Abstract
Background
Understanding trends in patient profiles and identifying predictors for adverse outcomes are key to improving the effectiveness of HIV care and treatment programs. Previous work in Kenya has documented findings from a rural setting. This paper describes trends in demographic and clinical characteristics of antiretroviral therapy (ART) treatment cohorts at a large urban, referral HIV clinic and explores treatment outcomes and factors associated with attrition during 12 years of follow-up.
Methods
This was a retrospective cohort analysis of HIV-infected adults who started ART between January 1, 2004, and September 30, 2015. ART-experienced patients and those with missing data were excluded. The Cochran–Armitage test was used to determine trends in baseline characteristics over time. Cox proportional hazards models were used to determine the effect of baseline characteristics on attrition.
Results
ART uptake among older adolescents (15–19 years), youth, and young adults increased over time (p=0.0001). Independent predictors for attrition included (adjusted hazard ratio [95% CI]) male sex: 1.30 (1.16–1.45), p=0.0001; age: 15–19 years: 1.83 (1.26–2.66), p=0.0014; 20–24 years: 1.93 (1.52–2.44), p=0.0001; and 25–29 years: 1.31 (1.11–1.54), p=0.0012; marital status – single: 1.27 (1.11–1.44), p=0.0005; and divorced/separated: 1.56 (1.30–1.87), p=0.0001; urban residence: 1.40 (1.20–1.64), p=0.0001; entry into HIV care following hospitalization: 1.31 (1.10–1.57), p=0.0026, or transfer from another facility: 1.60 (1.26–2.04), p=0.0001; initiation of ART more than 12 months after the date of HIV diagnosis: 1.36 (1.19–1.55), p=0.0001, and history of a current or past opportunistic infection (OI): 1.15 (1.02–1.30), p=0.0284.
Conclusion
Although ART uptake among adolescents and young people increased over time, this group was at increased risk for attrition. Single marital status, urban residence, history of hospitalization or OI, and delayed initiation of ART also predicted attrition. This calls for focused evidence-informed strategies to address attrition and improve outcomes.
Keywords: antiretroviral therapy, attrition, lost to follow-up, risk factors, electronic medical records, adolescents, urban
Introduction
The past decade has witnessed a remarkable increase in access to human immunodeficiency virus (HIV) prevention modalities and lifesaving antiretroviral therapy (ART) in resource-limited settings.1 This has led to notable declines in HIV incidence and HIV-related morbidity and mortality.2 In Kenya, the number of people living with HIV (PLHIV) receiving ART increased a 100-fold between 2003 and 2013.3,4 By 2015, more than 800,000 adults aged 15 years and above, and over 70,000 children aged 0–14 years were receiving ART.5 Annual acquired immunodeficiency syndrome (AIDS)-related deaths decreased by 65% between 2003 and 2013.6
In May 2014, the Joint United Nations Programme on HIV/AIDS announced ambitious new targets to have 90% of all PLHIV knowing their status, 90% of those diagnosed with HIV receiving effective ART, and 90% of those receiving ART achieving viral suppression, by the year 2020.7 To meet these targets, HIV programs must identify and address challenges through lessons learnt over the last decade and a half of the HIV/AIDS response in resource-scarce settings. For instance, the median CD4 of those initiating ART has only modestly increased.8 Patients starting ART at low CD4 cell counts have poorer treatment outcomes, including the risk of early mortality.9 Additionally, studies from ART programs in sub-Saharan Africa (SSA) report that a third of patients on ART are lost to care by 36 months, with most attrition occurring within the first year.10
Establishing temporal trends and identifying factors associated with adverse treatment outcomes in diverse settings are key to improving long-term effectiveness of ART programs in low- and middle-income countries.11 Data on temporal trends and treatment outcomes from a large HIV treatment site in rural Kenya demonstrated increased rates of retention in care.12 Although the urban environment presents unique challenges and opportunities in HIV management, there is a paucity of data on temporal trends from this setting. Besides, little has been published on this topic within the context of a large, urban, referral setting in Kenya. In view of this data gap, we sought to determine trends in demographic and clinical characteristics of adult HIV-infected ART cohorts over a 12-year period at a large urban referral HIV clinic in Nairobi, Kenya. We further described how these characteristics influence attrition.
Methods
Study setting and design
This was a retrospective cohort analysis of data, which were routinely collected during and prior to the implementation of the Partnership for Advanced Care and Treatment – Centres of Excellence (PACT-CoE) program. PACT-CoE was a US President’s Emergency Plan for AIDS Relief-funded project implemented at the ambulatory HIV Comprehensive Care Centre (CCC) of the Kenyatta National Hospital (KNH) from 2010 to 2015. KNH is Kenya’s largest national teaching and referral hospital. PACT-CoE’s principal aim was to build capacity and scope for sustainable HIV preventive and treatment services. Patients diagnosed with HIV access the CCC from several sources, including the on-site client- and provider-initiated testing and counseling services. Care at the CCC is provided by a team of primary and specialist providers. Outpatient HIV care is provided at no cost to patients, and encompasses ART as well as treatment of, and prophylaxis against, select opportunistic infections (OIs). Free laboratory tests provided include HIV-specific investigations (CD4 T cell count and HIV RNA/DNA assays), OI screening for tuberculosis (TB) and cryptococcus, and, where indicated, additional tests such as hemogram, lipid profile, liver and renal function tests. In-country ART initiation policy guidelines based on CD4 cell count thresholds have evolved during the follow-up period, starting with CD4 ≤200 cells/mm3 in 2002, and increasing to CD4 ≤250, CD4 ≤350, and CD4 ≤500 cells/mm3 in 2007, 2010, and 2014, respectively.
HIV care and treatment consisted of a standard minimum package of care that includes evidence-based interventions and ART. Currently, the recommended first-line ART comprises tenofovir (TDF), lamivudine, and efavirenz (EFV).13 Zidovudine and nevirapine are alternative nucleoside and non-nucleoside reverse transcriptase inhibitors for those unable to tolerate TDF and EFV, respectively.
Ethical consideration
Approval for the study was obtained from the KNH/University of Nairobi Ethics and Research Committee and the US Centers for Disease Control and Prevention, Associate Director for Science. The requirement for individual informed consent was waived by the committee, as the research involved no more than minimal risk; the waiver would not adversely affect the rights and welfare of the subjects; and it would be impracticable to conduct the research without the waiver.
Selection and description of participants
All patients ≥15 years who started ART between January 1, 2004, and September 30, 2015, were included in the analysis. Exclusion criteria were 1) patients missing the main outcome or explanatory variables of interest, such as sex, age/date of birth; 2) non-ART naïve patients – transferred to the clinic on ART; 3) starting ART before January 1, 2004, or after September 30, 2015; and 4) aged <15 years at ART start.
Data management and analysis
Data extraction
Data were extracted using an in-built data mining functionality of an electronic medical records (EMR) system designed for HIV care and treatment and exported to Microsoft Access for analysis in SAS.
Variable measurements
Key outcome variables that were extracted included post-ART initiation treatment status outcomes: 1) attrition (died or lost to follow-up [LTFU]); 2) retention on ART; and 3) transfer out of the facility. The main outcome variable of interest was attrition. This was defined as those who died or were LTFU (having no contact with the facility for at least 6 months). Retention was defined as those who were active on ART at the end of the follow-up period. Key explanatory variables included year of ART start (categorized into six groups: 2004–2006, 2007–2008, 2009–2010, 2011–2012, 2013–2014, and 2015), demographic characteristics such as sex, age at ART start (categorized into five age groups: 15–19, 20–24, 25–29, 30–54, and 55 years and older), marital status, and residence (urban/rural). Clinical characteristics included CD4 cell count (categorized into six groups: 0–50, 51–100, 101–250, 251–350, 351–499, and ≥500 cells/µL), World Health Organization (WHO) clinical stage (categorized into two: stages 1 and 2, stages 3 and 4), and OIs at ART start. Nondocumentation has previously been associated with poor outcomes among patients with HIV/AIDS.14 To assess for the role of missing data on covariates we created a “not documented” category for marital status, residence, care entry point, HIV diagnosis date, disease stage, and CD4 count.
Quality assurance
Extracted data were stripped of patient identifiers such as names, home address, and telephone number(s). In addition, the data analysts did not have access to the original data in the EMR and had no way of linking extracted records to any individual patient. Patient serial numbers were, however, maintained for ease of merging datasets from different sources.
Statistical analysis
Demographic and clinical characteristics that could potentially influence ARV treatment status outcomes were measured at the time of ART initiation, also called “baseline”. Descriptive analyses were performed for baseline characteristics. Categorical variables (age group, marital status, residence, care entry point, linkage to ART after HIV diagnosis, WHO stage, CD4 category, OIs and ART start regimen) were summarized using proportions. Continuous variables (age and CD4 count) were summarized using mean values and SDs or medians and interquartile ranges (IQRs) as appropriate. The Cochran–Armitage test for trend was used to determine trends in baseline characteristics over time. All statistical tests were two-sided, and p-values <0.05 were considered significant.
Cox proportional hazards models were used to determine the effect of baseline demographic and clinical characteristics on attrition. Unadjusted and adjusted hazard ratios (aHR) with 95% CIs and p-values were generated and used to determine the patient characteristics that were independently associated with ART attrition. Wald confidence limits were used for all Cox univariate/multivariate analyses. Data were analyzed using SAS software 9.2 (SAS Institute, Cary, NC, USA).
Results
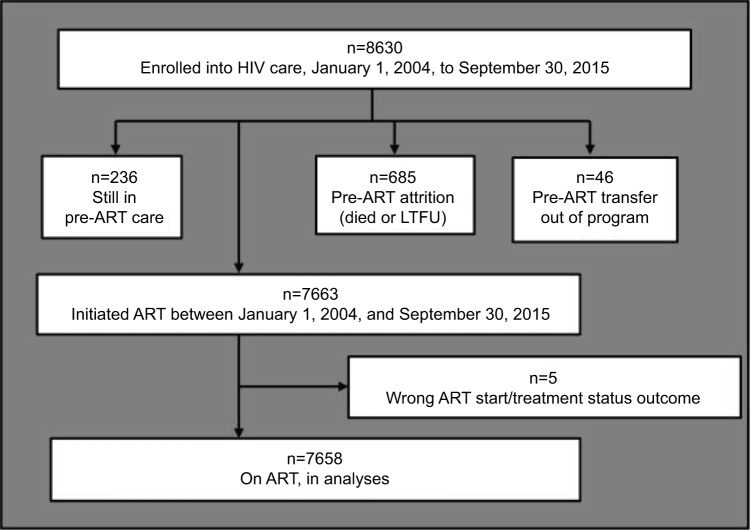
A total of 8630 patients were enrolled into care. Of these, 7663 were initiated on ART. After excluding those with a wrong ART start or treatment status outcome date, 7658 were included in the analyses (Figure 1).
Figure 1.
Schematic flow chart showing number of patients included in this analysis.
Abbreviations: ART, antiretroviral therapy; LTFU, lost to follow-up.
Patient characteristics at ART initiation
Baseline characteristics are presented in Table 1. Overall, 63.1% of the patient population was female, 56.1% was married, and 77.6% was urban residents. The mean age at baseline was 38.7 years (SD 9.7), and the most common point of entry into care was the on-site voluntary counseling and testing (VCT) center (42.3%). Thirty-six percent of all patients had an OI at baseline.
Table 1.
Baseline characteristics
| Baseline characteristics | Female, n=4831 | Male, n=2827 | Overall, n=7658 |
|---|---|---|---|
| Age (years) | |||
| Mean (SD) | 37.3 (9.5) | 41.1 (9.5) | 38.7 (9.7) |
| Age group, n (%), years | |||
| 15–19 | 60 (1.2) | 49 (1.7) | 109 (1.4) |
| 20–24 | 244 (5.1) | 52 (1.8) | 296 (3.9) |
| 25–29 | 720 (14.9) | 161 (5.7) | 881 (11.5) |
| 30–54 | 3550 (73.5) | 2331 (82.5) | 5881 (76.8) |
| ≥55 | 257 (5.3) | 234 (8.3) | 491 (6.4) |
| Marital status, n (%) | |||
| Single | 1373 (28.4) | 371 (13.1) | 1744 (22.8) |
| Married | 2213 (45.8) | 2081 (73.6) | 4294 (56.1) |
| Divorced/separated | 446 (9.2) | 145 (5.1) | 591 (7.7) |
| Widowed | 619 (12.8) | 155 (5.5) | 774 (10.1) |
| Not documented | 180 (3.7) | 75 (2.7) | 255 (3.3) |
| Residence, n (%) | |||
| Rural | 696 (14.4) | 390 (13.8) | 1086 (14.2) |
| Urban | 3684 (76.3) | 2262 (80.0) | 5946 (77.6) |
| Not documented | 451 (9.3) | 175 (6.2) | 626 (8.2) |
| Year of ART start, n (%) | |||
| 2004–2006 | 708 (14.7) | 437 (15.5) | 1145 (15.0) |
| 2007–2008 | 729 (15.1) | 397 (14.0) | 1126 (14.7) |
| 2009–2010 | 682 (14.1) | 425 (15.0) | 1107 (14.5) |
| 2011–2012 | 1108 (22.9) | 695 (24.6) | 1803 (23.5) |
| 2013–2014 | 1211 (25.1) | 662 (23.4) | 1873 (24.5) |
| 2015 | 393 (8.1) | 211 (7.5) | 604 (7.9) |
| Care entry point, n (%) | |||
| On-site VCT | 1956 (40.5) | 1284 (45.4) | 3240 (42.3) |
| PMTCT | 829 (17.2) | 366 (12.9) | 1195 (15.6) |
| TB clinic | 395 (8.2) | 276 (9.8) | 671 (8.8) |
| In-patient | 443 (9.2) | 259 (9.2) | 702 (9.2) |
| Other facilities | 180 (3.7) | 111 (3.9) | 291 (3.8) |
| Other sources | 597 (12.4) | 322 (11.4) | 919 (12.0) |
| Not documented | 431 (8.9) | 209 (7.4) | 640 (8.4) |
| Linkage to ART after HIV diagnosis (time [months] from HIV diagnosis to ART start) | |||
| Months to linkage | |||
| Within 3 months | 1132 (23.4) | 811 (28.7) | 1943 (25.4) |
| Within 6 months | 1362 (28.2) | 938 (33.2) | 2300 (30.0) |
| Within 12 months | 1699 (35.2) | 1129 (39.9) | 2828 (36.9) |
| HIV diagnosis date not documented | 1636 (33.9) | 898 (31.8) | 2534 (33.1) |
| Disease stage, n (%) | |||
| Stages 1 and 2 | 2052 (42.5) | 1035 (36.6) | 3087 (40.3) |
| Stages 3 and 4 | 1699 (35.2) | 1232 (43.6) | 2931 (38.3) |
| Not documented | 1080 (22.4) | 560 (19.8) | 1640 (21.4) |
| CD4 count (cells/μL) | |||
| Median (IQR) | 231 (109–384) | 189 (76–326) | 216 (95–359) |
| CD4 category, n (%) | |||
| 0–50 | 390 (8.1) | 331 (11.7) | 721 (9.4) |
| 51–100 | 308 (6.4) | 221 (7.8) | 529 (6.9) |
| 101–250 | 909 (18.8) | 581 (20.6) | 1490 (19.5) |
| 251–350 | 496 (10.3) | 297 (10.5) | 793 (10.4) |
| 351–500 | 427 (8.8) | 228 (8.1) | 655 (8.6) |
| >500 | 435 (9.0) | 160 (5.7) | 595 (7.8) |
| Not documented | 1866 (38.6) | 1009 (35.7) | 2875 (37.5) |
| OIs, n (%) | |||
| TB | 410 (8.5) | 371 (13.1) | 781 (10.2) |
| PCP | 172 (3.6) | 99 (3.5) | 271 (3.5) |
| Cryptococcal disease | 30 (0.6) | 21 (0.7) | 51 (0.7) |
| Oral candidiasis | 153 (3.2) | 81 (2.9) | 234 (3.1) |
| Esophageal candidiasis | 19 (0.4) | 8 (0.3) | 27 (0.4) |
| Kaposi’s sarcoma | 42 (0.9) | 33 (1.2) | 75 (1.0) |
| Other OIsa | 1268 (26.2) | 781 (27.6) | 2049 (26.8) |
| Any OIb | 1672 (34.6) | 1085 (38.4) | 2757 (36.0) |
| Start regimenc, n (%) | |||
| TDF-based regimen | 2274 (49.9) | 1337 (50.4) | 3611 (50.1) |
| AZT-based regimen | 1059 (23.2) | 634 (23.9) | 1693 (23.5) |
| Stavudine-based regimen | 1223 (26.8) | 680 (25.7) | 1903 (26.4) |
Notes:
Other OIs: OIs other than those listed in the table.
Any OI: OIs listed in table plus other OIs.
Start regimen: 451 patients (275 females and 176 males) had missing/wrong start regimen.
Abbreviations: ART, antiretroviral therapy; AZT, zidovudine; IQR, interquartile range; OI, opportunistic infection; PCP, pneumocystis pneumonia; PMTCT, prevention of mother-to-child transmission; TB, tuberculosis; TDF, tenofovir; VCT, voluntary counseling and testing.
Compared to women, men were, at ART initiation, older (41.1 years [SD 9.5] vs 37.3 years [SD 9.5]), more likely to be married (73.6% vs 45.8%), and had more advanced disease (WHO stage 3 or 4: 43.6% vs 35.2%; CD4 [median {IQR}]: 189 [76–326] vs 231 [109–384]).
Trends in baseline characteristics at ART initiation
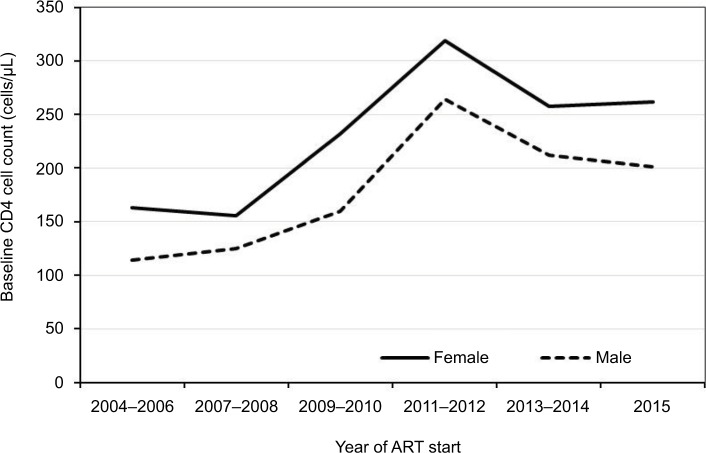
Trends in baseline characteristics stratified by 2-year cohorts are presented in Table 2A and B. The proportion of older adolescents (15–19 years), youth (20–24 years), and young adults (25–29 years) initiating ART increased during the observation period (p=0.0001; Table 2A). A similar trend was observed among those who were single or divorced (p=0.0001). While care entry through the prevention of mother-to-child transmission (PMTCT) and TB clinics declined (p=0.0001), the proportion of patients accessing care from the in-patient unit increased (p=0.0188). Similarly, care entry following referral from other facilities and other sources increased (p=0.0001). Declining trends were noted in the proportions of patients initiated on ART 3 and 6 months after HIV diagnosis (p=0.0001; Table 2B). The proportion of patients initiating ART with CD4 counts >350 cells/µL increased (p=0.0001), while those starting ART with advanced disease (WHO stage 3 or 4) declined over time (p=0.0001). Overall, the median CD4 cell count at ART initiation increased (Figure 2). The proportion of patients with oral candidiasis at ART initiation declined over time (p=0.0001), while that with other OIs or any OI increased (p=0.0001; Table 2B). Overall, nondocumentation of care entry point, disease stage, and CD4 count declined during the period of observation (p=0.0001). Nondocumentation of residence, however, increased (p=0.0001).
Table 2.
(A) Trends in baseline demographic characteristics by year of ART start
| Baseline characteristics | 2004–2006, n=1145 | 2007–2008, n=1126 | 2009–2010, n=1107 | 2011–2012, n=1803 | 2013–2014, n=1873 | 2015, n=604 | p-value |
|---|---|---|---|---|---|---|---|
| Sex | |||||||
| Female | 708 (61.8) | 729 (64.7) | 682 (61.6) | 1108 (61.5) | 1211 (64.7) | 393 (65.1) | 0.25 |
| Male | 437 (38.2) | 397 (35.3) | 425 (38.4) | 695 (38.6) | 662 (35.3) | 211 (34.9) | Ref |
| Marital status | |||||||
| Single | 230 (20.1) | 229 (20.3) | 203 (18.3) | 407 (22.6) | 507 (27.1) | 168 (27.8) | 0.0001 |
| Married | 684 (59.7) | 633 (56.2) | 707 (63.9) | 1067 (59.2) | 906 (48.4) | 297 (49.2) | Ref |
| Divorced | 70 (6.1) | 83 (7.4) | 62 (5.6) | 105 (5.8) | 204 (10.9) | 67 (11.1) | 0.0001 |
| Widowed | 126 (11.0) | 139 (12.3) | 106 (9.6) | 169 (9.4) | 182 (9.7) | 52 (8.6) | 0.65 |
| Not documented | 35 (3.1) | 42 (3.7) | 29 (2.6) | 55 (3.1) | 74 (4.0) | 20 (3.3) | 0.0675 |
| Age (years) | |||||||
| 15–19 | 5 (0.4) | 10 (0.9) | 15 (1.4) | 24 (1.3) | 42 (2.2) | 13 (2.2) | 0.0001 |
| 20–24 | 22 (1.9) | 24 (2.1) | 42 (3.8) | 67 (3.7) | 98 (5.2) | 43 (7.1) | 0.0001 |
| 25–29 | 105 (9.2) | 112 (10.0) | 130 (11.7) | 177 (9.8) | 274 (14.6) | 83 (13.7) | 0.0001 |
| 30–54 | 949 (82.9) | 907 (80.6) | 856 (77.3) | 1405 (77.9) | 1330 (71.0) | 434 (71.9) | Ref |
| ≥55 | 64 (5.6) | 73 (6.5) | 64 (5.8) | 130 (7.2) | 129 (6.9) | 31 (5.1) | 0.0646 |
| Residency | |||||||
| Rural | 240 (21.0) | 218 (19.4) | 181 (16.4) | 264 (14.6) | 147 (7.9) | 36 (6.0) | Ref |
| Urban | 820 (71.6) | 837 (74.3) | 868 (78.4) | 1406 (78.0) | 1515 (80.9) | 500 (82.8) | 0.0001 |
| Not documented | 85 (7.4) | 71 (6.3) | 58 (5.2) | 133 (7.4) | 211 (11.3) | 68 (11.3) | 0.0001 |
| Care entry point | |||||||
| VCT | 424 (37.0) | 448 (39.8) | 497 (44.9) | 742 (41.2) | 853 (45.5) | 276 (45.7) | Ref |
| PMTCT | 268 (23.4) | 228 (20.3) | 194 (17.5) | 296 (16.4) | 155 (8.3) | 54 (8.9) | 0.0001 |
| TB clinic | 133 (11.6) | 136 (12.1) | 132 (11.9) | 212 (11.8) | 51 (2.7) | 7 (1.2) | 0.0001 |
| In-patient | 103 (9.0) | 87 (7.7) | 71 (6.4) | 149 (8.3) | 209 (11.2) | 83 (13.7) | 0.0188 |
| Other facilities | 28 (2.5) | 25 (2.2) | 25 (2.3) | 65 (3.6) | 138 (7.4) | 10 (1.7) | 0.0001 |
| Other sources | 68 (5.9) | 76 (6.8) | 76 (6.9) | 145 (8.0) | 386 (20.6) | 168 (27.8) | 0.0001 |
| Not documented | 121 (10.6) | 126 (11.2) | 112 (10.1) | 194 (10.8) | 81 (4.3) | 6 (1.0) | 0.0001 |
|
| |||||||
| (B) Trends in baseline clinical characteristics by year of ART start
| |||||||
| Baseline characteristics | 2004–2006, n=1145 | 2007–2008, n=1126 | 2009–2010, n=1107 | 2011–2012, n=1803 | 2013–2014, n=1873 | 2015, n=604 | p-value |
|
| |||||||
| Time to linkage to ART (months) | |||||||
| 3 months | |||||||
| Within 3 months | 311 (27.2) | 437 (38.8) | 345 (31.2) | 218 (12.1) | 451 (24.1) | 181 (30.0) | Ref |
| After 3 months | 497 (43.4) | 342 (30.4) | 445 (40.2) | 1086 (60.2) | 633 (33.8) | 178 (29.5) | 0.0001 |
| 6 months | |||||||
| Within 6 months | 406 (35.5) | 484 (43.0) | 391 (35.3) | 315 (17.5) | 509 (27.2) | 195 (32.3) | Ref |
| After 6 months | 402 (35.1) | 295 (26.2) | 399 (36.0) | 989 (54.9) | 575 (30.7) | 164 (27.2) | 0.0001 |
| 12 months | |||||||
| Within 12 months | 539 (47.1) | 550 (48.9) | 472 (42.6) | 454 (25.2) | 591 (31.6) | 222 (36.8) | Ref |
| After 12 months | 269 (23.5) | 229 (20.3) | 318 (28.7) | 850 (47.1) | 493 (26.3) | 137 (22.7) | 0.0001 |
| Disease stage | |||||||
| WHO stages 1 and 2 | 208 (18.2) | 380 (33.8) | 449 (40.6) | 747 (41.4) | 924 (49.3) | 379 (62.8) | Ref |
| WHO stages 3 and 4 | 441 (38.5) | 494 (43.9) | 344 (31.1) | 833 (46.2) | 612 (32.7) | 207 (34.3) | 0.0001 |
| Not documented | 496 (43.3) | 252 (22.4) | 314 (28.4) | 223 (12.4) | 337 (18.0) | 18 (3.0) | 0.0001 |
| CD4 count | |||||||
| 0–50 | 105 (9.2) | 141 (12.5) | 61 (5.5) | 89 (4.9) | 241 (12.9) | 84 (13.9) | 0.0001 |
| 51–100 | 92 (8.0) | 122 (10.8) | 59 (5.3) | 79 (4.4) | 127 (6.8) | 50 (8.3) | 0.0001 |
| 101–250 | 202 (17.6) | 277 (24.6) | 226 (20.4) | 257 (14.3) | 391 (20.9) | 137 (22.7) | 0.24 |
| 251–350 | 78 (6.8) | 63 (5.6) | 96 (8.7) | 204 (11.3) | 291 (15.5) | 61 (10.1) | Ref |
| 351–500 | 33 (2.9) | 48 (4.3) | 46 (4.2) | 197 (10.9) | 227 (12.1) | 104 (17.2) | 0.0001 |
| >500 | 28 (2.5) | 34 (3.0) | 49 (4.4) | 212 (11.8) | 186 (9.9) | 86 (14.2) | 0.0001 |
| Not documented | 607 (53.0) | 441 (39.2) | 570 (51.5) | 765 (42.4) | 410 (21.9) | 82 (13.6) | 0.0001 |
| OIs | |||||||
| TB | |||||||
| No | 1037 (90.6) | 1016 (90.2) | 1019 (92) | 1651 (91.6) | 1619 (86.4) | 535 (88.6) | Ref |
| Yes | 108 (9.4) | 110 (9.8) | 88 (7.9) | 152 (8.4) | 254 (13.6) | 69 (11.4) | 0.0007 |
| PCP | |||||||
| No | 1109 (96.9) | 1099 (97.6) | 1076 (97.2) | 1689 (93.7) | 1824 (97.4) | 590 (97.7) | Ref |
| Yes | 36 (3.1) | 27 (2.4) | 31 (2.8) | 114 (6.3) | 49 (2.6) | 14 (2.3) | 0.54 |
| Cryptococcal disease | |||||||
| No | 1142 (99.7) | 1119 (99.4) | 1099 (99.3) | 1793 (99.5) | 1855 (99.0) | 599 (99.2) | Ref |
| Yes | 3 (0.3) | 7 (0.6) | 8 (0.7) | 10 (0.6) | 18 (1.0) | 5 (0.8) | 0.0489 |
| Oral candidiasis | |||||||
| No | 1081 (94.4) | 1083 (96.2) | 1085 (98.0) | 1756 (97.4) | 1832 (97.8) | 587 (97.2) | Ref |
| Yes | 64 (5.6) | 43 (3.8) | 22 (2.0) | 47 (2.6) | 41 (2.2) | 17 (2.8) | 0.0001 |
| Esophageal candidiasis | |||||||
| No | 1140 (99.6) | 1121 (99.6) | 1105 (99.8) | 1798 (99.7) | 1866 (99.6) | 601 (99.5) | Ref |
| Yes | 5 (0.4) | 5 (0.4) | 2 (0.2) | 5 (0.3) | 7 (0.4) | 3 (0.5) | 0.91 |
| Kaposi’s sarcoma | |||||||
| No | 1132 (98.9) | 1118 (99.3) | 1095 (98.9) | 1786 (99.1) | 1855 (99.0) | 597 (98.8) | Ref |
| Yes | 13 (1.1) | 8 (0.7) | 12 (1.1) | 17 (0.9) | 18 (1.0) | 7 (1.2) | 0.92 |
| Other OIs | |||||||
| No | 948 (82.8) | 914 (81.2) | 916 (82.8) | 1194 (66.2) | 1213 (64.8) | 424 (70.2) | Ref |
| Yes | 197 (17.2) | 212 (18.8) | 191 (17.3) | 609 (33.8) | 660 (35.2) | 180 (29.8) | 0.0001 |
| Any OI | |||||||
| No | 835 (72.9) | 805 (71.5) | 809 (73.1) | 1027 (57.0) | 1038 (55.4) | 387 (64.1) | Ref |
| Yes | 310 (27.1) | 321 (28.5) | 298 (26.9) | 776 (43.0) | 835 (44.6) | 217 (35.9) | 0.0001 |
Note: The p-values in bold font are those that met the significance threshold of <0.05.
Abbreviations: ART, antiretroviral therapy; OI, opportunistic infection; PCP, pneumocystis pneumonia; PMTCT, prevention of mother-to-child transmission; Ref, reference; TB, tuberculosis; VCT, voluntary counseling and testing; WHO, World Health Organization.
Figure 2.
Trends in median CD4 cell count at ART initiation.
Abbreviation: ART, antiretroviral therapy.
Treatment status outcomes
Overall, at the end of follow-up, 5835 (76.2%) patients were active (on ART), 1501 (19.6%) had either died or were LTFU (attrition), and 322 (4.2%) had transferred out. The median duration on ART was 37 months (IQR 16–83; Table 3).
Table 3.
Treatment status outcomes at the end of the observation period
| Year of ART start | n | Duration on ART (months) Median (IQR) | Retention % (95% CI) | Attrition % (95% CI) | Transfer out % (95% CI) |
|---|---|---|---|---|---|
| 2004–2006 | 1145 | 112 (106–119) | 82.9 (80.7–85.1) | 13.6 (11.6–15.6) | 3.5 (2.4–4.6) |
| 2007–2008 | 1126 | 90 (82–97) | 79.8 (77.4–82.1) | 15.9 (13.8–18.0) | 4.4 (3.2–5.5) |
| 2009–2010 | 1107 | 66 (59–72) | 75.3 (72.7–77.8) | 19.4 (17.1–21.8) | 5.3 (4.0–6.7) |
| 2011–2012 | 1803 | 36 (32–37) | 74.0 (72.0–76.1) | 21.8 (19.9–23.7) | 4.2 (3.2–5.1) |
| 2013–2014 | 1873 | 15 (9–22) | 68.6 (66.4–70.7) | 27.3 (25.3–29.4) | 4.1 (3.2–5.0) |
| 2015 | 604 | 3 (1–5) | 88.7 (86.2–91.3) | 7.8 (5.6–9.9) | 3.5 (2.0–4.9) |
| Overall | 7658 | 37 (16–83) | 76.2 (75.2–77.1) | 19.6 (18.7–20.5) | 4.2 (3.8–4.6) |
Note: The values in bold font reflect the combined treatment status outcomes.
Abbreviations: ART, antiretroviral therapy; IQR, interquartile range; Ref, reference.
Attrition
Attrition proportions are presented in Table 4. Overall, ART attrition was higher among males (21.8% [20.2%–23.3%]), compared to females; those who were single (21.9% [20.0%–23.8%]) or divorced/separated (23.5% [20.1%–26.9%]) compared to those who were married; patients entering care from the in-patient (23.4% [20.2%–26.5%]) or other facilities (27.1% [22.0%–32.3%]), compared to those entering care through the on-site VCT; patients initiating ART in WHO stages 3 and 4 (23.2% [21.7%–24.7%]), compared to WHO stages 1 and 2 (16.6% [15.3%–17.9%]); and patients with TB at ART initiation (23.4% [20.5%–26.4%]) compared to those without TB (19.2% [18.2%–20.1%]). Attrition was higher among young patients and declined with increasing age at ART start (adolescents [15–19 years] 28.4%, youth [20–24 years] 26.4% vs 19.0%–20.2% for patients aged 25 years and older). Patients with missing information on any of the covariates had higher attrition: marital status 20.4% vs 18.4%; residence 24.0% vs 17.3%; care entry point 25.8% vs 18.5%; duration taken to link to ART after HIV diagnosis 22.1% vs 18.2%–18.9%; WHO stage18.8% vs 16.6%; and CD4 count 22.0% vs 17.3%.
Table 4.
Attrition by baseline characteristics
| Baseline characteristics | n | Attrition % (95% CI) |
|---|---|---|
| Sex | ||
| Female | 4831 | 18.4 (17.3–19.5)* |
| Male | 2827 | 21.8 (20.2–23.3) |
| Age group (years) | ||
| 15–19 | 109 | 28.4 (20.0–36.9) |
| 20–24 | 296 | 26.4 (21.3–31.4) |
| 25–29 | 881 | 20.2 (17.6–22.9) |
| 30–54 | 5881 | 19.0 (18.0–20.0)* |
| ≥55 | 491 | 19.6 (16.0–23.1) |
| Marital status | ||
| Single | 1744 | 21.9 (20.0–23.8) |
| Married | 4294 | 18.4 (17.2–19.5)* |
| Divorced/separated | 591 | 23.5 (20.1–26.9) |
| Widowed | 774 | 18.2 (15.5–20.9) |
| Not documented | 255 | 20.4 (15.4–25.3) |
| Residence | ||
| Rural | 1086 | 17.3 (15.1–19.6)* |
| Urban | 5946 | 19.6 (18.6–20.6) |
| Not documented | 626 | 24.0 (20.6–27.3) |
| Care entry point | ||
| VCT | 3240 | 18.5 (17.2–19.9)* |
| PMTCT | 1195 | 18.2 (16.0–20.3) |
| TB | 671 | 17.6 (14.7–20.5) |
| In-patient | 702 | 23.4 (20.2–26.5) |
| Other facilities | 291 | 27.1 (22.0–32.3) |
| Other sources | 919 | 17.2 (14.8–19.6) |
| Not documented | 640 | 25.8 (22.4–29.2) |
| Linked to ART after HIV diagnosis | ||
| 3 months | ||
| Within 3 months | 1943 | 18.0 (16.3–19.7)* |
| After 3 months | 3181 | 18.6 (17.2–19.9) |
| 6 months | ||
| Within 6 months | 2300 | 18.6 (17.0–20.2)* |
| After 6 months | 2824 | 18.2 (16.7–19.6) |
| 12 months | ||
| Within 12 months | 2828 | 17.9 (16.5–19.3)* |
| After 12 months | 2296 | 18.9 (17.3–20.5) |
| HIV diagnosis date not documented | 2534 | 22.1 (20.5–23.8) |
| Disease stage | ||
| WHO stages 1 and 2 | 3087 | 16.6 (15.3–17.9)* |
| WHO stages 3 and 4 | 2931 | 23.2 (21.7–24.7) |
| Not documented | 1640 | 18.8 (16.9–20.7) |
| CD4 count | ||
| 0–50 | 1250 | 21.8 (19.5–24.0) |
| 51–100 | 977 | 19.5 (17.1–22.0) |
| 101–250 | 513 | 17.0 (13.7–20.2) |
| 251–350 | 793 | 17.3 (14.6–19.9)* |
| 351–500 | 658 | 13.7 (11.1–16.3) |
| >500 | 592 | 15.5 (12.6–18.5) |
| Not documented | 2875 | 22.0 (20.5–23.5) |
| OIs | ||
| TB | ||
| No | 6877 | 19.2 (18.2–20.1)* |
| Yes | 781 | 23.4 (20.5–26.4) |
| PCP | ||
| No | 7387 | 19.6 (18.7–20.5)* |
| Yes | 271 | 20.3 (15.5–25.1) |
| Cryptococcal disease | ||
| No | 7607 | 19.6 (18.7–20.5)* |
| Yes | 51 | 15.7 (5.7–25.7) |
| Oral candidiasis | ||
| No | 7424 | 19.6 (18.7–20.5)* |
| Yes | 234 | 20.1 (15.0–25.2) |
| Esophageal candidiasis | ||
| No | 7631 | 19.6 (18.7–20.5)* |
| Yes | 27 | 22.2 (6.5–37.9) |
| Kaposi’s sarcoma | ||
| No | 7583 | 19.6 (18.7–20.5)* |
| Yes | 75 | 20.0 (10.9–29.1) |
| Other OIs | ||
| No | 5609 | 19.1 (18.1–20.1)* |
| Yes | 2049 | 21.0 (19.3–22.8) |
| Any OI | ||
| No | 4901 | 18.9 (17.8–20.0)* |
| Yes | 2757 | 20.9 (19.4–22.4) |
Notes: The values in bold font are significant by virtue of the fact that the confidence intervals do not overlap with those of the respective reference categories. Reference categories are indicated by the * symbol.
Abbreviations: ART, antiretroviral therapy; OI, opportunistic infection; PCP, pneumocystis pneumonia; PMTCT, prevention of mother-to-child transmission; TB, tuberculosis; VCT, voluntary counseling and testing; WHO, World Health Organization.
aHR for ART attrition are presented in Table 5. Overall, baseline characteristics that were independently associated with attrition included (aHR [95% CI]) male sex: 1.30 (1.16–1.45), p=0.0001, compared to female; age: 15–19 years: 1.83 (1.26–2.66), p=0.0014; 20–24 years: 1.93 (1.52–2.44), p=0.0001; and 25–29 years: 1.31 (1.11–1.54), p=0.0012; compared to age 30–54 years; marital status – single: 1.27 (1.11–1.44), p=0.0005; and divorced/separated: 1.56 (1.30–1.87), p=0.0001, compared to married; urban residence: 1.40 (1.20–1.64), p=0.0001, compared to rural; entry into HIV care from the in-patient: 1.31 (1.10–1.57), p=0.0026, and from another facility: 1.60 (1.26–2.04), p=0.0001, compared to entry into HIV care from the on-site VCT; initiation of ART >12 months after the date of HIV diagnosis, and having an OI: 1.15 (1.02–1.30), p=0.0284. Nondocumentation of residence, care entry point, and date of HIV diagnosis were also associated with attrition.
Table 5.
Baseline characteristics associated with attrition (death or lost to follow-up) after ART initiation
| Variable | HR (95% CI) | p-value | aHR (95% CI) | p-value |
|---|---|---|---|---|
| Sex | ||||
| Female | Ref | Ref | Ref | Ref |
| Male | 1.18 (1.06–1.30) | 0.0019 | 1.30 (1.16–1.45) | 0.0001 |
| Age (years) | ||||
| 15–19 | 2.17 (1.52–3.11) | 0.0001 | 1.83 (1.26–2.66) | 0.0014 |
| 20–24 | 1.91 (1.52–2.40) | 0.0001 | 1.93 (1.52–2.44) | 0.0001 |
| 25–29 | 1.23 (1.05–1.44) | 0.0121 | 1.31 (1.11–1.54) | 0.0012 |
| 30–54 | Ref | Ref | Ref | Ref |
| ≥55 | 1.09 (0.88–1.34) | 0.4390 | 1.13 (0.91–1.39) | 0.28 |
| Marital status | ||||
| Single | 1.34 (1.18–1.51) | 0.0001 | 1.27 (1.11–1.44) | 0.0005 |
| Married | Ref | Ref | Ref | Ref |
| Divorced | 1.49 (1.24–1.79) | 0.0001 | 1.56 (1.30–1.87) | 0.0001 |
| Widowed | 0.97 (0.81–1.16) | 0.7423 | 1.04 (0.86–1.25) | 0.69 |
| Not documented | 1.16 (0.88–1.53) | 0.3030 | 1.13 (0.85–1.50) | 0.42 |
| Residency | ||||
| Rural | Ref | Ref | Ref | Ref |
| Urban | 1.39 (1.19–1.62) | 0.0001 | 1.40 (1.20–1.64) | 0.0001 |
| Not documented | 1.82 (1.47–2.25) | 0.0001 | 1.75 (1.41–2.18) | 0.0001 |
| Care entry point | ||||
| VCT | Ref | Ref | Ref | Ref |
| PMTCT | 0.81 (0.69–0.94) | 0.0066 | 0.85 (0.73–1.00) | 0.0452 |
| TB clinic | 0.78 (0.64–0.95) | 0.0153 | 0.79 (0.65–0.96) | 0.0192 |
| In-patient | 1.34 (1.12–1.59) | 0.0010 | 1.31 (1.10–1.57) | 0.0026 |
| Other facilities | 1.74 (1.38–2.20) | 0.0001 | 1.60 (1.26–2.04) | 0.0001 |
| Other sources | 1.24 (1.04–1.48) | 0.0156 | 1.20 (1.00–1.43) | 0.0496 |
| Not documented | 1.20 (1.01–1.42) | 0.0401 | 1.27 (1.06–1.52) | 0.0091 |
| Time to linkage to ART after HIV diagnosis | ||||
| Within 12 months | Ref | Ref | Ref | Ref |
| After 12 months | 1.23 (1.08–1.40) | 0.0018 | 1.36 (1.19–1.55) | 0.0001 |
| HIV diagnosis date not documented | 1.47 (1.30–1.66) | 0.0001 | 1.48 (1.31–1.67) | 0.0001 |
| Disease stage | ||||
| WHO stages 1 and 2 | Ref | Ref | Ref | Ref |
| WHO stages 3 and 4 | 1.19 (1.06–1.34) | 0.0030 | 1.10 (0.97–1.25) | 0.13 |
| Not documented | 0.75 (0.65–0.87) | 0.0001 | 0.72 (0.62–0.84) | 0.0001 |
| CD4 cell count (cells/μL) | ||||
| 0–50 | 1.38 (1.11–1.72) | 0.0037 | 1.18 (0.94–1.48) | 0.16 |
| 51–100 | 0.68 (0.52–0.90) | 0.0067 | 0.66 (0.50–0.87) | 0.0031 |
| 101–250 | 0.92 (0.75–1.13) | 0.4226 | 0.92 (0.75–1.13) | 0.42 |
| 251–350 | Ref | Ref | Ref | Ref |
| 351–499 | 0.91 (0.70–1.19) | 0.5010 | 0.84 (0.64–1.09) | 0.19 |
| ≥500 | 1.02 (0.79–1.33) | 0.8706 | 0.89 (0.68–1.16) | 0.37 |
| Not documented | 0.98 (0.81–1.17) | 0.7924 | 1.04 (0.86–1.26) | 0.66 |
| OI | ||||
| Any OI | 1.29 (1.17–1.44) | 0.0001 | 1.15 (1.02–1.30) | 0.0284 |
Note: Values in bold font are statistically significant (p <0.05).
Abbreviations: aHR, adjusted hazard ratio; ART, antiretroviral therapy; HR, hazard ratio; OI, opportunistic infection; PMTCT, prevention of mother-to-child transmission; Ref, reference; TB, tuberculosis; VCT, voluntary counseling and testing; WHO, World Health Organization.
Factors that rendered attrition less likely included (aHR [95% CI]) entry into care through the TB clinic: 0.79 (0.65–0.96), p=0.0192, compared to entry into care through the on-site VCT; and CD4 count 51–100: 0.66 (0.50–0.87), p=0.0031, compared to CD4 count 251–350.
Discussion
This study highlights priority areas for focused interventions to improve outcomes in this cohort of patients. The upward trend in ART uptake among adolescents and young people could be an indication of increased awareness and better diagnosis, or that HIV prevention interventions are not reaching these groups in the needed scale. The observed trend is consistent with population-based data reporting increasing incidence15 and entry into care and treatment1 in this age group. A recent study that followed up youth (15–24 years) in four SSA countries, including Kenya, documented a 4% increase in the proportion initiating ART.16 In another analysis, Koech et al found a decrease in the proportion of patients aged 10–14 and 15–19 years, and an increase in the proportion of 20–24-year-old patients initiating ART in Kenya.17 A study conducted in Tanzania documented a decline in the proportion of 10–14-year-olds and an increase in the proportion of 15–19- and 20–29-year-olds initiating ART.18 Although there are some similar trends, inconsistencies in age cutoffs and varied study settings limit conclusive comparisons.
ART uptake among males was consistently lower than that among females throughout the observation period. They also had lower CD4 counts at ART initiation. This implies that they are seeking care when already in advanced disease, putting them at risk for poor outcomes. This calls for program-level strategies that identify male patients in early stages of disease and link them to treatment.
We observed that the majority of patients entered care through the on-site VCT, suggesting the ongoing demand for these services. This can be attributed to increased awareness of testing that has been achieved through aggressive mass media campaigns countrywide. It will be crucial that the program prioritizes VCT accessibility. HIV programs in Tanzania demonstrated a significant increase in the proportion of patients entering care through the VCT.18 Other SSA countries have reported stable19 or declining20 proportions of patients accessing care and treatment from VCT centers.
Our analysis demonstrated a statistically significant decline in the proportion of patients initiating ART with advanced disease over time, mostly a reflection of changing ART initiation policies, which progressively increased the CD4 count thresholds for ART initiation. This trend could also be a reflection of more referrals from the on-site VCT center, which would typically be attended by healthier patients.18,21,22 Similar trends have been reported in other SSA countries23 including Rwanda.24
Overall, median CD4 counts at ART initiation increased over time. The observed upward trend can be explained by the adoption of routine universal testing coupled with revision of national guidelines, which changed ART eligibility from CD4 counts ≤200 cells/mm3 to ≤350 cells/µL in 201025 and later to 500 cells/µL in 2014.26 The trend is likely to be maintained during the implementation of the most current guidelines that recommend treatment for all HIV-positive clients irrespective of the CD4 count or clinical stage.27 Similar trends have been observed in other African countries23,24 and in Asia.28 However, a study in Ethiopia20 and a meta-analysis of studies from SSA countries29 found that CD4 counts at ART initiation remained stable over time.
There was a progressive increase in enrollment of adolescents, youth, and young adults (15–29 years), single persons, and immunologically healthier clients, all of which are associated with higher attrition. Other factors that were linked to attrition in this setting included male sex, being divorced or separated, care entry through the in-patient, referral from another facility, and history of an OI prior to initiation of ART.
Adolescents and youth face unique challenges in coping with a diagnosis of HIV infection, contributing to the higher attrition observed.16,30–32 Single persons and those who are separated or divorced may lack social support and hence are unable to adequately deal with the stigma and overall social and economic burden associated with HIV infection. They are, therefore, more prone to attrition.30 Consistent with previous work, being male in this cohort was associated with higher risk for attrition.31,33,34
Referral from a facility other than the study site was predictive of attrition. This being a referral center in a large metropolis, distances, transportation costs, and possibilities of change in employment may be responsible for higher attrition of in-bound referrals.32,35 Care entry from the inpatient units was independently associated with attrition. These patients are more likely to have advanced disease, hence more prone to mortality associated attrition. Any OI (history of or active) at ART initiation was predictive of attrition. The OIs listed are associated with stage 3 or 4 disease, suggesting that this is most likely mortality-associated attrition.
Contrary to findings of previous research,30,31,33,34 our study did not demonstrate that advanced disease (WHO stage 3 and 4) was predictive of mortality-associated attrition. However, this finding should be interpreted with caution because nondocumentation of clinical staging and underreporting of mortality may have contributed to this observation.
TB clinic as a care entry point conferred protection from attrition. Initiating ART prior to enrollment or in the same year of enrollment has been long known to result in improved retention,30,34 explaining the conferred protection from attrition among patients entering care through the TB clinic.
Strengths of this study include the reasonably large sample size, which rendered sufficient power for precise effect estimates, and the long duration of follow-up, which allowed for trend analysis over time. Limitations of the study are related to the data source. We used routinely collected clinical data, which are more prone to data quality barriers, including missing information, than data specifically collected for research purposes.36 The referral nature of KNH renders our study sample highly selective, impacting generalizability of our findings.
Conclusion
Although ART uptake among adolescents and young people increased over time, this group was at increased risk for attrition. Single marital status, urban residence, history of hospitalization or OI, and late initiation of ART also predicted attrition. These findings and current national data underscore the need for policies that call for intensified HIV preventive strategies targeting adolescents and young people, and those that focus on increasing awareness on the availability and importance of HIV counseling and testing services. At program level, there is an urgent need to implement evidence-based interventions that address attrition especially among adolescents, youth, males, and the other at-risk groups highlighted by these data. Finally, these findings reiterate the need to ensure completeness of documentation of health records for all patients.
Acknowledgments
Prof Zipporah W Ngumi, Principal Investigator, Cooperative Agreement U2GPS002182-01-05 for administrative support and advice; and Christopher Githu for extracting the analysis database from the electronic medical records system. The views presented in this publication are solely those of the authors and do not necessarily reflect the official position of the funding agencies. This research was supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the US Centers for Disease Control and Prevention (CDC) under the terms of the Cooperative Agreement Number U2GPS002182-01-05.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.How AIDS changed everything . MDG6: 15 years, 15 lessons of hope from the AIDS response. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS; 2015. [Google Scholar]; UNAIDS 2015. [Accessed November 25, 2015]. Available from: http://www.unaids.org/sites/default/files/media_asset/MDG6Report_en.pdf.
- 2.Global Update On HIV Treatment 2013: results, impact and opportunities. 2013. [Accessed November 12, 2015]. Available from: http://www.unaids.org/sites/default/files/sub_landing/files/20130630_treatment_report_en_3.pdf.
- 3.Gap Report 2014. [Accessed November 12, 2015]. Available from: http://www.unaids.org/sites/default/files/en/media/unaids/contentassets/documents/unaidspublication/2014/UNAIDS_Gap_report_en.pdf.
- 4.Kenya AIDS Response Progress Report 2014. [Accessed November 13, 2015]. Available from: http://www.unaids.org/sites/default/files/country/documents/KEN_narrative_report_2014.pdf.
- 5.Kenya Country Factsheet 2015. [Accessed May 15, 2017]. Available from: http://aidsinfo.unaids.org/
- 6.Kenya HIV Estimates 2014. [Accessed November 14, 2015]. Available from: http://healthservices.uonbi.ac.ke/sites/default/files/centraladmin/healthservices/HIV%20estimates%20report%20Kenya%202014.pdf.
- 7.90-90-90 An Ambitious treatment target to help end the AIDS epidemic. 2014. [Accessed November 14, 2015]. Available from: http://www.unaids.org/sites/default/files/media_asset/90-90-90_en_0.pdf.
- 8.Nash D, Wu Y, Elul B, Hoos D, El Sadr W. International Center for AIDS Care and Treatment Programs. Program-level and contextual-level determinants of low-median CD4+ cell count in cohorts of persons initiating ART in eight sub-Saharan African countries. AIDS. 2011;25(12):1523–1533. doi: 10.1097/QAD.0b013e32834811b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22(15):1897–1908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fox MP, Rosen S. Retention of adult patients on antiretroviral therapy in low- and middle-income countries: systematic review and meta-analysis 2008–2013. J Acquir Immune Defic Syndr. 2015;69(1):98–108. doi: 10.1097/QAI.0000000000000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grimsrud A, Balkan S, Casas EC, et al. Outcomes of antiretroviral therapy over a 10-year period of expansion: a multicohort analysis of African and Asian HIV programs. J Acquir Immune Defic Syndr. 2014;67(2):e55–e66. doi: 10.1097/QAI.0000000000000268. [DOI] [PubMed] [Google Scholar]
- 12.Ochieng-Ooko V, Ochieng D, Sidle JE, et al. Influence of gender on loss to follow-up in a large HIV treatment programme in western Kenya. 9. Vol. 88. Bull World Health Organ; 2010. pp. 681–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ministry of Health Kenya . Guidelines on Use of Antiretriviral Drugs for Treating and Preventing HIV Infection in Kenya. Nairobi, Kenya: NASCOP; 2016. [Google Scholar]
- 14.Pati R, Lahuerta M, Elul B, et al. Identifying Optimal Models of HIV Care in Mozambique Study Group. Factors associated with loss to clinic among HIV patients not yet known to be eligible for antiretroviral therapy (ART) in Mozambique. J Int AIDS Soc. 2013;16(1):18490. doi: 10.7448/IAS.16.1.18490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World AIDS Day Report 2012 [webpage on the Internet] UNAIDS; [Accessed November 25, 2015]. p. 2012. Available from: http://www.unaids.org/en/resources/campaigns/20121120_globalreport2012. [Google Scholar]
- 16.Lamb MR, Fayorsey R, Nuwagaba-Biribonwoha H, et al. High attrition before and after ART initiation among youth 15–24 years of age) enrolled in HIV care. AIDS. 2014;28(4):559–568. doi: 10.1097/QAD.0000000000000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koech E, Teasdale CA, Wang C, et al. Characteristics and outcomes of HIV-infected youth and young adolescents enrolled in HIV care in Kenya. AIDS. 2014;28(18):2729–2738. doi: 10.1097/QAD.0000000000000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nuwagaba-Biribonwoha H, Kilama B, Antelman G, et al. Identifying Optimal Models of HIV Care and Treatment in Sub-Saharan Africa Consortium, United Republic of Tanzania Reviewing progress: 7 year trends in characteristics of adults and children enrolled at HIV care and treatment clinics in the United Republic of Tanzania. BMC Public Health. 2013;(13):1016. doi: 10.1186/1471-2458-13-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lahuerta M, Lima J, Elul B, et al. Patients Enrolled in HIV care in Mozambique: baseline characteristics and follow-up outcomes. J Acquir Immune Defic Syndr. 2011;58(3):e75–e86. doi: 10.1097/QAI.0b013e31822ac0a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melaku Z, Lamb MR, Wang C, et al. Characteristics and outcomes of adult Ethiopian patients enrolled in HIV care and treatment: a multi-clinic observational study. BMC Public Health. 2015;15:462. doi: 10.1186/s12889-015-1776-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lahuerta M, Lima J, Nuwagaba-Biribonwoha H, et al. Factors associated with late antiretroviral therapy initiation among adults in Mozambique. PLoS One. 2012;7(5):e37125. doi: 10.1371/journal.pone.0037125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wachira J, Kimaiyo S, Ndege S, Mamlin J, Braitstein P. What is the impact of home-based HIV counseling and testing on the clinical status of newly enrolled adults in a large HIV care program in Western Kenya? Clin Infect Dis. 2012;54(2):275–281. doi: 10.1093/cid/cir789. [DOI] [PubMed] [Google Scholar]
- 23.Lahuerta M, Wu Y, Hoffman S. Multi-level determinants of late ART initiation in sub-Saharan Africa Team and; Identifying Optimal Models of HIV Care in sub-Saharan Africa Collaboration. Advanced HIV disease at entry into HIV care and initiation of antiretroviral therapy during 2006–2011 findings from four sub-saharan African countries. Clin Infect Dis. 2014;58(3):432–441. doi: 10.1093/cid/cit724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mutimura E, Addison D, Anastos K, et al. IeDEA Central Africa Collaboration Trends in and correlates of CD4+ cell count at antiretroviral therapy initiation after changes in national ART guidelines in Rwanda. AIDS. 2015;29(1):67–76. doi: 10.1097/QAD.0000000000000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National AIDS and STI Control Programme (NASCOP) MoH, Kenya . Guidelines for Antiretroviral Drug Therapy in Kenya. Kenya: MOH, GOK; 2011. [Google Scholar]
- 26.Ministry of Health Kenya . Guidelines on Use of Antiretroviral Drugs for Treating and Preventing HIV Infection in Kenya: A Rapid Advice. Nairobi, Kenya: NASCOP; 2014. [Google Scholar]
- 27.WHO [webpage on the Internet] Consolidated Guidelines on the use of Antiretroviral drugs for treating and preventing HIV infection: What’s New. 2015. [Accessed April 25, 2016]. Available from: http://www.who.int/hiv/pub/arv/policy-brief-arv-2015/en/
- 28.Kiertiburanakul S, Boettiger D, Lee MP, et al. TREAT Asia HIV Observational Databases (TAHOD); TREAT Asia Studies to Evaluate Resistance (TASER). Trends of CD4 cell count levels at the initiation of antiretroviral therapy over time and factors associated with late initiation of antiretroviral therapy among Asian HIV-positive patients. J Int AIDS Soc. 2014;(17):18804. doi: 10.7448/IAS.17.1.18804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siedner MJ, Ng CK, Bassett IV, Katz IT, Bangsberg DR, Tsai AC. Trends in CD4 count at presentation to care and treatment initiation in sub-Saharan Africa, 2002–2013: a meta-analysis. Clin Infect Dis. 2015;60(7):1120–1127. doi: 10.1093/cid/ciu1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alvarez-Uria G, Naik PK, Pakam R, Midde M. Factors associated with attrition, mortality, and loss to follow up after antiretroviral therapy initiation: data from an HIV cohort study in India. Global Health Action. 2013;6:21682. doi: 10.3402/gha.v6i0.21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zachariah R, Tayler-Smith K, Manzi M, et al. Retention and attrition during the preparation phase and after start of antiretroviral treatment in Thyolo, Malawi, and Kibera, Kenya: implications for programmes? Trans R Soc Trop Med Hyg. 2011;105(8):421–430. doi: 10.1016/j.trstmh.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 32.Govindasamy D, Ford N, Kranzer K. Risk factors, barriers and facilitators for linkage to antiretroviral therapy care: a systematic review. AIDS. 2012;26(16):2059–2067. doi: 10.1097/QAD.0b013e3283578b9b. [DOI] [PubMed] [Google Scholar]
- 33.Fox MP, Rosen S. Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007–2009: systematic review. Trop Med Int Health. 2010;15(Suppl 1):1–15. doi: 10.1111/j.1365-3156.2010.02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thida A, Tun ST, Zaw SK, et al. Retention and risk factors for attrition in a large public health ART program in Myanmar: a retrospective cohort analysis. PLoS One. 2014;9(9):e108615. doi: 10.1371/journal.pone.0108615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Kop ML, Ekström AM, Awiti-Ujiji O, et al. Factors associated with attrition from HIV care during the first year after antiretroviral therapy initiation in Kenya. J AIDS Clin Res. 2014;5:354. [Google Scholar]
- 36.Topp SM, Li MS, Chipukuma JM, et al. Does provider-initiated counselling and testing (PITC) strengthen early diagnosis and treatment initiation? Results from an analysis of an urban cohort of HIV-positive patients in Lusaka, Zambia. J Int AIDS Soc. 2012;15(2):17352. doi: 10.7448/IAS.15.2.17352. [DOI] [PMC free article] [PubMed] [Google Scholar]