Abstract
This study was aimed to investigate brain function connectivity in premature ejaculation (PE) patients using the functional connectivity density (FCD) and network property of resting-state functional magnetic resonance imaging. Twenty PE patients (mean age: 27.95 ± 4.52 years) and 15 normal controls (mean age: 27.87 ± 3.78 years) with no self-reported history of neurologic or psychiatric disease were enrolled in this study. International Index of Erectile Function-5 and Chinese Index of Sexual Function for Premature Ejaculation-5 questionnaires and self-reported intravaginal ejaculatory latency time (IELT) were obtained from each participant for symptom assessment. Two-sample t-tests (intergroup comparison) were applied in the short-range FCD (SFCD) analysis, long-range FCD (LFCD) analysis, region of interest–based analysis, and network topological organization analysis. Pearson correlation analysis was performed to correlate IELT with FCD or the network property. The patients with PE showed significantly decreased SFCD in the bilateral middle temporal gyrus, left orbitofrontal cortex, nucleus accumbens, fusiform, caudate, and thalamus (p < 0.05, AlphaSim-corrected). Notably, all these aforementioned brain areas are located in the dopamine pathway. In contrast, increased LFCD was observed in the left insula, Heschl's gyrus, putamen, bilateral precuneus, supplementary motor area, middle cingulate cortex, and anterior cingulate cortex in PE patients (p < 0.05, AlphaSim-corrected). In addition, the network topological analysis found reinforced network connectivity between several nodes. The degree of hub nodes increased in the patients with PE. IELT was positively correlated with SFCD and negatively correlated with LFCD or the degree of hub nodes (p < 0.05, Pearson correlation). In summary, our results are important for understanding the brain network in PE patients. The present findings indicate that PE patients have a significant synergism disorder across the region of dopamine pathway, which implied neuronal pathological changes might be related with the change of dopamine. The FCD and network property can serve as new disease severity biomarkers and therapeutic targets in PE.
Keywords: Premature ejaculation, Functional connectivity density, The dopamine system, The rewarding system
Highlights
-
•
PE patients have different patterns of FCD compared with normal controls.
-
•
The functional brain network efficiency has changed in PE patients.
-
•
The FCD and network property can serve as disease severity biomarkers.
1. Introduction
Ejaculatory dysfunction, particularly premature ejaculation (PE), is considered the most common type of male sexual disorder (Laumann et al., 2005; Saitz and Serefoglu, 2015), and affects 20–30% of men of all ages (Gur and Sikka, 2015). Using an evidence-based unified definition, the International Society for Sexual Medicine defines PE as a male sexual dysfunction characterized by ejaculation that always or nearly always occurs prior to or within 1 min of vaginal penetration from the first sexual experience (lifelong PE) or a clinically significant and bothersome reduction in latency time, often to approximately 3 min or less (acquired PE) (Serefoglu et al., 2014). However, the understanding of the epidemiology, pathophysiology, and management of this disorder remains limited (Serefoglu and Saitz, 2012).
Although the noninvasive and objective functional brain imaging techniques, like functional magnetic resonance imaging (fMRI) have largely developed in recent decades, there is scarce data on brain functional changes in patients with PE. In recent years, increasing attention has been focused on using task or resting-state functional magnetic resonance imaging (rs-fMRI) to detect brain response and activity in patients. Interestingly, several studies have used task-based fMRI to examine the brain response to visual sexual stimuli in healthy men and found an increased response in the parietal lobes, temporal lobes, parieto-occipital sulcus, superior occipital gyrus, anterior cingulate gyrus, insula, amygdala, and septal areas (Kim et al., 2006; Mallick et al., 2007; Mouras et al., 2003). These studies indicate that at a macro level, various brain areas are involved in the ejaculatory behavior process (Coolen et al., 1997). In addition, other studies with task-based fMRI demonstrate that the ejaculatory behavior process employs a complex interconnected network comprising the hypothalamic, diencephalic, and pontine areas (Gur and Sikka, 2015). However, how these brain areas interact during the ejaculatory behavior process and whether these brain areas are coordinated with some particular brain systems remain unclear.
The complex task paradigm is required in the aforementioned studies with task-based fMRI. However, based on functional connectivity density (FCD) mappings, rs-fMRI can detect intrinsic activity in the brain with simple cooperation of the subjects. In fact, data-driven FCD is ideal for exploratory analysis because it quantifies the strength of the local functional connectivity hubs (network nodes with high connectivity to nearby brain regions) and does not rely on priory hypothesis (Biswal et al., 1995; Tomasi and Volkow, 2010). Moreover, the graph theoretical analysis can provide a unique framework for measuring brain networks and has therefore gained popularity in neuroimaging and brain network studies. Notably, the graph theoretical analysis in highly connected hub regions of functional networks (Tomasi and Volkow, 2010, Tomasi and Volkow, 2012) can overcome the limitations of seed-based approaches for the identification of hubs in the human brain. Therefore, it would be valuable to investigate the activity disorder at a connected hub and network level.
Our previous study with combined rs-fMRI and task-based fMRI analysis has found aberrant brain responses and impaired functional integration in certain brain areas in PE patients (B. Zhang et al., 2017). However, it remains unclear whether the brain areas identified in the previous study can be integrated into some specific neuro systems. In this study, we used the rs-fMRI data from the previous datasets to explore pathophysiological mechanisms in PE by FCD and network-based graph theoretical analysis. We hypothesized that 1) patients with PE had different patterns of FCD mapping compared with normal controls (NCs). 2) In addition, the topological organization of brain network, such as network global or local efficiency, small-world property, or nodal degree, will be changed in functional connectivity networks composed of hubs in PE patients.
2. Materials and methods
2.1. Participants
From 2012 to 2014 in the Nanjing Drum Tower Hospital, 20 right hand-dominant patients with lifelong PE and 15 right hand-dominant healthy controls were enrolled in this study. The detailed information about these participants can be found in our previous work (B. Zhang et al., 2017). Briefly, the lifelong PE patients were diagnosed according to ISSM guidelines (Serefoglu et al., 2014): a) ejaculation that always or nearly always occurs prior to or within about 1 min of vaginal penetration; b) the inability to delay ejaculation on all or nearly all vaginal penetrations; and c) negative personal consequences such as distress, bother, frustration, and/or the avoidance of sexual intimacy. Fifteen healthy subjects were enrolled in this study as controls, with self-reported intravaginal ejaculatory latency time (IELT) of >3 min. The IELT was measured for the 4-week baseline period during which both patients and NCs were asked to have sexual intercourse at least 4 times. Patients with erectile dysfunction (International Index of Erectile Function [IIEF]-5 score < 21), reduced sexual desire, or inhibited male orgasm were excluded from the study. Moreover, patients with mental disorders, physical illnesses which affect ejaculatory function, abuse of alcohol, and any medical treatment for premature ejaculation in the past 6 months were excluded. All the subjects completed the following two questionnaires: IIEF-5 (Rhoden et al., 2002) and Chinese Index of Sexual Function for Premature Ejaculation (CIPE)-5 questionnaires (Yuan et al., 2004). This study was conducted according to the Declaration of Helsinki and approved by the institutional review boards of the Nanjing Drum Tower Hospital. Written informed consent was obtained from each subject.
2.2. Image acquisition
The fMRI experiment was performed with an Achieva 3.0 T (TX) MR system, and the acquisition parameters of resting state fMRI were set as follows: field of view (FOV) = 192 × 192 mm2; section thickness = 4 mm with no section gap; matrix = 64 × 64; repetition time (TR) = 2000 ms; echo time (TE) = 30 ms; and flip angle = 90°. A total of 230 volumes were acquired, and each volume included 35 transverse slices covering the whole brain. During the resting state fMRI scanning, each subject was requested to lie quietly with his eyes closed. In addition, the high-resolution 3D T1-weighted brain structural images were also acquired for each participant, and the acquisition parameters were set as follows: TR = 7600 ms; TE = 3400 ms; flip angle = 8°; FOV = 256 × 256 × 192 mm3; and slice thickness = 1 mm.
2.3. Image preprocessing
The fMRI data were processed with Data Processing Assistant for Resting-State fMRI, advanced edition [http://rfmri.org/DPARSFA] (Yan and Zang, 2010), which is based on Statistical Parametric Mapping (http://www.fil.ion.ucl.ac.uk/spm) and the toolbox for Data Processing & Analysis of Brain Imaging [DPABI, http://rfmri.org/DPABI] (Yan et al., 2016). Slice timing, head motion correction, and spatial normalization to the standard Montreal Neurological Institute (MNI) EPI template with a resolution of 3 × 3 × 3 mm3 were conducted. According to the study by Anderson et al. and Murphy et al., the global signal regression was not performed to avoid introducing distortions into the time-series data (Anderson et al., 2011; Murphy et al., 2009). The included subjects had head movement <3 mm translation or <3° angular rotation in any axis during fMRI scanning. Subsequently, the data were detrended to remove the linear trend of time courses and band-pass filtered (0.01–0.08 Hz).
2.4. FCD mapping
In the present study, the FCD definition was based on the previous study by Tomasi and Volkow, 2010, Tomasi and Volkow, 2012. Usually, the FCD is divided into short-range FCD (SFCD) and long-range FCD (LFCD). The SFCD reflects the FC between voxels within a local cluster (intraregional), while LFCD reflects the FC between a voxel within a local and the other without the local cluster (interregional) (Z. Zhang et al., 2017). The detailed computing procedure about SFCD and LFCD can be found in Tomasi's study (Tomasi and Volkow, 2011). In brief, to calculate the SFCD, we computed Pearson's correlations between the time course at x0 and those at its local neighbors. A voxel (xj) was added to the list of neighbors of x0 only if it was directed linked to x0 with the correlation factor R0j > 0.6. This calculation was repeated for all voxels that were adjacent to voxels that belonged to the list of neighbors of x0 in an iterative manner until no new neighbors could be added to the list. The SFCD of x0 was computed as the number of elements in the local functional connectivity cluster, k(x0). Subsequently, the calculation was initiated for a different x0. This ‘growing’ algorithm was provided by Yang et al. (2016). Similarly, the LFCD equated to the numbers of functional connections between the voxel x0 and all other voxels without the local cluster in the whole-brain gray matter. Finally, SFCD and LFCD maps were spatially smoothed (8 mm) in SPM12 (Wellcome Department of Imaging Neuroscience, University London College, UK) (Friston et al., 1994). The SFCD and LFCD were calculated in the gray matter with a total of 67,541 voxels in MNI space.
2.5. Region of interest–based analysis of SFCD and LFCD between PE patients and controls
A total of 42 regions of interest (ROIs) noted in the literature were selected (Tomasi and Volkow, 2012) and included in the network analysis (Tomasi and Volkow, 2010). Specifically, each ROI was an isotropic cubic mask containing 27 imaging voxels (0.73 mL) and defined at the coordinates of the cluster centers (Table 3). The average FCD value of each ROI was extracted from each individual map using DPARSFA. The coordinates of the masks remained fixed across subjects. The two-sample t-test was used to evaluate the differences between the PE patients and NCs, and a Bonferroni correction was applied to the data to adjust for the number of factors investigated.
Table 3.
Brain area between-group differences in SFCD and LFCD in 42 functional hubs.
| Region | BA | MNI coordinates |
SFCD |
LFCD |
||||
|---|---|---|---|---|---|---|---|---|
| x (mm) | y (mm) | z (mm) | t | p | t | p | ||
| Precuneus R | 7 | 3 | −63 | 36 | −1.75 | 0.09 | −2.34 | 0.03 |
| Mid. orbital frontal R | 11 | 0 | 54 | −9 | 0.50 | 0.60 | −1.52 | 0.14 |
| Sup. medial frontal R | 32 | 0 | 54 | 18 | −0.65 | 0.52 | −1.12 | 0.27 |
| Sup. medial frontal R | 9 | 0 | 51 | 42 | −1.57 | 0.13 | −2.31 | 0.03 |
| Ant. cingulate R | 32 | 6 | 18 | 39 | 1.27 | 0.18 | 2.33 | 0.02 |
| Mid. frontal R | 45 | 45 | 45 | 6 | 0.15 | 0.88 | 0.80 | 0.43 |
| Supramarginal R | 40 | 63 | −33 | 36 | 0.37 | 0.70 | 0.59 | 0.56 |
| Angular R | 39 | 48 | −66 | 30 | −1.66 | 0.11 | −0.49 | 0.62 |
| Inf. frontal R | 47 | 36 | 30 | 3 | −0.76 | 0.45 | 1.39 | 0.15 |
| Mid. frontal R | 46 | 33 | 45 | 24 | −0.68 | 0.50 | 0.48 | 0.63 |
| Nucleus accumbens R | 25 | 15 | 12 | −12 | −0.88 | 0.38 | −0.80 | 0.43 |
| Rectus R | 44 | 0 | 21 | −24 | −1.75 | 0.09 | −0.49 | 0.63 |
| Caudate R | 9 | 15 | 6 | −1.39 | 0.17 | −1.05 | 0.30 | |
| Post–central R | 3 | 42 | −21 | 51 | 0.44 | 0.66 | 1.03 | 0.29 |
| Thalamus R | 12 | −24 | 3 | −1.07 | 0.29 | 0.02 | 0.99 | |
| Hippocampus R | 27 | 21 | −30 | −6 | 0.00 | 1.00 | 0.46 | 0.63 |
| Insula R | 13 | 36 | −15 | 6 | 0.04 | 0.97 | 1.80 | 0.07 |
| Cerebellum R | 36 | −3 | −27 | −1.95 | 0.10 | −0.72 | 0.48 | |
| Pons R | 12 | −27 | −24 | −1.57 | 0.13 | −0.56 | 0.58 | |
| Sup. temporal R | 41 | 39 | −33 | 15 | 0.88 | 0.35 | 2.21 | 0.02 |
| Fusiform R | 37 | 39 | −48 | −18 | 0.46 | 0.65 | 0.54 | 0.59 |
| Mid. cingulate R | 23 | 12 | −12 | 36 | 1.27 | 0.19 | 2.92 | 0.00 |
| Inf. temporal R | 20 | 42 | −21 | −24 | −0.43 | 0.65 | 0.43 | 0.66 |
| Sup. temporal R | 22 | 69 | −18 | 6 | 1.49 | 0.11 | 1.69 | 0.08 |
| Pre–central R | 6 | −24 | −15 | 63 | −1.31 | 0.20 | 0.11 | 0.91 |
| Precuneus L | 23 | −3 | −57 | 24 | −1.98 | 0.06 | −2.43 | 0.02 |
| Pos. cingulate L | 23 | −3 | −39 | 27 | −2.26 | 0.03 | −2.07 | 0.05 |
| Inf. parietal L | 7 | −36 | −69 | 48 | −1.49 | 0.15 | −1.20 | 0.24 |
| Sup. frontal L | 9 | −21 | 36 | 51 | −2.31 | 0.03 | −0.52 | 0.61 |
| Mid. frontal L | 9 | −42 | 18 | 51 | −1.01 | 0.31 | −0.55 | 0.59 |
| Precentral L | 44 | −48 | 12 | 33 | −2.84 | 0.01 | −2.34 | 0.03 |
| Nucleus accumbens L | 25 | −12 | 9 | −9 | −2.63 | 0.01 | −1.03 | 0.31 |
| Inf. frontal L | 45 | −42 | 30 | 18 | −1.98 | 0.06 | −0.39 | 0.70 |
| Post–central L | 4 | −24 | −30 | 63 | −0.54 | 0.59 | 1.07 | 0.29 |
| Fusiform L | 37 | −24 | −36 | −33 | −2.28 | 0.03 | −0.87 | 0.39 |
| Hippocampus L | 27 | −18 | −30 | −3 | −1.58 | 0.12 | 0.40 | 0.69 |
| Fusiform L | 36 | −36 | 0 | −39 | −3.21 | 0.00 | −1.75 | 0.09 |
| Heschl's L | 13 | −42 | −24 | 12 | 0.00 | 1.00 | 0.96 | 0.34 |
| Sup. temporal L | 22 | −63 | −15 | 0 | 0.65 | 0.51 | 0.16 | 0.87 |
| Mid. cingulate L | 24 | −9 | 3 | 30 | −0.97 | 0.34 | −0.39 | 0.70 |
| Mid. temporal L | 21 | −45 | −45 | 15 | −1.53 | 0.14 | −0.85 | 0.40 |
| Calcarine L | 18 | −18 | −75 | 18 | 0.48 | 0.63 | −0.14 | 0.89 |
Statistical values were averaged in 3-mm isotropic (cubic) regions of interest (27 voxels) and centered at the Montreal Neurological Institute (MNI) coordinates (x, y, and z). Two sample t-tests were performed (Bonferroni corrected).
BA: Brodmann area; FCD: functional connectivity density; Inf.: inferior; Mid.: middle; Pos.: posterior; Sup.: superior.
Bold numbers indicates significance at p < 0.05.
2.6. Topological organization properties of functional connectivity network
For functional network analysis, 42 ROIs were used as nodes and the positive correlation coefficient was applied with a fisher-z-transformation (between each pair of these nodes) as edges. The weighed-network properties were calculated, with a sparsity range from 0.05 to 0.4 with step 0.01. The sparsity at 0.2 was selected due to the similar results ranging from 0.15 to 0.4. The global metrics examined included the clustering coefficient Cp, characteristic path length Lp (Watts and Strogatz, 1998), and network efficiency parameters (Latora and Marchiori, 2001), including the local efficiency Eloc and global efficiency Eglob. The node metrics examined included the node degree and betweenness centrality. Global efficiency quantifies the exchange of information across the entire network where information is concurrently exchanged. Local efficiency describes how well the information is exchanged by a node's neighbors (Achard and Bullmore, 2007). The clustering coefficient is defined as the number of edges that exist between a node's adjacent structures (Achard et al., 2006), and the node degree signifies the number of connections a node possesses. The network properties were calculated using GRETNA (http://www.nitrc.org/projects/gretna/) (Wang et al., 2015). The brain networks were visualized with the BrainNet Viewer (http://www.nitrc.org/projects/bnv/) (Xia et al., 2013).
2.7. Relationship among FCD, network properties, and clinical variables
Correlation analysis among the SFCD, LFCD, node degree, and clinical variables (IELT) was performed to determine whether the FCD and network property varied with disease progression in PE patients and healthy controls. A statistical significance level of p < 0.05 was used because the analysis was exploratory in nature.
2.8. Statistical analyses
The statistical analysis was performed using SPSS Version 16.0 (SPSS Inc. IL, USA) for the demographic data. The ages, IIEF-5, CIPE-5 and IELT were reported as the mean value ± the standard deviation, the marital status and educational level were reported as constituent ratio. Two-sample t-test as well as Chi-Squared test was used. p < 0.05 was accepted as a statistically significant value. Two-sample t-tests were also used to compare spontaneous brain activations between the PE and control groups (minimum statistical threshold voxel level at p < 0.001 and cluster level at p < 0.05, AlphaSim-corrected). The group difference was visualized using Resting-State fMRI Data Analysis Toolkit (http://restfmri.net/forum/index.php) (Song et al., 2011) A network-based statistical analysis (Zalesky et al., 2010) was applied to localize specific pairs of brain regions in which functional connectivity was altered in patients with PE at a significance level of p < 0.05.
3. Results
3.1. Demographic and clinical data
The demographic, psychiatric, and behavioral features in PE patients and controls are shown in Table 1. No significant differences in age (p = 0.95), education level (p = 0.97), or marital status (p = 0.77) were detected between PE patients and control subjects. PE patients (mean age: 23.75 ± 1.25 years) and healthy controls (mean age: 24.13 ± 0.74 years) all had normal erectile function [reference score of IIEF-5: 22-25 (Rosen et al., 1999)]. CPEI-5 and IELT showed a significant difference between these two groups (p < 0.01). The detailed information can be found in our previous work (B. Zhang et al., 2017).
Table 1.
Demographic and clinical characteristics of patients with PE and control subjects.
| PE patients (n = 20) | NC (n = 15) | p | |
|---|---|---|---|
| Age (year) | |||
| Mean ± SD | 27.95 ± 4.52 | 27.87 ± 3.78 | 0.95 |
| Marital status, N (%) | 0.77# | ||
| Single | 11 (55%) | 9 (60%) | |
| Married | 9 (45%) | 6 (40%) | |
| Education level, N (%) | 0.97# | ||
| Elementary | 4 (20%) | 2 (13.33%) | |
| High school | 10 (50%) | 6 (40%) | |
| University | 6 (30%) | 7 (46.67%) | |
| IIEF-5 score | |||
| Mean ± SD | 23.75 ± 1.25 | 24.13 ± 0.74 | 0.30 |
| CIPE-5 score | |||
| Mean ± SD | 9.00 ± 2.12 | 22.07 ± 2.02 | <0.01 |
| IELT (in min) | |||
| Mean ± SD | 0.86 ± 0.41 | 10.69 ± 6.84 | <0.01 |
Data from questionnaires were presented in terms of mean score (mean) and standard deviation (SD) in the premature ejaculation (PE) and healthy control (NC) groups.#: chi-squared test; without #: two-sample t-test.
IIEF-5: International Index of Erectile Function; CIPE-5: International Index of Erectile Function; IELT: intravaginal ejaculatory latency time.
3.2. SFCD and LFCD in patients with PE
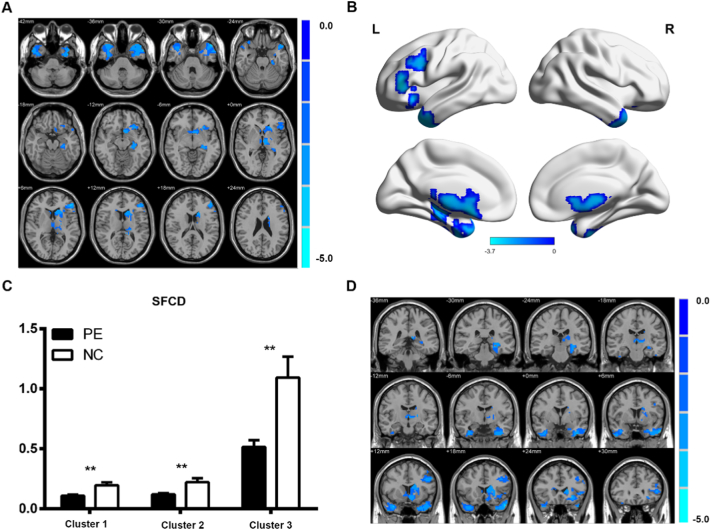
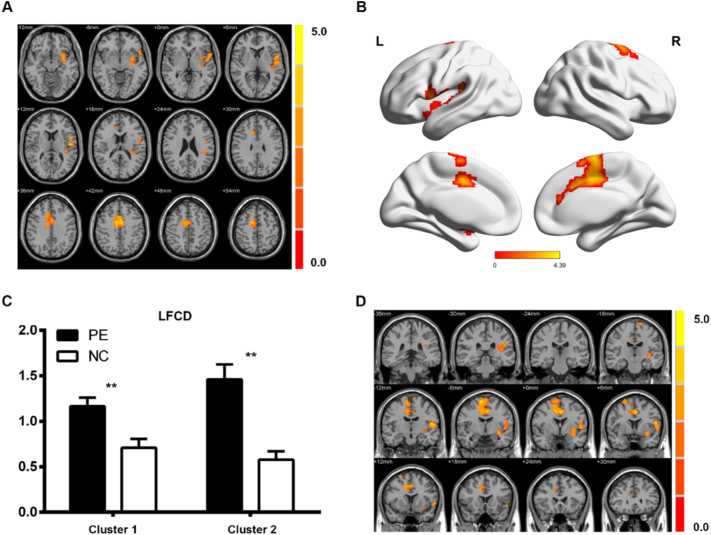
Compared with healthy controls, PE patients showed a significantly decreased SFCD in the bilateral middle temporal gyrus, bilateral fusiform, left orbitofrontal cortex (OFC), left caudate, and left thalamus (p < 0.05, AlphaSim-corrected); however, no significantly increased SFCD was found in PE patients (Fig. 1 and Table 2). In contrast, PE patients showed increased LFCD in the left insula, left Heschl's gyrus, left putamen, bilateral supplementary motor area (SMA), middle cingulate cortex (MCC), and anterior cingulate cortex (ACC) (p < 0.05, AlphaSim-corrected), but no significant reduction in LFCD was found in PE patients (Fig. 2 and Table 2).
Fig. 1.
Differences in SFCD between PE patients and NCs. The color bar indicates the T score level, which represents a decreased SFCD in PE patients compared with the NCs. (A) Axial view; (B) 3D view of different SFCD areas in the PE patients and NCs; (C) the SFCD value at the peak point of each cluster in the PE patients and NCs; (D) the coronal view.
**p < 0.01.
AlphaSim-corrected, significance level was set at p < 0.05 (voxel-level threshold p < 0.01, cluster-level threshold p < 0.05, cluster size = 387). NC, normal control; PE, premature ejaculation; SFCD, short-range functional connectivity density. Cluster 1: x: −39, y: −3, z: −39; Cluster 2: x: 36, y: 12, z: −33; Cluster 3: x: −48, y: 36, z: 6. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 2.
Differences in SFCD and LFCD between PE patients and NC subjects.
| Cluster | Cluster size (voxel) | MNI coordinates |
t value | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| SFCD | 1 | 986 | −39 | −3 | −39 | −3.70 |
| 2 | 581 | 36 | 12 | −33 | −3.35 | |
| 3 | 1681 | −48 | 36 | 6 | −3.58 | |
| LFCD | 1 | 482 | −54 | 6 | 15 | 3.54 |
| 2 | 755 | 12 | −6 | 66 | 4.39 | |
LFCD: Long-range functional connectivity density; SFCD: short-range functional connectivity density. Two-sample t-tests were used in this study.
Fig. 2.
Differences in LFCD between patients with PE and NCs. The color bar indicates the T score level, which represents an increased SFCD in the patients with PE compared with the NCs. (A) Axial view; (B) 3D view of the different LFCD areas between the patients with PE and NCs; (C) LFCD value at the peak point of each cluster in the patients with PE and NCs; (D) coronal view.
**p < 0.01.
AlphaSim-corrected, significant level was set at p < 0.05 (voxel-level threshold p < 0.01, cluster-level threshold p < 0.05, cluster size = 237). LFCD, long-range functional connectivity density. Cluster 1: x: −54, y: 6, z: 15; Cluster 2: x: 12, y: −6, z: 66. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.3. ROI-based analysis of SFCD and LFCD in PE patients and controls
The signals were extracted from 42 functional hub regions [defined by Tomasi and Volkow, 2012] of each subject's SFCD and LFCD map. Compared with control subjects, PE patients had decreased SFCD in the left posterior cingulum (t = −2.26, p = 0.03), left dorsolateral frontal gyrus (t = −2.31, p = 0.03), left nucleus accumbens (t = −2.63, p = 0.01), and left fusiform (t = −3.21, p < 0.01). In contrast, significantly increased LFCD was found in the bilateral precuneus (right: t = 2.34, p = 0.03; left: t = 2.43, p = 0.02), right ACC (t = 2.33, p = 0.02), and MCC (t = 2.29, p < 0.01) in PE patients (Table 3, Bonferroni-corrected). The results were consistent with those of the FCD analysis.
3.4. Topological organization properties of the functional connectivity network
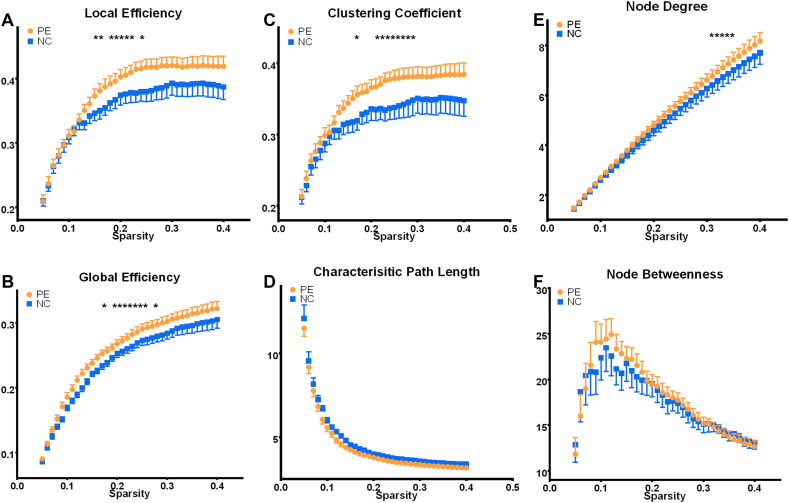
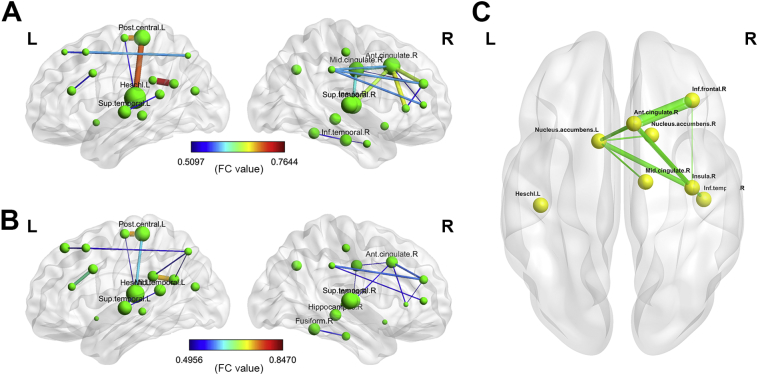
A statistically significant increase was observed in global efficiency, local efficiency, clustering coefficient, and node degree (p < 0.05), but no statistically significant differences were found in the characteristic path length and node betweenness in PE subjects compared with NCs (Fig. 3). Furthermore, the connectivity strengths between several nodes in the network were altered (p < 0.05; Fig. 4A and B). The connection among these nodes was reinforced in PE patients particularly in the right ACC, right inferior frontal gyrus, bilateral nucleus accumbens, right insula, left Heschl's gyrus, right MCC, and right inferior temporal gyrus. Moreover, the node degree in the right ACC, right inferior frontal gyrus, and right MCC significantly increased in the patients with PE (p < 0.05; Fig. 4C).
Fig. 3.
Graph theoretical and network-based statistical analysis. Patients with PE had significantly higher global efficiency (A), local efficiency (B), clustering coefficient (C), and node degree (E), but no statistically significant differences in the characteristic path length (D) and node betweenness (F) were found between PE patients and NCs.
*p < 0.05.
Fig. 4.
Network patterns in patients with PE and NCs. (A) Network pattern of patients with PE (node size represents the degree of the node, and the edge size represents the correlation coefficient between the connected nodes). One-sample t-test at p < 0.05. (B) Network pattern of NCs (node size represents the degree of the node, and the edge size represents the correlation coefficient between the connected nodes). One-sample t-test at p < 0.05. (C) Network edges and nodes in the patients with PE and NCs (the edge size represents p value between PE and NC groups and the nodes, which were significantly different between PE patients and controls, p < 0.05. The degree of the presented nodes was significantly different between PE patients and controls, p < 0.05). r, Correlation coefficient.
3.5. Correlation between clinical scores and brain activity
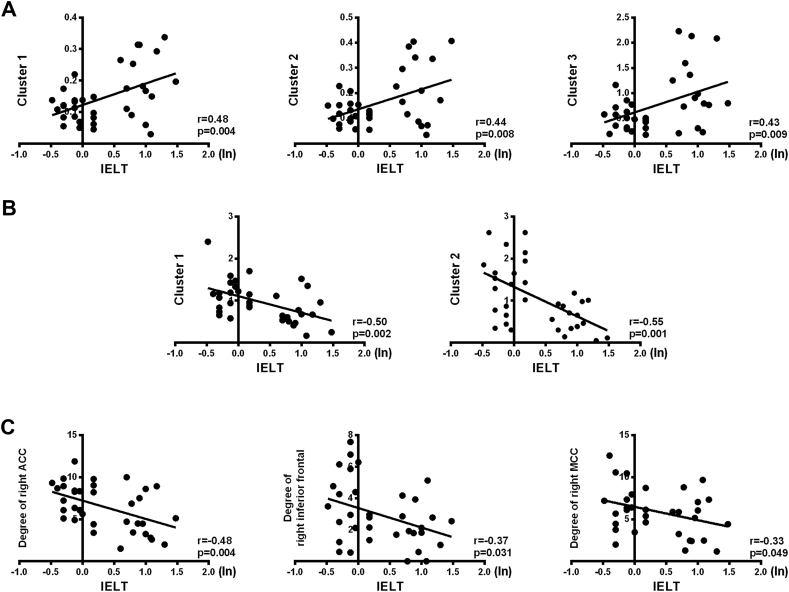
The IELT score was significantly positively correlated with the SFCD at three clusters (p < 0.05, AlphaSim-corrected; Cluster 1: x: −39, y: −3, z: −39; Cluster 2: x: 36, y: 12, z: −33; and Cluster 3: x: −48, y: 36, z: 6; Fig. 5A), and a negative correlation was observed between the IELT score and LFCD at two clusters (p < 0.05, AlphaSim-corrected; Cluster 1: x: −54, y: 6, z: 15; and Cluster 2: x: 12, y: −6, z: 66) (Fig. 5B). In addition, the node degree, which increased in PE patients compared with the controls in three brain regions (the right ACC, right inferior frontal gyrus, and right MCC), was negatively correlated with IELT (p < 0.05; Fig. 5C).
Fig. 5.
Correlation between clinical scores and brain activity. (A) Correlation between SFCD and IELT (Cluster 1: x: −39, y: −3, z: −39; Cluster 2: x: 36, y: 12, z: −33; and Cluster 3: x: −48, y: 36, z: 6). (B) Correlation between LFCD and IELT (Cluster 1: x: −54, y: 6, z: 15; and Cluster 2: x: 12, y: −6, z: 66). (C) Correlation between the node degree (right ACC, right inferior frontal gyrus, and right MCC) and IELT. IELT, Intravaginal ejaculatory latency time.
4. Discussion
This study explored the difference between PE patients and normal control from voxel level, ROI level to whole brain network using fMRI. The results showed that patients with PE had a significant disorder within several brain areas which were largely overlapped with a previously reported dopamine pathway (Costumero et al., 2013; Haber and Knutson, 2010). Locally, comparing to NCs, PE weakened connectivity within brain regions in dopamine pathway were revealed by SFCD on voxel level in PE patients, and several non-dopamine pathway brain regions which previously reported as DP related regions showed increased LFCD. Furthermore, ROI level analysis showed enhanced function connectivity between dopamine pathway brain regions and these non-dopamine pathway brain regions. On the whole brain level, the brain network metrics such as network efficiency, etc. were increased in the PE group.
In this study, we found a significantly decreased SFCD in the bilateral middle temporal gyrus, bilateral fusiform, left OFC, left caudate and left thalamus. ROI-based analyses also showed the similar results with decreased SFCD in the left nucleus accumbens and left fusiform. These results indicated that the connectivity were decreased among these adjacent regions, which were more closely cooperated as a functional network in NC, and are primarily located along the dopamine pathways (Costumero et al., 2013; Haber and Knutson, 2010; Ikemoto, 2007).
Based on currently available neurobiological knowledge on ejaculation, Waldinger speculated that lifelong PE is mediated by a more complex interaction of the central nervous system and the endocrinological system (Waldinger, 2014). It would have to include serotonergic and other neurotransmitter and endocrinological processes, like increased dopaminergic neurotransmission (Waldinger, 2014). Dopamine is vital in copulation (Romanelli et al., 2010) and has been shown to facilitate ejaculation by the activation of brain excitatory areas (Peeters and Giuliano, 2008). Men with longer tandem repeat lengths in the dopamine transporter gene have been shown to have increased transcription of the dopamine transporter protein, which is responsible for the reuptake of dopamine back into the presynaptic neuron, thereby reducing synaptic dopamine activity (Santtila et al., 2010). The current study found that PE patients have disrupted SFCD within dopamine, and moreover, the SFCD was positively related with IELT score. This provided macro connectivity level evidences on these dopamine mechanisms of PE. The decreased SFCD found in the brain regions in the dopamine pathway may indicate reduced brain activation. The decreased brain activity can reduce the reuptake of dopamine back into the presynaptic neuron (Santtila et al., 2010), thus lead to sustainable synaptic dopamine activation, and may eventually result in PE. Notably, the SFCD changed in nucleus accumbens and fusiform gyrus may interrupt the dopamine releasing (Sanna et al., 2017), dopamine signal receiving (Lee et al., 2015) and dopamine related neural activity (Lee et al., 2015). The SFCD and ROI analysis results may imply the damaged cortex in the dopamine system might be the pathological basis underlying PE.
The present results also indicate that LFCD is reinforced in the left insula, left Heschl's gyrus, left putamen, bilateral SMA, MCC, and ACC. As expected, the ROI based analysis also finds the same results. Many previous studies have found that these brain regions are associated with the dopamine system (Costumero et al., 2013; Edmiston et al., 2013; Sescousse et al., 2013), which may increase dopamine neurotransmission (Tomasi and Volkow, 2012). The ejaculatory reflex is regulated by neurochemicals, and several neurotransmitter systems have been implicated in this process. The central serotonin- and dopamine-containing neurons are primarily involved through their neurotransmitters. The increased connection between the dopamine system and other brain areas may increase dopamine neurotransmission, and the increased dopamine neurotransmission could promote brain activity (Saitz and Serefoglu, 2015), lower the threshold of ejaculation, and make the brain more sensitive to erotic stimuli.
The functional connectivity network found that the PE patients showed increased global efficiency, local efficiency, clustering coefficient, and node degree compared with the NCs. The increased clustering coefficient and local efficiency indicate increased local segregation of the network. This is consistent with the results of the LFCD and ROI-based analysis. Furthermore, the lower the IELT score, the greater the node degree has been found in the right ACC, right inferior frontal gyrus, and right MCC.
The main limitation of this study is self-report IELT. IELT was measured for the 4-week baseline period during which both patients and NCs were asked to have sexual intercourse at least 4 times to reduce the error. Stopwatch technique instead of self-report should be used to get more accurate IELT. However, Chinese culture is conservative in terms of sexuality, and it is very reluctant to admit that one's sexual ability problems to their sex partner especially for the Chinese male. Thus it is difficult to persuade the patients to join the experiment and get them good cooperation if using stop watch method. In the future study, we should persuade the subjects to cooperate with us and use the stop watch technology. The sample size of this study is small, and more subjects should be included to reflect the entire PE population. Moreover, detailed data on clinical and demographical characteristics of the participants should been obtained. It would be also advisable to have complete questionnaires from patients with PE rather than to rely on self-reporting technique.
In conclusion, this study demonstrated weakened connectivity between the inner brain regions in the dopamine system and strengthened connectivity between the dopamine system and other brain areas in PE patients. It is believed that these findings may enhance the understanding of PE pathology and will provide a new approach to this medical issue for the development of new therapy strategies.
Acknowledgments
The authors thank Albert C. Yang for providing the “growing” algorithm to calculate short-range FCD.
This work was supported by the Jiangsu Science and Technology Department (BL2014001, Y. Dai), the National Natural Science Foundation of China (81571040, 81300925, B. Zhang., 81471643, B. Zhu), the Natural Science Foundation of Jiangsu Province (BK20131085, B. Zhang), the National and Provincial postdoctoral project (BE179 and 1501076A, BZ), the project of the sixth peak of talented people (WSN-O50, BZ), the key project of Nanjing Health Bureau (ZKX14027, BZ), and social development project of science and technology in Jiangsu Province (BE2016605, B. Zhang). The funders had no any roles in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Yutian Dai, Email: 13913957628@163.com.
Bing Zhang, Email: zhangbing_nanjing@nju.edu.cn.
References
- Achard S., Bullmore E. Efficiency and cost of economical brain functional networks. PLoS Comput. Biol. 2007;3 doi: 10.1371/journal.pcbi.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achard S., Salvador R., Whitcher B., Suckling J., Bullmore E. A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. J. Neurosci. 2006;26:63–72. doi: 10.1523/JNEUROSCI.3874-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J.S., Druzgal T.J., Lopez-Larson M., Jeong E.K., Desai K., Yurgelun-Todd D. Network anticorrelations, global regression, and phase-shifted soft tissue correction. Hum. Brain Mapp. 2011;32:919–934. doi: 10.1002/hbm.21079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B., Zerrin Yetkin F., Haughton V.M., Hyde J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Coolen L.M., Olivier B., Peters H.J., Veening J.G. Demonstration of ejaculation-induced neural activity in the male rat brain using 5-HT 1A agonist 8-OH-DPAT. Physiol. Behav. 1997;62:881–891. doi: 10.1016/s0031-9384(97)00258-8. [DOI] [PubMed] [Google Scholar]
- Costumero V., Barros-Loscertales A., Bustamante J.C., Ventura-Campos N., Fuentes P., Rosell-Negre P., Avila C. Reward sensitivity is associated with brain activity during erotic stimulus processing. PLoS One. 2013;8 doi: 10.1371/journal.pone.0066940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmiston E.K., Mchugo M., Dukic M.S., Smith S.D., Abou-Khalil B., Eggers E., Zald D.H. Enhanced visual cortical activation for emotional stimuli is preserved in patients with unilateral amygdala resection. J. Neurosci. 2013;33:11023–11031. doi: 10.1523/JNEUROSCI.0401-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J., Holmes A.P., Worsley K.J., Poline J., Frith C.D., Frackowiak R.S. Statistical parametric maps in functional imaging: a general linear approach. Hum. Brain Mapp. 1994;2:189–210. [Google Scholar]
- Gur S., Sikka S.C. The characterization, current medications, and promising therapeutics targets for premature ejaculation. Andrology. 2015;3:424–442. doi: 10.1111/andr.12032. [DOI] [PubMed] [Google Scholar]
- Haber S.N., Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens–olfactory tubercle complex. Brain Res. Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Sohn D., Cho Y., Yang W., Lee K., Juh R., Ahn K., Chung Y., Han S., Lee K. Brain activation by visual erotic stimuli in healthy middle aged males. Int. J. Impot. Res. 2006;18:452–457. doi: 10.1038/sj.ijir.3901449. [DOI] [PubMed] [Google Scholar]
- Latora V., Marchiori M. Efficient behavior of small-world networks. Phys. Rev. Lett. 2001;87 doi: 10.1103/PhysRevLett.87.198701. [DOI] [PubMed] [Google Scholar]
- Laumann E.O., Nicolosi A., Glasser D.B., Paik A., Gingell C., Moreira E., Wang T. Sexual problems among women and men aged 40–80 y: prevalence and correlates identified in the Global Study of Sexual Attitudes and Behaviors. Int. J. Impot. Res. 2005;17:39–57. doi: 10.1038/sj.ijir.3901250. [DOI] [PubMed] [Google Scholar]
- Lee S.W., Jeong B.S., Choi J., Kim J.W. Sex differences in interactions between nucleus accumbens and visual cortex by explicit visual erotic stimuli: an fMRI study. Int. J. Impot. Res. 2015;27:161–166. doi: 10.1038/ijir.2015.8. [DOI] [PubMed] [Google Scholar]
- Mallick H., Tandon S., Jagannathan N., Gulia K., Kumar V. Brain areas activated after ejaculation in healthy young human subjects. Indian J. Physiol. Pharmacol. 2007;51:81. [PubMed] [Google Scholar]
- Mouras H., Stoléru S., Bittoun J., Glutron D., Pélégrini-Issac M., Paradis A.-L., Burnod Y. Brain processing of visual sexual stimuli in healthy men: a functional magnetic resonance imaging study. NeuroImage. 2003;20:855–869. doi: 10.1016/S1053-8119(03)00408-7. [DOI] [PubMed] [Google Scholar]
- Murphy K., Birn R.M., Handwerker D.A., Jones T.B., Bandettini P.A. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? NeuroImage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters M., Giuliano F. Central neurophysiology and dopaminergic control of ejaculation. Neurosci. Biobehav. Rev. 2008;32:438–453. doi: 10.1016/j.neubiorev.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Rhoden E., Telöken C., Sogari P., Souto C.V. The use of the simplified International Index of Erectile Function (IIEF-5) as a diagnostic tool to study the prevalence of erectile dysfunction. Int. J. Impot. Res. 2002;14:245. doi: 10.1038/sj.ijir.3900859. [DOI] [PubMed] [Google Scholar]
- Romanelli R.J., Williams J.T., Neve K.A. Dopamine receptor signaling: intracellular pathways to behavior. In: Neve K.A., editor. The Dopamine Receptors. Humana Press; Totowa, NJ: 2010. pp. 137–173. [Google Scholar]
- Rosen R.C., Cappelleri J.C., Smith M.D. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction[J] Int. J. Impot. Res. 1999;11(6):319. doi: 10.1038/sj.ijir.3900472. [DOI] [PubMed] [Google Scholar]
- Saitz T.R., Serefoglu E.C. Advances in understanding and treating premature ejaculation. Nat. Rev. Urol. 2015;12:629–640. doi: 10.1038/nrurol.2015.252. [DOI] [PubMed] [Google Scholar]
- Sanna F., Bratzu J., Piludu M.A., Corda M.G., Melis M.R., Giorgi O., Argiolas A. Dopamine, noradrenaline and differences in sexual behavior between Roman high and low avoidance male rats: a microdialysis study in the medial prefrontal cortex. Front. Behav. Neurosci. 2017;11:108. doi: 10.3389/fnbeh.2017.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santtila P., Jern P., Westberg L., Walum H., Pedersen C.T., Eriksson E., Kenneth Sandnabba N. The dopamine transporter gene (DAT1) polymorphism is associated with premature ejaculation. J. Sex. Med. 2010;7:1538–1546. doi: 10.1111/j.1743-6109.2009.01696.x. [DOI] [PubMed] [Google Scholar]
- Serefoglu E.C., Saitz T.R. New insights on premature ejaculation: a review of definition, classification, prevalence and treatment. Asian J. Androl. 2012;14:822–829. doi: 10.1038/aja.2012.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serefoglu E.C., Mcmahon C.G., Waldinger M.D., Althof S.E., Shindel A., Adaikan G., Becher E.F., Dean J., Giuliano F., Hellstrom W.J. An evidence-based unified definition of lifelong and acquired premature ejaculation: report of the second International Society for Sexual Medicine Ad Hoc Committee for the Definition of Premature Ejaculation. J. Sex. Med. 2014;11:1423–1441. doi: 10.1111/jsm.12524. [DOI] [PubMed] [Google Scholar]
- Sescousse G., Barbalat G., Domenech P., Dreher J.C. Imbalance in the sensitivity to different types of rewards in pathological gambling. Brain. 2013;136:2527–2538. doi: 10.1093/brain/awt126. [DOI] [PubMed] [Google Scholar]
- Song X.-W., Dong Z.-Y., Long X.-Y., Li S.-F., Zuo X.-N., Zhu C.-Z., He Y., Yan C.-G., Zang Y.-F. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D., Volkow N.D. Functional connectivity density mapping. Proc. Natl. Acad. Sci. U. S. A. 2010;107:9885–9890. doi: 10.1073/pnas.1001414107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D., Volkow N.D. Functional connectivity hubs in the human brain. NeuroImage. 2011;57:908–917. doi: 10.1016/j.neuroimage.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D., Volkow N.D. Aging and functional brain networks. Mol. Psychiatry. 2012;17(471) doi: 10.1038/mp.2011.81. (549-458) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldinger M.D. Ejaculatio praecox, erectio praecox, and detumescentia praecox as symptoms of a hypertonic state in lifelong premature ejaculation: a new hypothesis. Pharmacol. Biochem. Behav. 2014;121:189–194. doi: 10.1016/j.pbb.2013.12.004. [DOI] [PubMed] [Google Scholar]
- Wang J., Wang X., Xia M., Liao X., Evans A., He Y. GRETNA: a graph theoretical network analysis toolbox for imaging connectomics. Front. Hum. Neurosci. 2015;9 doi: 10.3389/fnhum.2015.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts D.J., Strogatz S.H. Collective dynamics of ‘small-world’ networks. Nature. 1998;393:440. doi: 10.1038/30918. [DOI] [PubMed] [Google Scholar]
- Xia M., Wang J., He Y. BrainNet viewer: a network visualization tool for human brain connectomics. PLoS One. 2013;8 doi: 10.1371/journal.pone.0068910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C., Zang Y. DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front. Syst. Neurosci. 2010;4:13. doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C.-G., Wang X.-D., Zuo X.-N., Zang Y.-F. DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics. 2016:1–13. doi: 10.1007/s12021-016-9299-4. [DOI] [PubMed] [Google Scholar]
- Yang A.C., Tsai S.J., Liu M.E., Huang C.C., Lin C.P. The Association of Aging with White Matter Integrity and Functional Connectivity Hubs. Front. Aging Neurosci. 2016;8:143. doi: 10.3389/fnagi.2016.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y.-M., Xin Z.-C., Jiang H., Guo Y.-J., Liu W.-J., Tian L., Zhu J.-C. Sexual function of premature ejaculation patients assayed with Chinese Index of Premature Ejaculation. Asian J. Androl. 2004;6:121–126. [PubMed] [Google Scholar]
- Zalesky A., Fornito A., Bullmore E.T. Network-based statistic: identifying differences in brain networks. NeuroImage. 2010;53:1197–1207. doi: 10.1016/j.neuroimage.2010.06.041. [DOI] [PubMed] [Google Scholar]
- Zhang B., Lu J., Xia J., Wang F., Li W., Chen F., Han Y., Chen Y., Zhu B., Qing Z. Functional insights into aberrant brain responses and integration in patients with lifelong premature ejaculation. Sci. Rep. 2017;7:460. doi: 10.1038/s41598-017-00421-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Liao M., Yao Z., Hu B., Xie Y., Zheng W., Hu T., Zhao Y., Yang F., Zhang Y. Frequency-specific functional connectivity density as an effective biomarker for adolescent generalized anxiety disorder. Front. Hum. Neurosci. 2017;11 doi: 10.3389/fnhum.2017.00549. [DOI] [PMC free article] [PubMed] [Google Scholar]