economía y Competitividad, (Madrid, Spain and FONDOS FEDER); CB16/12/00400 grant (CIBERONC, Instituto de Salud Carlos III, Ministerio de Economía y Competitividad, (Madrid, Spain and FONDOS FEDER); the FIS PI06/0824-FEDER, PS09/02430-FEDER, PI12/00905-FEDER, DTS15/00119-FEDER, PI16/00787-FEDER and PI17/00399-FEDER grants, from the Fondo de Investigación Sanitaria of Instituto de Salud Carlos III; the GRS206/A/08 grant, (Ayuda al Grupo GR37 de Excelencia, SAN/1778/2009) from the Gerencia Regional de Salud (Consejería de Educación and Consejería de Sanidad of Castilla y León, Valladolid, Spain) and the SA079U14 grant (Consejería de Educación and Consejería de Sanidad of Castilla y León, Valladolid, Spain). ML Gutiérrez is supported by grant PTA2014-09963-I from the Instituto de Salud Carlos III.
References
- 1.Rai KR, Jain P. Chronic lymphocytic leukemia (CLL)-Then and now. Am J Hematol. 2016;91(3):330–340. [DOI] [PubMed] [Google Scholar]
- 2.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute – Working Group 1996 guidelines. Blood. 2008;111(12):5446–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scarfo L, Ferreri AJM, Ghia P. Chronic lymphocytic leukaemia. Crit Rev Oncol Hematol. 2016;104:169–182. [DOI] [PubMed] [Google Scholar]
- 4.Bachow SH, Lamanna N. Evolving strate gies for the treatment of chronic lymphocytic leukemia in the upfront setting. Curr Hematol Malig Rep. 2016;11(1):61–70. [DOI] [PubMed] [Google Scholar]
- 5.Chiorazzi N. Implications of new prognostic markers in chronic lymphocytic leukemia. Hematol Am Soc Hematol Educ Progr. 2012;2012:76–87. [DOI] [PubMed] [Google Scholar]
- 6.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon (France): IARC; 2017. [Google Scholar]
- 7.Rawstron AC, Shanafelt T, Lanasa MC, et al. Different biology and clinical outcome according to the absolute numbers of clonal B-cells in monoclonal B-cell lymphocytosis (MBL). Cytometry B Clin Cytom. 2010;78(Suppl. 1):19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rawstron AC, Bennett FL, O’Connor SJM, et al. Monoclonal B-cell lymphocytosis and chronic lymphocytic leukemia. N Engl J Med. 2008;359(6):575–583. [DOI] [PubMed] [Google Scholar]
- 9.Vardi A, Dagklis A, Scarfo L, et al. Immunogenetics shows that not all MBL are equal: the larger the clone, the more similar to CLL. Blood. 2013;121(22):4521–4528. [DOI] [PubMed] [Google Scholar]
- 10.Fazi C, Scarfo L, Pecciarini L, et al. General population low-count CLL-like MBL persists over time without clinical progression, although carrying the same cytogenetic abnormalities of CLL. Blood. 2011;118(25):6618–6625. [DOI] [PubMed] [Google Scholar]
- 11.Shim YK, Middleton DC, Caporaso NE, et al. Prevalence of monoclonal B-cell lymphocytosis: a systematic review. Cytometry B Clin Cytom. 2010;78(Suppl 1):10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nieto WG, Almeida J, Romero A, et al. Increased frequency (12%) of circulating chronic lymphocytic leukemia-like B-cell clones in healthy subjects using a highly sensitive multicolor flow cytometry approach. Blood. 2009;114(1):33–37. [DOI] [PubMed] [Google Scholar]
- 13.Scarfo L, Fazi C, Ghia P. MBL versus CLL: how important is the distinction? Hematol Oncol Clin North Am. 2013;27(2):251–265. [DOI] [PubMed] [Google Scholar]
- 14.Nieto WG, Teodosio C, López A, et al. Non-CLL-like monoclonal B-Cell lymphocytosis in the general population: Prevalence and phenotypic/genetic characteristics. Cytometry B Clin Cytom. 2010;78(Suppl. 1):24–34. [DOI] [PubMed] [Google Scholar]
- 15.Casabonne D, Almeida J, Nieto WG, et al. Common infectious agents and monoclonal B-cell lymphocytosis: a cross-sectional epidemiological study among healthy adults. PLoS One. 2012;7(12):e52808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalina T, Flores-Montero J, van der Velden VHJ, et al. EuroFlow standardization of flow cytometer instrument settings and immunophenotyping protocols. Leukemia. 2012;26(9):1986–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flores-Montero J, Sanoja-Flores L, Paiva B, et al. Next Generation Flow for highly sensitive and standardized detection of minimal residual disease in multiple myeloma. Leukemia. 2017;31(10):2094–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quijano S, López A, Rasillo A, et al. Impact of trisomy 12, del(13q), del(17p), and del(11q) on the immunophenotype, DNA ploidy status, and proliferative rate of leukemic B-cells in chronic lymphocytic leukemia. Cytometry B Clin Cytom. 2008;74(3):139–149. [DOI] [PubMed] [Google Scholar]
- 19.Efron B, Tibshirani R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistial accuracy. Stat Sci. 1986;1(1):54–77. [Google Scholar]
- 20.Landgren O, Albitar M, Ma W, et al. B-cell clones as early markers for chronic lymphocytic leukemia. N Engl J Med. 2009; 360(7):659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shanafelt TD, Ghia P, Lanasa MC, Landgren O, Rawstron AC. Monoclonal B-cell lymphocytosis (MBL): biology, natural history and clinical management. Leukemia. 2010;24(3):512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shim YK, Vogt RF, Middleton D, et al. Prevalence and natural history of monoclonal and polyclonal B-cell lymphocytosis in a residential adult population. Cytometry B Clin Cytom. 2007;72(5):344–353. [DOI] [PubMed] [Google Scholar]
- 23.Matos DM, Furtado FM, Falcao RP. Monoclonal B-cell lymphocytosis in individuals from sporadic (non-familial) chronic lymphocytic leukemia families persists over time, but does not progress to chronic B-cell lymphoproliferative diseases. Rev Bras Hematol Hemoter. 2015;37(5):292–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481(7381):306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henriques A, Rodriguez-Caballero A, Nieto WG, et al. Combined patterns of IGHV repertoire and cytogenetic/molecular alterations in monoclonal B lymphocytosis versus chronic lymphocytic leukemia. PLoS One. 2013;8(7):e67751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanchez M-L, Almeida J, Gonzalez D, et al. Incidence and clinicobiologic characteristics of leukemic B-cell chronic lymphoproliferative disorders with more than one B-cell clone. Blood. 2003;102(8):2994–3002. [DOI] [PubMed] [Google Scholar]
- 27.Kern W, Bacher U, Schnittger S, et al. Flow cytometric identification of 76 patients with biclonal disease among 5523 patients with chronic lymphocytic leukaemia (B-CLL) and its genetic characterization. Br J Haematol. 2014;164(4):565–569. [DOI] [PubMed] [Google Scholar]
- 28.Jacobs KB, Yeager M, Zhou W, et al. Detectable clonal mosaicism and its relationship to aging and cancer. Nat Genet. 2012;44(6):651–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Machiela MJ, Zhou W, Caporaso N, et al. Mosaic 13q14 deletions in peripheral leukocytes of non-hematologic cancer cases and healthy controls. J Hum Genet. 2016;61(5):411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shanafelt TD, Kay NE, Rabe KG, et al. Brief report: natural history of individuals with clinically recognized monoclonal B-cell lymphocytosis compared with patients with Rai 0 chronic lymphocytic leukemia. J Clin Oncol. 2009;27(24):3959–3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kern W, Bacher U, Haferlach C, et al. Monoclonal B-cell lymphocytosis is closely related to chronic lymphocytic leukaemia and may be better classified as early-stage CLL. Br J Haematol. 2012;157(1):86–96. [DOI] [PubMed] [Google Scholar]
- 32.Strati P, Shanafelt TD. Monoclonal B-cell lymphocytosis and early-stage chronic lymphocytic leukemia: diagnosis, natural history, and risk stratification. Blood. 2015; 126(4):454–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shanafelt TD, Kay NE, Rabe KG, et al. Survival of patients with clinically identified monoclonal B-cell lymphocytosis (MBL) relative to the age- and sex-matched general population. Leukemia. 2012; 26(2):373–376. [DOI] [PubMed] [Google Scholar]
- 34.Landau DA, Tausch E, Taylor-Weiner AN, et al. Mutations driving CLL and their evolution in progression and relapse. Nature. 2015;526(7574):525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barrio S, Shanafelt TD, Ojha J, et al. Genomic characterization of high-count MBL cases indicates that early detection of driver mutations and subclonal expansion are predictors of adverse clinical outcome. Leukemia. 2017;31(1):170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agathangelidis A, Ljungström V, Scarfò L, et al. Highly similar genomic landscapes in monoclonal B-cell lymphocytosis and ultra-stable chronic lymphocytic leukemia with low frequency of driver mutations. Haematologica. 2018. February 15 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Criado I, Muñoz-Criado S, Rodríguez-Caballero A, et al. Host virus and pneumococcus-specific immune responses in high-count monoclonal B-cell lymphocytosis and chronic lymphocytic leukemia: implications for disease progression. Haematologica. 2017;102(7):1238–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.te Raa GD, Tonino SH, Remmerswaal EBM, et al. Chronic lymphocytic leukemia specific T-cell subset alterations are clone-size dependent and not present in monoclonal B lymphocytosis. Leuk Lymphoma. 2012;53(11):2321–2325. [DOI] [PubMed] [Google Scholar]
- 39.Ghia P, Prato G, Stella S, Scielzo C, Geuna M, Caligaris-Cappio F. Age-dependent accumulation of monoclonal CD4+CD8+ double positive T lymphocytes in the peripheral blood of the elderly. Br J Haematol. 2007;139(5):780–790. [DOI] [PubMed] [Google Scholar]
- 40.Purroy N, Wu CJ. Coevolution of leukemia and host immune cells in chronic lymphocytic leukemia. Cold Spring Harb Perspect Med. 2017;7(4):a026740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Os A, Bürgler S, Ribes AP, et al. Chronic lymphocytic leukemia cells are activated and proliferate in response to specific T helper cells. Cell Rep. 2013;4(3):566–77. [DOI] [PubMed] [Google Scholar]
- 42.Ramsay AG, Clear AJ, Fatah R, Gribben JG. Multiple inhibitory ligands induce impaired T-cell immunologic synapse function in chronic lymphocytic leukemia that can be blocked with lenalidomide: establishing a reversible immune evasion mechanism in human cancer. Blood. 2012; 120(7):1412–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riches JC, Davies JK, McClanahan F, et al. T cells from CLL patients exhibit features of T-cell exhaustion but retain capacity for cytokine production. Blood. 2013; 121(9):1612–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morrison VA. Infectious complications of chronic lymphocytic leukaemia: pathogenesis, spectrum of infection, preventive approaches. Best Pract Res Clin Haematol. 2010;23(1):145–153. [DOI] [PubMed] [Google Scholar]
- 45.Moreira J, Rabe KG, Cerhan JR, et al. Infectious complications among individuals with clinical monoclonal B-cell lymphocytosis (MBL): a cohort study of newly diagnosed cases compared to controls. Leukemia. 2013;27(1):136–141. [DOI] [PubMed] [Google Scholar]
- 46.Forconi F, Moss P. Perturbation of the normal immune system in patients with CLL. Blood. 2015;126(5):573–81. [DOI] [PubMed] [Google Scholar]
- 47.Solomon BM, Chaffee KG, Moreira J, et al. Risk of non-hematologic cancer in individuals with high-count monoclonal B-cell lymphocytosis. Leukemia. 2016; 30(2):331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371(26):2488–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Genovese G, Kahler AK, Handsaker RE, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371(26):2477–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.