Abstract
Background
Epilepsy surgery for focal cortical dysplasia type II (FCD II) offers good chances for seizure freedom, but remains a challenge with respect to lesion detection, defining the epileptogenic zone and the optimal resection strategy. Integrating results from magnetic source imaging from magnetoencephalography (MEG) with magnetic resonance imaging (MRI) including MRI postprocessing may be useful for optimizing these goals.
Methods
We here present data from 21 adult FCD II patients, investigated during a 10 year period and evaluated including magnetic source imaging. 16 patients had epilepsy surgery, i.e. histopathologically verified FCD II, and a long follow up. We present our analysis of epileptogenic zones including MEG in relation to structural data according to MRI data and relate these results to surgical outcomes.
Results
FCD II in our cohort was characterized by high MEG yield and localization accuracy and MEG showed impact on surgical success-rates. MEG source localizations were detected in 95.2% of patients and were as close as 12.3 ± 8,1 mm to the MRI-lesion. After a mean follow up of >3 years, we saw >80% Engel I outcomes, with more favourable outcomes when the MEG source was completely resected (Fishers exact test 0,033).
Conclusion
We argue for a high value of conducting a combined MEG-MRI approach in the presurgical workup and the resection strategy in patients with FCD II related epilepsy.
Keywords: Focal cortical dysplasia; Magnetic source imaging, epilepsy surgery
Highlights
-
•
Focal cortical dysplasia Type II (FCD II) is a highly epileptogenic brain lesion.
-
•
Magnetic source imaging (MSI) is able to image lesion-related epileptogenicity in FCD II.
-
•
MSI detected sources close to the lesion in 95% of patients.
-
•
Seizure outcomes were better if the MEG-marked MAP-lesion was resected.
-
•
Integrating MSI into the diagnostic and therapeutic strategy is useful in FCD II – surgery.
1. Introduction
Focal cortical dysplasia (FCD), as recognized by Taylor, Bruton, Falconer and Corsellis is a unique type of lesion in epilepsy pathology (Taylor et al., 1971, Kasper et al., 2010). Taylor's focal cortical dysplasia, i.e. FCD II A and B according to current histopathological classifications (Blümcke et al., 2011), is characterized by pathognomonic tissue features of dysplastic neurons often accompanied by “balloon cells” (Blümcke et al., 2017, Aronica et al., 2012, Taylor et al., 1971). A large body of evidence has shown that FCD II has an intrinsic epileptogenic potential: epileptic discharges do arise from the lesion itself as shown by lesional samplings with subdural, electrocorticographic and intracerebral recordings (Dubeau et al., 1998, Palmini et al., 1991, Chassoux et al., 2012), single cell electrophysiological techniques (Cepeda et al., 2003), as well as previous data using magnetoencephalography (MEG) (Morioka 1999, Bast 2004, Widjaja et al., 2008, Ishii, 2008, Sueda et al., 2010, Wilenius et al., 2013, Wang et al., 2014). Overlap of irritative zones and seizure onset zones has been reported for FCD in high rates (Bartolomei et al., 2016). Extratemporal epileptogenic zones prevail in FCD II due to predilection for cortical areas outside the temporal lobes (Blümcke et al., 2017, Guerrini et al., 2015, Aronica et al., 2012), predominating in frontal and pericentral localisations (Urbach et al., 2002). Accordingly, seizure semiology often appears complex and surface EEG recordings may not show localized epileptic discharges or ictal patterns at all. Epilepsy surgery offers good chances for FCD II patients (Rössler et al., 2017), but localizing the epileptogenic zone and surgery is a challenge in FCD II (Guerrini et al., 2015, Fauser and Zentner, 2012) since lesions often reside in close relation to eloquent cortex and lesion borders are not exactly defined by MRI (Tassi et al., 2001). Currently, many FCD II lesions are visualized presurgically by optimized magnetic resonance imaging (MRI) (Urbach et al., 2002, Wagner et al., 2011, Martin et al., 2017). However, when subtle and small, then often residing within deep parts of cerebral sulci (Besson et al., 2008), FCD II can escape visual MRI analysis even at experienced referral centers (Bernasconi et al., 2011, Von Oertzen et al., 2002). FCD II likely represents the most frequent cause of “nonlesional” focal epilepsy (Bernasconi et al., 2011, Bonini et al., 2017). Sophisticated MRI postprocessing methods have been developed in order to increase FCD II detection rates after normal MRI readings (Martin et al., 2017, Bernasconi et al., 2011, Wagner et al., 2011, Huppertz et al., 2005, Antel et al., 2003, Bernasconi et al., 2001) including the morphometric analysis program (MAP, Huppertz et al., 2005). Since MRI/MAP results represent pure structural information and may provide false positive findings (Huppertz et al., 2005), supporting concordant evidence from other data including methods depicting epileptogenicity is needed in order to plan an epilepsy-surgical strategy. We here will demonstrate high efficacy and accuracy of magnetoencephalography (MEG) in FCD II and argue for surgically targeting the MEG-MRI-lesion, for this seems to facilitate excellent results.
2. Patients and methods
Patients were selected from the database of the epilepsy surgery program at Erlangen Epilepsy Center, Friedrich-Alexander University Erlangen-Nuremberg, Germany. All patients had been investigated for pharmacoresistant focal epilepsy from 2006 to 2016 in order to determine eligibility for epilepsy surgery. Patients selected for this study had either a histopathological verified diagnosis of FCD II and/or characteristic MRI signs of FCD II. MRI criteria were: focal cortical thickening, grey-white matter blurring, white matter signal increase and/or transmantle sign (Wagner et al., 2011). In addition to comprehensive epilepsy workup (Kral et al., 2002) including non-invasive-video-EEG, MRI imaging and neuropsychological testing, all patients had received a MEG using the same whole-head system (see Methods). A total of 21 patients were included in the study. Selected patients had invasive recordings in advance to resective surgery (n = 14). All surgical patients had a follow up of at least 1 year.
2.1. MRI acquisition and morphometric analysis (MAP)
High resolution MRI imaging had been performed using a 3 T Magnetom Trio (Siemens Medical Solutions, Erlangen Germany) with a 32 channel head coil. Sequences included the following: 1) FLAIR (3D-FLAIR, 1 × 1 × 1 cm; TR 4000, TE 388, matrix 258 × 256), 2) T1 magnetization prepared rapid gradient echo (MPRAGE, 1 × 1 × 1cm, TR 2300, TE 2.98, matrix 256 × 256). 3D MPRAGE sequences were used to perform morphometric analysis using the MAP software (morphometric analysis program) by Huppertz et al. (2005). The software compares an individual patient's structural MRI to a scanner-specific database of normal controls. Distribution of grey and white matter is analyzed voxel-wise and yields maps of selected feature parameters of cortical thickness and grey-white boundary sharpness highlighting typical signs of FCD (Huppertz et al., 2005). All feature maps are also combined into a “combined z-score” map. Only these combined-z-score maps were used for statistical evaluation in the presented study. Anatomical lesion localizations were rated visually and categorized according to Wang (Wang et al., 2014). We depicted the FCD by using MAP at a z-score threshold > 3 in order to calculate distances between the MEG source localization (see below) and the FCD. Identification of the real FCD was validated based on postsurgical diagnosis and the synopsis of all available findings (i.e. video-EEG, semiology, imaging).
2.2. MEG acquisition and analysis
MEG was acquired using a whole-head 248 channel magnetometer system for patient investigations after 2010 (Magnes 3600WH, 4D-Neuroimaging, San Diego, CA, USA) and a two sensor gradiometer system with 2 × 37 channels before (Magnes II, 4D-Neuroimaging). Duration of recordings varied depending on the clinical needs and respective investigation, e.g. for functional mapping and/or epileptic focus localization. Recordings were conducted in supine position. Patients were asked to keep their eyes closed. If they fell asleep, they were not woken up before of the end of the complete recording. With the Magnes 3600WH system, data were acquired using a sample rate of 508 Hz and an analogue 0.1 Hz high pass filter. Noise reduction was performed offline, taking reference gradiometers and magnetometers into account (manufacturer's software). Recordings with the Magnes II system utilized a sample rate of 520 Hz, an analogue 1 Hz high pass filter and no offline noise reduction. For analysis, an additional digital 1–70 Hz and a 50 Hz notch filter were applied. Epileptic discharges were manually identified and selected by an experience reviewer (SR). Subsequently, all identified patterns were averaged and submitted to source analysis utilizing single moving dipole localization in spherical volume conductors (Curry 7, Compumedics Neuroscan, Singen, Germany). All patients only presented with a single focal type of spike, i.e. a subclassification of spikes into groups with similar topography and morphology was not necessary. Channels for analysis were restricted to 37 channels centered on the steepest gradient at the spike peak using data from Magnes 3600WH. Dipole localization methodology with both systems was thus comparable. This approach, suggested by the system manufacturer and also used in other studies (Stefan et al., 2003) removes channels distant from the maximal gradient, which contain mostly unrelated data, thus improving the appropriateness of a single dipole model for source analysis. Only resulting dipoles with deviation between measured and modelled field of <30% were analyzed further. The single best dipole per patient in terms of minimal deviation was superimposed on individual 3D MP-RAGE MRI, and MAP. If two dipoles resulted in equal deviation, the earlier was used.
2.3. Analysis of spatial relationship between MEG-source, MAP-lesion and postOP-situs
Both MEG and MAP utilize the same 3D MP-RAGE dataset as part of their respective workflow for visualization of MEG localizations and as a basis for computation of statistical MAP maps. Standard coregistration procedures for MEG using fiducials at the nasion and the left and right preauricular point were applied. MEG results were displayed on MAP maps using Curry 7 software. Concordance of MEG results and MAP findings were compared on a sublobar level using a classification scheme according to Wang et al. (2014) and Knowlton (2006), which included the following regions: frontopolar, dorsolateral frontal (superior), dorsolateral frontal (inferior), mesial frontal, anteroparietal, posteroparietal (superior), posteroparietal (inferior), mesial parietal, lateral occipital, mesial occipital, temporopolar, lateral temporal, mesial (neocortical) temporal, anterior insular, posterior insular, anterior cingulate, posterior cingulate and central area (see Table 1, Table 2). In patients with epilepsy surgery, the relation of MEG localizations, MAP finding and resection cavity was evaluated using postoperative MR images, which were acquired either immediately after the resection using intraoperative 1.5 T MRI (Sommer et al., 2013) or 3 T MRI at 6 months after surgery. Patient 15 had to be excluded from this analysis, since although undergoing epilepsy surgery, postoperative MRIs were not available. The degree of resection was evaluated visually: The MEG localization was considered to be resected completely, if it was located within the resection volume. It was classified as partially resected if the localization was located at the border of the resection (within 2 cm) and not resected if otherwise. Resection extent of MAP findings was related to MAP above the significance threshold of z > 3 (“MEG marked MAP”). (See Table 3.)
Table 1.
Clinical summary.
| Part 1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| ID | M/F | Histology | Age ate sz onset | Epilepsy duration | Lesion site | Side | BOS | Semiology |
| 1 | F | FCD IIA | 2 | 28 | Frontal lateral | Right | No | No aura; Tonic arm left asymm. Tonic, versive to right; Rarely Tonic-clonic |
| 2 | F | FCD IIB | 17 | 4 | Parietal postcentral | Left | Yes | Dysesthesia & paresis arm/hand right |
| 3 | M | n.a | 16 | 6 | Frontal | Left | No | Clonic right hand |
| 4 | F | FCD IIA | 2 | 37 | Frontal G. front. Med | Right | No | Vocalization, tachycardia, complex-motor (trunk and proximal muscles) from sleep |
| 5 | F | n.a | 11 | 16 | Prefrontal | Left | No | Somatosensory leg right evolving tt motor leg right, one initial TCS |
| 6 | F | n.a | 9 | 31 | Prefrontal | Left | No | Versive to right |
| 7 | F | n.a | 6 | 22 | Frontal G. front. Med | Left | Yes | (Unspecific aura), vocalization, complex-motor evolving to asymmetric tonic; most from sleep |
| 8 | M | FCD IIB | 7 | 29 | Parietal postcentral | Left | No | Somatosensory: haptic + cold-goose flash non-lateralized; in sleep: vocalization, blinking, complex-motor |
| 9 | F | FCD IIB | 5 | 27 | Frontal lat-opercular | Right | No | No aura, face grimacing, blinking; complex-motor; often ictal speech; evolution to tonic-clonic (versive left) |
| 10 | F | FCD IIB | 7 | 23 | Temp-occ lateral | Right | No | Headache + optic sensations + vomiting; accompanied by nystagmus/oscillopsia; +automotor in sleep; tonic-clonic: single |
| 11 | F | FCD IIB | 12 | 24 | Parieto-occipital | Right | No | Dysmnestic aura (visual-scenic), out-of-body experience; evolving to dialeptic; one initial TCS |
| 12 | F | FCD IIA | 17 | 12 | Frontal precentral | Left | No | Clonic right face; nocturnal hypermotor |
| 13 | M | FCD IIB | 4 | 29 | Frontal G. front inf. | Right | No | (unspecific aura); nocturnal complex motor |
| 14 | M | FCD IIA | 24 | 23 | Parietal mesial | Left | No | Assymetr. tonic, shouting; inconstant sensory right leg |
| 15 | F | FCD IIB | 6 | 33 | Insular | Left | No | Hypermotor |
| 16 | M | FCD IIB | 7 | 10 | Frontobasal | Right | No | Autonomic – behavioural symtoms; ictal speech |
| 17 | W | n.a | 6 | 14 | Frontal lateral opercular | Right | Yes | Cephalic aura - somatosensory arm left, complex-motor; no generalizations |
| 18 | W | FCD IIB | 13 | 29 | Frontal mesial, SSMA | Right | Yes | Complex-motor from sleep |
| 19 | W | FCD IIA | 1 | 26 | Frontal premotor | Left | No | Clonic right arm and assymmetric tonic |
| 20 | M | FCD II nos | 4 | 17 | Parietal mesial | Right | Yes | Sensory left leg and visual - assymetric tonic |
| 21 | W | FCD IIB | 3 | 33 | Frontal dorsal | Left | No | Hypermotor from sleep and unspecific aurae during day |
| FCD = focal cortical dysplasia; FCD nos = FCD not otherwise specified (due to fragmented tissue); SSMA = supplementary sensomotor area. BOS = bottom-of-sulcus-FCD. | ||||||||
| Part 2 | ||||||||
|---|---|---|---|---|---|---|---|---|
| ID | 1st MRIread | Surface EEG spikes | Surface EEG: ictal patterns | Invasive | Surgery | Age at surgery | Outcome Engel | Timepoint (months) |
| 1 | Neg | No | Decrement only | Yes | Yes | 32 | 3A | 24 |
| 2 | + | No | Decrement evolving to pattern C3-P3 | Yes | Yes | 21 | 1A | 24 |
| 3 | + | No | No pattern | n.a | No | No surgery | n.a | n.a |
| 4 | + | Frequent C4, P4 | Rhythmic spikes F4 C4 or decrement followed by fast Beta C4, Cz | Yes | Yes | 41 | 1C | 48 |
| 5 | + | Frequent over midline Fz, Cz | Fast activity midline | Yes | No | No surgery | n.a | n.a |
| 6 | + | F3-C3 | Rhythmic pattern C3-P3 | n.a | No | No surgery | n.a | n.a |
| 7 | + | Rare, F3, Fz | Rhythmic spikes f left F3, Fz preictally à Beta Fz F3 20 Hz | n.a | No | No surgery | n.a | n.a |
| 8 | + | Rare, left parietal P3, P5 | Left parietal P3, P5 12–14 Hz | Yes | Yes | 36 | 1A | 60 |
| 9 | + | Very rare | Non lateralized decrement | No | Yes | 32 | 1A | 48 |
| 10 | + | Rare; periictally many occ-temp right | Temp-occ r | No | Yes | 33 | 1D | 45 |
| 11 | + | Par-occ r | Decrement evolving to right par-occ | No | Yes | 33 | 1A | 36 |
| 12 | + | No | No pattern | Yes | Yes | 29 | 1A | 36 |
| 13 | (+) | Frequent FP2, F4 | Right lateral | Yes | Yes | 33 | 1A | 42 |
| 14 | Neg | F3-C3 | Fast activity C3, Cz | Yes | Yes | 47 | 3A | 24 |
| 15 | (+) | Rare, left temporal | Centrotemporal delta-then bilateral | Yes | Yes | 39 | 1B | 91 |
| 16 | (+) | FP2, F4 | None or frontal right | Yes | Yes | 17 | 1B | 36 |
| 17 | + | Frequent F8 in sleep | No clear ictal pattern | No | No | No surgery | n.a | n.a |
| 18 | Neg | No | No pattern | Yes | Yes | 42 | 1A | 30 |
| 19 | (+) | Sharp wave C3, Cz | Subclinical C3, Cz; clinical sz 50% without pattern; other fast C3-P3 | Yes | Yes | 27 | 3A | 48 |
| 20 | Neg | Rare sharp wave P4, Pz | Rhythmic sharp waves P4-C4-Cz | Yes | Yes | 21 | 1A | 18 |
| 21 | (+) | Polyspikes F3, FP1 | Regional frontocentral left | Yes | Yes | 36 | 1A | 12 |
Electrode naming according to 10–20 system; n.a = not applicable.
1st MRIread refers to the initial visual MRI interpretation: +: clear-cut FCD II; (+); suspected FCD II; neg: no lesion.
Table 2.
Results of MR – MAP – MEG analysis.
| ID | Side | MR Location (Wang) | MEG Location (Wang) | Duration (min.) | Spikes (number) | Distance to real FCD (mm) |
|---|---|---|---|---|---|---|
| 1 | Right | DLF inf. | DLF inf. | 20 | 10 | 14.5 |
| 2 | Left | DLF inf. | DLF inf. | 12 | 11 | 6.9 |
| 3 | Left | DLF sup. | DLF sup. | 20 | 62 | 0.6 |
| 4 | Right | DLF inf. | DLF inf. | 20 | 22 | 17.8 |
| 5 | Left | DLF sup. | DLF sup. | 20 | 5 | 1.6 |
| 6 | Left | DLF sup. | DLF sup. | 20 | 5 | 21.8 |
| 7 | Left | DLF inf. | DLF inf. | 20 | 24 | 24.8 |
| 8 | Left | PP | PP | 20 | 5 | 8.7 |
| 9 | Right | FP, AI | AI | 20 | 18 | 10.6 |
| 10 | Right | LO | LO | 20 | 45 | 2.2 |
| 11 | Right | AP | AP | 20 | 54 | 10.1 |
| 12 | Left | DLF sup. | DLF sup. | 20 | 12 | 6.3 |
| 13 | Right | DLF inf. | DLF inf. | 20 | 49 | 11.7 |
| 14 | Left | PM | PM | 20 | 7 | 4.5 |
| 15 | Left | PI | PI | 20 | 33 | 12.2 |
| 16 | Right | FP | FP | 20 | 88 | 9.6 |
| 17 | Right | DLF inf. | DLF inf. | 40 | 11 | 14.5 |
| 18 | Right | DLF sup. | DLF sup. | 40 | 14 | 12.2 |
| 19 | Left | FM | n.a | n.a | 0 | n.a |
| 20 | Right | FM | PM | 40 | 51 | 33.0 |
| 21 | Left | DLF sup. | DLF sup | 40 | 33 | 2.2 |
| Mean 28.1 | Mean 12.3 |
FP = fronto-polar; FM = frontal mesial; AI = antero-insular; PI = postero-insular; DLF dorso-lateral frontal; PP = postero-parietal.
AP = antero-parietal; PM = parietal mesial; LO = lateral occipital; LT = lateral temporal; inf. = inferior; sup. = superior.
Table 3.
Resection of MEG/MAP - classification.
| ID | MEG resected | MEG-marked MAP resected |
|---|---|---|
| 1 | No | Partial |
| 2 | Partial | Complete |
| 3 | n.a | n.a |
| 4 | Complete | (Complete) |
| 5 | n.a | n.a |
| 6 | n.a | n.a |
| 7 | n.a | n.a |
| 8 | Complete | Partial |
| 9 | Partial | Complete |
| 10 | Complete | Complete |
| 11 | Partial | Complete |
| 12 | Partial | Partial |
| 13 | Complete | Complete |
| 14 | No | No |
| 15 | n.a | n.a |
| 16 | Complete | Complete |
| 17 | n.a | n.a |
| 18 | Complete | (Complete) |
| 19 | n.a | n.a |
| 20 | No | Complete |
| 21 | Complete | Complete |
n.a = not applicable.
2.4. Outcome
Seizure outcome was scored according to Engel's classification at last available follow up.
2.5. Statistical analysis
Differences of MEG and MAP resection in patients with Engel 1 (completely or almost seizure free) versus Engel 2–4 (persisting seizures) outcomes were compared using Fisher's exact test. Sensitivity, specificity, positive and negative predictive values were calculated based on concordance of MEG localizations with the resection volume in seizure free patients vs. patients with persisting seizures. A localization was classified as a positive if the localization was within 2 cm of the resection (corresponding to complete or partial resection, see above), negative otherwise. Specificity thus is based on localizations outside this volume in patients with persisting seizures (Engel 2–4). Note that this approach may overestimate specificity (Rikir et al., 2014). Differences of MEG distances to FCD between patients with BOS and other FCDs were evaluated using a t-test. Statistical analysis was performed using IBM SPSS Statistics 24.0 (Armonk, NY, USA).
3. Results
3.1. Clinical data
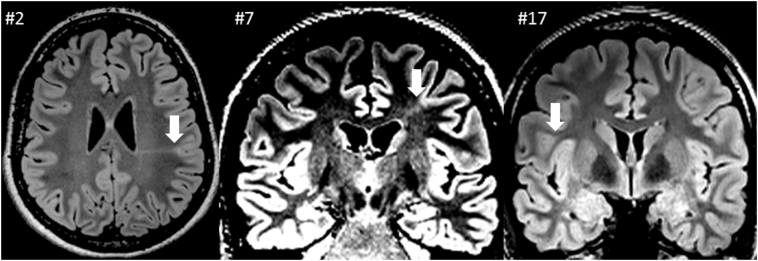
Twenty-one patients were eligible for this study. Twelve patients showed a clear FCD II suspect lesion by conventional visual MRI inspection alone. In five patients, MAP analysis gave the main clue for FCD suspicion, and lesions were detected by visual MRI re-view. In four patients (patients 1, 14, 18, 20), FCD II was suspected on the basis of MAP mainly. 5 lesions were classified as bottom-of-sulcus dysplasia (BOS), i.e. patients 2, 7, 17, 18, 20 (see Fig. 1 for illustrative examples). Lesions were localized as follows: frontal (14), parietal (4), parietooccipital (1), temporooccipital (1) and insular (1). Right/Left ratio was 10/11. Mean age at presurgical evaluation in this FCD II patient cohort was 31.1 years, mean epilepsy duration at time of evaluation was 22.5 years (range 4–37). Age at epilepsy onset ranged from 1 to 24 years (mean 8.5). Operated patients had a mean age at surgery of 32.4 years. One patient underwent surgery at another center (patient 15). Mean follow up time was 38.9 months. Seizures in all patients were reported as daily/multiple per day, the majority occurring from sleep, all but one patient had no secondary generalizations. Surface EEG recordings did not show any interictal spike-wave activity in five patients. Semiology contained helpful signs indicating at least hemispheric lateralization in fourteen patients. In seven patients, semiology was inconclusive for lateralization or lobe. Sixteen patients underwent resective epilepsy surgery, 14/16 after invasive recordings with subdural and/or depth electrodes. Two patients had a second surgery (patients 1 and 11), 3 and 7 years after the 1st surgery, respectively. Patient #15 underwent surgery at another institution, data about resection extent were not available. Respectively, this patient was excluded for comparison of resection and seizure outcome. 81.3% (13/16) had excellent outcomes (Engel 1) at last follow up. Clinical data and findings of our patient group are summarized in Table 1.
Fig. 1.
Illustrative MRI examples from bottom-of-sulcus dysplasias (BOS), all FLAIR (see arrows); patient IDs as indicated.
3.2. Imaging results from MEG-MRI coregistration
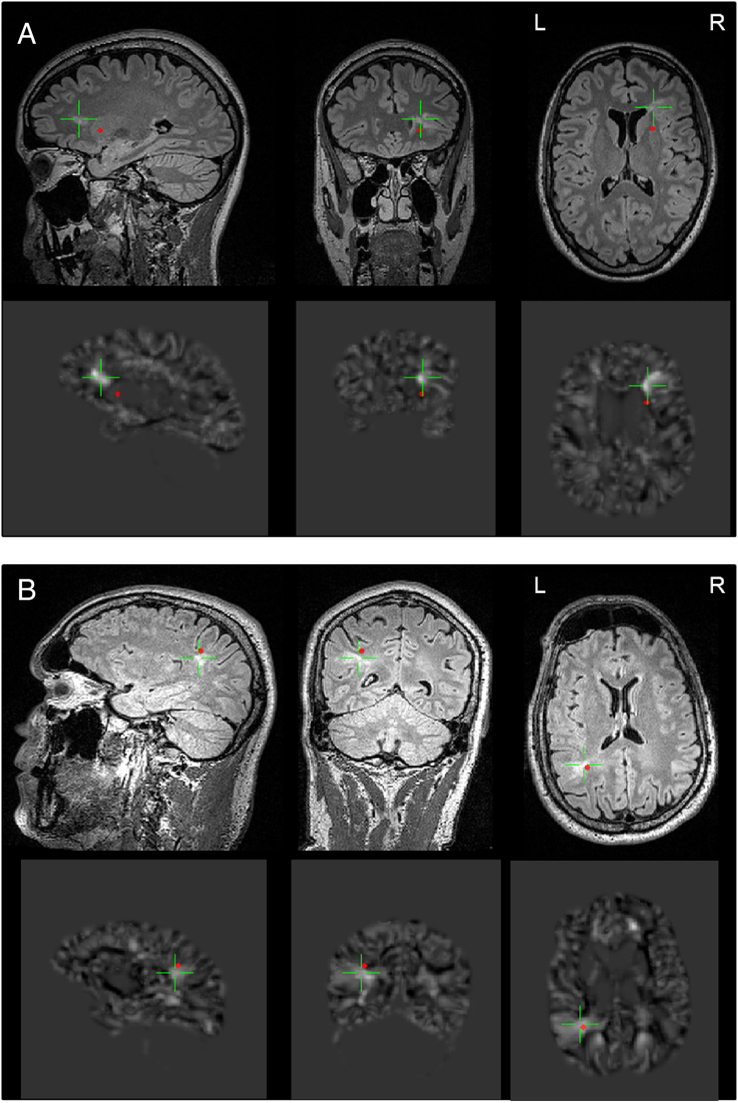
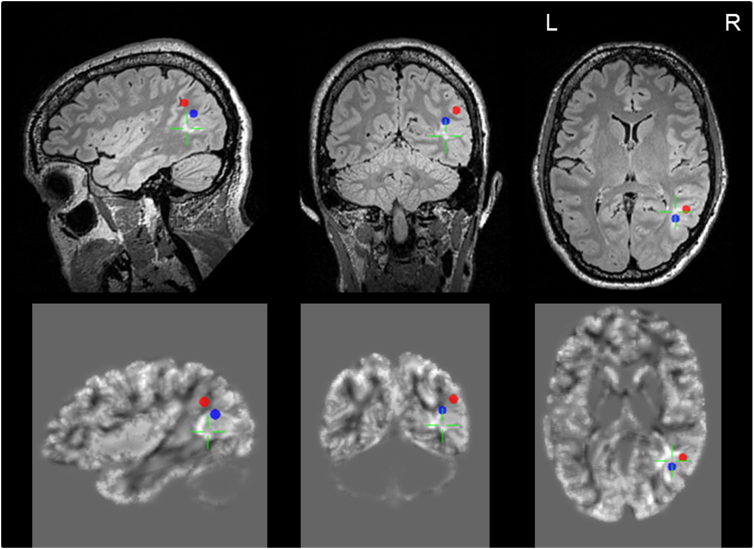
MEG analysis revealed a circumscript source localization of interictal activity in 20 of 21 patients (95.2%), one patient had no detectable spikes during the MEG session. Mean spike frequency during MEG-measurement was 29.9 per 20 min, all cases showed at least 5 spikes except for patient #19 with no spikes. Source localizations as determined by MEG analysis had close spatial relationship to lesional sites as determined by MAP in all these 20 patients. The mean distance of dipole localization to outer border of MAP abnormality at the FCD site was 12.3 ± 8,1 mm, all but three values were below 20 mm. Distances to BOS FCDs were larger on average compared to other FCDs (18.3 vs. 9.0 mm, p = 0.025). Details are summarized in Table 2, examples are illustrated in Fig. 2. In patient 10, a non-motor focal seizure occurred during MEG acquisition, suitable for source analysis of ictal hypersynchronous activity, illustrating close spatial relation of interictal and ictal sources (Fig. 3).
Fig. 2.
A/B MEG-MRI and MEG-MAP coregistrations, illustrative examples. Upper rows: 3 lesional planes from 3D-FLAIR; Lower Rows: 3 corresponding planes from MAP; red dot indicates source localization from MEG, green cursor is focused on the FCD. A: patient 9; B: patient 8 (see Table 1, Table 2).
Fig. 3.
Ictal (red dot) and interictal (blue dot) MEG sources in close proximity to each other and the lesion as illustrated in multiplanar FLAIR (upper row), and MAP (lower row); patient 10. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. MEG localizations versus resection cavities and surgical outcome
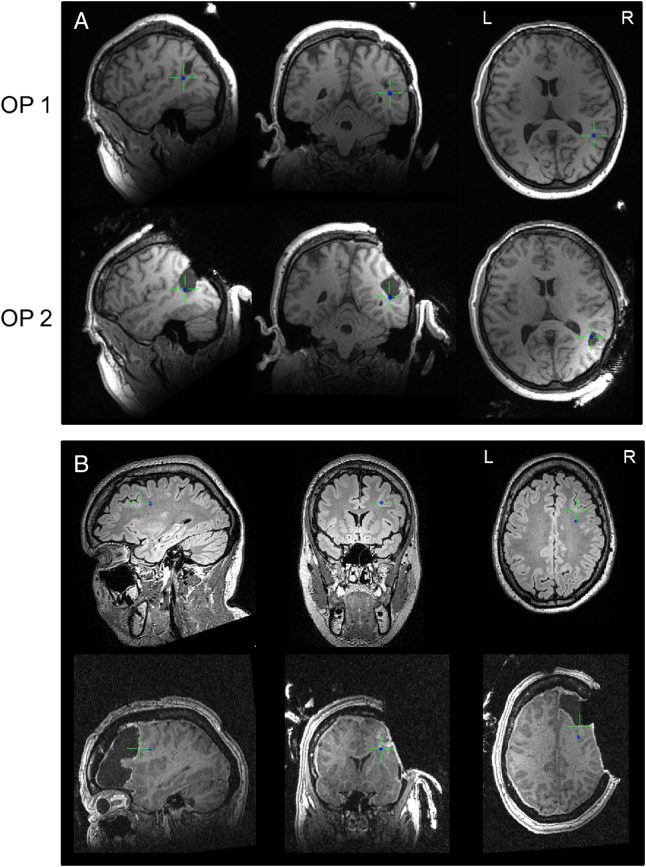
Results of evaluating the resection of the MEG source are shown in Tables 4 and 5. We included 14 of 16 operated patients in this analysis, since a single patient had no MEG source (patient 19) and in one patient postsurgical MRIs were unavailable (patient 15). When the MEG source was ranked resected, as well as when the MEG-marked MAP volume was ranked resected, patients showed better surgical outcomes according to Engel (p = 0.033 each, Fishers exact test). Sensitivity of MEG localizations for the resection in seizure free patients was 92% with a specificity of 100%. Positive and negative predictive value were 100% and 67%. Illustrative patients with persisting seizures after a surgery sparing the MEG source by 1st surgery are shown in Fig. 4, one patient achieving seizure freedom by 2nd surgery including the MEG source.
Tables 4 and 5.
Resection of MEG sources versus seizure outcome.
| MEG resected | ||||
|---|---|---|---|---|
| No | Partially | Completely | Fisher's exact test | |
| Engel 1 | 1 | 4 | 7 | 0.033 |
| Engel > 1 | 2 | 0 | 0 | |
| MEG marked MAP maximum resected | ||||
|---|---|---|---|---|
| Engel 1 | 0 | 2 | 10 | 0.033 |
| Engel > 1 | 1 | 1 | 0 | |
Fig. 4.
A/B. MEG sources in relation to resection cavities in patients with two surgeries: A: patient 11; continuing seizures after 1st surgery, sustained seizure freedom after 2nd surgery years later. As illustrated, the 2nd surgery (lower row, intraoperative MRI) included the MEG-source, which had remained unresected by the 1st intervention; B: patient 1: persisting seizures after 2nd surgery, MEG source in unresected edge area despite large resection volume.
4. Discussion
FCD II, also known as Taylor's focal cortical dysplasia, is a highly epileptogenic neocortical lesion (Blümcke et al., 2017; Aronica et al., 2012). The FCD II related seizure disorder is often characterized by a pharmacoresistant course after early-life epilepsy onset and high seizure burdens with seizures predominating in sleep (Nobili et al., 2007, Nobili et al., 2009, Losurdo et al., 2014), strikingly illustrated also by our patient cohort (mean age at epilepsy onset 8.4 years; seizures multiple per day in all). Failure to visualize a lesion is a negative predictor of surgical success in epilepsy overall (Bien et al., 2009). Visualizing the lesion and defining lesion extent is a challenge in FCD II. Epilepsy surgery can achieve seizure freedom in a significant percentage of FCD II patients (Fauser and Zentner, 2012, Bien et al., 2013, Losurdo 2014, Rössler et al., 2017). The crucial point is to reliably (i) detect FCD II as the underlying etiology in these epilepsies and (ii) identify the epileptogenic zone including its spatial relations to the structural lesion (Fauser and Zentner, 2012, McIntosh et al., 2012). According to our study these goals can be supported by combining MRI, MRI post-processing, such as MAP, and MEG, as shown here by excellent surgical outcomes with 81.3% reaching Engel 1 compared to published series (for reference see Fauser and Zentner, 2012) including patients with prolonged duration of pharmacoresistant epilepsy. According to our data, targeting the MEG-source seems particularly successful in FCD II.
MEG analysis in our patient cohort demonstrated a close spatial relationship between MEG source and FCD II lesion with a circumscript source localization in the majority of patients, corroborating earlier studies reporting good sensitivity and localization accuracy for MEG in FCD II (Widjaja et al., 2008, Ishii et al., 2008, Sueda et al., 2010, Wilenius et al., 2013, Mu et al., 2014, Wang et al., 2012, Wang et al., 2014). In our cohort, MEG sensitivity was as high as 95.2% in FCD II, compared to 70% reported from unselected epilepsy series (Stefan et al., 2003). Moreover, dipole localizations were within or close to the lesion site in our cohort, matching reports of very active and localized epileptogenicity for FCD II compared to other etiologies (Palmini et al., 1991, Morioka et al., 1999, Bast et al., 2004, Ishii et al., 2008, Blenkmann et al., 2012). MEG-MAP analysis in this study was facilitated by preselecting patients with clear-cut FCD II, validated by classic MRI and histopathology in most cases. Interestingly, the MEG source was not constantly found in the center or within the MR lesion, but rather next to edge areas. Of note in this context is that tissue extent in FCD II has been shown to exceed the area of MRI visible lesion, with dysmorphic neurons beyond the borders of the MRI lesion (Tassi et al., 2001). Areas of signal increase in FLAIR seem to correlate to balloon cell rich parts of the FCD, which in turn are more likely to be electrically silent (Cepeda et al., 2003; Chassoux et al., 2012). Therefore, our observation still is compatible with an intralesional MEG-source. The close proximity of MEG sources to the FCD site in our cohort are contrasted by a recent study describing also more remote MEG clusters in some patients, especially concerning small BOS FCDs (Nakajima et al., 2016). These authors suggest closed-field effects due to the random orientation of neurons within the FCD, low density of neural cells and small sizes of especially BOS FCDs as potential reasons for remote localizations due to lower signal-to-noise ratios (SNR). Furthermore, they used single moving dipole analysis of single spikes (Nakajima et al., 2016). As a consequence, noise and ongoing, unrelated background activity likely had a stronger impact on the results as our averaged spike approach, which provides and overall better SNR (Bast et al., 2004). Whereas distances between MEG and the lesion were significantly different between BOS and non-BOS FCDs, BOS FCDs also showed closely related MEG-sources in our study. We also utilized local channel groups centered on the gradient of interest, which further excludes unrelated activity, e.g. in contralateral sensors. The combination of these two strategies may provide access especially to early components of the activity, which may not have propagated as much from the sources in or close to the FCDs. Epileptogenic areas in terms of both interictal irritative zones and ictal onset zones in FCD II are closely related (Bartolomei et al., 2016), as illustrated by our patient 10, where source localizations of interictal spiking as well as seizure onset were highly concordant (Fig. 3), again illustrating intrinsic epileptogenicity for FCD II (Cepeda et al., 2003, Morioka et al., 1999, Dubeau et al., 1998, Palmini et al., 1995).
Recently, some reports already did imply a potential for combining different diagnostic approaches in FCD II including MRI/MAP, MEG and depth stereo EEG (SEEG): Implantation of a depth electrode into a patient's MAP-lesion, located within a neocortical compartment suggested by semiology, led to good localizing data and successful surgery (Wellmer et al., 2010). In another FCD II patient receiving invasive recordings with simultaneous MEG as a final step of evaluating a MAP lesion, localizing data were highly concordant (Wang et al., 2012). Histopathological workup in both cases revealed FCD II and seizure freedom achieved (Wellmer et al., 2010; Wang et al., 2012). In another series, resection of “densely clustered” MEG sources in MRI-negative cases related to a postsurgical histopathology of underlying FCD II in some patients and achieved good outcomes (Wilenius et al., 2013). In further three patients, MEG was highly concordant to the localization of occult FCD II in insular epilepsy (Heers et al., 2012). Indeed, patient 3 from the latter study is identical to patient 15 in our cohort. Another recent study also reported on linking MAP and MEG data in FCDs (Wang et al., 2014). In these studies, MAP analysis had been performed in retrospect on preoperative MRIs from patients after surgery for MRI negative epilepsy in order to investigate the value of MAP results versus preOP-MEG data (Wang et al., 2014): while positive correlations between favourable seizure outcomes and complete resection of the MEG source and/or MAP lesion were reported (Wang et al., 2014), comparison to our series is difficult due to population heterogeneity in terms of pathology (only two cases of FCD II were included), epilepsy syndrome (high percentage of temporal lobe epilepsy), and <50% of patients showing a MAP abnormality overall (Wang et al., 2014).
In the same way, a recent report on relationships between structural, magnetic source and stereo-EEG data in FCDs (Bouet et al., 2017) did include very different epilepsies and lesions, but no FCD IIB respectively. According to our data, linking MRI and MEG offers a powerful tool in FCD II related epilepsy. We selected cases with clear-cut FCD II lesions, the majority proven by histopathology as “gold standard”, since here lesions were expected to be shown by MAP. Our data show that combining MRI-postprocessing and MEG effectively provides accurate and concordant localizing data in a high percentage of FCD II related epilepsies. That a circumscript MEG-source can support better identification of a MRI-lesion during thorough imaging review has been demonstrated (Moore et al., 2002, Funke et al., 2011). In a “MRI negative” scenario, therefore, a localized MEG source matching with an abnormality seen in MAP only, is likely to indicate underlying FCD II, especially in extratemporal epilepsy displaying certain clinical characteristics, e.g. frequent nocturnal seizures, at least in an adult cohort as ours. Automatization of this kind of analysis, however, might be difficult since MAP can display false positives and abnormal z-values outside the true FCD. Whether there are signal characteristics in MEG-data, that might specifically indicate underlying FCD II-pathology is an important aspect for future research.
Optimal planning of resective surgery and/or targeting intracranial electrode placement in FCD II so far largely relies on information where the lesion resides. MEG technology appears as an ideal tool for visualizing FCD II-related epileptogenicity: (i) MEG is noninvasive (ii) MEG shows excellent spatial and temporal resolution; (iii) MEG results can be coregistered to other individual data in three-dimensional space; (iv) MEG can detect epileptic activity even if surface EEG fails and (v) MEG can help predicting invasive EEG localization (Stefan et al., 2003, Fischer et al., 2005, Ebersole and Ebersole, 2010, Knowlton et al., 2009; Stefan et al., 2011, Wilenius et al., 2013, Englot et al., 2015). Overall, MEG coregistration is not difficult (see Methods). In contrast, surface EEG techniques may fail in FCD II: (i) extratemporal seizures often show a complex semiology without clearly localizing symptoms, (ii) surface EEG often lacks interictal epileptic activity or clear ictal seizure patterns (Chassoux et al., 2012, Zakaria et al., 2012), at least when standard 10-20-EEG is considered. Therefore, invasive recordings are often chosen (Fauser and Zentner, 2012), and have been discussed to delineate the resective strategy in FCD II best (Chassoux, 2008). Incorporating MEG sources into surgical planning in our view could improve rates of surgical success in FCD II, as suggested by previous reports (Wellmer et al., 2010, Heers et al., 2012), and offers potential to optimize but also limit the amount of invasive recording and resection volumes in the future by better targeting (Wellmer et al., 2010). An interesting target for future research would be to investigate (and compare to MSI results) electric source imaging (ESI) specifically for FCD II, including high-density EEG (HD-ESI). Some studies using ESI for localizing diagnostics for epilepsy surgery did include FCD patients (Abdallah et al., 2017, Russo et al., 2016, Lascano et al., 2016, Rikir et al., 2014, Brodbeck et al., 2011). From these reports, that did include quite heterogenous etiologies, it seems that ESI can be particularily successful in FCD II-related epilepsies. However, no study so far focussed on tissue-proven FCD II. So far, only one conceptual technical analytic study compared different source localization methods from both HD-EEG and MEG for epileptic activities including clinical application on two patients reported with FCD (Chowdhury et al., 2016).
Surgical strategy in FCD II is warranting a more complex approach than pure “lesionectomy” since the tissue lesion in FCD II is usually larger than seen on MRI (Tassi et al., 2001), epileptogenicity appears not homogenously distributed within an individual FCD II lesion (Boonyapisit et al., 2003), and seizure onset zones could relate to parts of large FCD II only (Marusic et al., 2002 and our patient 8, see Fig. 2). The utility of surgically targeting of MEG clusters in epilepsy surgery has been suggested by earlier studies including FCD II (Vadera et al., 2013, Mu et al., 2014, Nakajima et al., 2016). MEG seems more suitable than SPECT-techniques for highlighting FCD II related epileptogenic zones, since MEG is noninvasive, independent of a seizure, and has better properties concerning resolution in time and space (Knowlton, 2006). MEG data could be very helpful in cases with MRI-suspicion of FCD II but missing concordant electroclinical data, as also shown by the various patients from our cohort displaying inconclusive data by standard presurgical methods. We further suggest to integrate MEG into a diagnostic algorithm for patients presenting with “MRI-negative” epilepsy (Wang et al., 2014, Englot et al., 2015, Delev et al., 2017), especially in extratemporal epilepsies with frequent, sleep-bound seizures, since a MAP lesion in this context is highly indicative of FCD II (Nobili et al., 2007, Nobili et al., 2009). Lesions delineated by MAP only and marked by a MEG source by this means can become eligible to a resective strategy. Patients regarded no surgical candidates using standard methods could get the chance of seizure freedom after a long duration of pharmacoresistant seizures. In summary, our data demonstrate MEG as a very strong tool in FCD II related epilepsy. A comprehensive non-invasive approach including MEG could help to achieve better outcomes in the future.
Disclosure/conflict of interest
Dr. Rampp serves as consultant for Elekta Oy, Helsinki, Finland. The other authors have no conflicts of interest.
Acknowledgements
We thank Martina Rzonsa for excellence in MEG acquisition.
Parts of this research were supported by the Deutsche Forschungsgemeinschaft (DFG RA2061/1-1, WO1425/7-1), the FoRUM research fund of the medical faculty, Ruhr-University Bochum, Germany (F831-2014) and the EU FP7 DESIRE grant (no. 60253). The present work was performed in partial fulfillment of the requirements for obtaining the degree "Dr. rer. biol. hum." of Angelika Mennecke.
Contributor Information
Burkhard S. Kasper, Email: burkhard.kasper@uk-erlangen.de.
Karl Rössler, Email: karl.roessler@uk-erlangen.de.
Hajo M. Hamer, Email: hajo.hamer@uk-erlangen.de.
Arnd Dörfler, Email: arnd.doerfler@uk-erlangen.de.
Ingmar Blümcke, Email: ingmar.bluemcke@uk-erlangen.de.
Roland Coras, Email: roland.coras@uk-erlangen.de.
Julie Roesch, Email: julie.roesch@uk-erlangen.de.
Angelika Mennecke, Email: angelika.mennecke@uk-erlangen.de.
Jörg Wellmer, Email: joerg.wellmer@kk-bochum.de.
Björn Sommer, Email: bjoern.sommer@paracelsus-kliniken.de.
Bogdan Lorber, Email: bogdan.lorber@kclj.si.
Johannes D. Lang, Email: johannes.lang@uk-erlangen.de.
Wolfgang Graf, Email: wolfgang.graf@uk-erlangen.de.
Hermann Stefan, Email: hermann.stefan@t-online.de.
Stefan Schwab, Email: stefan.schwab@uk-erlangen.de.
Michael Buchfelder, Email: michael.buchfelder@uk-erlangen.de.
Stefan Rampp, Email: stefan.rampp@uk-erlangen.de.
References
- Abdallah C., Maillard L.G., Rikir E., Jonas J., Thiriaux A., Gavaret M., Bartolomei F., Colnat-Coulbois S., Vignal J.P., Koessler L. Localizing value of electrical source imaging: frontal lobe, malformations of cortical development and negative MRI related epilepsies are the best candidates. Neuroimage Clin. 2017;16:319–329. doi: 10.1016/j.nicl.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antel S.B., Collins D.L., Bernasconi N., Andermann F., Shinghal R., Kearney R.E., Arnold D.L., Bernasconi A. Automated detection of focal cortical dysplasia lesions using computational models of their MRI characteristics and texture analysis. NeuroImage. 2003;19:1748–1759. doi: 10.1016/s1053-8119(03)00226-x. [DOI] [PubMed] [Google Scholar]
- Aronica E., Becker A.J., Spreafico R. Malformations of cortical development. Brain Pathol. 2012;22:380–401. doi: 10.1111/j.1750-3639.2012.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomei F., Trébuchon A., Bonini F., Lambert I., Gavaret M., Woodman M., Giusiano B., Wendling F., Bénar C. What is the concordance between the seizure onset zone and the irritative zone? A SEEG quantified study. Clin. Neurophysiol. 2016;127:1157–1162. doi: 10.1016/j.clinph.2015.10.029. [DOI] [PubMed] [Google Scholar]
- Bast T., Oezkan O., Rona S., Stippich C., Seitz A., Rupp A., Fauser S., Zentner J., Rating D., Scherg M. EEG and MEG source analysis of single and averaged interictal spikes reveals intrinsic epileptogenicity in focal cortical dysplasia. Epilepsia. 2004;45:621–631. doi: 10.1111/j.0013-9580.2004.56503.x. [DOI] [PubMed] [Google Scholar]
- Bernasconi A., Antel S.B., Collins D.L., Bernasconi N., Olivier A., Dubeau F., Pike G.B., Andermann F., Arnold D.L. Texture analysis and morphological processing of magnetic resonance imaging assist detection of focal cortical dysplasia in extra-temporal partial epilepsy. Ann. Neurol. 2001;49:770–775. [PubMed] [Google Scholar]
- Bernasconi A., Bernasconi N., Bernhardt B.C., Schrader D. Advances in MRI for ‘cryptogenic’ epilepsies. Nat. Rev. Neurol. 2011;7:99–108. doi: 10.1038/nrneurol.2010.199. [DOI] [PubMed] [Google Scholar]
- Besson P., Andermann F., Dubeau F., Bernasconi A. Small focal cortical dysplasia lesions are located at the bottom of a deep sulcus. Brain. 2008;131:3246–3255. doi: 10.1093/brain/awn224. [DOI] [PubMed] [Google Scholar]
- Bien C.G., Szinay M., Wagner J., Clusmann H., Becker A.J., Urbach H. Characteristic and surgical outcomes in patients with refractory magnetic resonance imaging-negative epilepsies. Arch. Neurol. 2009;66:1491–1499. doi: 10.1001/archneurol.2009.283. [DOI] [PubMed] [Google Scholar]
- Bien C.G., Raabe A.L., Schramm J., Becker A., Urbach H., Elger C.E. Trends in presurgical evaluation and surgical treatment of epilepsy at one centre from 1988–2009. J. Neurol. Neurosurg. Psychiatry. 2013;84:54–61. doi: 10.1136/jnnp-2011-301763. [DOI] [PubMed] [Google Scholar]
- Blenkmann A., Seifer G., Princich J.P., Consalvo D., Kochen S., Muravchik C. Association between equivalent current dipole source localization and focal cortical dysplasia in epilepsy patients. Epilepsy Res. 2012;98:223–231. doi: 10.1016/j.eplepsyres.2011.09.018. [DOI] [PubMed] [Google Scholar]
- Blümcke I., Thom M., Aronica E., Armstrong D.D., Vinters H.V., Palmini A., Jacques T.S., Avanzini G., Barkovich A.J., Battaglia G., Becker A., Cepeda C., Cendes F., Colombo N., Crino P., Cross J.H., Delalande O., Dubeau F., Duncan J., Guerrini R., Kahane P., Mathern G., Najm I., Ozkara C., Raybaud C., Represa A., Roper S.N., Salamon N., Schulze-Bonhage A., Tassi L., Vezzani A., Spreafico R. The clinicopathologic spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia. 2011;52:158–174. doi: 10.1111/j.1528-1167.2010.02777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blümcke I., Spreafico R., Haaker G., Coras R., Kobow K., Bien C.G., Pfäfflin M., Elger C., Widman G., Schramm J., Becker A., Braun K.P., Leijten F., Baayen J.C., Aronica E., Chassoux F., Hamer H., Stefan H., Rössler K., Thom M., Walker M.C., Sisodiya S.M., Duncan J.S., AW McEvoy, Pieper T., Holthausen H., Kudernatsch M., Meencke H.J., Kahane P., Schulze-Bonhage A., Zentner J., Heiland D.H., Urbach H., Steinhoff B.J., Bast T., Tassi L., Lo Russo G., Özkara C., Oz B., Krsek P., Vogelgesang S., Runge U., Lerche H., Weber Y., Honavar M., Pimentel J., Arzimanoglou A., Ulate-Campos A., Noachtar S., Hartl E., Schijns O., Guerrini R., Barba C., Jacques T.S., Cross J.H., Feucht M., Mühlebner A., Grunwald T., Trinka E., Winkler P.A., Gil-Nagel A., Toledano Delgado R., Mayer T., Lutz M., Zountsas B., Garganis K., Rosenow F., Hermsen A., von Oertzen T.J., Diepgen T.L., Avanzini G., EEBB Consortium Histopathological findings in brain tissue obtained during epilepsy surgery. N. Engl. J. Med. 2017;377(17):1648–1656. doi: 10.1056/NEJMoa1703784. [DOI] [PubMed] [Google Scholar]
- Bonini F., McGonigal A., Scavarda D., Carron R., Régis J., Dufour H., Péragut J.C., Laguitton V., Villeneuve N., Chauvel P., Giusiano B., Trébuchon A., Bartolomei F. Predictive factors of surgical outcome in frontal lobe epilepsy explored with stereoelectroencephalography. Neurosurgery. 2017 Jun 29 doi: 10.1093/neuros/nyx342. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Boonyapisit K., Najm I., Klem G., Ying Z., Burrier C., LaPresto E., Nair D., Bingaman W., Prayson R., Lüders H. Epileptogenicity of focal malformations due to abnormal cortical development: direct electrocorticographic-histopathologic correlations. Epilepsia. 2003;44:69–76. doi: 10.1046/j.1528-1157.2003.08102.x. [DOI] [PubMed] [Google Scholar]
- Bouet R., Mauguière F., Daligault S., Isnard J., Guenot M., Bertrand O., Jung J. The relationship between morphological lesion, magnetic source imaging, and intracranial stereo-electroencephalography in focal cortical dysplasia. Neuroimage Clin. 2017;15:71–79. doi: 10.1016/j.nicl.2017.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodbeck V., Spinelli L., Lascano A.M., Wissmeier M., Vargas M.I., Vulliemoz S., Pollo C., Schaller K., Michel C.M., Seeck M. Electroencephalographic source imaging: a prospective study of 152 operated epileptic patients. Brain. 2011;134:2887–2897. doi: 10.1093/brain/awr243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C., Hurst R.S., Flores-Hernández J., Hernández-Echeagaray E., Klapstein G.J., Boylan M.K., Calvert C.R., Jocoy E.L., Nguyen O.K., André V.M., Vinters H.V., Ariano M.A., Levine M.S., Mathern G.W. Morphological and electrophysiological characterization of abnormal cell types in pediatric cortical dysplasia. J. Neurosci. Res. 2003;72:472–486. doi: 10.1002/jnr.10604. [DOI] [PubMed] [Google Scholar]
- Chassoux F. Malformation of cortical development: which strategy is best? Neurochirurgie. 2008;54(3):272–281. doi: 10.1016/j.neuchi.2008.02.056. [DOI] [PubMed] [Google Scholar]
- Chassoux F., Landré E., Mellerio C., Turak B., Mann M.W., Daumas-Duport C., Chiron C., Devaux B. Type II focal cortical dysplasia: electroclinical phenotype and surgical outcome related to imaging. Epilepsia. 2012;53:349–358. doi: 10.1111/j.1528-1167.2011.03363.x. [DOI] [PubMed] [Google Scholar]
- Chowdhury R.A., Merlet I., Birot G., Kobayashi E., Nica A., Biraben A., Wendling F., Lina J.M., Albera L., Grova C. Complex patterns of spatially extended generators of epileptic activity: comparison of source localization methods cMEM and 4-ExSo-MUSIC on high resolution EEG and MEG data. NeuroImage. 2016;143:175–195. doi: 10.1016/j.neuroimage.2016.08.044. [DOI] [PubMed] [Google Scholar]
- Delev D., Quesada C.M., Grote A., Boström J.P., Elger C., Vatter H., Surges R. A multimodal concept for invasive diagnostics and surgery based on neuronavigated voxel-based morphometric MRI postprocessing data in previously nonlesional epilepsy. J. Neurosurg. 2017 Jun;16:1–9. doi: 10.3171/2016.12.JNS161676. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Dubeau F., Palmini A., Fish D., Avoli M., Gambardella A., Spreafico R., Andermann F. The significance of electrocorticographic findings in focal cortical dysplasia: a review of their clinical, electrophysiological and neurochemical characteristics. Electroencephalogr. Clin. Neurophysiol. Suppl. 1998;48:77–96. [PubMed] [Google Scholar]
- Ebersole J.S., Ebersole S.M. Combining MEG and EEG source modeling in epilepsy evaluations. J. Clin. Neurophysiol. 2010;27:360–371. doi: 10.1097/WNP.0b013e318201ffc4. [DOI] [PubMed] [Google Scholar]
- Englot D.J., Nagarajan S.S., Imber B.S., Raygor K.P., Honma S.M., Mizuiri D., Mantle M., Knowlton R.C., Kirsch H.E., Chang E.F. Epileptogenic zone localization using magnetoencephalography predicts seizure freedom in epilepsy surgery. Epilepsia. 2015;56:949–958. doi: 10.1111/epi.13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauser S., Zentner J. Management of cortical dysplasia in epilepsy. Adv. Tech. Stand. Neurosurg. 2012;38:137–163. doi: 10.1007/978-3-7091-0676-1_7. [DOI] [PubMed] [Google Scholar]
- Fischer M.J., Scheler G., Stefan H. Utilization of magnetoencephalography results to obtain favourable outcomes in epilepsy surgery. Brain. 2005 Jan;128(Pt 1):153–157. doi: 10.1093/brain/awh333. [DOI] [PubMed] [Google Scholar]
- Funke M.E., Moore K., Orrison W.W., Jr, Lewine J.D. The role of magnetoencephalography in "nonlesional" epilepsy. Epilepsia. 2011;52(suppl. 4):10–14. doi: 10.1111/j.1528-1167.2011.03144.x. [DOI] [PubMed] [Google Scholar]
- Guerrini R., Duchowny M., Jayakar P., Krsek P., Kahane P., Tassi L., Melani F., Polster T., Andre V.M., Cepeda C., Krueger D.A., Cross J.H., Spreafico R., Cosottini M., Gotman J., Chassoux F., Ryvlin P., Bartolomei F., Bernasconi A., Stefan H., Miller I., Devaux B., Najm I., Giordano F., Vonck K., Barba C., Blumcke I. Diagnostic methods and treatment options for focal cortical dysplasia. Epilepsia. 2015;56:1669–1686. doi: 10.1111/epi.13200. [DOI] [PubMed] [Google Scholar]
- Heers M., Rampp S., Stefan H., Urbach H., Elger C.E., von Lehe M., Wellmer J. MEG-based identification of the epileptogenic zone in occult peri-insular epilepsy. Seizure. 2012;21:128–133. doi: 10.1016/j.seizure.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Huppertz H.J., Grimm C., Fauser S., Kassubek J., Mader I., Hochmuth A., Spreer J., Schulze-Bonhage A. Enhanced visualization of blurred gray-white matter junctions in focal cortical dysplasia by voxel-based 3D MRI analysis. Epilepsy Res. 2005;67:35–50. doi: 10.1016/j.eplepsyres.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Ishii R., Canuet L., Ochi A., Xiang J., Imai K., Chan D., Iwase M., Takeda M., Snead O.C., 3rd, Otsubo H. Spatially filtered magnetoencephalography compared with electrocorticography to identify intrinsically epileptogenic focal cortical dysplasia. Epilepsy Res. 2008;81:228–232. doi: 10.1016/j.eplepsyres.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Kasper B.S., Taylor D.C., Janz D., Kasper E.M., Maier M., Williams M.R., Crow T.J. Neuropathology of epilepsy and psychosis: the contributions of J.A.N. Corsellis. Brain. 2010 Dec;133(Pt 12):3795–37805. doi: 10.1093/brain/awq235. [DOI] [PubMed] [Google Scholar]
- Knowlton R.C. The role of FDG-PET, ictal SPECT, and MEG in the epilepsy surgery evaluation. Epilepsy Behav. 2006;8:91–101. doi: 10.1016/j.yebeh.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Knowlton R.C., Razdan S.N., Limdi N., Elgavish R.A., Killen J., Blount J., Burneo J.G., Ver Hoef L., Paige L., Faught E., Kankirawatana P., Bartolucci A., Riley K., Kuzniecky R. Effect of epilepsy magnetic source imaging on intracranial electrode placement. Ann. Neurol. 2009;65(6):716–723. doi: 10.1002/ana.21660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral T., Clusmann H., Urbach J. Preoperative evaluation for epilepsy surgery (Bonn Algorithm) Zentralbl. Neurochir. 2002;63:106–110. doi: 10.1055/s-2002-35826. [DOI] [PubMed] [Google Scholar]
- Lascano A.M., Perneger T., Vulliemoz S., Spinelli L., Garibotto V., Korff C.M., Vargas M.I., Michel C.M., Seeck M. Yield of MRI, high-density electric source imaging (HD-ESI), SPECT and PET in epilepsy surgery candidates. Clin. Neurophysiol. 2016;127:150–155. doi: 10.1016/j.clinph.2015.03.025. [DOI] [PubMed] [Google Scholar]
- Losurdo A., Proserpio P., Cardinale F., Gozzo F., Tassi L., Mai R., Francione S., Castana L., Lo Russo G., Casaceli G., Sartori I., Della Marca G., Cossu M., Nobili L. Drug-resistant focal sleep related epilepsy: results and predictors of surgical outcome. Epilepsy Res. 2014 Jul;108(5):953–962. doi: 10.1016/j.eplepsyres.2014.02.016. [DOI] [PubMed] [Google Scholar]
- Martin P., Winston G.P., Bartlett P., de Tisi J., Duncan J.S., Focke N.K. Voxel-based magnetic resonance image postprocessing in epilepsy. Epilepsia. 2017;58:1653–1664. doi: 10.1111/epi.13851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusic P., Najm I.M., Ying Z., Prayson R., Rona S., Nair D., Hadar E., Kotagal P., Bej M.D., Wyllie E., Bingaman W., Lüders H. Focal cortical dysplasias in eloquent cortex: functional characteristics and correlation with MRI and histopathologic changes. Epilepsia. 2002;43:27–32. doi: 10.1046/j.1528-1157.2002.00801.x. [DOI] [PubMed] [Google Scholar]
- McIntosh A.M., Averill C.A., Kalnins R.M., Mitchell L.A., Fabinyi G.C., Jackson G.D., Berkovic S.F. Long-term seizure outcome and risk factors for recurrence after extratemporal epilepsy surgery. Epilepsia. 2012;53:970–978. doi: 10.1111/j.1528-1167.2012.03430.x. [DOI] [PubMed] [Google Scholar]
- Moore K.R., Funke M.E., Constantino T., Katzman G.L., Lewine J.D. Magnetoencephalographically directed review of high-spatial-resolution surface-coil MR images improves lesion detection in patients with extratemporal epilepsy. Radiology. 2002;225:880–887. doi: 10.1148/radiol.2253011597. [DOI] [PubMed] [Google Scholar]
- Morioka T., Nishio S., Ishibashi H., Muraishi M., Hisada K., Shigeto H., Yamamoto T., Fukui M. Intrinsic epileptogenicity of focal cortical dysplasia as revealed by magnetoencephalography and electrocorticography. Epilepsy Res. 1999;33:177–187. doi: 10.1016/s0920-1211(98)00096-5. [DOI] [PubMed] [Google Scholar]
- Mu J., Rampp S., Carrette E., Roessler K., Sommer B., Schmitt F.C., De Tiège X., Hamer H., Boon P., Pauli E., Bluemcke I., Zhou D., Buchfelder M., Stefan H. Clinical relevance of source location in frontal lobe epilepsy and prediction of postoperative long-term outcome. Seizure. 2014;23:553–559. doi: 10.1016/j.seizure.2014.04.006. [DOI] [PubMed] [Google Scholar]
- Nakajima M., Widjaja E., Baba S., Sato Y., Yoshida R., Tabei M., Okazaki A., Sakuma S., Holowka S.A., Ochi A., Carter Snead I.I.I.O., Rutka J.T., Drake J.M., Shiraishi H., Doesburg S., Otsubo H. Remote MEG dipoles in focal cortical dysplasia at bottom of sulcus. Epilepsia. 2016;57:1169–1178. doi: 10.1111/epi.13399. [DOI] [PubMed] [Google Scholar]
- Nobili L., Francione S., Mai R., Cardinale F., Castana L., Tassi L., Sartori I., Didato G., Citterio A., Colombo N., Galli C., Lo Russo G., Cossu M. Surgical treatment of drug-resistant nocturnal frontal lobe epilepsy. Brain. 2007;130:561–573. doi: 10.1093/brain/awl322. [DOI] [PubMed] [Google Scholar]
- Nobili L., Cardinale F., Magliola U., Cicolin A., Didato G., Bramerio M., Fuschillo D., Spreafico R., Mai R., Sartori I., Francione S., Lo Russo G., Castana L., Tassi L., Cossu M. Taylor's focal cortical dysplasia increases the risk of sleep-related epilepsy. Epilepsia. 2009;50:2599–2604. doi: 10.1111/j.1528-1167.2009.02169.x. [DOI] [PubMed] [Google Scholar]
- Palmini A., Andermann F., Olivier A., Tampieri D., Robitaille Y., Andermann E., Wright G. Focal neuronal migration disorders and intractable partial epilepsy: a study of 30 patients. Ann. Neurol. 1991;30:741–749. doi: 10.1002/ana.410300602. [DOI] [PubMed] [Google Scholar]
- Palmini A., Gambardella A., Andermann F., Dubeau F., da Costa J.C., Olivier A., Tampieri D., Gloor P., Quesney F., Andermann E. Intrinsic epileptogenicity of human dysplastic cortex as suggested by corticography and surgical results. Ann. Neurol. 1995;37:476–487. doi: 10.1002/ana.410370410. [DOI] [PubMed] [Google Scholar]
- Rikir E., Koessler L., Gavaret M., Bartolomei F., Colnat-Coulbois S., Vignal J.P., Vespignani H., Ramantani G., Maillard L.G. Electrical source imaging in cortical malformation-related epilepsy: a prospective EEG-SEEG concordance study. Epilepsia. 2014;55:918–932. doi: 10.1111/epi.12591. [DOI] [PubMed] [Google Scholar]
- Rössler K., Kasper B.S., Heynold E., Coras R., Sommer B., Rampp S., Hamer H.M., Blümcke I., Buchfelder M. Intraoperative MR imaging and neuronavigation during resection of FCD Type II in adult epilepsy surgery offers better seizure outcome. World Neurosurg. 2017;S1878–8750(17):31607–31608. doi: 10.1016/j.wneu.2017.09.100. (Sep 23, Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Russo A., Lallas M., Jayakar P., Miller I., Hyslop A., Dunoyer C., Resnick T., Duchowny M. The diagnostic utility of 3D-ESI rotating and moving dipole methodology in the pre-surgical evaluation of MRI-negative childhood epilepsy due to focal cortical dysplasia. Epilepsia. 2016 Sep;57(9):1450–1457. doi: 10.1111/epi.13454. [DOI] [PubMed] [Google Scholar]
- Sommer B., Grummich P., Coras R., Kasper B.S., Blumcke I., Hamer H.M., Stefan H., Buchfelder M., Roessler K. Integration of functional neuronavigation and intraoperative MRI in surgery for drug-resistant extratemporal epilepsy close to eloquent brain areas. Neurosurg. Focus. 2013 Apr;34 doi: 10.3171/2013.2.FOCUS12397. [DOI] [PubMed] [Google Scholar]
- Stefan H., Hummel C., Scheler G., Genow A., Druschky K., Tilz C., Kaltenhäuser M., Hopfengärtner R., Buchfelder M., Romstöck J. Magnetic brain source imaging of focal epileptic activity: a synopsis of 455 cases. Brain. 2003;126:2396–2405. doi: 10.1093/brain/awg239. [DOI] [PubMed] [Google Scholar]
- Stefan H., Rampp S., Knowlton R.C. Magnetoencephalography adds to the surgical evaluation process. Epilepsy Behav. 2011;20(2):172–177. doi: 10.1016/j.yebeh.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Sueda K., Takeuchi F., Shiraishi H., Nakane S., Asahina N., Kohsaka S., Nakama H., Otsuki T., Sawamura Y., Saitoh S. MEG time-frequency analyses for pre- and post-surgical evaluation of patients with epileptic rhythmic fast activity. Epilepsy Res. 2010;88:100–107. doi: 10.1016/j.eplepsyres.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Tassi L., Pasquier B., Minotti L., Garbelli R., Kahane P., Benabid A.L., Battaglia G., Munari C., Spreafico R. Cortical dysplasia: electroclinical, imaging, and neuropathologic study of 13 patients. Epilepsia. 2001;42:1112–1123. doi: 10.1046/j.1528-1157.2001.00501.x. [DOI] [PubMed] [Google Scholar]
- Taylor D.C., Falconer M.A., Bruton C.J., Corsellis J.A. Focal dysplasia of the cerebral cortex in epilepsy. J. Neurol. Neurosurg. Psychiatry. 1971;34:369–387. doi: 10.1136/jnnp.34.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbach H., Scheffler B., Heinrichsmeier T., von Oertzen J., Kral T., Wellmer J., Schramm J., Wiestler O.D., Blümcke I. Focal cortical dysplasia of Taylor's balloon cell type: a clinicopathological entity with characteristic neuroimaging and histopathological features, and favorable postsurgical outcome. Epilepsia. 2002;43:33–40. doi: 10.1046/j.1528-1157.2002.38201.x. [DOI] [PubMed] [Google Scholar]
- Vadera S., Jehi L., Burgess R.C., Shea K., Alexopoulos A.V., Mosher J., Bingaman W. Correlation between magnetoencephalography-based “clusterectomy” and postoperative seizure freedom. Neurosurg. Focus. 2013;34(6) doi: 10.3171/2013.4.FOCUS1357. [DOI] [PubMed] [Google Scholar]
- Von Oertzen J., Urbach H., Jungbluth S., Kurthen M., Reuber M., Fernández G., Elger C.E. Standard magnetic resonance imaging is inadequate for patients with refractory focal epilepsy. J. Neurol. Neurosurg. Psychiatry. 2002;73:643–647. doi: 10.1136/jnnp.73.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J., Weber B., Urbach H., Elger C.E., Huppertz H.J. Morphometric MRI analysis improves detection of focal cortical dysplasia type II. Brain. 2011;134:2844–2854. doi: 10.1093/brain/awr204. [DOI] [PubMed] [Google Scholar]
- Wang Z.I., Jones S.E., Ristic A.J., Wong C., Kakisaka Y., Jin K., Schneider F., Gonzalez-Martinez J.A., Mosher J.C., Nair D., Burgess R.C., Najm I.M., Alexopoulos A.V. Voxel-based morphometric MRI post-processing in MRI-negative focal cortical dysplasia followed by simultaneously recorded MEG and stereo-EEG. Epilepsy Res. 2012;100:188–193. doi: 10.1016/j.eplepsyres.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.I., Alexopoulos A.V., Jones S.E., Najm I.M., Ristic A., Wong C., Prayson R., Schneider F., Kakisaka Y., Wang S., Bingaman W., Gonzalez-MArtines J.A., Burgess R.C. Linking MRI postprocessing with magnetic source imaging in MRI-negative epilepsy. Ann. Neurol. 2014;75:759–770. doi: 10.1002/ana.24169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellmer J., Parpaley Y., von Lehe M., Huppertz H.J. Integrating magnetic resonance imaging postprocessing results into neuronavigation for electrode implantation and resection of subtle focal cortical dysplasia in previously cryptogenic epilepsy. Neurosurgery. 2010;66:187–194. doi: 10.1227/01.NEU.0000359329.92781.B7. [DOI] [PubMed] [Google Scholar]
- Widjaja E., Otsubo H., Raybaud C., Ochi A., Chan D., Rutka J.T., Snead O.C., 3rd, Halliday W., Sakuta R., Galicia E., Shelef I., Chuang S.H. Characteristics of MEG and MRI between Taylor's focal cortical dysplasia (type II) and other cortical dysplasia: surgical outcome after complete resection of MEG spike source and MR lesion in pediatric cortical dysplasia. Epilepsy Res. 2008;82:147–155. doi: 10.1016/j.eplepsyres.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Wilenius J., Medvedovsky M., Gaily E., Metsähonkala L., Mäkelä J.P., Paetau A., Valanne L., Paetau R. Interictal MEG revelas focal cortical dysplasias: special focus on patients with no visible MRI lesions. Epilepsy Res. 2013;105:337–348. doi: 10.1016/j.eplepsyres.2013.02.023. [DOI] [PubMed] [Google Scholar]
- Zakaria T., Noe K., So E., Cascino G.D., Wetjen N., Van Gompel J.J., Marsh W.R., Meyer F.B., Giannini C., Watson R.E., Worrell G.A. Scalp and intracranial EEG in medically intractable extratemporal epilepsy with normal MRI. ISRN Neurol. 2012;2012:942849. doi: 10.5402/2012/942849. [DOI] [PMC free article] [PubMed] [Google Scholar]