Abstract
The kidney allocation system (KAS) altered pediatric candidate prioritization. We determined KAS’s impact on pediatric kidney recipients by examining delayed graft function (DGF) rates from 2010 to 2016. A propensity score-matched pediatric recipients pre- and post-KAS. A semiparametric decomposition analysis estimated the contributions of KAS-related changes in donor characteristics and dialysis time on DGF rate. The unadjusted odds of DGF were 69% higher post-KAS for young (<10 years at listing) recipients (N = 1153, P = .02) but were not significantly increased for older pediatric (10-17 years at listing) recipients (N = 2624, P = .48). Post-KAS, young recipients received significantly fewer pediatric (<18 years) donor kidneys (21% vs 32%, P < .01) and had longer median pretransplant dialysis time (603 vs 435 days, P < .01). After propensity score matching, post-KAS status increased the odds of DGF in young recipients 71% (OR 1.71, 95% CI 1.01-2.46). In decomposition analysis, 24% of the higher DGF rate post-KAS was attributable to donor characteristics and 19% to increased recipient dialysis time. In a confirmatory survival analysis, DGF was associated with a 2.2 times higher risk of graft failure (aHR2.28, 95% CI 1.46-3.54). In conclusion, KAS may lead to worse graft survival outcomes in children. Allocation changes should be considered.
Keywords: classification systems, delayed graft function, pediatric transplantation, kidney (allograft) function/dysfunction, waitlist management
1 |. INTRODUCTION
The Organ Procurement and Transplantation Network (OPTN) implemented a substantial redesign to the national transplant allocation system for deceased donor kidneys on December 4, 2014. The new kidney allocation system (KAS) was designed with adult candidates in mind. The aim was to increase transplantation of highly sensitized patients, to decrease geographic variability in access, and to increase organ longevity by matching high-quality kidneys with adult candidates with longer expected post-transplant survival.1 Pediatric candidates were not the focus of the policy aims, but nevertheless, the pool of kidneys offered to pediatric candidates was altered. Prior to KAS, pediatric candidates received priority for all kidneys from deceased donors under the age of 35 years (Share-35). Post-KAS, pediatric candidates receive priority for the top 35% Kidney Donor Profile Index (KDPI) organs after highly sensitized adults and former living donors.2 The motivation for this shift was an attempt to preserve the pediatric advantage while maintaining a uniform allocation system using KDPI.
However, the predictive accuracy of KDPI is modest at best (c = 0.6) and was derived using only adult recipients.2,3 Physiologically, younger (<10 years of age) pediatric patients are significantly different from both adolescents and adults.4 Therefore, it is not surprising that it has been recently demonstrated that KDPI is even less accurate for pediatric recipients.5 Kidney Donor Profile Index has also been shown to be inaccurate in its assessment of young pediatric donor kidneys, typically assigning them KDPI >35 percentile scores, which is worse than their actual measured graft function.6 Under KAS, pediatric candidates do not have priority for KDPI >35 kidneys and, therefore, pediatric candidates do not have consistent access to kidneys from young pediatric donors. Not only might these kidneys outperform adult kidneys in children, but also certain young pediatric candidates require a pediatric kidney for anatomical reasons.7 Furthermore, the changes in pediatric priority under KAS have led to a lower overall offer rate for pediatric candidates.8 Therefore, the overall impact on kidney graft survival following the switch from Share-35 to top 35% KDPI for pediatric recipients is unknown.
At this point after KAS implementation, important transplant outcomes such as long-term graft survival cannot be determined. However, delayed graft function (DGF), which has been previously associated with worse outcomes in pediatric kidney recipients,9-11 can be assessed and may be an appropriate surrogate marker for graft survival. We aimed to 1) confirm the association of DGF with graft survival in the pediatric transplant population; 2) determine the effect of KAS on pediatric recipients by examining the rate of DGF before and after the policy was implemented; and 3) determine the factors that may contribute to any change in DGF rate post-KAS.
2 |. METHODS
2.1 |. Data source, population, and outcomes
This study used OPTN data as of March 1st, 2017 obtained from the United Network for Organ Sharing (UNOS). Data on recipient, donor, and transplant factors for all kidney-alone deceased donor kidney transplants to pediatric recipients from 2006 to 2016 were collected. We divided pediatric recipients a priori into 2 previously identified cohorts: young (<10 years of age at listing) and adolescent (10-17 years of age at listing)12 as we hypothesized that young pediatric recipients would be uniquely harmed by KAS. Recipient factors collected included gender, age, size, diagnosis, medical comorbidities, and sensitization data. Donor factors included age (categorized as young pediatric, adolescent, adult), race, comorbidities, cause of death, terminal creatinine, and size. Transplant factors included dialysis time, time spent on the waitlist, number of HLA mismatches, and cold ischemia time. The primary outcomes of the study were DGF and graft survival. Delayed graft function was defined by need for dialysis in the first-week post-kidney transplant.13
2.2 |. Statistical analysis
2.2.1 |. Association of DGF with graft survival
First, we determined the relationship between DGF and graft failure in the pediatric recipient population. Using data from all deceased donor kidney transplants in pediatric recipients from January 1st, 2006 to December 31st, 2016, Kaplan-Meier estimates of graft survival were generated for young and adolescent recipients with and without DGF after transplant. The entire post-Share 35 period was included to extend follow-up time and generate more precise 5-year graft survival estimates. To eliminate the influence of surgical technique and/or graft thrombosis, we excluded recipients with early graft loss (permanent graft failure within the first week after transplant) from the graft survival analysis.
To evaluate whether DGF was truly harmful independent of other risk factors for graft failure, we estimated the adjusted hazard ratio of graft failure for DGF using a Cox proportional hazards model with year of transplant and previously identified risk factors for graft failure in pediatric recipients as covariates.14
2.2.2 |. Pre-post KAS comparison
Pre-post KAS comparisons for all donor, and transplant factors using linear regression with robust standard errors clustered by transplant center. Quantile regression with robust standard errors was used to compare medians. An incidence rate ratio exact test was used to compare the rate of transplant in the 2 periods. Results were considered significant at the P < .05 level.
For the main estimate of the effect of KAS on DGF, propensity score nearest-neighbor matching was used to match pediatric deceased donor kidney transplant recipients post-KAS (December 4th, 2014-December 31st, 2016) to pediatric recipients in the 5-year pre-KAS (January 1st, 2010-December 3rd, 2014) based on recipient characteristics.15 In our propensity score, recipient factors previously associated with DGF16,17 were included to reduce bias based on changes in recipient characteristics over time. We performed balancing tests on our matched cohort with an absolute standardized difference of <10% being considered well balanced.18 As a sensitivity analysis, we also estimated the adjusted odds of DGF post-KAS vs pre-KAS using a multivariable logistic regression model with all recipient factors as covariates. To account for a potential “bolus” effect post-KAS, we estimated the DGF rate separately in 2015 and 2016.
2.3 |. Decomposition analysis
We did not control for changes in donor characteristics and transplant factors with our propensity score matching analysis as changes in these variables are consequences (or downstream effects) of KAS. Instead, we used changes in donor and transplant factors to determine the mechanism by which KAS increased the DGF rate with the DiNardo-Fortin-Lemieux (DFL) method.19 The DFL method is a semiparametric approach to decompose mean differences in outcomes between 2 groups into the percentage of the difference attributable to differences in covariates between the groups. The main advantage of the DFL method over the Oaxaca-Blinder method20 is that no parametric assumptions are made about the functional form of the relationship between the outcome and the covariates. The DFL method uses a propensity score and inverse probability weighting to estimate the counter-factual outcome that would have occurred if post-KAS recipients had received the same kidneys with the same amount of dialysis time as pre-KAS recipients. In other words, the DFL method calculates the rate of DGF that pediatric recipients transplanted after 12/4/2014 would have experienced if they had been allocated kidneys under Share-35 rather than KAS. Sequentially adding sets of variables to the propensity score allows for the estimation of the relative contributions of donor and transplant factors to change in the DGF rate.
2.4 |. Trends in multiorgan transplants
Multiorgan transplant candidates get priority over pediatric candidates for high-quality low KDPI kidneys.21 Given the increasing number of multiorgan transplants over time, one may hypothesize that this indirectly affected the quality of organs being offered to pediatric candidates. Therefore, we examined trends in the multiorgan transplants from 2010 to 2016 on all deceased donor transplants to both adult and pediatric recipients. To test the hypothesis that multiorgan transplants are utilizing proportionally more high-quality kidneys over time, we performed a nonparametric test for trend22 in the percentage of KDPI <35 transplants used in multiorgan transplants from 2010 to 2016.
All analyses were performed with STATA, version 15 (StataCorp, College Station, TX, USA).
3 |. RESULTS
3.1 |. DGF and graft survival
From 2006 to 2016, there were 1746 deceased donor kidney-alone transplants to young pediatric recipients (<10 years old at listing) and 4180 transplants to adolescent recipients (10-17 years) with graft survival greater than 7 days. In unadjusted Kaplan-Meier survival analysis, young recipients with DGF had significantly lower 1-year (89% vs 97%), 3-year (78% vs 92%), and 5-year (73% vs 86%) survival than recipients without DGF (Figure 1, P < .01 for all comparisons). In the Cox proportional hazards model adjusting for other risk factors for graft failure and year of transplant, the adjusted hazard ratio for DGF was 2.28 (95% CI 1.46-3.54, P < .01, Table S1). Among adolescent recipients, the association of DGF with graft failure was of smaller magnitude but still statistically significant (aHR 1.63, 95% CI 1.30-2.05, P < .01).
FIGURE 1.
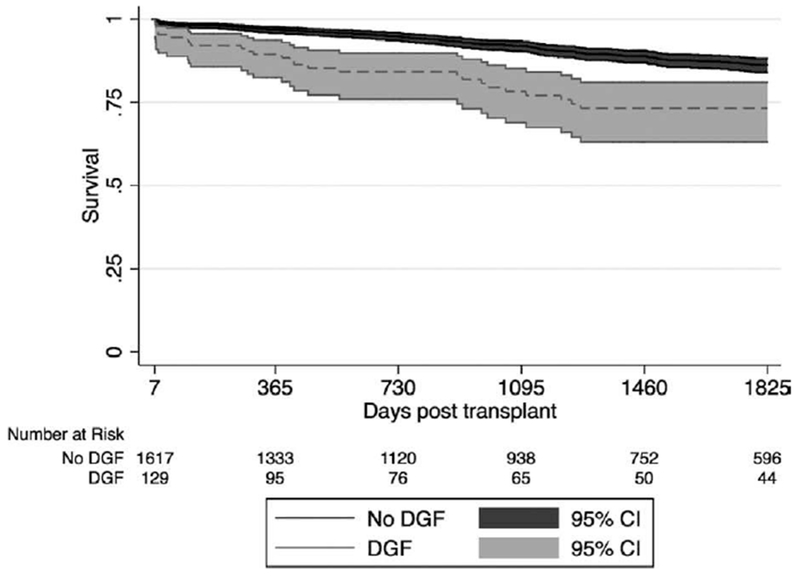
Graft survival for young pediatric recipients by delayed graft function (DGF), 2006-2016. Kaplan-Meier survival estimates and 95% CI are displayed for each group. Grafts with DGF had significantly lower 1-y (89% vs 97%), 3-y (78% vs 92%), and 5-y (73% vs 86%) survival than grafts without DGF (P < .01 for all comparisons)
3.2 |. DGF rates pre- and post-KAS by pediatric recipient age
From 2010 to 2016, there were 1153 transplants to young pediatric recipients (<10 years) and 2624 transplants to adolescent recipients (10-17 years). The rate of DGF pre- and post-KAS by initial age is displayed in Figure 2. Delayed graft function occurred in 7.47% of adolescent recipients pre-KAS and 8.28% post-KAS (P = .48), whereas DGF occurred in 5.96% of young pediatric recipients pre-KAS and 9.67% post-KAS (P = .02). For young pediatric recipients, the odds of DGF were 69% higher post-KAS (OR 1.69, 95% CI 1.07-2.67). The increase in DGF for young pediatric recipients was not limited to transplants performed early after KAS implementation. The rate of DGF for young pediatric recipients was 8.6% in year 1 post-KAS and 10.6% in year 2 post-KAS. Among non-preemptively transplanted young pediatric patients, the increased risk of DGF post-KAS was (OR 1.70, 95% CI 1.04-2.78), similar to the OR for all young pediatric recipients. The rate of primary graft nonfunction (defined by failure within 90 days per OPTN policy)21 was similar in both time periods for both groups (1.8% vs 1.2% for adolescents, P = .18 and 2.9% vs 3.0% for young recipients, P = .45).
FIGURE 2.
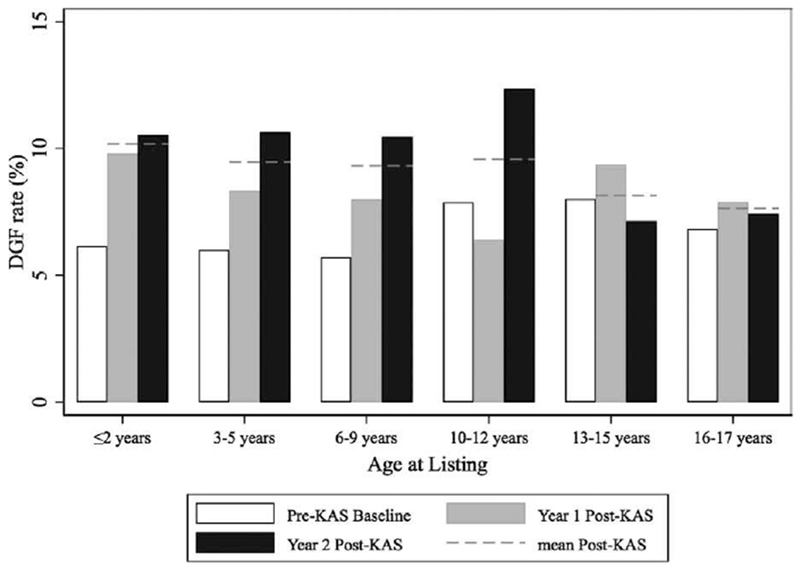
Delayed graft function (DGF) rates pre-post KAS by age of listing for pediatric recipients. (white = pre-KAS, grey = year 1 post-KAS, black = year 2 post-KAS, dashed line = mean post-KAS). Delayed graft function was defined by need for dialysis in the first week after transplant. Young pediatric recipients (<10 y at listing) had DGF 5.96% of the time pre-KAS and 9.67% of the time post-KAS (P = .024). Older pediatric recipients (≥10 y at listing had DGF 7.47% of the time pre-KAS and 8.28% of the time post-KAS (P = .477)
3.3 |. Recipient, transplant, and donor characteristics pre-post KAS
Young pediatric recipients were similar in most demographic categories pre- and post-KAS (Table 1). The percentage of highly sensitized recipients increased from 0% to 2.2% but the final calculated panel reactive antibodies (CPRA) was not significantly different (mean 8.2 vs 9.3, P = .43). Transplant factors (eg, HLA mismatches, cold ischemia time) were not significantly different pre- and post-KAS.
TABLE 1.
Recipient factors pre-post KAS for young pediatric recipients (<10 y at listing)
| pre-KAS 1/1/2010-12/3/2014 | post-KAS 12/3/2014-12/31/2016 | P-value* | |
|---|---|---|---|
| Mean transplants per year | 160.3 | 175.5 | .077 |
|
| |||
| Male per year (%) | 103.8 (64.8) | 104.1 (59.3) | .056 |
|
| |||
| Age at transplant | |||
|
| |||
| <5 y old per year (%) | 86.5 (54) | 82 (47) | .031 |
| 6-10 y old per year (%) | 69.3 (43.2) | 82 (47) | |
| 10 + y old per year (%) | 4.5 (2.8) | 10.6 (6.1) | |
|
| |||
| Race | |||
|
| |||
| White per year (%) | 63.2 (39.4) | 73.8 (42) | .26 |
| Black per year (%) | 39.6 (24.7) | 34.7 (19.8) | |
| Hispanic per year (%) | 44.3 (27.6) | 53.5 (30.5) | |
| Other per year (%) | 13.2 (8.2) | 13.5 (7.7) | |
|
| |||
| Size | |||
|
| |||
| Mean weight in kg ± SD | 17 ± 7.6 | 17.7 ± 7.8 | .14 |
|
| |||
| Mean height in cm ± SD | 96.9 ± 20 | 99.6 ± 19.2 | .04 |
|
| |||
| Mean BMI ± SD | 17.5 ± 2.9 | 17.3 ± 3.4 | .46 |
|
| |||
| Diagnosis | |||
|
| |||
| Congenital or acquired obstructive uropathy per year (%) | 28.6 (17.9) | 29.4 (16.8) | .64 |
|
| |||
| Focal segmental glomerulosclerosis per year (%) | 14.0 (8.7) | 15.9 (9.1) | .84 |
|
| |||
| Hypoplasia, dysplasia, dysgenesis, or agenesis per year (%) | 32.5 (20.3) | 40 (22.8) | .39 |
|
| |||
| Sensitization | |||
|
| |||
| Highly sensitized (CPRA ≥99) per year (%) | 0 (0) | 3.9 (2.2) | <.001 |
|
| |||
| History of previous transplant per year (%) | 8.3 (5.2) | 9.6 (5.5) | .82 |
|
| |||
| Mean final CPRA ± SD | 8.2 ± 20.1 | 9.3 ± 22.2 | .43 |
|
| |||
| Transplant factors | |||
|
| |||
| Mean number of HLA mismatches ± SD | 4.5 ± 1.3 | 4.5 ± 1.1 | .90 |
|
| |||
| Preemptive transplant per year (%) | 47 (29.4) | 39 (22.5) | .025 |
|
| |||
| Mean dialysis time in days ± SD | 546 ± 560 | 742 ± 758 | <.001 |
|
| |||
| Mean time on waitlist in days ± SD | 360 ± 435.5 | 449 ± 555 | .020 |
|
| |||
| Median cold ischemia time in hours [IQR] | 11.4 [8.1-15.3] | 11 [7.9-15.8] | .369 |
Recipient and transplant factors for all deceased donor kidney transplants to child recipient under the age of 10 at listing before and after the implementation of KAS. For categorical variables, mean number of yearly transplants in each category is reported with percentage of yearly lists in parentheses. For continuous variables, mean ± SD or median with [intraquartile range] are reported.
Robust standard errors clustered by transplant center calculated using linear regression for continuous variables and multinomial logit for categorical variables. Quantile regression with robust standard errors used to compare medians. Incidence rate ratio exact test was used to compare the rate of transplant in the 2 periods. Significant results at the P < .05 level are bolded.
Despite similar recipient characteristics, the rate of preemptive transplants decreased from 29.4% to 22.5% (P = .03) and the mean time spent on the wait list for young pediatric recipients increased 89 days post-KAS compared to pre-KAS (P = .02). Young pediatric recipients spent an average of 359 days on the waitlist pre-KAS, 415 days in year 1 post-KAS, and 480 days in year 2 post-KAS (Figure 3). The number of young pediatric recipients who spent over a year on the waitlist before transplant increased from 32.6% to 39.3% (P = .03; Figure S1). The mean dialysis time increased from 547 days pre-KAS to 715 days in year 1 post-KAS to 766 days in year 2 post-KAS (P < .01 for pre-post difference; Figure 4).
FIGURE 3.
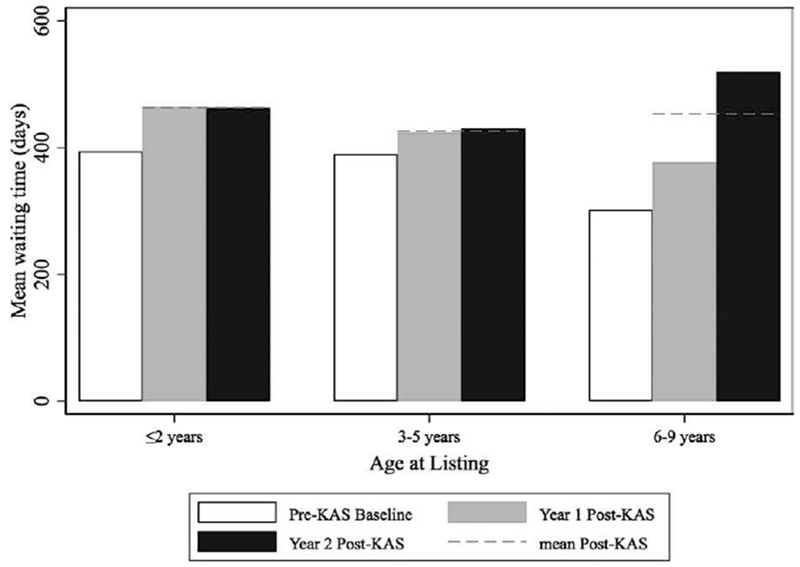
Mean time on wait list by age of listing for young pediatric recipients, pre-post KAS. Waiting time was calculated as actually time spent on the waitlist (white = pre-KAS, grey = year 1 post-KAS, black = year 2 post-KAS, dashed line = mean post-KAS). Young pediatric recipients spent an average of 359 d on the waitlist pre-KAS, 415 d in year 1 post-KAS, and 480 d in year 2 post-KAS (P = .02 for pre-post KAS difference)
FIGURE 4.
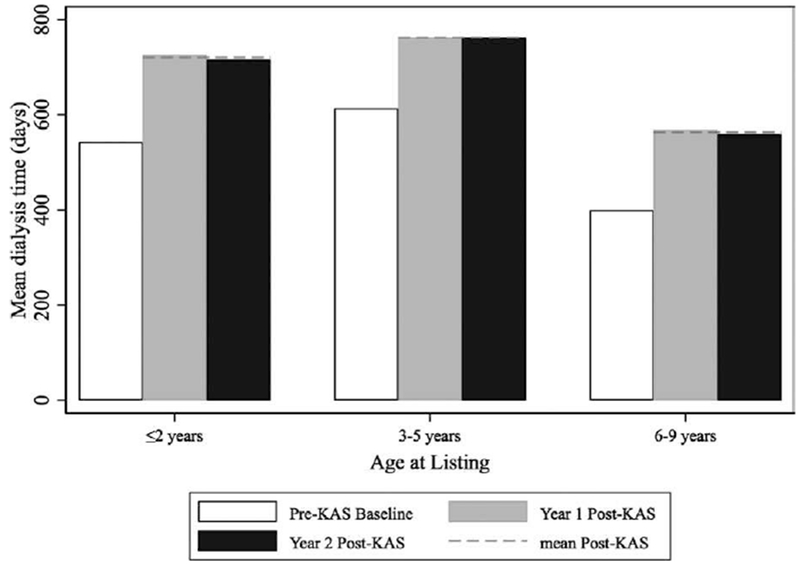
Mean dialysis time by age of listing for young pediatric recipients, pre-post KAS. Mean dialysis by age at the time of initial listing and policy (white = pre-KAS, grey = year 1 post-KAS, black = year 2 post-KAS, dashed line = mean post-KAS). Overall for young pediatric recipients, the mean dialysis time increased from 547 d pre-KAS to 715 d in year 1 post KAS to 766 d in year 2 post-KAS (P < .001 for pre-post difference)
Young pediatric recipients transplanted post-KAS received organs from deceased donors with a significantly different age and size (height and weight) distribution (P < .01, Table 2). After KAS, young pediatric recipients received 34% fewer deceased donor kidneys from pediatric donors overall (32% pre-KAS vs 21% post-KAS, P < .01), including a 76% decrease in deceased donor kidneys from young pediatric donors (7% pre-KAS vs 1.7% post-KAS, P < .01 (Table 2, Figure 5A). Instead, young pediatric recipients received 727% more kidneys from donors over the age of 35 (1.1% pre-KAS vs 9.1% post-KAS, P < .01).
TABLE 2.
Donor factors pre-post KAS for young pediatric recipients
| prior to KAS1/1/2010-12/3/2014 | post-KAS12/3/2014-12/3/2016 | P-value* | |
|---|---|---|---|
| Mean transplants per year | 160.3 | 175.5 | .077 |
|
| |||
| Donor age | |||
|
| |||
| 0-5 y old (%) | 5.1 (3.2) | 0.5 (0.3) | <.001 |
| 6-9 y old (%) | 6.1 (3.8) | 2.4 (1.4) | |
| 10-17 y old (%) | 39.6 (24.7) | 33.8 (19.2) | |
| 18-24 y old (%) | 61.7 (38.5) | 75.2 (42.9) | |
| 25-29 y old (%) | 26.6 (16.6) | 25.6 (14.6) | |
| 30-34 y old (%) | 19.3 (12) | 22.2 (12.6) | |
| 35 + y old (%) | 1.8 (1.1) | 15.9 (9.1) | |
|
| |||
| Donor race | |||
|
| |||
| White (%) | 91.4 (57) | 98.4 (56) | .16 |
| Black (%) | 27.6 (17.2) | 24.6 (14) | |
| Hispanic (%) | 32.9 (20.5) | 45.8 (26.1) | |
|
| |||
| Other | 8.3 (5.2) | 6.8 (3.8) | |
|
| |||
| Donor hypertension (%) | 3 (1.9) | 2.9 (1.6) | .7 |
|
| |||
| Donor diabetes (%) | 1.2 (0.8) | 0 (0) | .1 |
|
| |||
| DCD (%) | 0 (0) | 0.5 (0.3) | .14 |
|
| |||
| Donor hepatitis C (%) | 0 (0) | 0 (0) | – |
|
| |||
| Donor cause of death | |||
|
| |||
| CVA (%) | 37.4 (23.3) | 50.6 (28.8) | .08 |
| Anoxia (%) | 16.0 (10) | 14.5 (8.2) | |
| Head trauma (%) | 102.4 (63.9) | 108.0 (61.5) | |
| Other (%) | 4.4 (2.8) | 2.6 (1.4) | |
|
| |||
| Mean donor creatinine in mg/dL ± SD | 0.83 ± 0.37 | 0.86 ± 0.35 | .38 |
|
| |||
| Mean weight kg ± SD | 69.7 ± 21.1 | 74.1 ± 18.9 | .002 |
|
| |||
| Mean height in cm ± SD | 166.7 ± 19.4 | 170.8 ± 12.2 | <.001 |
|
| |||
| Mean BMI ± SD | 24.4 ± 5.2 | 25.2 ± 5.3 | .047 |
|
| |||
| Kidney donor profile index (2015 reference) | 18.3 ± 16 | 15.6 ± 11.5 | .013 |
Donor information from deceased donor kidney transplants to child recipients under the age of 10 at listing before and after the implementation of KAS. For categorical variables, number of yearly transplants in each category is reported with percentage of yearly lists in parentheses. For continuous variables, mean ± SD or median with [intra quartile range] are reported. Young pediatric recipients were significantly less likely to receive a kidney from pediatric donors (21% vs 32%, P < .001) and received organs from larger donors based on height, weight, and BMI.
Robust standard errors clustered by transplant center calculated using linear regression for continuous variables and multinomial logit for categorical variables. Incidence rate ratio exact test was used to compare the rate of transplant in the 2 periods. Significant results at the P < .05 level are bolded.
FIGURE 5.
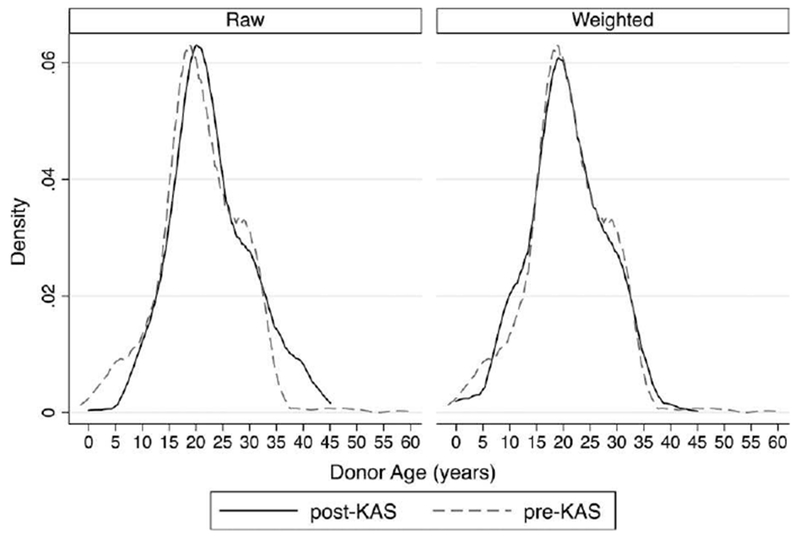
Donor age distribution for young pediatric recipients, pre-post KAS. A (left). The raw sample demonstrates how after KAS, young pediatric recipients received substantially fewer deceased donor kidneys from pediatric donors (32% pre-KAS vs 21% post-KAS, P < .01) and young pediatric donors (8.7% vs 3.8%, P < .01). B (right). In the propensity score-weighted sample, the post-KAS transplants are weighted to match the covariate distribution of the pre-KAS transplants. The donor age distributions have significantly better matching in the weighted sample by down-weighting >35 y of age and up-weighting pediatric donors (see Table S3 for standardized differences)
The mean KDPI (2015 reference) for kidneys transplanted into young pediatric recipients decreased from 18.3% pre-KAS to 15.6% post-KAS (P = .01; box plot of distribution of KDPI pre- and post-KAS displayed in Figure S2).
3.4 |. Adjusted DGF increase for young recipients
The unadjusted odds of DGF among young pediatric recipients were 69% higher post-KAS (OR 1.69, 95% CI 1.07-2.67; Table 3). After adjustment for recipient factors in multivariable logistic regression, the odds were 75% higher (aOR 1.75, 95% CI 1.09-2.82, Table S2). In the propensity score-matched cohorts (N = 354 for each group), the odds of DGF were 71% higher after KAS (aOR 1.71, 95% CI 1 1.01-2.46). Balancing tests indicated good covariate balance with standardized differences were all <10% indicating successful matching of post-KAS recipients to similar pre-KAS recipients (Table S3, Figure S3). There were 8 highly sensitized (CPRA ≥99%) young pediatric recipients transplanted post-KAS that had to be excluded from the propensity score cohort as there were no highly sensitized recipients transplanted in the pre-KAS period. One of the 8 highly sensitized young recipients post-KAS developed DGF.
TABLE 3.
Effect of KAS on young pediatric recipients
| Odds ratio of DGF (post-KAS vs pre-KAS) | 95% CI | |
|---|---|---|
| Unadjusted (N = 1143) | 1.69 | (1.07-2.67) |
|
| ||
| Adjusted (multivariable logistic regression) (N = 1143) | 1.75 | (1.09-2.82) |
|
| ||
| Propensity score-matched cohort (N = 708) | 1.71 | (1.01-2.46) |
Odds of DGF post-KAS relative to pre-KAS in the unadjusted sample, adjusted (multivariable logistic regression) model, and nearest-neighbor propensity score-matched cohort. Covariates used for adjustment or matching included recipient age, sex, race, renal diagnosis (congenital/acquired uropathy, FSGS, hypoplasia/dysplasia/dysgenesis/agenesis, other) history of previous transplant and sensitization (end CPRA). See Table S1 for the full multivariable logistic regression model and Table S2 for balancing in propensity score-matched cohort.
3.5 |. Decomposition analysis
The results of the decomposition analysis are displayed in Table 4. Differences in donor factors (size and age) explained 24% of the increased odds of DGF post-KAS, and duration of dialysis prior to transplant explained 19% of the increased odds of DGF post-KAS. Dialysis time, donor age, and donor size were well balanced in the propensity score-weighted sample distributions with standardized differences of <10% for all deciles for each covariate (Table S4, Figure 5B).
TABLE 4.
Decomposition of pre-post KAS DGF rate for young pediatric recipients
| Odds ratio of DGF (post-KAS vs pre-KAS) | 95% CI | % Odds difference explained, cumulative | |
|---|---|---|---|
| Sample (N = 1143) | 1.68 | (1.06-2.66) | – |
|
| |||
| Weighted by donor age/size (N = 1138) | 1.52 | (0.95-3.46) | 24% |
|
| |||
| Weighted by donor age/size and duration of recipient dialysis (N = 1138) | 1.37 | (0.90-2.66) | 43% |
Semiparametric decomposition of the increased odds of DGF post-KAS via the DiNardo, Fortin, and Lemieux method. This method uses a propensity score and inverse probability weighting to estimate the counterfactual outcome if post-KAS recipients had received kidneys from the same donors with the same amount of dialysis time as pre-KAS recipients. Each row represents a set of variables added to the propensity score and the resulting reduction in the odds of DGF can be interpreted as the percent of the post-KAS DGF explained by the variables. See Figure 5 and Table S3 for balancing of covariates in the final model. Donor size variables included age, height, weight, and BMI.
3.6 |. Trends in multiorgan transplants
From 2010 to 2016, the absolute number of KDPI <35 kidneys used in multiorgan transplants increased (from 906 to 1145 during 2010-2016), driven mainly by increased liver-kidney transplants (from 205 to 374 during 2010-2016). However, the number of total DDKT also increased (11 799-14 443, Table S5) and the absolute number of KDPI <35 kidneys transplanted increased (3724-4801). There was no significant trend in the percentage of KDPI <35 kidneys used by multiorgan transplants during the 2010-2016 period (Table S6, P = .73). On average, 19.4% of KDPI <35 kidneys were used by multiorgan transplants during the study period (range 18.6%-19.9%).
4 |. DISCUSSION
In this UNOS database analysis of 3777 deceased donor kidney transplants in pediatric recipients, DGF was clearly associated with significantly worse graft outcomes. Among young pediatric recipients, DGF was associated with a 13% reduction in 5-year graft survival, similar to the drop in graft survival seen when adult recipients experience DGF.23,24 Kidney allocation system implementation was associated with a 70% increase in odds of DGF for young (<10 years at listing) pediatric recipients, but no increase in DGF for adolescent recipients. Young pediatric recipients had similar baseline characteristics in the pre- and post-KAS eras and after propensity score matching based on recipient characteristics, a similar magnitude of KAS associated increase in DGF was observed. In decomposition analysis, increases in donor age and size and recipient dialysis time each explained substantial portions of the post-KAS DGF risk.
The fact that DGF rates significantly increased for young pediatric recipients post-KAS implementation is an important warning sign that this vulnerable patient population is not being well served by KAS. The OPTN has already reported a 40% increase in DGF in the overall deceased donor-recipient pool, which was attributed to a “bolus” of transplantation of adult candidates with long dialysis times and high sensitization.11,13 Fortunately, for the adult population, the increase in DGF is steadily trending down and is expected to return to pre-KAS rates.13 We demonstrate an even larger relative increase in DGF risk for young pediatric recipients that were not attributable to changes in recipient sensitization and did not trend down in year 2 post-KAS. While there were a few transplants to highly sensitized young pediatric recipients post-KAS (N = 8), overall young pediatric recipients were not highly sensitized in either period, with similar low mean CPRAs. So, not surprisingly, after propensity score matching based on CPRA (and all other recipient factors), the post-KAS DGF increase was unchanged. In addition, deceased donor transplants in adult recipients have a significant increase in organ cold-times with increased national sharing under KAS that could contribute to DGF. In contrast, the pediatric cohort has not seen a cold ischemic time change, most likely because the majority of kidneys being transplanted into pediatric recipients are from local deceased donors.
The post-KAS shift in the donor age and size distributions are a direct result of the way KDPI scores donor kidneys. For DGF to increase in pediatric recipients despite a decrease in their donor’s mean KDPI provides evidence that KDPI, a tool developed for and with adult recipients is not accurate when applied to young pediatric recipients. In the context of fewer offers for pediatric candidates post-KAS,8 perhaps transplant surgeons are falsely reassured by the “good” KDPIs of older donors and changing practices to accept organs that may be a poor physiologic fit for some young candidates.4 Our decomposition analysis confirms that this KAS-related shift toward larger and older donors played a significant role in the DGF increase.
The decomposition analysis also revealed that longer dialysis times contributed to the DGF increase among young pediatric recipients. Because KAS gave additional priority to candidates whose initiation of dialysis preceded the time of their waitlist registration, a small shift toward longer dialysis time among pediatric recipients was expected. However, the longer mean wait times (actual time on the list) in both year 1 and year 2 post-KAS, as opposed to the short-lived bolus effect seen in overall in transplant recipients,11,13 imply that there are other reasons why children are waiting longer for an appropriate organ. One explanation for the longer waiting time involves decreased access to appropriate donor kidneys. Young children received fewer organs from pediatric donors (especially young pediatric donors) under KAS because small kidneys are mostly classified as sequence C by KDPI (ie, KDPI >35),6 for which children have no priority. Young candidates who have clinical scenarios necessitating small donor kidneys, such as inferior vena cava thrombosis, small abdominal cavities, or baseline hypotension, may be spending a long time waiting for the right offer, leading to increased DGF when they finally get an organ. Further analysis of offer and acceptance/rejection data should be performed to explore the reasons behind the longer waiting time. However, regardless of the exact cause, the persistence of longer wait times in years 1 and 2 post-KAS is troubling given the poor survival, growth, cognitive, and developmental outcomes of children on dialysis.25,26
We believe our results provide strong evidence for the need to modify the current kidney allocation system to better assess donor kidney quality for children. Because KDPI was derived for adult recipients, a potential solution would involve developing a novel donor ranking system specifically for young pediatric recipients and allocating young pediatric candidates the best organs for them according to this metric. Alternatively, a scoring system specifically designed to accurately score pediatric donor kidneys6 could be implemented to ensure these organs are offered and allocated, when appropriate, to the pediatric recipient pool. Finally, the simplest solution would involve giving young pediatric candidates priority for pediatric donors. This policy would increase the donor pool for children with kidneys that are more suitable for pediatric recipients and give centers their own autonomy to match donor to recipient characteristics. A modification of KAS should be formally developed and simulated to ensure that kidneys with the maximal graft longevity are offered to children, the transplant population with the potential to live the longest.
4.1 |. Study limitations
Our study has several limitations. First, the true value of an allocation system is best measured with graft and patient survival, but it is too early to use these measures to assess the impact of KAS. Instead, we use DGF, a known surrogate for graft survival confirmed to be independently associated with graft failure in our sample,9,10 which can be meaningfully assessed with the limited time post-KAS implementation. Second, while the propensity score-matched analysis is based on measured recipient factors, it is possible young pediatric recipients are different after KAS implementation in ways not captured by the OPTN database, which could explain the increased DGF rate. Third, in decomposition analysis, about half of the increased post-KAS DGF risk remains unexplained. This is likely to be due, at least in part, to lack of overlap of donor covariate distributions that were not equalized with weighting. However, the final inverse propensity score-weighted odds ratio is not significantly different than 1, indicating that there was no significant difference in DGF pre- and post-KAS after weighting by donor age, size, and dialysis time. This result supports our position that KAS poorly extrapolates to pediatric recipients.
Finally, inherent in any sequential time period analysis is the possibility of concurrent events that could explain the longitudinal trend. For example, global donor quality could have coincidentally worsened post-KAS in some way unrelated to KAS itself. We investigated the specific hypothesis that increasing multiorgan transplantation consumed more high quality, low KDPI organs and, therefore, reduced the pool of high-quality organs for pediatric patients. While there was an increase in the absolute number of low KDPI kidneys used in multiorgan transplants during the study period, we found no significant trend in the proportion of KDPI <35 kidneys used in multiorgan transplants. Therefore, the DGF increase among young pediatric recipients seen in this paper is unlikely to be attributable to utilization of low KDPI kidneys by multiorgan transplants. Furthermore, if donor kidney quality for young pediatric candidates is worsening over time, KAS is not detecting it because the mean KDPI of donors transplanted into young pediatric recipients actually decreased post-KAS.
5 |. CONCLUSIONS
The implementation of KAS was associated with a 70% increase in odds of DGF for young (<10 years at listing) pediatric recipients of deceased donor kidneys. The increase in DGF can be attributed to changes such as a shift toward larger and older donors and longer dialysis times for recipients. Delayed graft function in young pediatric recipients is associated with significantly worse graft survival, suggesting that KAS will ultimately lead to worse graft survival outcomes in children. A change in kidney allocation policy should be considered to re-establish the full pediatric advantage that Share-35 attained.
Supplementary Material
ACKNOWLEDGEMENTS
The data reported here have been supplied by the United Network for Organ Sharing as the contractor for the Organ Procurement and Transplantation Network. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the OPTN or the U.S. Government. This work was supported in part by Health Resources and Services Administration contract 234-2005-370011C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Funding information
Supported in part by Institutional Clinical and Translational Science Award grant UL1 RR024999; PI: Dr. Julian Solway. William Parker is supported by a NIHT32 Training Grant, number 5T32HL007605-32.
Footnotes
CONFLICT OF INTEREST
The authors of this manuscript have no conflicts of interests to disclose.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
REFERENCES
- 1. Friedewald JJ Samana CJ Kasiske BL et al. The kidney allocation system Surg Clin North Am 2013. 93 1395–1406 [DOI] [PubMed] [Google Scholar]
- 2.OPTN Kidney Committee. [Accessed January 15, 2018];A Guide to Calculating and Interpreting the Kidney Donor Profile Index (KDPI) https://optn.transplant.hrsa.gov/media/1512/guide_to_calculating_interpreting_kdpi.pdf.
- 3. Rao PS Schaubel DE Guidinger MK et al. A comprehensive risk quantification score for deceased donor kidneys: the kidney donor risk index Transplantation 2009. 88 231–236 [DOI] [PubMed] [Google Scholar]
- 4. Salvatierra J Singh T Shifrin R et al. Successful transplantation of adult-sized kidneys into infants requires maintenance of high aortic blood flow Transplantation 1998. 66 819–823 [DOI] [PubMed] [Google Scholar]
- 5. Nazarian SM Peng AW Duggirala B et al. The kidney allocation system does not appropriately stratify risk of pediatric donor kidneys: implications for pediatric recipients Am J Transplant 2018. 18 574–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Parker WF Thistlethwaite JR Ross LF Kidney donor profile index does not accurately predict the graft survival of pediatric deceased donor kidneys Transplantation 2016. 100 2471–2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Salvatierra O Concepcion W Sarwal MM Renal transplantation in children with thrombosis of the inferior vena cava requires careful assessment and planning Pediatr Nephrol 2008. 23 2107–2109 [DOI] [PubMed] [Google Scholar]
- 8. Formica RN A critical assessment on kidney allocation systems Transplant Rev 2017. 31 61–67 [DOI] [PubMed] [Google Scholar]
- 9. Sert I Yavascan Ö Tugmen C et al. A retrospective analysis of long-term graft survival in 61 pediatric renal transplant recipients: a single-center experience Ann Transplant 2013. 18 497–504 [DOI] [PubMed] [Google Scholar]
- 10. Cesca E Ghirardo G Kiblawi R Murer L Gamba P Zanon GF Delayed graft function in pediatric deceased donor kidney transplantation: donor-related risk factors and impact on two-yr graft function and survival: a single-center analysis Pediatr Transplant 2014. 18 357–362 [DOI] [PubMed] [Google Scholar]
- 11. Stewart DE Kucheryavaya AY Klassen DK Turgeon NA Formica RN Aeder MI Changes in deceased donor kidney transplantation one year after KAS implementation Am J Transplant 2016. 16 1834–1847 [DOI] [PubMed] [Google Scholar]
- 12. Opelz G Influence of recipient and donor age in pediatric renal transplantation collaborative transplant study Transpl Int 1988. 1 95–98 [DOI] [PubMed] [Google Scholar]
- 13. [Accessed June 29, 2017];Analysis shows kidney allocation system achieving key goals - OPTN. https://optn.transplant.hrsa.gov/news/analysis-shows-kidney-allocation-system-achieving-key-goals/
- 14. Gill J Dong J Rose C Gill JS The risk of allograft failure and the survival benefit of kidney transplantation are complicated by delayed graft function Kidney Int 2016. 89 1331–1336 [DOI] [PubMed] [Google Scholar]
- 15. Hwang AH Cho YW Cicciarelli J Mentser M Iwaki Y Hardy BE Risk factors for short- and long-term survival of primary cadaveric renal allografts in pediatric recipients: a UNOS analysis Transplantation 2005. 80 466–470 [DOI] [PubMed] [Google Scholar]
- 16. D’Agostino RB Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group Stat Med 1998. 17 2265–2281 [DOI] [PubMed] [Google Scholar]
- 17. Irish WD Ilsley JN Schnitzler MA Feng S Brennan DC A risk prediction model for delayed graft function in the current era of deceased donor renal transplantation Am J Transplant 2010. 10 2279–2286 [DOI] [PubMed] [Google Scholar]
- 18. Ounissi M Cherif M Abdallah TB et al. Risk factors and consequences of delayed graft function Saudi J Kidney Dis Transpl 2013. 24 243–246 [DOI] [PubMed] [Google Scholar]
- 19. Austin PC Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples Stat Med 2009. 28 3083–3107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. DiNardo J Fortin NM Lemieux T Labor market institutions and the distribution of wages, 1973-1992: a semiparametric approach Econometrica 1996. 64 1001–1044 [Google Scholar]
- 21. Oaxaca R Male-female wage differentials in urban labor markets Int Econ Rev 1973. 14 693–709 [Google Scholar]
- 22.OPTN Kidney Committee. [Accessed January 17, 2017];Policy 8: Allocation of Kidneys. OPTN website. https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf#nameddest=Policy_08.
- 23. Cuzick J A Wilcoxon-type test for trend Stat Med 1985. 4 87–90 [DOI] [PubMed] [Google Scholar]
- 24. Quiroga I McShane P Koo DDH et al. Major effects of delayed graft function and cold ischaemia time on renal allograft survival Nephrol Dial Transplant 2006. 21 1689–1696 [DOI] [PubMed] [Google Scholar]
- 25. Shroff R Ledermann S Long-term outcome of chronic dialysis in children Pediatr Nephrol 2009. 24 463–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bacchetta J Harambat J Cochat P Salusky IB Wesseling-Perry K The consequences of chronic kidney disease on bone metabolism and growth in children Nephrol Dial Transplant 2012. 27 3063–3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


