Abstract
Multiple concussions, particularly in contact sports, have been associated with cognitive deficits, psychiatric impairment and neurodegenerative diseases like chronic traumatic encephalopathy. We used volumetric and deformation-based morphometric analyses to test the hypothesis that repeated concussions may be associated with smaller regional brain volumes, poorer cognitive performance and behavioural symptoms among former professional football players compared to healthy controls. This study included fifty-three retired Canadian Football League players, 25 age- and education-matched healthy controls, and controls from the Cambridge Centre for Aging and Neuroscience database for validation. Volumetric analyses revealed greater hippocampal atrophy than expected for age in former athletes with multiple concussions than controls and smaller left hippocampal volume was associated with poorer verbal memory performance in the former athletes. Deformation-based morphometry confirmed smaller bilateral hippocampal volume that was associated with poorer verbal memory performance in athletes. Repeated concussions may lead to greater regional atrophy than expected for age.
Keywords: Sport-related concussion, Mild traumatic brain injury, Deformation based morphometry, Aging
Highlights
-
•
Age had a greater effect on hippocampal volume loss in athletes than in controls.
-
•
Years playing professional football related to smaller hippocampi and amygdalae.
-
•
Smaller hippocampal volume was related to lower verbal memory scores.
1. Introduction
There is a high incidence of concussions, particularly among players of contact sports, with an estimated 1.6 to 3.8 million sports-related concussions occurring each year in the United States alone (Langlois et al., 1991). Professional players of contact sports will experience hits to the head but not all will report having a concussion. While most concussive events resolve within weeks, at least 10% of patients experience prolonged symptoms known as post-concussion syndrome (Hiploylee et al., 2017). Recently, there is growing concern that repeated concussions can cause late life mild cognitive impairment, an earlier onset of Alzheimer's disease (Abner et al., 2014) or the neurodegenerative disease called chronic traumatic encephalopathy (CTE) (Omalu et al., 2005; McKee et al., 2009). The majority of CTE cases have been reported in athletes involved in contact sports, including boxing, football, hockey, rugby, wrestling and soccer (Omalu et al., 2005; McKee et al., 2009; Omalu et al., 2006, Omalu et al., 2010).
Neuronal damage from traumatic brain injury (TBI) has been associated with cerebral atrophy in studies of mild, moderate and severe brain injury (Shenton et al., 2012; Green et al., 2014; Cole et al., 2015). Normal aging is also associated with mild brain volume loss and some cognitive deficits (Cole et al., 2015; Bigler et al., 1997). Accelerated cognitive decline may occur as a result of mild, moderate or severe TBI, and exacerbate deficits associated with the normal aging process (Tremblay et al., 2013). Memory impairment is one of the most frequent cognitive complaints following mild, moderate and severe TBI (Rabinowitz and Levin, 2014). Verbal memory impairment may result from injury to the left medio-temporal and hippocampal regions (Frisk and Milner, 1990) while deficits in visuospatial memory may be associated with these regions in the right hemisphere (Smith and Milner, 1981). Moreover, post-concussive symptoms include behaviour and personality changes, such as depression, apathy, impulsivity and aggression (Malia et al., 1995), which have been associated with generalized and regional brain atrophy in various study populations (Matthies et al., 2012; Tajima-Pozo et al., 2015).
Several neuroimaging techniques have been used to examine whether symptoms resulting from multiple concussions are associated with smaller brain volume and impairment in function (Goswami et al., 2016; Ilvesmaki et al., 2014; Meier et al., 2016; Multani et al., 2016; Tremblay et al., 2014; Strain et al., 2015). However, results from these studies have been mixed; while some identify structural and functional brain changes associated with symptoms in both acute and chronic concussed populations (Goswami et al., 2016; Meier et al., 2016; Multani et al., 2016; Strain et al., 2015), other studies report no abnormalities (Ilvesmaki et al., 2014; Tremblay et al., 2014).
Severe, moderate and mild TBI are associated with long-term damage to the brain (McKee et al., 2009; Corsellis et al., 1973). We hypothesize that long-term damage can result from mild TBI and contribute to measurable brain atrophy and that this atrophy will be associated with cognitive deficits and behavioural changes. Using structural segmentation and deformation-based morphometry (DBM) (Ashburner et al., 1998) analyses, the current study compares the effect of multiple concussions on regional brain volumes in retired professional athletes from the Canadian Football League (ex-CFL) with non-athlete control subjects with no history of concussion. As the sample size of our control group was small, a larger control population from the Cambridge Centre for Aging and Neuroscience database was leveraged for further analysis. We also investigated the relationship between regional brain volume and memory and personality changes. We predict that ex-CFL will show greater focal atrophy of the hippocampi and amygdala, and these regions will be associated with poorer memory performance and personality changes compared to controls.
2. Materials and methods
2.1. Participants
This study included 53 ex-CFL players (mean age = 55.6 ± 12.9 years), most of whom report multiple concussions, and 25 healthy age- and education-matched male non-concussed controls (mean age = 50.8 ± 10.0 years), recruited from the general population. Athletes played for one or more seasons with the CFL. Informed consent was obtained, and the study was approved by the University Health Network research ethics board.
Age-matched male controls from the Cambridge Centre for Aging and Neuroscience (Cam-CAN, N = 321, mean age of 58.1 ± 16.0 years, range 30–85) were used for validation due to the small size of the local healthy control group. Data were obtained from the Cam-CAN repository (available at http://www.mrc-cbu.cam.ac.uk/datasets/camcan/) (Shafto et al., 2014; Taylor et al., 2017).
The median number of self-reported concussions in the ex-CFL group was 4 (Table 1). Exclusion criteria included: neurological disorders prior to concussions (e.g. seizure disorder), systemic illnesses known to affect the brain (e.g. diabetes and lupus), a history of psychotic disorder, known developmental disorders, and history of migraines. Similar criteria were used for study and Cam-CAN controls (Taylor et al., 2017). Concussion exposure was based on players' recall of injury during a semi-structured interview in accordance with the Zurich Guidelines on Concussions (McCrory et al., 2013). Absence of concussions in the control group was verified through interview with control subjects.
Table 1.
Subject demographics.
| Ex-CFL (n = 53) | Study controls (n = 25) | Cam-CAN (n = 321) | p (Ex-CFL vs study controls) | |
|---|---|---|---|---|
| Age (mean ± SD) | 55.6 ± 12.9 | 50.8 ± 10.0 | 58.1 ± 16.0 | 0.112+ |
| Education (years, mean ± SD) | 16.0 ± 1.7 | 16.0 ± 1.9 | – | 0.926 |
| Vascular risk factorsa | 20/53 (38%) | 4/24 (17%) | – | 0.110 |
| No. concussions (median, IQR) | 4.0, 3.0–8.5 | 0 | – | – |
| No. years in CFL (median, IQR) | 9.0, 5.0–11.0 | – | – | – |
| RAVLT short delay (mean score ± SD) | 8.8 ± 3.6 | 9.2 ± 2.2 | – | 0.497 |
| RAVLT long delay (mean score ± SD) | 8.1 ± 3.9 | 8.9 ± 2.8 | – | 0.298 |
| RVDLT long delay (mean score ± SD) | 9.2 ± 3.6 | 9.2 ± 2.4 | – | 0.977 |
| PAI aggression (T-score ± SD) | 49.9 ± 10.0 | 43.5 ± 6.2 | – | 0.001 |
| PAI irritability (T-score ± SD) | 49.3 ± 9.4 | 43.7 ± 8.3 | – | 0.014 |
| PAI depression (T-score ± SD) | 48.9 ± 10.4 | 44.7 ± 9.7 | – | 0.098 |
| APOE-e4 allele frequency | 21.0% | 19.0% | – | – |
| Genotype | ||||
| e2/e2, e2/e3, e3/e3 | 29 (60.4%) | 15 (62.5%) | – | 0.864 |
| e2/e4, e3/e4 | 18 (37.5%) | 9 (37.5%) | – | 1.000 |
| e4/e4 | 1 (2.1%) | 0 (0%) | – | 1.000 |
| Right hippocampal volume (mean CC ± SD) | 4.3 ± 0.8 | 4.5 ± 0.5 | 4.1 ± 0.6 | 0.238⁎,+ |
| Left hippocampal volume (mean CC ± SD) | 4.3 ± 0.8 | 4.5 ± 0.6 | 4.2 ± 0.6 | 0.129+ |
| Right amygdala volume (mean CC ± SD) | 1.6 ± 0.4 | 1.7 ± 0.2 | – | 0.167 |
| Left amygdala volume (mean CC ± SD) | 1.7 ± 0.4 | 1.8 ± 0.2 | – | 0.375 |
| Ventricle volume (mean CC ± SD) | 34.1 ± 19.8 | 26.7 ± 8.9 | 38.5 ± 21.4 | 0.078+ |
Ex-CFL = retired Canadian Football League players; IQR = interquartile range; RAVLT = Rey Auditory Verbal Learning Test; RVDLT = Rey Visual Design Learning Test; PAI=Personality Assessment Inventory.
Vascular risk factors include hypertension, diabetes, smoking, hypercholesterolemia, and heart disease.
p < 0.05 between Ex-CFL and Cam-CAN.
p < 0.05 between study controls and Cam-CAN.
2.2. Neuropsychological assessment
All participants underwent an extensive neuropsychological test battery comprising a series of cognitive and behavioural assessments. Memory was assessed by the Rey Auditory Verbal Learning Test (RAVLT) (Rey, 1964), which is a test of verbal learning and memory; and by the Rey Visual Design Learning Test (RVDLT) (Spreen and Strauss, 1991) assessing visual learning and memory. For the RAVLT, participants were asked to repeat 15 unrelated words over five consecutive trials, after which an interference list is presented and recalled. Subjects are then asked to recall the original list after this short delay, and again after a 20-minute long delay. The number of words recalled after the short and after the long delay were the primary behavioural outcome measures. For the RVDLT, participants were presented with 15 stimulus cards with geometric design (over five consecutive trials), and asked to draw all designs they could recall after each trial. Twenty minutes after completing the final trial, participants were asked to redraw as many of the 15 designs they could recall. The number of accurately drawn figures was the primary behavioural outcome measure.
Symptoms measured by the Personality Assessment Inventory (PAI) (Morey, 1991) were included for analysis, correcting for age. The PAI was chosen to measure personality changes frequently associated with concussion. Aggression and irritability were chosen for analysis as the PAI symptoms most relevant to concussion. The PAI is a comprehensive and informative self-report questionnaire of adult personality and psychopathology, and contains 344 items scored on a 4-point scale: F = false, ST = slightly true; MT = mainly true; VT = very true. This assessment contains 22 full scales (four validity scales, 11 clinical scales, five treatment scales, and two interpersonal scales) with 10 of these scales further subdivided into 31 conceptually derived subscales. T-scores are based on a census matched standardization sample of 1000 normal adults.
2.3. Neuroimaging
2.3.1. Image acquisition
Participants underwent a whole-brain scan using a T1-weighted inversion recovery prepped, 3-dimensional IR-FSPGR (inversion fast spoiled gradient echo) sequence at 3 Tesla (GE Signa HDx, Milwaukee, WI, USA) with the following parameters: 180 axial slices, 1 × 1 × 1-mm voxels, 256 × 256 matrix size, 25.6-cm field of view, flip angle = 158°, echo time = 3 ms, repetition time = 7.8 ms, inversion time = 450 ms.
Cam-CAN participants underwent T1-weighted MPRAGE (magnetization prepared rapid acquisition gradient echo) sequence at 3 Tesla (Siemens TIM Trio scanner with a 32-channel head coil) with the following acquisition parameters: 1 × 1 × 1-mm voxels, field of view = 256 × 240 × 192, flip angle = 9°, echo time = 2.99 ms, repetition time = 2250 ms, inversion time = 900 ms.
2.3.2. Pre-processing
T1-weighted scans of the subjects were pre-processed through our standard pipeline. Image denoising (Coupe et al., 2008), intensity non-uniformity correction (Sled et al., 1998), and image intensity normalization into range (0−100) using histogram matching were performed.
2.3.3. Deformation-based morphometry
DBM analysis was performed using MNI MINC tools. Pre-processed images were first linearly (using a 9-parameter rigid registration) (Collins et al., 1994) and then non-linearly warped (Collins et al., 1995) to an average template brain of 152 healthy young individuals (MNI-ICBM-152). The local deformation obtained from the non-linear transformations was used as a measure of tissue expansion or atrophy. DBM was used to examine the relationship between brain volume and performance on a memory task. Voxel-wise deformation maps were correlated with RAVLT short delay scores and corrected for multiple comparisons using False Discovery Rate (FDR), thresholded at q = 0.05.
2.3.4. Analysis of subcortical structures
All images were first linearly (using a 9-parameter rigid registration) and then nonlinearly registered to an average template (MNI ICBM152) as part of the ANIMAL software (Collins and Evans, 1997; Collins and Pruessner, 2010). The deep structures, i.e., thalami, ventricles, putamen, and caudate, were segmented as part of the validated ANIMAL software by warping segmentations from ICBM152 back to each subject using the obtained nonlinear transformations. The hippocampi and amygdala were segmented using a validated automated patch-based label-fusion technique (Collins and Pruessner, 2010). The method selects the most similar templates from a library of labelled MRI template images, and combines them with majority voting scheme to assign the highest weighted label to a given voxel to generate a discrete segmentation. Quality control was performed on the individual registered images as well as the automated structure segmentations by visual inspection. The volumes of the structures were then calculated from the segmentations in the ICBM152 space, i.e. the values were scaled by a scaling factor inversely proportional to the intracranial volume to account for differences in head sizes.
2.4. APOE genotyping
For all study participants, genomic DNA was extracted from whole blood using Qiagen kits. The two single nucleotide polymorphisms in APOE (rs7412 and rs429358) defining the APOE-e2, -e3 and -e4 alleles were genotyped as previously described (Saunders et al., 1993).
2.5. Statistical analysis
Statistical analyses were performed using MATLAB R2015b software (MATLAB, Natick, MA, USA). The relationship between hippocampal volume and age was examined by multiple linear regression. Partial correlations (two-tailed) were calculated between hippocampal, amygdala and ventricular volumes and years playing football, correcting for education, and between these volumes and RAVLT short and long delay scores and RVDLT long delay scores, correcting for age and education. A Student's t-test for independent samples was used to compare age, education, RAVLT and RVDLT scores, PAI scores for aggression, irritability and depression symptoms, and hippocampal, amygdala and ventricular volume between the ex-CFL players and the control group. Chi-square or Fisher's exact test were used to compare APOE genotype and vascular risk factors, between ex-CFL players and controls. Pearson correlations (two-tailed) were also calculated between behavioural symptoms (aggression and irritability) and amygdala volume, correcting for age. All images were linearly transformed into the same space before analysis, thus accounting for head size. The significance level for all analyses was set at p < 0.05 (two-tailed). Bonferroni correction for multiple comparisons was performed.
2.6. Linear regression model
Linear regression was used to measure the relationship between age and right and left hippocampal volumes in ex-CFL, study controls and control participants from the Cam-CAN database. Different intercepts and interactions with age were allowed for each cohort. The linear regression model was:
where “:” indicates an interaction between the two terms, H is the volume of the hippocampi (left or right hippocampus), β0 is the intercept term for the Cam-CAN cohort, β1 is the additional intercept for the study controls cohort, and β2 is the additional intercept for the ex-CFL cohort. Similarly, β3 is the linear slope for age for Cam-CAN cohort, and β4 and β5 are the additional slopes for study controls and ex-CFL cohort, respectively. A negative β value indicates a relatively steeper slope (added to the negative slope for age estimated from the Cam-CAN cohort) for the respective cohort. Similarly, a positive β value indicates a less steep slope in comparison with the Cam-CAN cohort. The estimated parameters of the model are reported in Table 3. Table 3 reports the linear regression model estimates after z-scoring all variables to enable comparisons between estimates of each variable examined. Results have been plotted without z-scoring variables to show actual data points for each subject for ease of interpretability.
Table 3.
Linear regression for left and right hippocampal volume with age in ex-CFL players, study controls and Cam-CAN controls, where “:” indicates an interaction between the two terms.
| Variable | Left hippocampus |
Right hippocampus |
||||
|---|---|---|---|---|---|---|
| β | 95% CI | p | β | 95% CI | p | |
| InterceptCam-CAN controls | −0.046 | −0.14 to 0.05 | 0.350 | −0.077 | −0.17 to 0.02 | 0.112 |
| Cohortstudy controls | 0.412 | 0.01 to 0.82 | 0.047 | 0.603 | 0.21 to 1.00 | 0.003 |
| Cohortex-CFL players | 0.119 | −0.13 to 0.37 | 0.347 | 0.213 | −0.03 to 0.46 | 0.084 |
| AgeCam-CAN controls | −0.484 | −0.58 to −0.39 | <0.001 | −0.479 | −0.57 to −0.39 | <0.001 |
| Age: Cohortstudy controls | 0.169 | −0.35 to 0.69 | 0.528 | 0.078 | −0.43 to 0.59 | 0.765 |
| Age: Cohortex-CFL players | −0.351 | −0.64 to −0.06 | 0.017 | −0.431 | −0.71 to −0.15 | 0.003 |
Ex-CFL = retired Canadian Football League players.
3. Results
Subject demographics are listed in Table 1. There were no significant differences between athletes and controls in age, education, or memory score. There was also no difference in the proportion of APOE-e4 allele carriers between the groups. Number of years playing professional football was significantly related to smaller left and right hippocampus and left and right amygdala volumes, but not with ventricular volume (Table 2). After Bonferroni correction for multiple comparisons, only correlations with the left and right amygdala remained significant (p < 0.01). There was no correlation between career years and self-reported number of concussions (r = −0.01, p = 0.93).
Table 2.
Correlation coefficients for years playing professional football in ex-CFL players.
| Region | Years played in CFL |
|
|---|---|---|
| r | p | |
| Left hippocampus | −0.262 | 0.030 |
| Right hippocampus | −0.326 | 0.009 |
| Left amygdala | −0.395 | 0.002 |
| Right amygdala | −0.373 | 0.003 |
| Ventricle | 0.181 | 0.901 |
CFL = retired Canadian Football League.
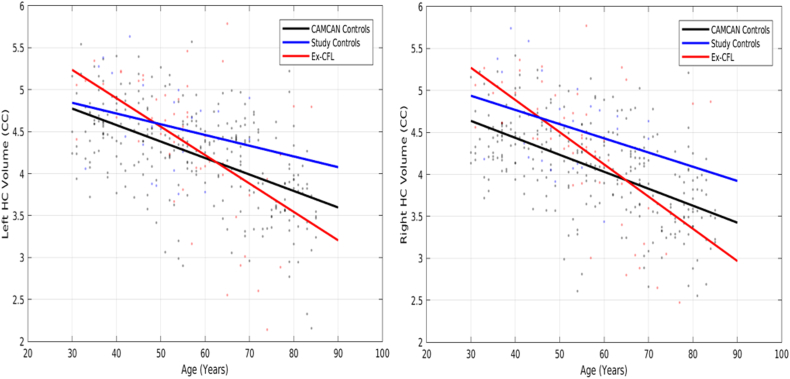
Linear regression showed a statistically significant association between age and the volumes of both the left hippocampus and the right hippocampus in Cam-CAN controls. This association was significantly different (i.e. steeper slope for both the left hippocampus and the right hippocampus) in the ex-CFL players, but not for study controls (Table 3; Fig. 1).
Fig. 1.
Linear regression model results showing the relationship between left and right hippocampal volumes and age in ex-CFL players, study controls and Cam-CAN controls. Modelling of age and left and right hippocampal volumes show a much steeper effect of age on hippocampal volumes in the former CFL players compared to both study controls and Cam-CAN controls. Dots represent the data points for each subject, and lines represent the linear regression model estimates for these data points.
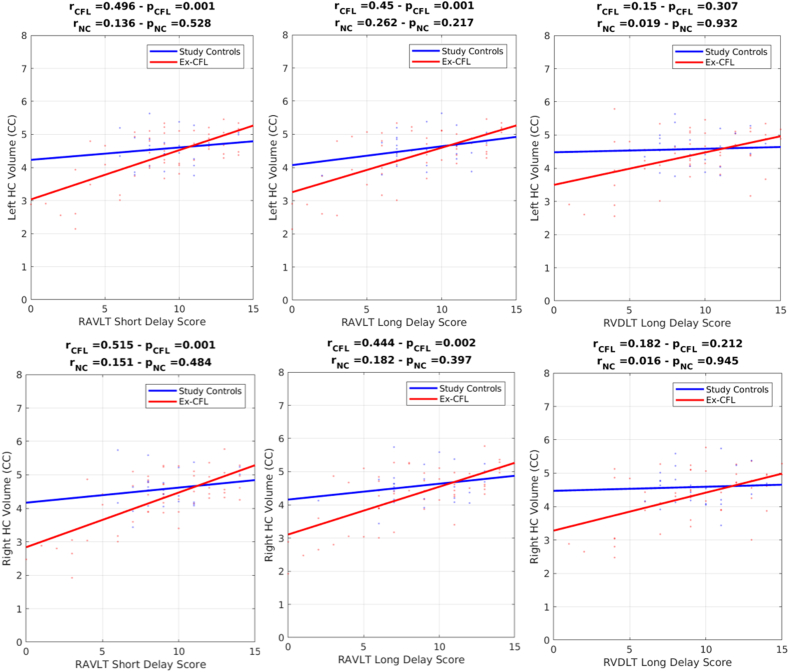
To assess whether hippocampal volume was associated with memory function, we investigated the relationship between left hippocampal volume and performance on the RAVLT (verbal memory task) and right hippocampal volume and RVDLT (visual memory task). The ex-CFL group, but not the study control group, showed a significant relationship between smaller left hippocampal volume and poorer word recall performance on the RAVLT short delay (r = 0.496, p = 0.001) and RAVLT long delay scores (r = 0.447, p = 0.001), and between smaller right hippocampal volume and poorer word recall performance on the RAVLT short delay (r = 0.515, p = 0.001) and RAVLT long delay scores (r = 0.444, p = 0.002) (Fig. 2). These correlations remained significant after Bonferroni correction (p < 0.016). There were no significant correlations between RVDLT long delay scores and right hippocampal volume in either the ex-CFL or control groups (Fig. 2). There were no significant differences between ex-CFL players and normal controls on RAVLT short delay scores (p = 0.497) long delay scores (p = 0.298), or RVDLT long delay scores (p = 0.977) (Table 1).
Fig. 2.
Pearson correlation graphs in ex-CFL and controls between left and right hippocampal volumes and the RAVLT short delay total score, the RAVLT long delay total score and the RVDLT long delay total score. Significant relationships were found between left and right hippocampal (HC) volumes and RAVLT short and long delay scores in the ex-CFL but not in the study controls.
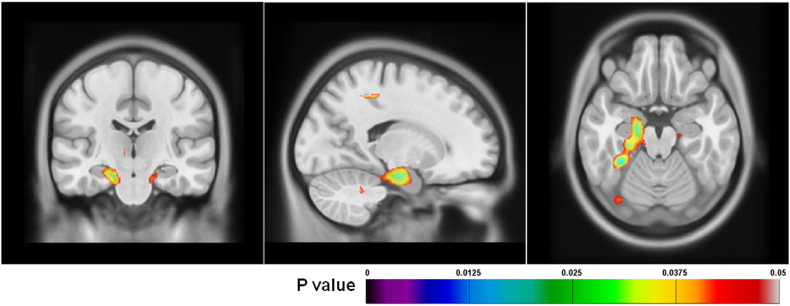
DBM analysis was used to identify which areas across the brain may be associated with memory performance. Correlations with DBM maps also showed a significant relationship with RAVLT short delay in the ex-CFL players (Fig. 3), where poorer scores on recall after a short delay were associated with smaller left and right hippocampal regions, after FDR correction (r = 0.552, p = 0.026; r = 0.492, p = 0.041, respectively). There were no regions significantly associated with RAVLT short delay scores in the control group.
Fig. 3.
Deformation-based morphometry showing regions associated with RAVLT short delay scores in ex-CFL players. FDR (q = 0.05) corrected p value map for regions associated with RAVLT short delay scores in ex-CFL players. Colours represent p-values and indicate the left medial temporal lobe, including primarily the hippocampus, parahippocampus, and entorhinal cortex.
The relationship between amygdala volume and aggression and irritability personality traits from the PAI were also examined in ex-CFL players. Left and right amygdala volumes were not significantly associated with T-scores for aggression (r = 0.162, p = 0.255; r = 0.206, p = 0.147, respectively) and irritability (r = 0.204, p = 0.150; r = 0.192, p = 0.177, respectively) measured by the PAI. Ex-CFL showed higher T-scores on the PAI for aggression (p = 0.001) and irritability (p = 0.014) in this study compared to control subjects.
4. Discussion
Using DBM and volumetric analyses, this study examined the career of CFL athletes as a proxy for repeated concussions on regional brain volumes, cognitive performance and behavioural symptoms among former professional football players compared to healthy controls. Volumetric analysis demonstrated a significantly greater effect of age on brain volume reduction among retired professional football players compared with healthy controls. More specifically, the association of age with hippocampal volume reduction was significantly greater in the ex-CFL cohort compared to the study controls and Cam-CAN control subjects. Our findings confirm an association of advanced age with decreased brain volume, and this appears to be amplified in the ex-CFL group. Moreover, smaller left hippocampal volume was associated with worse performance on the short and long delay verbal memory scores of the RAVLT among ex-CFL. In the ex-CFL players, DBM analysis also showed smaller bilateral hippocampal volume associated with poorer verbal memory performance. Although ex-CFL scored significantly higher than controls for symptoms of irritability and aggression on the PAI compared to controls, they were not in an elevated range (Morey, 1991). We did not find any relationship between PAI irritability and aggression scores and amygdala volume in our ex-CFL or control groups. This study is novel in using structural and DBM analyses that reveal exaggerated hippocampal volume loss and memory impairment in former professional athletes with multiple concussions.
Number of career years playing football with the CFL was associated with smaller hippocampi and amygdala volumes and larger ventricular volume in the ex-CFL. Career years were examined as a proxy for number of concussions because concussion history was self-reported by athletes and therefore a less reliable measure as a result of recall bias. Greater years playing professional football increases exposure to concussions and may contribute to the effect of age on brain volume observed in our ex-CFL group. In addition, many concussions may go unrecognized by athletes and/or their coaches as symptoms generally resolve on their own (Daneshvar et al., 2011). In our study, the four players reporting no concussions each played between 9 and 12 years in the CFL. It is possible that professional players of contact sports will experience hits to the head but may not report having a concussion, especially if they do not experience persistent post-concussive symptoms. As well, since concussion can be associated with both anterograde and retrograde amnesia, the players may have forgotten them (Cantu, 2001). The absence of correlation between career years and self-reported number of concussions may be because this study only assessed professional career years and did not capture the total years that each athlete may have played football or other sport (i.e. since childhood). We also do not have a measure of subconcussive blows. It is possible that other factors, including lifestyle, may contribute to the relationship between career years and hippocampal volume loss and these should be considered in future studies.
Previous studies have found evidence of cerebral atrophy in individuals with mild, moderate and severe TBI (Levine et al., 2008; MacKenzie et al., 2002). In particular, ventricular enlargement and volume loss of the hippocampi and thalami have been reported in studies of mild, moderate and severe TBI (Bigler et al., 1997; Wilde et al., 2006). Due to its location in the middle cranial fossa, the hippocampus is situated in a region vulnerable to injury (Shenton et al., 2012), and hippocampal atrophy following mild, moderate and severe TBI has been reported in both animal (Hicks et al., 1993; Dempsey and Raghavendra Rao, 2003) and human studies (Maxwell et al., 2003; Singh et al., 2014). The current study suggests that the effect of age on hippocampal volume loss is greater in those who have experienced repeated concussions. Concussion severity may also be an important determinant of hippocampal atrophy (Strain et al., 2015; Zagorchev et al., 2016). In a group of retired National Football League players, older retired athletes with history of concussion with loss of consciousness had smaller hippocampal volumes than control subjects and smaller right hippocampal volume than concussed athletes who did not experience loss of consciousness (Strain et al., 2015). Concussion severity has also been associated with late-life cognitive impairment (Guskiewicz et al., 2005). Moreover, hippocampal atrophy is associated with memory impairment in normal aging as well as in various neurodegenerative diseases including Alzheimer's disease and frontotemporal lobar degeneration. As would be expected, we found a relationship between smaller bilateral hippocampal volume and poorer performance on both early and delayed recall of a verbal memory task in ex-CFL players, and this relationship was greater in ex-CFL compared to study controls.
APOE protein is involved in lipid metabolism; and the APOE-e4 allele increases risk of Alzheimer's disease in a dose-dependent fashion (by three times for heterozygotes), whereas the APOE-e2 allele confers a protective benefit (Saunders et al., 1993). Some studies have suggested that APOE-e4 allele may be also associated with less efficient cognitive processing in concussed athletes (Merritt et al., 2017). However, in the current study, we did not find a significant difference between ex-CFL and study control subjects in APOE genotype or allele frequency.
The presence of vascular risk factors may also contribute to hippocampal atrophy, structural brain aging and cognitive decline (Debette et al., 2011). We examined hypertension, diabetes, smoking, hypercholesterolemia, and heart disease in ex-CFL subjects and study controls but did not find a significant difference between groups in the number of subjects with one or more of these vascular risk factors (p = 0.110).
Irritability and aggression are often reported in cases of CTE (McKee et al., 2009), and their relationship to amygdala volume was examined in this study. Significantly higher T-scores for both symptoms were found in ex-CFL compared to controls, but they were within the normal range in both groups. The difference in scores may be due to pre-existing character traits for individuals who choose a career in football. We did not find a significant association between amygdala volume and PAI T-scores for aggression and irritability. Increased risk of depression has also been associated with multiple concussions (Guskiewicz et al., 2007). We did not find a significant difference in our study between ex-CFL and control group on PAI T-scores for depression (p = 0.098), and both groups had scores within the normal range. Nevertheless, clinical assessment of depression may be better able to detect this symptom compared to this self-report measure.
At present, few studies have looked at regional cerebral atrophy among retired athletes with a history of multiple concussions. Results from these studies have been mixed, with some detecting no significant abnormalities in concussed athletes (Ilvesmaki et al., 2014). Most studies also include participants with a range of mild to severe TBI with few studies having looked at structural brain changes in concussion alone. Here, we show differences in brain volume in a group of athletes who were exposed to repetitive head impacts and a history of multiple concussions only.
The current study uses DBM to explore cross-sectional changes in brain volume that may result from repeated exposure to concussive head injury. DBM is a whole brain analysis that allows the examination of macroscopic differences across the entire brain by comparing the position of each voxel to a standard brain and so may be more sensitive to subtle volume changes (Ashburner et al., 1998; Gaser et al., 2001). Studies have examined the trajectory of grey matter atrophy with age using both linear and nonlinear models with mixed results and variation among structures (Fjell et al., 2013). Further work is needed to better understand the effect of age on atrophy in healthy and concussed populations.
Our study is limited in its ability to detect causal and temporal relationships in regional cerebral atrophy over time due to its cross-sectional design. Future studies should include longitudinal designs that can better assess causal relationships. In addition, the number of concussions was self-reported by ex-CFL players and these numbers are therefore subject to response and recall bias. Ex-CFL players were also self-referred and it is possible that those with specific neurobehavioural symptoms are more likely to choose to participate in this study. Participants in the current study were restricted to male professional football players and our findings may not apply to other groups including females and non-athletes. Age since retirement may also be a confounder in our analysis, however it would be difficult to de-correlate with age. Other confounders including family history of dementia, alcohol and substance abuse, and use of performance enhancing drugs were not included in our analysis but may be important modulators of the cross-sectional age-related changes in volume observed in this study, and future work should assess the potential contribution of these confounders.
This study reports exaggerated hippocampal volume loss and memory impairment in former professional athletes who report only concussions and no moderate or severe traumatic brain injury (TBI). We have demonstrated greater age effects on hippocampal volume in ex-CFL players. Moreover, we showed that smaller hippocampal volume is associated with the number of career years playing for the CFL. Taken together, these findings suggest that multiple concussions may contribute to pathology associated with greater age-related atrophy and result in earlier focal atrophy, although longitudinal studies are needed to determine the essence of this relationship. Future studies that track structural, cognitive and behavioural progression over time can provide additional insight into the effects of concussions on brain structure and function.
Acknowledgments
Acknowledgements
This study was funded by the Physicians' Services Incorporated Foundation and the Toronto General and Western Hospital Foundation through specific donations to the Canadian Concussion Centre. We thank Leo Ezerins of the CFLAA for his help in recruiting participants. Data collection and sharing for this project was provided by the Cambridge Centre for Aging and Neuroscience (CamCAN). CamCAN funding was provided by the UK Biotechnology and Biological Sciences Research Council (grant number BB/H008217/1), together with support from the UK Medical Research Council and University of Cambridge, UK. KM funded by CIHR. DLC received a grant from the André Carron Family. DLC and MCT contributed to study concept and design. KM, MD, AT, MWH, MKA, AE, NM, MK, R Goswami, RW, CT, R Green, BC, KD, MG, CS, ER, DM, and MCT contributed to data acquisition and analysis. KM, MD and MCT contributed to preparing the manuscript. We thank all the participants for the generous contribution of their time.
Competing interests
There are no competing interests to report.
References
- Abner E.L., Nelson P.T., Schmitt F.A., Browning S.R., Fardo D.W., Wan L., Jicha G.A., Cooper G.E., Smith C.D., Caban-Holt A.M., Van Eldik L.J., Kryscio R.J. Self-reported head injury and risk of late-life impairment and AD pathology in an AD center cohort. Dement. Geriatr. Cogn. Disord. 2014;37(5–6):294–306. doi: 10.1159/000355478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J., Hutton C., Frackowiak R., Johnsrude I., Price C., Friston K. Identifying global anatomical differences: deformation-based morphometry. Hum. Brain Mapp. 1998;6(5–6):348–357. doi: 10.1002/(SICI)1097-0193(1998)6:5/6<348::AID-HBM4>3.0.CO;2-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigler E.D., Blatter D.D., Anderson C.V., Johnson S.C., Gale S.D., Hopkins R.O., Burnett B. Hippocampal volume in normal aging and traumatic brain injury. AJNR Am. J. Neuroradiol. 1997;18(1):11–23. [PMC free article] [PubMed] [Google Scholar]
- Cantu R.C. Posttraumatic retrograde and anterograde amnesia: pathophysiology and implications in grading and safe return to play. J. Athl. Train. 2001;36(3):244–248. [PMC free article] [PubMed] [Google Scholar]
- Cole J.H., Leech R., Sharp D.J., Alzheimer's Disease Neuroimaging Initiative Prediction of brain age suggests accelerated atrophy after traumatic brain injury. Ann. Neurol. 2015;77(4):571–581. doi: 10.1002/ana.24367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins D.L., Evans A.C. Animal: validation and applications of nonlinear registration-based segmentation. Int. J. Pattern Recognit. Artif. Intell. 1997;11:1271. [Google Scholar]
- Collins D.L., Pruessner J.C. Towards accurate, automatic segmentation of the hippocampus and amygdala from MRI by augmenting ANIMAL with a template library and label fusion. NeuroImage. 2010;52(4):1355–1366. doi: 10.1016/j.neuroimage.2010.04.193. [DOI] [PubMed] [Google Scholar]
- Collins D.L., Neelin P., Peters T.M., Evans A.C. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J. Comput. Assist. Tomogr. 1994;18(2):192–205. [PubMed] [Google Scholar]
- Collins D.L., Evans A.C., Holmes C., Peters T.M. Automatic 3D segmentation of neuro-anatomical structures from MRI. Comp. Image Vis. 1995;3:139–152. [Google Scholar]
- Corsellis J.A., Bruton C.J., Freeman-Browne D. The aftermath of boxing. Psychol. Med. 1973;3:270–303. doi: 10.1017/s0033291700049588. [DOI] [PubMed] [Google Scholar]
- Coupe P., Yger P., Prima S., Hellier P., Kervrann C., Barillot C. An optimized blockwise nonlocal means denoising filter for 3-D magnetic resonance images. IEEE Trans. Med. Imaging. 2008;27(4):425–441. doi: 10.1109/TMI.2007.906087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneshvar D.H., Nowinski C.J., McKee A., Cantu R.C. The epidemiology of sport-related concussion. Clin. Sports Med. 2011;30(1):1–17. doi: 10.1016/j.csm.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debette S., Seshadri S., Beiser A., Au R., Himali J.J., Palumbo C., Wolf P.A., DeCarli C. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77(5):461–468. doi: 10.1212/WNL.0b013e318227b227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey R.J., Raghavendra Rao V.L. Cytidinediphosphocholine treatment to decrease traumatic brain injury-induced hippocampal neuronal death, cortical contusion volume, and neurological dysfunction in rats. J. Neurosurg. 2003;98(4):867–873. doi: 10.3171/jns.2003.98.4.0867. [DOI] [PubMed] [Google Scholar]
- Fjell A.M., Westlye L.T., Grydeland H., Amlien I., Espeseth T., Reinvang I., Raz N., Holland D., Dale A.M., Wahlhovd K.B., Alzheimer Disease Neuroimaging Initiative Critical ages in the life course of the adult brain: nonlinear subcortical aging. Neurobiol. Aging. 2013;34(10):2239–2247. doi: 10.1016/j.neurobiolaging.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisk V., Milner B. The role of the left hippocampal region in the acquisition and retention of story content. Neuropsychologia. 1990;28:349–359. doi: 10.1016/0028-3932(90)90061-r. [DOI] [PubMed] [Google Scholar]
- Gaser C., Nenadic I., Buchsbaum B.R., Hazlett E.A., Buchsbaum M.S. Deformation-based morphometry and its relation to conventional volumetry of brain lateral ventricles in MRI. NeuroImage. 2001;13(6 Pt 1):1140–1145. doi: 10.1006/nimg.2001.0771. [DOI] [PubMed] [Google Scholar]
- Goswami R., Dufort P., Tartaglia M.C., Green R.E., Crawley A., Tator C.H., Wennberg R., Mikulis D.J., Keightley M., Davis K.D. Frontotemporal correlates of impulsivity and machine learning in retired professional athletes with a history of multiple concussions. Brain Struct. Funct. 2016;221:1911–1925. doi: 10.1007/s00429-015-1012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R.E., Colella B., Maller J.J., Bayley M., Glazer J., Mikulis D.J. Scale and pattern of atrophy in the chronic stages of moderate-severe TBI. Front. Hum. Neurosci. 2014;8 doi: 10.3389/fnhum.2014.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guskiewicz K.M., Marshall S.W., Bailes J., McCrea M., Cantu R.C., Randolph C., Jordan B.D. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery. 2005;57(4):719–726. doi: 10.1093/neurosurgery/57.4.719. [DOI] [PubMed] [Google Scholar]
- Guskiewicz K.M., Marshall S.W., Bailes J., McCrea M., Harding H.P., Jr., Matthews A., Mihalik J.R., Cantu R.C. Recurrent concussion and risk of depression in retired professional football players. Med. Sci. Sports Exerc. 2007;39(6):903–909. doi: 10.1249/mss.0b013e3180383da5. [DOI] [PubMed] [Google Scholar]
- Hicks R.R., Smith D.H., Lowenstein D.H., Saint Marie R., McIntosh T.K. Mild experimental brain injury in the rat induces cognitive deficits associated with regional neuronal loss in the hippocampus. J. Neurotrauma. 1993;10(4):405–414. doi: 10.1089/neu.1993.10.405. [DOI] [PubMed] [Google Scholar]
- Hiploylee C., Dufort P.A., Davis H.S., Wennberg R.A., Tartaglia M.C., Mikulis D., Hazrati L.N., Tator C.H. Longitudinal study of postconcussion syndrome: not everyone recovers. J. Neurotrauma. 2017;34(8):1511–1523. doi: 10.1089/neu.2016.4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilvesmaki T., Luoto T.M., Hakulinen U., Brander A., Ryymin P., Eskola H., Iverson G.L., Ohman J. Acute mild traumatic brain injury is not associated with white matter change on diffusion tensor imaging. Brain. 2014;137(Pt 7):1876–1882. doi: 10.1093/brain/awu095. [DOI] [PubMed] [Google Scholar]
- Langlois J.A., Rutland-Brown W., Wald M.M. The epidemiology and impact of traumatic brain injury: a brief overview. J. Head Trauma Rehabil. 1991;21(5):375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- Levine B., Kovacevic N., Nica E.I., Cheung G., Gao F., Schwartz M.L., Black S.E. The Toronto traumatic brain injury study: injury severity and quantified MRI. Neurology. 2008;70(10):771–778. doi: 10.1212/01.wnl.0000304108.32283.aa. [DOI] [PubMed] [Google Scholar]
- MacKenzie J.D., Siddiqi F., Babb J.S., Bagley L.J., Mannon L.J., Sinson G.P., Grossman R.I. Brain atrophy in mild or moderate traumatic brain injury: a longitudinal quantitative analysis. AJNR Am. J. Neuroradiol. 2002;23(9):1509–15.23. [PMC free article] [PubMed] [Google Scholar]
- Malia K., Powell G., Torode S. Personality and psychosocial function after brain injury. Brain Inj. 1995;9(7):697–712. doi: 10.3109/02699059509008226. [DOI] [PubMed] [Google Scholar]
- Matthies S., Rusch N., Weber M., Lieb K., Philipsen A., Tuescher O., Ebert D., Hennig J., van Elst L.T. Small amygdala-high aggression? The role of the amygdala in modulating aggression in healthy subjects. World J. Biol. Psychiatry. 2012;13(1):75–81. doi: 10.3109/15622975.2010.541282. [DOI] [PubMed] [Google Scholar]
- Maxwell W.L., Dhillon K., Harper L., Espin J., MacIntosh T.K., Smith D.H., Graham D.I. There is differential loss of pyramidal cells from the human hippocampus with survival after blunt head injury. J. Neuropathol. Exp. Neurol. 2003;62(3):272–279. doi: 10.1093/jnen/62.3.272. [DOI] [PubMed] [Google Scholar]
- McCrory P., Meeuwisse W., Aubry M., Cantu B., Dvorak J., Echemendia R., Engebretsen L., Johnston K., Kutcher J., Raftery M. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2013. Br. J. Sports Med. 2013;47(5):250–258. doi: 10.1136/bjsports-2013-092313. [DOI] [PubMed] [Google Scholar]
- McKee A.C., Cantu R.C., Nowinski C.J., Hedley-Whyte T., Gavett B.E., Budson A.E., Santini V.E., Lee H.S., Kubilus C.A., Stern R.A. Chronic traumatic encephalopathy in athletes: progressive tauopathy following repetitive head injury. J. Neuropathol. Exp. Neurol. 2009;68(7):709–735. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier T.B., Bergamino M., Bellgowan P.S., Teague T.K., Ling J.M., Jeromin A., Mayer A.R. Longitudinal assessment of white matter abnormalities following sports-related concussion. Hum. Brain Mapp. 2016;37(2):833–845. doi: 10.1002/hbm.23072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt V.C., Rabinowitz A.R., Arnett P.A. The influence of the apolipoprotein E (APOE) gene on subacute post-concussion neurocognitive performance in college athletes. Arch. Clin. Neuropsychol. 2017;24:1–11. doi: 10.1093/arclin/acx051. [DOI] [PubMed] [Google Scholar]
- Morey L.C. Psychological Assessment Resources. 1991. Personality Assessment Inventory professional manual. [Google Scholar]
- Multani N., Goswami R., Khodadadi M., Ebraheem A., Davis K.D., Tator C.H., Wennberg R., Mikulis D.J., Ezerins L., Tartaglia M.C. The association between white-matter tract abnormalities, and neuropsychiatric and cognitive symptoms in retired professional football players with multiple concussions. J. Neurol. 2016;263(7):1332–1341. doi: 10.1007/s00415-016-8141-0. [DOI] [PubMed] [Google Scholar]
- Omalu B.I., DeKosky S.T., Minster R.L., Kamboh M.I., Hamilton R.L., Wecht C.H. Chronic traumatic encephalopathy in a National Football League player. Neurosurgery. 2005;57(1):128–134. doi: 10.1227/01.neu.0000163407.92769.ed. [DOI] [PubMed] [Google Scholar]
- Omalu B.I., DeKosky S.T., Hamilton R.L., Minster R.L., Kamboh M.I., Shakir A.M., Wecht C.H. Chronic traumatic encephalopathy in a national football league player: part II. Neurosurgery. 2006;59(5):1086–1092. doi: 10.1227/01.NEU.0000245601.69451.27. (discussion 92-3) [DOI] [PubMed] [Google Scholar]
- Omalu B.I., Fitzsimmons R.P., Hammers J., Bailes J. Chronic traumatic encephalopathy in a professional American wrestler. J. Forensic Nurs. 2010;6(3):130–136. doi: 10.1111/j.1939-3938.2010.01078.x. [DOI] [PubMed] [Google Scholar]
- Rabinowitz A.R., Levin H.S. Cognitive sequelae of traumatic brain injury. Psychiatr. Clin. N. Am. 2014;37(1):1–11. doi: 10.1016/j.psc.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey A. Presses Universitaires de France; Paris: 1964. L'examen Clinique en Psychologie. [Google Scholar]
- Saunders A.M., Strittmatter W.J., Schmechel D., George-Hyslop P.H., Pericak-Vance M.A., Joo S.H., Rosi B.L., Gusella J.F., Crapper-MacLachlan D.R., Alberts M.J. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer's disease. Neurology. 1993;43(8):1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- Shafto M.A., Tyler L.K., Dixon M., Taylor J.R., Rowe J.B., Cusack R., Calder A.J., Marslen-Wilson W.D., Duncan J., Dalgleish T. The Cambridge Centre for Ageing and Neuroscience (Cam-CAN) study protocol: a cross-sectional, lifespan, multidisciplinary examination of healthy cognitive ageing. BMC Neurol. 2014;14(14):204. doi: 10.1186/s12883-014-0204-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton M.E., Hamoda H.M., Schneiderman J.S., Bouix S., Pasternak O., Rathi Y., Vu M.A., Purohit M.P., Helmer K., Koerte I., Lin A.P., Westin C.F., Kikinis R., Kubicki M., Stern R.A., Zafonte R. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav. 2012;6(2):137–192. doi: 10.1007/s11682-012-9156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R., Meier T.B., Kuplicki R., Savitz J., Mukai I., Cavanagh L., Allen T., Teague T.K., Nerio C., Polanski D. Relationship of collegiate football experience and concussion with hippocampal volume and cognitive outcomes. JAMA. 2014;311(18):1883–1888. doi: 10.1001/jama.2014.3313. [DOI] [PubMed] [Google Scholar]
- Sled J.G., Zijdenbos A.P., Evans A.C. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans. Med. Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Smith M.L., Milner B. The role of the right hippocampus in the recall of spatial location. Neuropsychologia. 1981;19:781–793. doi: 10.1016/0028-3932(81)90090-7. [DOI] [PubMed] [Google Scholar]
- Spreen O., Strauss E. Oxford University Press; New York, Oxford: 1991. A Compendium of Neuropsychological Tests: Administration, Norms and Commentary. [Google Scholar]
- Strain J.F., Womack K.B., Didehbani N., Spence J.S., Conover H., Hart J., Jr., Kraut M.A., Cullum C.M. Imaging correlates of memory and concussion history in retired National Football League Athletes. JAMA Neurol. 2015;72(7):773–780. doi: 10.1001/jamaneurol.2015.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima-Pozo K., Ruiz-Manrique G., Yus M., Arrazola J., Montanes-Rada F. Correlation between amygdala volume and impulsivity in adults with attention-deficit hyperactivity disorder. Acta Neuropsychiatr. 2015;27(6):362–367. doi: 10.1017/neu.2015.34. [DOI] [PubMed] [Google Scholar]
- Taylor J.R., Williams N., Cusack R., Auer T., Shafto M.A., Dixon M., Tyler L.K., Cam-Can, Henson R.N. The Cambridge Centre for Ageing and Neuroscience (Cam-CAN) data repository: structural and functional MRI, MEG, and cognitive data from a cross-sectional adult lifespan sample. NeuroImage. 2017;144(Pt B):262–269. doi: 10.1016/j.neuroimage.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay S., De Beaumont L., Henry L.C., Boulanger Y., Evans A.C., Bourgouin P., Poirier J., Theoret H., Lassonde M. Sports concussions and aging: a neuroimaging investigation. Cereb. Cortex. 2013;23(5):1159–1166. doi: 10.1093/cercor/bhs102. [DOI] [PubMed] [Google Scholar]
- Tremblay S., Beaule V., Proulx S., Tremblay S., Marjanska M., Doyon J., Lassonde M., Theoret H. Multimodal assessment of primary motor cortex integrity following sport concussion in asymptomatic athletes. Clin. Neurophysiol. 2014;125(7):1371–1379. doi: 10.1016/j.clinph.2013.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde E.A., Bigler E.D., Pedroza C., Ryser D.K. Post-traumatic amnesia predicts long-term cerebral atrophy in traumatic brain injury. Brain Inj. 2006;20(7):695–699. doi: 10.1080/02699050600744079. [DOI] [PubMed] [Google Scholar]
- Zagorchev L., Meyer C., Stehle T., Wenzel F., Young S., Peters J., Weese J., Paulsen K., Garlinghouse M., Ford J. Differences in regional brain volumes two months and one year after mild traumatic brain injury. J. Neurotrauma. 2016;33:29–34. doi: 10.1089/neu.2014.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]