Abstract
Objective
The caregivers of patients with head and neck cancer (HNC) may suffer from impaired psychological well-being and a decreased quality of life (QOL) related to the chronic burden of caring for patients’ physical conditions and their mood changes. In this study, we aimed to compare the psychological well-being and QOL between spouse caregivers and non-spouse caregivers of patients with HNC over a 6-month follow-up period.
Patients and methods
This study was conducted using a prospective design with consecutive sampling. We recruited study subjects from the outpatient combined treatment clinic of HNC at a medical center in Southern Taiwan. The Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, fourth edition was carried out by a trained senior psychiatrist to diagnose caregivers. Furthermore, one research assistant collected the caregivers’ demographic characteristics, clinical data, and clinical rating scales, including the Short Form 36 (SF-36) Health Survey, Hospital Anxiety and Depression Scale (HADS), and Family Appearance, Pulse, Grimace, Activity, and Respiration index at the patients’ pretreatment, as well as their 3- and 6-month follow-up appointments.
Results
Of the 143 subjects that successfully completed the study, two-thirds of caregivers were spouses. During the 6-month follow-up period, spouse caregivers demonstrated significantly higher rates of depression diagnosis (p=0.032), higher scores in the depression subscale of HADS (HADS-D) (p=0.010), and lower SF-36 mental component summary (MCS) scores (p=0.007) than non-spouse caregivers. Furthermore, during those 6 months, HADS-D (p=0.007) and the anxiety subscale of HADS scores (p<0.001) significantly decreased, while SF-36 MCS scores significantly increased (p=0.015).
Conclusion
The mental health of spouse caregivers of HNC patients was more severely affected than that of non-spouse caregivers during the observed 6-month follow-up period. Therefore, clinicians need to pay more attention to caregivers’ psychological distress during patient care, especially for spouse caregivers.
Keywords: spouse caregiver, psychological well-being, quality of life, head and neck cancer, follow-up study
Introduction
Head and neck cancer (HNC) is among the 10 most common cancers in the world, with ~540,000 new cases and 271,000 deaths every year worldwide and a 5-year mortality rate of ~50%.1 The caregivers of cancer patients may suffer from impaired psychological well-being (including anxiety, depression, or family support) and a decreased quality of life (QOL) related to the chronic burden of caring for patients’ physical conditions and their mood changes.2,3
In the past, studies that generally used self-rated questionnaires have reported that the prevalence of depression in caregivers for cancer patients varied from 4.5% to 82.2%.4–7 However, few studies have investigated the morbidity of depression in caregivers of HNC patients, which has been found to range from 9.7% to 14.7%.4,8 Compared to studies of the morbidity of depression in cancer caregivers, epidemiological studies of anxiety in cancer caregivers are quite rare. Park et al conducted a nationwide survey of patient–family caregiver dyads in Korea and found that the prevalence of anxiety in family caregivers reached 38.1%.7
Previous studies have also shown that caregivers that experienced depression demonstrated impaired family support compared to nondepressed caregivers.9,10 Our prospective study followed caregivers of HNC patients for 6 months and revealed that impaired family support is a risk for depression among cancer caregivers.11 Yeh et al12 explored the psychological well-being, quality of caregiver–patient relationship, and family support on the health of family caregivers for cancer patients, observing that a lack of family support had a significant negative correlation with caregivers’ health. These studies suggest that family support not only affects caregivers’ moods but also has an influence on their physical health.
Several researchers have reported the impact that providing care to HNC patients has on the QOL of those caregivers.4,13,14 Furthermore, some cross-sectional studies have even confirmed the correlation between depression and QOL in these caregivers.4,8 A study of 897 cancer family caregivers from Korea found that the QOL of caregivers is associated with anxiety.7 In our longitudinal study, we followed depression, anxiety, and QOL, and their interaction in caregivers of HNC patients and found that the caregivers’ QOL significantly improved over the 6-month follow-up period, and we observed a lower mental component of the Short Form 36 (SF-36) Health Survey score at baseline to be a predictor of depressive disorders after 6 months.11
Spouses are often the primary and most valuable source of support and care for cancer patients.15 The cancer experience is not only a stressful event for patients but also for their spouses.16 When faced with the patient’s exasperating disease, ongoing caregiving responsibilities, and the fear of losing their loved ones, spouses can tend to feel quite distressed.17 Previous studies have demonstrated that spousal caregivers of cancer patients are at greater risk of depression and various physical conditions (eg, coronary heart disease and stroke) when compared to the general population.18–20 These prior studies have suggested that medical professionals should pay close attention to the mental health and QOL of the spouses of cancer patients. However, few studies have conducted a spouse/non-spouse caregiver comparison of QOL and psychological well-being in HNC patients.
Based on the aforementioned literature review, some studies have detected correlations among depression, anxiety, family support, and QOL in caregivers of HNC patients.4,8,11,13,14 Nevertheless, prospective studies comparing those same factors between spouse caregivers and non-spouse caregivers of HNC patients are rare. The aim of this present study was to compare the psychological well-being (depression, anxiety, family support) and QOL between spouse caregivers and non-spouse caregivers of HNC patients during a 6-month follow-up period.
Patients and methods
Subjects
The study protocol was approved by the institutional review board of Chang Gung Hospital in Taiwan (reference number: 99-3723B). All procedures performed in this study involving participants were in accordance with the ethical standards of the institutional and/or national research committee and with the Declaration of Helsinki. We obtained written informed consent from the patients and their caregivers.
In this prospective study, we adopted a consecutive sampling design and recruited subjects from the outpatient combined clinic for HNC from Kaohsiung Chang Gung Memorial Hospital (KCGMH). Of the four medical centers in Southern Taiwan, KCGMH is the largest, and provided services to 5,000 cancer patients between February 2012 and January 2013. The caregivers had to meet the following inclusion criteria: 1) taking care of patients with newly diagnosed, untreated HNC; 2) aged 20 years or over; 3) living with the patients and taking care of their daily needs; and 4) able to verbalize and write. Exclusion criteria for the patients were as follows: 1) a previous history of malignancy or 2) recurrent HNC, while the exclusion criterion for caregivers was a previous history of malignancy.
Assessments
Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV), clinician version (SCID-CV)
Psychiatric diagnoses were performed using the SCID-CV interview, a structured diagnostic interview based on DSM-IV criteria.21 This interview was carried out by a clinician or mental health professional with the relevant professional training. The diagnosis was supplemented by the DSM-IV criteria for primary insomnia (which was not included in the SCID). The SCID interview carried out by a trained psychiatrist is considered a “gold standard” of psychiatric diagnosis.
Hospital Anxiety and Depression Scale (HADS)
HADS is a 14-item self-administered questionnaire that assesses the severity of anxiety and depression.22 It is commonly used in hospital practices (including cancer patients) and primary care, as well as for the general population.23 Seven items are used to evaluate anxiety, and seven other items are used to evaluate depression. Each item has four possible responses (scored from 0 to 3); the anxiety (HADS-A) and depression (HADS-D) subscales of HADS–each have independent measures.22 In this study, HADS was carried out by a trained research assistant.
SF-36
SF-36 was designed to evaluate functional health, well-being, and QOL in population surveys.24 It consists of eight health domains: physical functioning, role limitations due to physical problems, bodily pain, general health, vitality, social functioning, role limitations due to emotional problems, and mental health. A standard scoring algorithm combines scores into two summary scores for the physical component summary (PCS) and the mental component summary (MCS).25 The Taiwanese version of SF-36 was validated by Lu et al26 and has been widely adopted to measure QOL in studies in Taiwan, as well as those of other Asian countries.27 In the present study, SF-36 was performed by a trained research assistant.
Family Appearance, Pulse, Grimace, Activity, and Respiration (APGAR) index
The family APGAR index, which was developed by Smilkstein,28 assesses a family member’s perception of family function by examining his/her satisfaction with family relationships. It includes five parameters of adaptation, partnership, growth, affection, and resolution with a three-point scale that ranges from 0 (hardly ever) to 2 (almost always). Total scores range from 0 to 10, where a higher score represents a higher level of family functioning. A trained research assistant administered the family APGAR index.
Procedures
The human research ethics committee of Chang Gung Memorial Hospital approved this study. The study procedures were as follows: 1) patients referred from the HNC outpatient clinic and their caregivers were asked to provide written informed consent; 2) caregivers attended the appointment with their related patients and were identified as fulfilling the inclusion criteria; 3) SCID was used by a senior psychiatrist (Dr Y Lee) to reach a psychiatric diagnosis; and then 4) a trained research assistant collected the patients’ demographic and clinical data, the caregivers’ demographic data, and clinical rating scales data, including HADS, SF-36, and the Family APGAR index using in-person interviews. Furthermore, the above questionnaires and psychiatric diagnostic interviews were repeated at the 3- and 6-month follow-up appointments.
Statistical analyses
Data were analyzed using the Statistical Package for the Social Sciences for Windows (version 16.0; SPSS Inc., Chicago, IL, USA). Variables are presented as either mean ± standard deviation (SD) or frequency. We adopted either a chi-square (χ2) test or independent t-test to compare the characteristics of the spouse and non-spouse caregiver groups. All statistical tests were two-tailed, and differences of p<0.05 were considered statistically significant.
We analyzed longitudinal data using generalized estimating equations (GEEs) in which the maximum likelihood estimation method and auto-regression covariance matrix were the primary analytic strategies. When compared to traditional approaches (ie, repeated-measure analysis of variance), these techniques are more effective at handling missing data.29,30 We used the GEE to examine the potential effects on the two caregiver groups (spouse vs non-spouse), the potential time effect, and the potential interaction effects of caregivers and time on caregivers’ psychometrics during the 6-month treatment period. The diagnosis of depression and HADS, APGAR, PCS, and MCS of the SF-36 scores were set as dependent variables, and the caregiver groups and follow-up time were set as the independent variables. To control for the potential confounding effects of caregivers’ age, gender, education levels, past history of depression, and patients’ chemotherapy, we set these characteristics as covariates in the GEE models. Bonferroni correction was used to adjust multiple tests.
Results
Of the 143 caregivers included in the study, 76.2% (N=109) were female. The average age of the subjects was 47.0±11.4 years, and their mean education level was 10.5±3.9 years. Of the included caregivers, 76.2% were married, and 58.7% were employed at the time (Table 1). Of the recruited subjects, 91 (63.6%) caregivers were the patients’ spouse, while the other 52 (36.4%) caregivers were not spouses of the patients. Spouse caregivers were more likely to be female (p<0.001), elderly (p<0.001), with lower education levels (p=0.001), tobacco users (p=0.003), betel nut users (p=0.037), and with a higher rate of a psychiatric disorder (p=0.003) than the non-spouse caregivers. The psychiatric disorders of caregivers considered in this study include depressive disorders, adjustment disorder, alcohol abuse, anxiety disorder not otherwise specified, and primary insomnia. The majority of caregivers had a psychiatric disorder after taking care of the cancer patients. The course of experiencing these psychiatric disorders ranged from 1 to 6 months. Although many caregivers suffered from a psychiatric disorder, most of them were still capable of caring for the patient without significant impairment to their functioning. Furthermore, patients cared for by non-spouse family members had higher rates of receiving chemotherapy (p=0.043). The most common familial relationship among the caregivers in our study was wives (58.0%), followed by children (23.1%), parents (6.3%), and husbands (5.6%).
Table 1.
Demographic and clinical characteristics between spouse and non-spouse caregivers in patients with head and neck cancer
| Variables | Spouse (N=91) | Non-spouse (N=52) | Total (N=143) | χ2/t-testa | p-value |
|---|---|---|---|---|---|
| Gender | 31.007 | <0.001*** | |||
| Male | 8 (8.8) | 26 (50.0) | 34 (23.8) | ||
| Female | 83 (91.2) | 26 (50.0) | 109 (76.2) | ||
| Age, years, mean ± SD | 49.9±8.9 | 41.7±13.3 | 47.1±11.4 | 4.417 | <0.001*** |
| Age demarcated | 14.713 | <0.001*** | |||
| ≥50 | 53 (58.2) | 13 (25.0) | 66 (46.2) | ||
| <50 | 38 (41.8) | 39 (75.0) | 77 (53.8) | ||
| Education | 13.042 | 0.001*** | |||
| Under elementary school | 27 (29.7) | 6 (11.5) | 33 (23.1) | ||
| High school | 54 (59.3) | 29 (55.8) | 83 (58.0) | ||
| College or above | 10 (11.0) | 17 (32.7) | 27 (18.9) | ||
| Education, years, mean ± SD | 9.6±3.8 | 12.0±3.7 | 10.4±3.9 | −3.715 | 0.001** |
| Unemployment | 38 (41.8) | 21 (40.4) | 59 (41.3) | 0.026 | 0.872 |
| Combine other medications | 32 (35.2) | 12 (23.1) | 44 (30.8) | 2.270 | 0.132 |
| Comorbid diseases | 45 (49.5) | 17 (32.7) | 62 (43.4) | 3.784 | 0.052 |
| Hypnotics use | 12 (13.2) | 4 (7.7) | 16 (11.2) | 1.005 | 0.316 |
| Alcoholism | 7 (7.7) | 8 (15.4) | 15 (10.5) | 2.085 | 0.149 |
| Smoking | 10 (11.0) | 16 (30.8) | 26 (18.2) | 8.703 | 0.003** |
| Betel nut | 3 (3.3) | 7 (13.5) | 10 (7.0) | 5.257 | 0.037** |
| Past history of depression | 8 (8.8) | 1 (1.9) | 9 (6.3) | 2.647 | 0.156 |
| Family history of depression | 8 (8.8) | 2 (3.8) | 10 (7.0) | 1.244 | 0.328 |
| Time since cancer diagnosis (weeks) | 3.7±1.6 | 3.5±0.9 | 3.6±1.4 | 1.011 | 0.314 |
| Depressive disorder | 16 (17.6) | 5 (9.6) | 21 (14.7) | 1.696 | 0.195 |
| Psychiatric disorder | 36 (39.6) | 8 (15.4) | 44 (30.8) | 9.079 | 0.003** |
| Patient treatment | |||||
| Operation | 54 (59.3) | 27 (51.9) | 81 (56.6) | 0.741 | 0.389 |
| Chemotherapy | 51 (56.0) | 38 (73.1) | 89 (62.2) | 4.085 | 0.043* |
| Radiotherapy | 55 (60.4) | 39 (75.0) | 94 (65.7) | 3.115 | 0.078 |
| CCRT | 48 (52.7) | 34 (65.4) | 82 (57.3) | 2.160 | 0.142 |
| Patient cancer stage | 0.631 | 0.427 | |||
| Early (stage I and II) | 43 (47.3) | 21 (40.4) | 64 (44.8) | ||
| Advance (stage III and IV) | 48 (52.7) | 31 (59.6) | 79 (55.2) | ||
| Patients’ cancer sites | 0.094 | 0.759 | |||
| Nasopharynx | 19 (20.9) | 12 (23.1) | 31 (21.7) | ||
| Others | 70 (79.1) | 40 (76.9) | 112 (78.3) |
Notes:
p<0.05,
p<0.01,
p<0.001.
Independent t-test.
Abbreviation: CCRT, concurrent chemoradiotherapy.
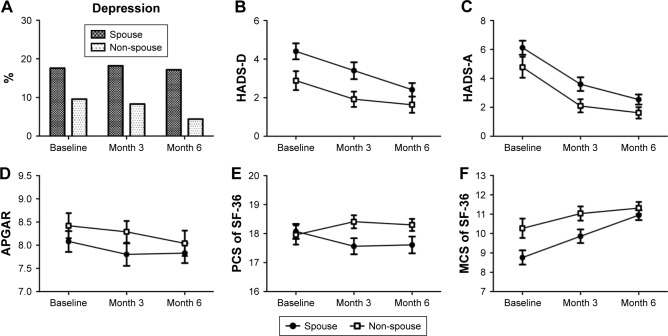
Figure 1 demonstrates the trends of depression diagnosis, HADS, APGAR, PCS, and MCS of the SF-36 scores of caregivers during the 6-month follow-up period. The effects of the two different caregiver groups and time on the aforementioned psychometrics are provided in Table 2. After controlling for the confounding effects of caregivers’ age, gender, education levels, history of depression, and patients’ chemotherapy, spouse caregivers had significantly elevated rates of being diagnosed with depression (p=0.032), greater HADS-D scores (p=0.010), and lower SF-36 MCS scores (p=0.007) than non-spouse caregivers during the 6-month follow-up period. Regarding the time effects, HADS-D (p=0.007) and HADS-A (p<0.001) scores significantly decreased, while SF-36 MCS scores significantly increased (p=0.015). Furthermore, caregivers and time had a significant interaction effect on SF-36 PCS scores (p=0.045). After the Bonferroni correction, spouse caregivers had significantly lower SF-36 MCS scores than non-spouse caregivers, and HADS-D and HADS-A scores significantly decreased during the 6-month follow-up.
Figure 1.
Trends of depression (A), HADS-D score (B), HADS-A score (C), APGAR score (D), PCS of SF-36 score (E) and MCS of SF-36 score (F) in caregivers of patients with head and neck cancer during a 6-month follow-up.
Abbreviations: APGAR, Appearance, Pulse, Grimace, Activity, and Respiration; HADS, Hospital Anxiety and Depression Scale; HADS-D, depression subscale of HADS; HADS-A, anxiety subscale of HADS; SF-36, Short Form 36; PCS, physical component summary; MCS, mental component summary.
Table 2.
Main effects of caregiver (spouse vs non-spouse) and time, and the interaction effect of caregiver and time on psychological well-being during a 6-month follow-up
| Psychological well-being | Spouse vs non-spouse
|
Time
|
Caregiver* time
|
|||
|---|---|---|---|---|---|---|
| B (95% CI) | p-value | B (95% CI) | p-value | B (95% CI) | p-value | |
| Depression | 1.00 (0.08, 1.92) | 0.032 | −0.44 (−1.19, 0.31) | 0.247 | 0.43 (−0.37, 1.23) | 0.292 |
| HADS-D | 2.22 (0.52, 3.92) | 0.010 | −0.66 (−1.14, −0.18) | 0.007* | −0.34 (−0.97, 0.29) | 0.291 |
| HADS-A | 1.95 (−0.35, 4.25) | 0.096 | −1.59 (−2.27, −0.92) | <0.001* | −0.21 (−1.05, 0.63) | 0.626 |
| APGAR | −0.42 (−1.38, 0.53) | 0.385 | −0.17 (−0.48, 0.14) | 0.287 | 0.02 (−0.36, 0.39) | 0.927 |
| PCS of the SF-36 | 0.24 (−0.83, 1.31) | 0.656 | 0.15 (−0.13, 0.42) | 0.290 | −0.37 (−0.74, −0.01) | 0.045 |
| MCS of the SF-36 | −2.28 (−3.93, −0.64) | 0.007* | 0.54 (0.10, 0.97) | 0.015 | 0.56 (−0.04, 1.15) | 0.067 |
Notes: Data were analyzed using generalized estimating equations models, controlling for age, gender, education levels, past history of depression, and patients’ chemotherapy.
Significant correlation remains after Bonferroni correction (p=0.05/6=0.0083).
Abbreviations: APGAR, Appearance, Pulse, Grimace, Activity, and Respiration; HADS, Hospital Anxiety and Depression Scale; HADS-D, depression subscale of HADS; HADS-A, anxiety subscale of HADS; SF-36, Short Form 36; PCS, physical component summary; MCS, mental component summary.
Discussion
This prospective study is among the first to examine the psychological well-being and QOL differences between spouse caregivers and non-spouse caregivers in HNC patients. We found that spouse caregivers were more likely to be female and elderly, less educated, and prone to psychiatric disorders than non-spouse caregivers. Spouse caregivers also demonstrated a significantly higher morbidity of depressive disorder, a greater severity of depression, and a worse mental component of QOL than non-spouse caregivers. The severity of depression and anxiety significantly decreased while the QOL mental component significantly improved over time. Furthermore, caregivers and time had a significant interaction effect on the physical component of QOL.
Of our subjects, most of the spouses were wives (wife: husband =10:1), while most of the non-spouse subjects were children (children: parents =3.7:1). Such demographic data can explain why spouse caregivers are more elderly and less educated than non-spouse caregivers. In regard to psychiatric diagnosis, the depressive disorder morbidity of spouse caregivers was 17.0% at both the index and the 6-month follow-up; meanwhile, the depressive disorder morbidity of non-spouse caregivers at the index and the 6-month follow-up was 10.3% and 4.5%, respectively. The adjustment disorder morbidity of spouse caregivers at the index and the 6-month follow-up was 20.2% and 1.1%, respectively, whereas the adjustment disorder morbidity of non-spouse caregivers at the index and the 6-month follow-up was 6.9% and 0, respectively. Therefore, we can suppose that spouse caregivers were more concerned for and distressed by their partner’s physical and even mental conditions and thus experienced more psychiatric disorders than non-spouse caregivers.
Wang et al31 carried out a meta-analytic study to examine the female preponderance in depressive disorders and discovered that women in the nonclinical population reported higher levels of depressive symptoms than men. Other studies have previously reported that unemployed adults were at an elevated risk of developing major depression.26 Therefore, being female and being unemployed are two risk factors for developing depressive disorder.31,32 Among our spouse caregivers, 91% were females and 42% were unemployed. This demographic characteristic of spouse caregivers can partially explain why spouse caregivers had a significantly higher morbidity of depressive disorders, as well as a greater severity of depression than non-spouse caregivers.
In comparing spouse caregivers and non-spouse caregivers, we also found that spouse caregivers’ QOL mental component was significantly worse than that of non-spouse caregivers. Prior studies have shown that the degree of QOL was inversely correlated to the severity of depression in the caregivers of cancer patients.11,33 Therefore, it is not unexpected that the QOL of our caregivers, both spouse and non-spouse, negatively correlated to their depression severity. However, few studies have reported that the QOL of spouse caregivers was worse than that of non-spouse caregivers when caring for patients, especially in regard to the mental component of QOL. More large-scale studies should be performed in the future to confirm our findings.
Regarding the time effect, our results indicate that caregivers’ depression and anxiety symptoms were inversely correlated to the QOL mental dimension in HNC caregivers during the 6-month follow-up period. Caregivers of newly diagnosed HNC patients face various treatment options during the first 6 months after diagnosis, and their resulting anxiety and depression symptoms are subsequently reduced through stress coping techniques and cognitive reconstruction.11,33 The physical component of QOL in non-spouse caregivers was significantly better than that of spouse caregivers during the 6-month follow-up period (Figure 1). Although spouse caregivers’ QOL mental component improved after the 6-month follow-up period, their QOL physical component decreased during the same time. This finding may indicate that the physical condition of spouse caregivers may deteriorate due to exhaustion after providing care for half a year. This hypothesis is understandable since most of the spouse caregivers in our sample werê50 years old.
Sterba et al performed a systemic review based on QOL in HNC patient–caregiver dyads. Of the 12 studies they reviewed, only one study detected a correlation between the QOL of spouses and their depression/psychiatric diagnosis.4,13 Furthermore, Zwahlen et al conducted a study to examine the mental health of 31 oral cancer patients and their wives. They found that 1) patients and their wives with a psychiatric diagnosis had a lower global QOL and 2) a higher marital quality in wives was associated with a higher QOL and lower rate of depression.4,34 These studies imply that spouse caregivers’ QOL is conversely correlated to the rate of depression in spouse caregivers of HNC patients. To the best of our knowledge, no studies have yet compared the psychological well-being and QOL between spouse caregivers and non-spouse caregivers of HNC patients. Additional studies in the future are necessary to confirm our results.
Study strengths and limitations
The present study has several strengths, including its prospective study design, the use of a structured clinical interview by psychiatrists, and being the first study to compare psychological well-being and QOL between spouse caregivers and non-spouse caregivers of HNC patients. Nevertheless, this study also has certain limitations that need to be considered when interpreting these data. First, consecutive sampling may result in a sampling bias. Second, we recruited our samples from a general hospital, which may not be representative of the general population. Third, our sample size was relatively small, so larger and longer-term follow-up studies with caregivers of patients with various types of cancer are needed in the future to better understand the psychological well-being and QOL between spouse caregivers and non-spouse caregivers. Fourth, we neither did evaluate the details of patients’ psychological well-being, QOL, or regimen for their HNC after the baseline assessment, nor did we examine the financial status of the caregivers. Such factors may potentially be important regarding caregivers’ psychological well-being and QOL.35 Finally, if Bonferroni correction had been applied to adjust for multiple testing in the GEE models (Table 2), some of our significant findings (ie, depression and HADS-D) would no longer be significant, which indicates that our sample size may not be sufficient to detect differences in depression between spousal and non-spousal caregivers.
Conclusion
Our findings suggest that depression and the mental component of QOL of spouse caregivers among HNC patients were more severe than that of non-spouse caregivers during the observed 6-month follow-up period. Clinicians should pay more attention to spouse caregivers’ mental health when providing patient care.
Acknowledgments
This study was supported by a grant from the Kaohsiung Chang Gung Memorial Hospital, Taiwan (CMRPG8A0581). The funding agent had no role in the study design, study performance, and the decision to submit the report.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Fan CY, Chao HL, Lin CS, et al. Risk of depressive disorder among patients with head and neck cancer: a nationwide population-based study. Head Neck. 2018;40(2):312–323. doi: 10.1002/hed.24961. [DOI] [PubMed] [Google Scholar]
- 2.Edwards B, Clarke V. The psychological impact of a cancer diagnosis on families: the influence of family functioning and patients’ illness characteristics on depression and anxiety. Psychooncology. 2004;13(8):562–576. doi: 10.1002/pon.773. [DOI] [PubMed] [Google Scholar]
- 3.Rivera HR. Depression symptoms in cancer caregivers. Clin J Oncol Nurs. 2009;13(2):195–202. doi: 10.1188/09.CJON.195.202. [DOI] [PubMed] [Google Scholar]
- 4.Zwahlen RA, Dannemann C, Gratz KW, et al. Quality of life and psychiatric morbidity in patients successfully treated for oral cavity squamous cell cancer and their wives. J Oral Maxillofac Surg. 2008;66(6):1125–1132. doi: 10.1016/j.joms.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Asai M, Akechi T, Nakano T, et al. Psychiatric disorders and background characteristics of cancer patients’ family members referred to psychiatric consultation service at National Cancer Center Hospitals in Japan. Palliat Support Care. 2008;6(3):225–230. doi: 10.1017/S1478951508000369. [DOI] [PubMed] [Google Scholar]
- 6.Vanderwerker LC, Laff RE, Kadan-Lottick NS, McColl S, Prigerson HG. Psychiatric disorders and mental health service use among caregivers of advanced cancer patients. J Clin Oncol. 2005;23(28):6899–6907. doi: 10.1200/JCO.2005.01.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park B, Kim SY, Shin JY, et al. Prevalence and predictors of anxiety and depression among family caregivers of cancer patients: a nationwide survey of patient–family caregiver dyads in Korea. Support Care Cancer. 2013;21(10):2799–2807. doi: 10.1007/s00520-013-1852-1. [DOI] [PubMed] [Google Scholar]
- 8.Lee Y, Lin PY, Chien CY, Fang FM. Prevalence and risk factors of depressive disorder in caregivers of patients with head and neck cancer. Psychooncology. 2015;24(2):155–161. doi: 10.1002/pon.3619. [DOI] [PubMed] [Google Scholar]
- 9.de Araujo AA, Reboucas Barbosa RA, de Menezes MS, de Medeiros II, de Araujo RF, Jr, de Medeiros CA. Quality of life, family support, and comorbidities in institutionalized elders with and without symptoms of depression. Psychiatr Q. 2016;87(2):281–291. doi: 10.1007/s11126-015-9386-y. [DOI] [PubMed] [Google Scholar]
- 10.Cao X, Jiang X, Li X, Lo MC, Li R, Dou X. Perceived family functioning and depression in bereaved parents in China after the 2008 Sichuan earthquake. Arch Psychiatr Nurs. 2013;27(4):204–209. doi: 10.1016/j.apnu.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Lee CY, Lee Y, Wang LJ, Chien CY, Fang FM, Lin PY. Depression, anxiety, quality of life, and predictors of depressive disorders in caregivers of patients with head and neck cancer: a six-month follow-up study. J Psychosom Res. 2017;100:29–34. doi: 10.1016/j.jpsychores.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Yeh PM, Wierenga ME, Yuan SC. Influences of psychological well-being, quality of caregiver–patient relationship, and family support on the health of family caregivers for cancer patients in Taiwan. Asian Nurs Res. 2009;3(4):154–166. doi: 10.1016/S1976-1317(09)60027-X. [DOI] [PubMed] [Google Scholar]
- 13.Sterba KR, Zapka J, Cranos C, Laursen A, Day TA. Quality of life in head and neck cancer patient–caregiver dyads: a systematic review. Cancer Nurs. 2016;39(3):238–250. doi: 10.1097/NCC.0000000000000281. [DOI] [PubMed] [Google Scholar]
- 14.Vickery LE, Latchford G, Hewison J, Bellew M, Feber T. The impact of head and neck cancer and facial disfigurement on the quality of life of patients and their partners. Head Neck. 2003;25(4):289–296. doi: 10.1002/hed.10206. [DOI] [PubMed] [Google Scholar]
- 15.Hodgkinson K, Butow P, Hunt GE, Wyse R, Hobbs KM, Wain G. Life after cancer: couples’ and partners’ psychological adjustment and supportive care needs. Support Care Cancer. 2007;15(4):405–415. doi: 10.1007/s00520-006-0148-0. [DOI] [PubMed] [Google Scholar]
- 16.Butler LD, Field NP, Busch AL, Seplaki JE, Hastings TA, Spiegel D. Anticipating loss and other temporal stressors predict traumatic stress symptoms among partners of metastatic/recurrent breast cancer patients. Psychooncology. 2005;14(6):492–502. doi: 10.1002/pon.865. [DOI] [PubMed] [Google Scholar]
- 17.Carmack Taylor CL, Badr H, Lee JH, et al. Lung cancer patients and their spouses: psychological and relationship functioning within 1 month of treatment initiation. Ann Behav Med. 2008;36(2):129–140. doi: 10.1007/s12160-008-9062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braun M, Mikulincer M, Rydall A, Walsh A, Rodin G. Hidden morbidity in cancer: spouse caregivers. J Clin Oncol. 2007;25(30):4829–4834. doi: 10.1200/JCO.2006.10.0909. [DOI] [PubMed] [Google Scholar]
- 19.Kim Y, Duberstein PR, Sörensen S, Larson MR. Levels of depressive symptoms in spouses of people with lung cancer: effects of personality, social support, and caregiving burden. Psychosomatics. 2005;46(2):123–130. doi: 10.1176/appi.psy.46.2.123. [DOI] [PubMed] [Google Scholar]
- 20.Ji J, Zöller B, Sundquist K, Sundquist J. Increased risks of coronary heart disease and stroke among spousal caregivers of cancer patients. Circulation. 2012;125(14):1742–1747. doi: 10.1161/CIRCULATIONAHA.111.057018. [DOI] [PubMed] [Google Scholar]
- 21.First MB, Gibbon M, Sptizer RL, Williams JBW. Structured Clinical Interview for DSM-IV-Clinician Version (SCID-CV) (User’s Guide and Interview) Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- 22.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell AJ, Meader N, Symonds P. Diagnostic validity of the Hospital Anxiety and Depression Scale (HADS) in cancer and palliative settings: a meta-analysis. J Affect Disord. 2010;126(3):335–348. doi: 10.1016/j.jad.2010.01.067. [DOI] [PubMed] [Google Scholar]
- 24.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 25.Taft C, Karlsson J, Sullivan M. Do SF-36 summary component scores accurately summarize subscale scores? Qual Life Res. 2001;10(5):395–404. doi: 10.1023/a:1012552211996. [DOI] [PubMed] [Google Scholar]
- 26.Lu JF, Tseng HM, Tsai YJ. Assessment of health-related quality of life in Taiwan (I): development and psychometric testing of SF-36 Taiwan version. Taiwan J Public Health. 2003;22(6):501–511. [Google Scholar]
- 27.Tseng HM, Lu JF, Gandek B. Cultural issues in using the SF-36 health survey in Asia: results from Taiwan. Health Qual Life Outcomes. 2003;1:72. doi: 10.1186/1477-7525-1-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smilkstein G. The family APGAR: a proposal for a family function test and its use by physicians. J Fam Pract. 1978;6(6):1231–1239. [PubMed] [Google Scholar]
- 29.Kenward MG, Lesaffre E, Molenberghs G. An application of maximum likelihood and generalized estimating equations to the analysis of ordinal data from a longitudinal study with cases missing at random. Biometrics. 1994;50(4):945–953. [PubMed] [Google Scholar]
- 30.Zhang H, Paik MC. Handling missing responses in generalized linear mixed model without specifying missing mechanism. J Biopharm Stat. 2009;19(6):1001–1017. doi: 10.1080/10543400903242761. [DOI] [PubMed] [Google Scholar]
- 31.Wang K, Lu H, Cheung EF, Neumann DL, Shum DH, Chan RC. “Female preponderance” of depression in non-clinical populations: a meta-analytic study. Front Psychol. 2016;7:1398. doi: 10.3389/fpsyg.2016.01398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bebbington P. The assessment and epidemiology of affective disorder. In: Checkley S, editor. The Management of Depression. Oxford: Blackwell Science; 1998. [Google Scholar]
- 33.Roing M, Hirsch JM, Holmstrom I. Living in a state of suspension – a phenomenological approach to the spouse’s experience of oral cancer. Scand J Caring Sci. 2008;22(1):40–47. doi: 10.1111/j.1471-6712.2007.00525.x. [DOI] [PubMed] [Google Scholar]
- 34.Jenewein J, Zwahlen RA, Zwahlen D, Drabe N, Moergeli H, Buchi S. Quality of life and dyadic adjustment in oral cancer patients and their female partners. Eur J Cancer Care (Engl) 2008;17(2):127–135. doi: 10.1111/j.1365-2354.2007.00817.x. [DOI] [PubMed] [Google Scholar]
- 35.Perlis ML, Smith LJ, Lyness JM, et al. Insomnia as a risk factor for onset of depression in the elderly. Behav Sleep Med. 2006;4(2):104–113. doi: 10.1207/s15402010bsm0402_3. [DOI] [PubMed] [Google Scholar]